Oxidation in Poultry Feed: Impact on the Bird and the Efficacy of Dietary Antioxidant Mitigation Strategies
Abstract
1. Introduction
2. Oxidation in Poultry Feed
3. Oxidation of Dietary Fats
4. Effects of Oxidation on Meat Quality
5. Oxidation of Non-Lipid Feed Components
6. In Vivo Effects of Feed Oxidation
7. Causative Factors for Oxidative Stress in Broilers
7.1. Nutrition
7.2. Physiological/Pathological Causes
7.3. Environmental Causes
8. The Effects of Oxidative Stress
8.1. Performance
8.2. Gut Health
8.3. Inflammation
8.4. Other Effects
9. Mitigating Feed Oxidation
9.1. Synthetic Antioxidants
9.2. Natural Antioxidants
9.3. Polyphenols
10. Mitigating the Effects of Oxidative Stress in Poultry
10.1. Endogenous and Exogenous Antioxidants
10.2. Effects of Antioxidants on Performance
10.3. Effects of Antioxidants on Oxidative Status
10.4. Effects of Antioxidants on Stress Parameters
10.5. Effect of Antioxidants on Gut Health
10.6. Effect of Antioxidants on Meat Quality
| Author | Antioxidant | Effect |
|---|---|---|
| Sheeshy et al. (1993) [176] | alpha-tocopherol acetate 5, 25, 65, 180 mg/kg | 65 and 180 mg/kg significantly improved the stability of raw and cooked meat during frozen storage |
| Niu et al. (2017) [179] | alpha-tocopherol acetate 0, 100 and 200 mg/kg | Increased TAC in breast meat, linear increase with dose of mRNA expression of SOD and GSH-Px |
| Cui et al. (2018) [39] | Moringa oleifera 0, 1, 2, 5, 10 and 15% | Inclusion of antioxidant (Moringa oleifera) reduced MDA in breast meat and significantly increase Plasma T-AOC linearly in line with dose |
| Wen et al. (2019) [150] | Betaine 0, 1000 mg/kg under heat stress | Betaine significantly reduced MDA in meat and significantly increased SOD, glutathione and glutathione peroxidase in meat |
| Zhao et al. (2019) [41] | Eucommia ulmoides leaf 500 and 1000 mg/kg feed, under heat stress | Inclusion of 1000 mg Eucommia ulmoides leaf significantly reduced cooking and drip loss % and MDA in the meat. Increased saturated fatty acids in breast meat. |
| Shen et al. (2019) [153] | Bamboo leaf extract (0, 1, 2, 3, 4, 5 g/kg feed) | Drip loss linearly improved in line with dose, shear force significantly reduced with 2 and 3 g/kg of bamboo leaf extract. MDA linearly reduced in meat in line with dose |
| Turcu et al. (2020) [175] | Grape pomace 0, 3, 6% white grape and 0, 3, 6% red grape | Reduced TBARS in thigh meat with all grape pomace treatments. 3% white grape pomace significantly reduced TBARS in breast meat |
| Chang et al. (2020) [155] | Chitosan oligosaccharide 200 mg/kg under heat stress | Reduced MDA, cooking loss %, increased glutathione peroxidase and superoxide dismutase in meat |
| Lu et al. (2019) [183] | Taurine 5 g/kg under heat stress | Reduced MDA and drip loss % of meat. Reduced circulating levels of ROS |
| Leskovec et al. (2019) [181] | Alpha-tocopherols 200 IU/kg, ascorbic acid 250 mg/kg, selenium 0.2 mg/kg or combination | Alpha-tocopherols and alpha tocopherols combined with SE and ascorbic acid significantly increased a-tocoherol content in breast meat and significantly reduced MDA. No effect of ascorbic or Se alone. |
| Dev et al. (2020) [180] | Lactobacillius acidophius (LAB) 106 CFU/g feed −107 CFU/g feed and mannan-oligosaccharides (MOS) 0.1–0.2% | Water holding capacity and extract release volume significantly increased with 0.2% MOS and LAB 106 CFU and 0.2% MOS and 107 CFU LAB. |
| Adeyemi et al. (2021) [184] | Morinda lucida leaf powder 0.1, 0.1%, BHA 0.02% BHA | Reduced TBARS and drip loss with all antioxidant addition. No significant difference between BHA and Morinda lucida |
| Lu et al. (2007) [185] | Manganese 0, 100, 200 mg/kg | Mn significantly upregulated superoxide dismutase. Reduced MDA in leg muscle |
| Cai et al. (2012) [182] | Nano-selenium (0, 0.3, 0.5, 1.0, 2.0 mg/kg) | Significantly increased glutathione peroxidase activity in muscle (0.3 mg/kg), significantly improved drip loss % in line with increased dose, and significant increase in T-AOC compared to untreated control. |
11. The Future of Antioxidant Supplementation and Prevention of In Vivo Oxidative Tress
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1996, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Dugan, L.L.; Choi, D.W. Excitotoxicity, free radicals, and cell membrane changes. Ann. Neurol. 1994, 35, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Wasowicz, E.; Gramza, A.; Hes, M.; Jelen, H.; Korczak, J.; Malecka, M.; Mildner-Szkudlarz, S.; Rudzinska, M.; Samotyja, U.; Zawirska-Wojtasiak, R. Oxidation of lipids in food. Pol. J. Food Nutr. Sci. 2004, 13, 87–100. [Google Scholar]
- Luna, C.; Estevez, M. Oxidative damage to food and human serum proteins: Radical-mediated oxidation vs. glycol-oxidation. Food Chem. 2018, 267, 111–118. [Google Scholar] [CrossRef]
- Skibsted, L.H. Understanding oxidation processes in foods. In Oxidation in Foods and Beverages and Antioxidant Applications; Woodhead Publishing: Cambridge, UK, 2010; pp. 3–35. [Google Scholar]
- Fellenburg, M.; Speisky, H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. Worlds Poult. Sci. J. 2006, 62, 53–70. [Google Scholar] [CrossRef]
- Mozuraityte, R.; Kristinova, V.; Rustad, T. Oxidation of Food Components. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 186–192. [Google Scholar]
- Jędrejek, D.; Levic, J.; Wallace, J.; Oleszek, W. Animal by-products for feed: Characteristics, European regulatory framework, and potential impacts on human and animal health and the environment. J. Anim. Feed Sci. 2016, 25, 189–202. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Bierinckx, K.; di Benedetto, M. Fats and oils in pig nutrition: Factors affecting digestion and utilization. Anim. Feed Sci. Technol. 2021, 277, 114950. [Google Scholar] [CrossRef]
- Halbaut, L.; Barbé, C.; Aróztegui, M.; de la Torre, C. Oxidative stability of semi-solid excipient mixtures with corn oil and its implication in the degradation of vitamin A. Int. J. Pharm. 1997, 147, 31–40. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Tavárez, M.; Boler, D.; Bess, K.; Zhao, J.; Yan, F.; Dilger, A.; McKeith, F.; Killefer, J. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 2011, 90, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.L.; Baptista, M.S. Mechanisms of Photosensitized Lipid Oxidation and Membrane Permeabilization. ACS Omega 2019, 4, 21636–21646. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.; Kaupp, M. The highest oxidation states of the transition metal elements. Coord. Chem. Rev. 2009, 253, 606–624. [Google Scholar] [CrossRef]
- Obando, M.; Papastergiadis, A.; Li, S.; De Meulenaer, B. Impact of Lipid and Protein Co-oxidation on Digestibility of Dairy Proteins in Oil-in-Water (O/W) Emulsions. J. Agric. Food Chem. 2015, 63, 9820–9830. [Google Scholar] [CrossRef]
- Dow, C. How Much Saturated Fat Is in Oils and Animal Fats-Nutrition Action | Center for Science in the Public Interest. 2017. Available online: https://www.cspinet.org/ (accessed on 20 July 2021).
- Lin, C.F.; Asghar, A.; Gray, J.I.; Buckley, D.J.; Booren, A.M.; Crackel, R.L.; Flegal, C.J. Effects of oxidised dietary oil and antioxidant supplementation on broiler growth and meat stability. Br. Poult. Sci. 1989, 30, 855–864. [Google Scholar] [CrossRef]
- Wiseman, J.; Edmunds, B.; Shepperson, N. The apparent metabolisable energy of sunflower oil and sunflower acid oil for broiler chickens. Anim. Feed Sci. Technol. 1992, 36, 41–51. [Google Scholar] [CrossRef]
- Kimura, T.; Iida, K.; Takei, Y. Mechanisms of adverse effect of air-oxidized soy bean oil-feeding in rats. J. Nutr. Sci. Vitaminol. 1984, 30, 125–133. [Google Scholar] [CrossRef]
- Engberg, R.M.; Borsting, C.F. Inclusion of oxidized fish oil in mink diets. 2. The influence on performance and health considering histopathological, clinical-chemical, and haematological indices. J. Anim. Physiol. Anim. Nutr. 1994, 72, 146–157. [Google Scholar] [CrossRef]
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Crespo, N.; Esteve-Garcia, E. Nutrient and fatty acid deposition in broilers fed different dietary fatty acid profiles. Poult. Sci. 2002, 81, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Cortinas, L.; Villaverde, C.; Galobart, J.; Baucells, M.D.; Codony, R.; Barroeta, A.C. Fatty Acid Content in Chicken Thigh and Breast as Affected by Dietary Polyunsaturation Level. Poult. Sci. 2004, 83, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrea, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity. 2017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5551541/#B26 (accessed on 5 August 2022).
- Hubert, S.M. Energy metabolism and sources of oxidative stress in wooden breast—A review. F1000Research 2020, 9, 319. [Google Scholar] [CrossRef]
- Cai, K.; Shao, W.; Chen, X.; Campbell, Y.L.; Nair, M.N.; Suman, S.P.; Beach, C.M.; Guyton, M.C.; Schilling, M.W. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult. Sci. 2018, 97, 337–346. [Google Scholar] [CrossRef]
- Owens, C.M. Woody Breast Meat. In Proceedings of the 69th Reciprocal Meat Conference Proceedings, San Angelo, TX, USA, 18–23 June 2016. [Google Scholar]
- Sihvo, H.-K.; Immonen, K.; Puolanne, E. Myodegeneration with Fibrosis and Regeneration in the Pectoralis Major Muscle of Broilers. Vet. Pathol. 2013, 51, 619–623. [Google Scholar] [CrossRef]
- Lilburn, M.; Griffin, J.; Wick, M. From muscle to food: Oxidative challenges and developmental anomalies in poultry breast muscle. Poult. Sci. 2019, 98, 4255–4260. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Lee, E.J.; Ahn, D.U. Consumption of Oxidized Oil Increases Oxidative Stress in Broilers and Affects the Quality of Breast Meat. J. Agric. Food Chem. 2010, 59, 969–974. [Google Scholar] [CrossRef]
- Griffin, J.R.; Moraes, L.; Wick, M.; Lilburn, M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying Pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Gao, J.; Lin, H.; Wang, X.J.; Song, Z.G.; Jiao, H.C. Vitamin E supplementation alleviates the oxidative stress induced by dexamethasone treatment and improves meat quality in broiler chickens. Poult. Sci. 2010, 89, 318–327. [Google Scholar] [CrossRef]
- Voljč, M.; Levart, A.; Žgur, S.; Salobir, J. The effect of α-tocopherol, sweet chestnut wood extract and their combination on oxidative stress in vivo and the oxidative stability of meat in broilers. Br. Poult. Sci. 2013, 54, 144–156. [Google Scholar] [CrossRef]
- Pozzo, L.; Cavallarin, L.; Antoniazzi, S.; Guerre, P.; Biasibetti, E.; Capucchio, M.T.; Schiavone, A. Feeding a diet contaminated with ochratoxin A for broiler chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 2. Effects on meat quality, oxidative stress, residues and histological traits. J. Anim. Physiol. Anim. Nutr. 2013, 97, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yin, B.; Xu, J.; Bao, E. Rosemary Reduces Heat Stress by Inducing CRYAB and HSP70 Expression in Broiler Chickens. Oxidative Med. Cell. Longev. 2018, 2018, 7014126. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-M.; Wang, J.; Lu, W.; Zhang, H.-J.; Wu, S.-G.; Qi, G.-H. Effect of dietary supplementation with Moringa oleifera leaf on performance, meat quality, and oxidative stability of meat in broilers. Poult. Sci. 2018, 97, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.; Alkhedaide, A.Q.; Ramadan, A.A.; Hafez, A.-E.S.E.; Hussein, M.A. Potential impact of stocking density on growth, carcass traits, indicators of biochemical and oxidative stress and meat quality of different broiler breeds. Poult. Sci. 2021, 100, 101442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, W.; Liu, H. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019, 98, 3040–3049. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ahn, D.U. Lipid and Protein Oxidation of Chicken Breast Rolls as Affected by Dietary Oxidation Levels and Packaging. J. Food Sci. 2011, 76, C612–C617. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ma, C.W.; Ahn, D.U. Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poult. Sci. 2011, 90, 1348–1357. [Google Scholar] [CrossRef]
- Mutryn, M.F.; Brannick, E.M.; Fu, W.; Lee, W.R.; Abasht, B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genom. 2015, 16, 399. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Brøndum, J.; Byrne, D.V.; Bak, L.S.; Bertelsen, G.; Engelsen, S.B. Warmed-over flavour in porcine meat-a combined spectroscopic, sensory and chemometric study. Meat Sci. 2000, 54, 83–95. [Google Scholar] [CrossRef]
- Min, B.; Corray, J.; Ahn, D.U. Endogenous factors affecting oxidative stability of beef loin, pork loin and chicken and thigh meats. J. Food. Sci. 2008, 73, C439–C446. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2021, 10, 40. [Google Scholar] [CrossRef]
- Elkassabany, M.; Hoseney, R.C. Ascorbic acid in wheat flour dough. II rheological effects. Cereal Chem. 1980, 57, 88–91. [Google Scholar]
- Cumbee, B.; Hildebrand, D.F. Soybean flour lipoxygenase isozymes effects on wheat flour rheological and breadmaking properties. J. Food Sci. 1997, 62, 281–283. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Quan, M. Effects on Protein oxidation on gelatinization, characteristics, during rice storage. J. Cereal Sci. 1997, 75, 228–233. [Google Scholar] [CrossRef]
- Heinonen, M.; Gürbüz, G.; Ertbjerg, P. Oxidation of Proteins. In Chemical Changes during Processing and Storage of Foods; Academic Press: Cambridge, MA, USA, 2021; pp. 85–123. [Google Scholar]
- Saripinar-Aksu, D.; Aksu, T.; Onel, S.E. Does inclusion at low levels of organically complexed minerals versus inorganic forms create weakness in performance or antioxidant defence in broiler diets. Int. J. Poult. Sci. 2012, 11, 666–672. [Google Scholar] [CrossRef]
- Salami, S.; Majoka, M.A.; Saha, S.; Garber, A.; Gabarrou, J.-F. Efficacy of Dietary Antioxidants on Broiler Oxidative Stress, Performance and Meat Quality: Science and Market. Avian Biol. Res. 2015, 8, 65–78. [Google Scholar] [CrossRef]
- Dierick, N.; Decuypere, J. Endogenous lipolysis in feedstuffs and compound feeds for pigs: Effects of storage time and conditions and lipase and/or emulsifier addition. Anim. Feed Sci. Technol. 2002, 102, 53–70. [Google Scholar] [CrossRef]
- Cadogan, D.J.; Choct, M.; Campbell, R.G. Effects of storage time and exogenous xylanase supplementation of new season wheats on the performance of young male pigs. Can. J. Anim. Sci. 2003, 83, 105–112. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidants in Poultry Nutrition and Reproduction: An Update. Antioxidants 2020, 9, 105. [Google Scholar] [CrossRef]
- Ryszawa, N.; Kawczyñska-Dród, A.; Pryjma, J.; Czesnikiewicz-Guzik, M.; Adamek-Guzik, T.; Naruszewicz, M.; Korbut, R.; Guzik, T.J. Effects of novel plant antioxidants. J. Physiol. Pharmacol. 2006, 57, 611–626. [Google Scholar] [PubMed]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; McPhee, D.J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and miRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Gagne, F. Oxidative Stress, Biochemical Ecotoxicity, Principals and Methods; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Nishida, N.; Arizumi, T.; Takita, M.; Kitai, S.; Yada, N.; Hagiwara, S.; Inoue, T.; Minami, Y.; Ueshima, K.; Sakurai, T.; et al. Reactive Oxygen Species Induce Epigenetic Instability through the Formation of 8-Hydroxydeoxyguanosine in Human Hepatocarcinogenesis. Dig. Dis. 2013, 31, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Manilla, H.A.; Husvéth, F. N-3 fatty acid enrichment and oxidative stability of broiler chicken (A review). Acta Aliment. 1999, 28, 235–249. [Google Scholar] [CrossRef]
- Surai, P.F. Natural antioxidants in poultry nutrition: New developments. In Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, France, 26–30 August 2007; pp. 669–675. [Google Scholar]
- Engberg, R.M.; Lauridsen, C.; Jensen, S.K.; Jakobsen, K. Inclusion of Oxidized Vegetable Oil in Broiler Diets. Its Influence on Nutrient Balance and on the Antioxidative Status of Broilers. Poult. Sci. 1996, 75, 1003–1011. [Google Scholar] [CrossRef]
- Dibner, J.; Atwell, C.; Kitchell, M.; Shermer, W.; Ivey, F. Feeding of oxidized fats to broilers and swine: Effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Anim. Feed Sci. Technol. 1996, 62, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Wang, Q.-H.; Zhang, J.-L.; Li, S.; Wang, X.-L.; Xu, S.-W. Effects of Oxidative Stress on Immunosuppression Induced by Selenium Deficiency in Chickens. Biol. Trace Elem. Res. 2012, 149, 352–361. [Google Scholar] [CrossRef]
- Juniper, D.; Bertin, G. Effects of dietary selenium supplementation on tissue selenium distribution and glutathione peroxidase activity in Chinese Ring Necked Pheasants. Animal 2013, 7, 562–570. [Google Scholar] [CrossRef][Green Version]
- Gou, Z.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Jiang, Z.Y.; Zheng, C.; Ding, F.Y.; Jiang, S.Q. Effects of oxidative stress induced by high dosage of dietary iron ingested on intestinal damage and caecal microbiota in Chinese Yellow broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Cui, H.; Peng, X.; Fang, J.; Wang, K.; Cui, W.; Liu, X. Dietary Vanadium Induces Oxidative Stress in the Intestine of Broilers. Biol. Trace Elem. Res. 2011, 145, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, Z.; Erdogan, S.; Celik, S.; Unlu, A. Effects of Ascorbic Acid on Cadmium-Induced Oxidative Stress and Performance of Broilers. Biol. Trace Elem. Res. 2005, 104, 19–32. [Google Scholar] [CrossRef]
- Huang, J.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wu, B. The Association between Splenocyte Apoptosis and Alterations of Bax, Bcl-2 and Caspase-3 mRNA Expression, and Oxidative Stress Induced by Dietary Nickel Chloride in Broilers. Int. J. Environ. Res. Public Health 2013, 10, 7310–7326. [Google Scholar] [CrossRef]
- Cinar, M.; Yildirim, E.; Yigit, A.; Yalcinkaya, I.; Duru, O.; Kisa, U.; Atmaca, N. Effects of Dietary Supplementation with Vitamin C and Vitamin E and Their Combination on Growth Performance, Some Biochemical Parameters, and Oxidative Stress Induced by Copper Toxicity in Broilers. Biol. Trace Elem. Res. 2014, 158, 186–196. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Zhao, Y.-Y.; Xiong, B.; Cui, X.-S.; Kim, N.-H.; Xu, Y.-X.; Sun, S.-C. Mycotoxin-Containing Diet Causes Oxidative Stress in the Mouse. PLoS ONE 2013, 8, e60374. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.O.; Loureiro-Bracarense, A.-P.; Oswald, I.P. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.J. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves in Nrf2 pathway in primary broiler hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Aly, S.E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J. Agric. Food Chem. 2003, 51, 2409–2414. [Google Scholar] [CrossRef]
- Allen, P.C.; Fetterer, R.H. Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry. Clin. Microbiol. Rev. 2002, 15, 58–65. [Google Scholar] [CrossRef]
- Wang, L.; Piao, X.; Kim, S.; Shen, Y.; Lee, H. Effects of Forsythia suspensa Extract on Growth Performance, Nutrient Digestibility, and Antioxidant Activities in Broiler Chickens Under High Ambient Temperature. Poult. Sci. 2008, 87, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Bottje, W.; Enkvetchakul, B.; Moore, R.; Mcnew, R. Effect of α-Tocopherol on Antioxidants, Lipid Peroxidation, and the Incidence of Pulmonary Hypertension Syndrome (Ascites) in Broilers. Poult. Sci. 1995, 74, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Benzer, F.; Yilmaz, S. Effects on oxidative stress and antioxidant enzyme activities of experimentally induced Ornithobacterium rhinotracheale infection in broilers. J. Anim. Vet. Adv. 2009, 8, 548–553. [Google Scholar]
- Ohtsuka, A.; Ohtani, T.; Horiguchi, H.; Kojima, H.; Hayashi, K. Vitamin E Reduces Glucocorticoid-Induced Growth Inhibition and Lipid Peroxidation in Rats. J. Nutr. Sci. Vitaminol. 1998, 44, 237–247. [Google Scholar] [CrossRef]
- Eid, Y.; Ohtsuka, A.; Hayashi, K. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br. Poult. Sci. 2003, 44, 127–132. [Google Scholar] [CrossRef]
- Dillard, C.J.; Litov, R.E.; Savin, W.M. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. 1978, 45, 927–932. [Google Scholar] [CrossRef]
- Vollaard, N.B.; Shearman, J.P.; Cooper, C.E. Exercise induced oxidative stress. Sport. Med. 2012, 35, 1045–1062. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Vet. Sci. 2019, 6, 60. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6409315/#B19 (accessed on 5 August 2022). [CrossRef]
- Altan, Ö.; Pabuçcuoğlu, A.; Altan, A.; Konyalioğlu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; DeGroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Tang, X.; Lu, Q.; Sa, R.; Zhang, H. Proteome changes in the small intestinal mucosa of broilers (Gallus gallus) induced by high concentrations of atmospheric ammonia. Proteome Sci. 2015, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, R.; Su, Y.; Bi, Y.; Li, X.; Zhang, X.; Li, J.; Bao, J. Effects of Acute Cold Stress After Long-Term Cold Stimulation on Antioxidant Status, Heat Shock Proteins, Inflammation and Immune Cytokines in Broiler Heart. Front. Physiol. 2018, 9, 1589. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, S.S.; Hassanin, K.M.; Youssef, I.M. The effect of dietary supplementation of some antioxidants on performance, oxidative stress and blood parameters in broilers under natural summer conditions. J. World’s Poult. Res. 2014, 4, 10–19. [Google Scholar]
- Li, W.; Wei, F.; Xu, B.; Sun, Q.; Deng, W.; Ma, H.; Bai, J.; Li, S. Effect of stocking density and alpha-lipoic acid on the growth performance, physiological and oxidative stress and immune response of broilers. Asian-Australas. J. Anim. Sci. 2019, 32, 1914–1922. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Jiang, S.; Mo, Y.; Zhou, G.; Yang, L. Consumption of Oxidized Soybean Oil Increased Intestinal Oxidative Stress and Affected Intestinal Immune Variables in Yellow-feathered Broilers. Asian-Australas. J. Anim. Sci. 2015, 28, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chi, Q.; Hu, X.; Cong, Y.; Li, S. Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia. Ecotoxicol. Environ. Saf. 2019, 183, 109578. [Google Scholar] [CrossRef]
- Ahmad, M.; Chand, N.; Khan, R.U.; Ahmad, N.; Khattak, I.; Naz, S. Dietary supplementation of milk thistle (Silybum marianum): Growth performance, oxidative stress, and immune response in natural summer stressed broilers. Trop. Anim. Health Prod. 2020, 52, 711–715. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, S.; Zhou, M. Effect of the flavonoid baicalein as a feed additive on growth performance, immunity and antioxidant capacity of broiler chickens. Poult. Sci. 2019, 98, 2790–2799. [Google Scholar] [CrossRef]
- Tan, L.; Rong, D.; Yang, Y.; Zhang, B. Effect of Oxidized Soybean Oils on Oxidative Status and Intestinal Barrier Function in Broiler Chickens. Braz. J. Poult. Sci. 2018, 20, 333–342. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, J.; Zhang, B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020, 99, 4892–4903. [Google Scholar] [CrossRef]
- Min, Y.N.; Yang, H.L.; Xu, Y.X.; Gao, Y.P. Effects of dietary supplementation of symbiotic on growth performance, intestinal morphology, sIgA content and antioxidant capacities of broilers. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol Impairs Hepatic and Intestinal Gene Expression of Selected Oxidative Stress, Tight Junction and Inflammation Proteins in Broiler Chickens, but Addition of an Adsorbing Agent Shifts the Effects to the Distal Parts of the Small Intestine. PLoS ONE 2013, 8, e69014. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A., Jr.; Bracarense, A.P.F. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon 2020, 185, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Panaite, T.D.; Saracila, M.; Papuc, C.P.; Predescu, C.N.; Soica, C. Influence of Dietary Supplementation of Salix alba Bark on Performance, Oxidative Stress Parameters in Liver and Gut Microflora of Broilers. Animals 2020, 10, 958. [Google Scholar] [CrossRef]
- Sun, Y.; Ni, A.; Jiang, Y.; Li, Y.; Huang, Z.; Shi, L.; Xu, H.; Chen, C.; Li, D.; Han, Y.; et al. Effects of Replacing In-feed Antibiotics with Synergistic Organic Acids on Growth Performance, Health, Carcass, and Immune and Oxidative Statuses of Broiler Chickens Under Clostridium perfringens Type A Challenge. Avian Dis. 2020, 64, 393–400. [Google Scholar] [CrossRef]
- Hoover, J. Mechanistic Understanding of Leaky Gut Syndrome in Heat Stressed Broiler Chickens. Master’s Thesis, University of Arkansas, Fayetteville, Arkansas, 2020. [Google Scholar]
- Naidoo, V.; McGaw, L.; Bisschop, S.; Duncan, N.; Eloff, J. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008, 153, 214–219. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Zhang, H.-J.; Wu, S.-G.; Qiu, K.; Fu, Y.; Qi, G.-H.; Wang, J. Supplemental Xylooligosaccharide Modulates Intestinal Mucosal Barrier and Cecal Microbiota in Laying Hens Fed Oxidized Fish Oil. Front. Microbiol. 2021, 12, 635333. [Google Scholar] [CrossRef]
- Kountouras, J.; Boziki, M.; Polyzos, S.A.; Katsinelos, P.; Gavalas, E.; Zeglinas, C.; Tzivras, D.; Romiopoulos, I.; Giorgakis, N.; Anastasiadou, K.; et al. Impact of reactive oxygen species generation on Helicobacter pylori-related extragastric diseases: A hypothesis. Free Radic. Res. 2017, 51, 73–79. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Tabler, T.W.; Greene, E.S.; Orlowski, S.K.; Hiltz, J.Z.; Anthony, N.B.; Dridi, S. Intestinal Barrier Integrity in Heat-Stressed Modern Broilers and Their Ancestor Wild Jungle Fowl. Front. Vet. Sci. 2020, 7, 249. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Gehring, J.; Trepka, B.; Klinkenberg, N.; Bronner, H.; Schleheck, D.; Polarz, S. Sunlight-Triggered Nanoparticle Synergy: Teamwork of Reactive Oxygen Species and Nitric Oxide Released from Mesoporous Organosilica with Advanced Antibacterial Activity. J. Am. Chem. Soc. 2016, 138, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-R.; Li, X.; Awati, A.; Bento, H.; Zhang, H.; Bontempo, V. Effect of an essential oils blend on growth performance, and selected parameters of oxidative stress and antioxidant defence of Escherichia coli challenged piglets. J. Anim. Feed Sci. 2017, 26, 38–43. [Google Scholar] [CrossRef]
- Frankič, T.; Pajk, T.; Rezar, V.; Levart, A.; Salobir, J. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem. Toxicol. 2006, 44, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Dadak, A.; Hess, M.; Böhm, J. Single and Combined Effects of Deoxynivalenol Mycotoxin and a Microbial Feed Additive on Lymphocyte DNA Damage and Oxidative Stress in Broiler Chickens. PLoS ONE 2014, 9, e88028. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Dadak, A.; Gille, L.; Staniek, K.; Hess, M.; Böhm, J. Genotoxic effects of deoxynivalenol in broiler chickens fed low-protein feeds. Poult. Sci. 2012, 91, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Haider, M.S.; Nazar, M.; Mansoor, M.K.; Zhang, H.; Tang, Z.; Li, Y. Potential molecular mechanism of ascites syndrome in broilers. World’s Poult. Sci. J. 2022, 78, 689–704. [Google Scholar] [CrossRef]
- Cawthon, D.; Beers, K.; Bottje, W.G. Electron Transport Chain Defect and Inefficient Respiration May Underlie Pulmonary Hypertension Syndrome (Ascites)-Associated Mitochondrial Dysfunction in Broilers. Poult. Sci. 2001, 80, 474–484. [Google Scholar] [CrossRef]
- Bottje, W.G.; Wideman, R.F. Potential role of free radicals in the etiology of pulmonary hypertension syndrome. Poult. Avian. Biol. Rev. 1995, 6, 211–231. [Google Scholar]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Hamid, S.B.A.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Register of Feed Additives (Online) Category (europa.eu). 2003. Available online: https://food.ec.europa.eu/safety/animal-feed/feed-additives/eu-register_en (accessed on 25 August 2022).
- German, J.B.; Traber, M.G. Nutrients and Oxidation: Actions, Transport, and Metabolism of Dietary Antioxidants. In Handbook of Vitamins, 3rd ed.; Rucker, R.B., Suttie, J.W., McCormick, D.B., Machlin, L.J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 569–588. [Google Scholar]
- Draper, H.H. Nutritional modulation of oxygen radical pathology. Adv. Nutr. Res. 1990, 8, 119–145. [Google Scholar] [PubMed]
- Wu, L.; Yin, W.; Tang, K.; Li, D.; Shao, K.; Zuo, Y.; Ma, J.; Liu, J.; Han, H. Enzymatic biosensor of horseradish peroxidase immobilized on Au-Pt nanotube/Au-graphene for the simultaneous determination of antioxidants. Anal. Chim. Acta 2016, 933, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Liu, L.; Li, Z.; Yang, Q.; Zhu, W.; Zhang, W.; Wang, J. Highly specific and sensitive determination of propyl gallate in food by a novel fluorescence sensor. Food Chem. 2018, 256, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pashtetsky, V.; Ostapchuk, P.; Il’yasov, R.; Zubochenko, D.; Kuevda, T. Use of Antioxidants in Poultry Farming. Conference Proceedings on Innovations in Agricultural and Rural Development 2019. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/341/1/012042/pdf (accessed on 7 August 2022).
- Halpner, A.D.; Handelman, G.J.; Belmont, C.A.; Harris, J.M.; Blumberg, J.B. Protection by vitamin C of oxidant-induced loss of vitamin E in rat hepatocytes. J. Nutr. Biochem. 1998, 9, 355–359. [Google Scholar] [CrossRef]
- Panda, A.K.; Cherian, G. Role of Vitamin E in Counteracting Oxidative Stress in Poultry. J. Poult. Sci. 2014, 51, 0130134. [Google Scholar] [CrossRef]
- Taulescu, C.; Mihaiu, M.; Bele, C.; Matea, C.; Dan, S.D.; Mihaiu, R.; Lapusan, A. Antioxidant Effect of Vitamin E and Selenium on Omega-3 Enriched Poultry Meat. Bulletin of the University of Agricultural Sciences & Veterinary Medicine Cluj-Napoca. Vet. Med. 2011, 68, 293–299. [Google Scholar]
- Pompeu, M.A.; Cavalcanti, L.; Toral, F. Effect of vitamin E supplementation on growth performance, meat quality, and immune response of male broiler chickens: A meta-analysis. Livest. Sci. 2018, 208, 5–13. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Eder, K.; Grünthal, G.; Kluge, H.; Hirche, F.; Spilke, J.; Brandsch, C. Concentrations of cholesterol oxidation products in raw, heat-processed and frozen-stored meat of broiler chickens fed diets differing in the type of fat and vitamin E concentrations. Brit. J. Nutr. 2005, 93, 633–643. [Google Scholar] [CrossRef]
- Surai, P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Brit. Poult. Sci. 2000, 41, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Urso, U.R.A.; Dahlke, F.; Maiorka, A.; Bueno, I.J.M.; Schneider, A.F.; Surek, D.; Rocha, C. Vitamin E and selenium in broiler breeder diets: Effect on live performance, hatching process, and chick quality. Poult. Sci. 2015, 94, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Rahman, Z.; Javed, I.; Muhammad, F. Supplementation of vitamins, probiotics and proteins on oxidative stress, enzymes and hormones in post-moult male broiler breeders. Arch. Anim. Breed. 2013, 56, 607–616. [Google Scholar] [CrossRef]
- Khan, R.; Rahman, Z.-U.; Javed, I.; Muhammad, F. Effect of vitamins, probiotics and protein on semen traits in post-molt male broiler breeders. Anim. Reprod. Sci. 2012, 135, 85–90. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Javan, A.J.; Nikmanesh, A.; Keykhosrravy, K.; Maftoon, S.; Amin Zare, M.; Bayani, M.; Parsiemehr, M.; Raeisi, M. Effect of Citric Acid Dipping Treatment on Bioactive Components and Antioxidant Properties of Slices Button Mushrooms (Agaricus bisporus). J. Food Qual. Hazard. Control 2015, 2, 20–25. [Google Scholar]
- Di Palma, L.; Mecozzi, R. Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater. 2007, 147, 768–775. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Del Campo, J.; Amiot, M.J.; Nguyen-The, C. Antimicrobial Effect of Rosemary Extracts. J. Food Prot. 2000, 63, 1359–1368. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2005, 40, 223–231. [Google Scholar] [CrossRef]
- Ahmad, A.; Singhal, U.; Hossain, M.M.; Islam, N.; Rizvi, I. The role of the endogenous antioxidant enzymes and malondialdehyde in essential hypertension. J. Clin. Diagn. Res. JCDR 2013, 7, 987. [Google Scholar] [PubMed]
- De Grande, A.; Leleu, S.; Delezie, E.; Rapp, C.; De Smet, S.; Goossens, E.; Haesebrouck, F.; Van Immerseel, F.; Ducatelle, R. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult. Sci. 2020, 99, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.; Altan, Ö.; Acikgoz, Z.; Baysal, Ş.H.; Şeremet, Ç. Effects of oxidised oil and vitamin E on performance and some blood traits of heat-stressed male broilers. S. Afr. J Anim. Sci. 2011, 41, 288–296. [Google Scholar] [CrossRef][Green Version]
- Hossini-Vashan, S.J.; Golian, A.; Yaghobfar, A. Growth, immune, antioxidant, and bone responses of heat stressed broilers fed diets supplemented with tomato pomace. Int. J Biometeorol. 2016, 60, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Liu, Y.; Ye, Y.; Tao, Z.; Cheng, Z.; Wang, T.; Zhou, Y. Effects of gingerols-rich extract of ginger on growth performance, serum metabolites, meat quality and antioxidant activity of heat-stressed broilers. J. Therm. Biol. 2020, 89, 102544. [Google Scholar] [CrossRef]
- Rajani, J.; Torshizi, M.K.; Rahimi, S. Control of ascites mortality and improved performance and meat shelf-life in broilers using feed adjuncts with presumed antioxidant activity. Anim. Feed Sci. Technol. 2011, 170, 239–245. [Google Scholar] [CrossRef]
- Alian, H.A.; Samy, H.M.; Ibrahim, M.T.; Mahmoud, M. Nanoselenium effect on growth performance, carcass traits, antioxidant activity, and immune status of broilers. Environ. Sci. Pollut. Res. 2020, 27, 38607–38616. [Google Scholar] [CrossRef]
- Boostani, A.; Sadeghi, A.A.; Mousavi, S.N.; Chamani, M.; Kashan, N. The effects of organic, inorganic, and nano-selenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Sci. Vet. 2015, 43, 1–6. [Google Scholar]
- Shen, M.; Zhang, L.; Chen, Y.; Zhang, Y.; Han, H.; Niu, Y.; He, J.; Cheng, Y.; Wang, T. Effects of bamboo leaf extract on growth performance, meat quality, and meat oxidative stability in broiler chickens. Poult. Sci. 2019, 98, 6787–6796. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, M.; Xu, Q.; Chen, Y.; Wen, C.; Zhou, Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018, 75, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Lu, Y.; Lan, R. Chitosan oligosaccharide as an effective feed additive to maintain growth performance, meat quality, muscle glycolytic metabolism, and oxidative status in yellow-feather broilers under heat stress. Poult. Sci. 2020, 99, 4824–4831. [Google Scholar] [CrossRef] [PubMed]
- Hussan, F.; Krishna, D.; Preetam, V.C.; Reddy, P.B.; Gurram, S. Dietary supplementation of nano zinc oxide on performance, carcass, serum and meat quality parameters of commercial broilers. Biol. Trace Elem. Res. 2022, 200, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Chen, Y.; Leng, Z.; Ding, L.; Wang, T.; Zhou, Y. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J. Sci. Food Agric. 2018, 99, 620–623. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.H.; Zheng, X.C.; Zhang, H.; Zhang, J.F.; Zhang, L.L.; Zhou, Y.M.; Wang, T. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2017, 96, 1159–1166. [Google Scholar] [CrossRef]
- Karadas, F.; Erdoğan, S.; Kor, D.; Oto, G.; Uluman, M. The Effects of Different Types of Antioxidants (Se, Vitamin E and Carotenoids) in Broiler Diets on the Growth Performance, Skin Pigmentation and Liver and Plasma Antioxidant Concentrations. Rev. Bras. Cienc. Avic. 2016, 18, 101–116. [Google Scholar] [CrossRef]
- Akhavast, A.R.; Daneshyar, M. Effects of Rosemary (Rosmarinus Officinalis) Extract on Performance, Antioxidant Ability and Blood Gas Indices of Broiler Chickens Treated with Sodium Nitrite in Drinking Water. Iran. J Appl. Anim Sci. 2017, 7, 471–477. [Google Scholar]
- Yildirim, B.A.; Tunc, M.A.; Gül, M.; Yildirim, F.; Yıldız, A. The effect of Rosemary (Rosmarinus officinalis L.) extract supplemented into broiler diets, on performance and blood parameters. GSC Biol. Pharm. Sci. 2018, 2, 1–9. [Google Scholar]
- Liu, H.S.; Mahfuz, S.U.; Wu, D.; Shang, Q.H.; Piao, X.S. Effect of chestnut wood extract on performance, meat quality, antioxidant status, immune function and cholesterol metabolism in broilers. Poult. Sci. 2020, 99, 4488–4495. [Google Scholar] [CrossRef]
- Ouyang, K.; Xu, M.; Jiang, Y.; Wang, W. Effects of alfalfa flavonoids on broiler performance, meat quality, and gene expression. Can. J. Anim. Sci. 2016, 96, 332–341. [Google Scholar] [CrossRef]
- Schiavone, A.; Guo, K.; Tassone, S.; Gasco, L.; Hernandez, E.; Denti, R.; Zoccarato, I. Effects of a Natural Extract of Chestnut Wood on Digestibility, Performance Traits, and Nitrogen Balance of Broiler Chicks. Poult. Sci. 2008, 87, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Biagia, G.; Cipollini, I.; Paulicks, B.R.; Roth, F.X. Effect of tannins on growth performance and intestinal ecosystem in weaned piglets. Arch. Anim. Nutr. 2010, 64, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, J.; Mahfuz, S.; Piao, X. Effects of Hydrolysable Tannins as Zinc Oxide Substitutes on Antioxidant Status, Immune Function, Intestinal Morphology, and Digestive Enzyme Activities in Weaned Piglets. Animals 2020, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Starčević, K.; Krstulović, L.; Brozić, D.; Maurić, M.; Stojević, Z.; Mikulec, Ž.; Bajić, M.; Mašek, T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Hayirli, A.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Komorowski, J.R.; Sahin, K. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult. Sci. 2017, 96, 4317–4324. [Google Scholar] [CrossRef]
- Pan, L.; Ma, X.; Zhao, P.; Shang, Q.; Long, S.; Wu, Y.; Piao, X. Forsythia suspensa extract attenuates breast muscle oxidative injury induced by transport stress in broilers. Poult. Sci. 2018, 97, 1554–1563. [Google Scholar] [CrossRef]
- Sarsour, A.; Persia, M. Effects of sulfur amino acid supplementation on broiler chickens exposed to acute and chronic cyclic heat stress. Poult. Sci. 2022, 101, 101952. [Google Scholar] [CrossRef]
- Chen, S.; Xue, Y.; Shen, Y.; Ju, H.; Zhang, X.; Liu, J.; Wang, Y. Effects of different selenium sources on duodenum and jejunum tight junction network and growth performance of broilers in a model of fluorine-induced chronic oxidative stress. Poult. Sci. 2021, 101, 101664. [Google Scholar] [CrossRef]
- Burin Junior, A.M. Intestinal Integrity, Oxidative Stress, and Immune Competence of Broilers Exposed to Heat Stress and Supplemented with Zn Amino Acid Complex. Ph.D. Dissertation, Universidade de São Paulo, Sao Paulo, Brazil, 2019. [Google Scholar]
- Sarker, T.; Wan, X.; Yang, H.; Wang, Z. Dietary Lycopene Supplementation Could Alleviate Aflatoxin B1 Induced Intestinal Damage through Improving Immune Function and Anti-Oxidant Capacity in Broilers. Animals 2021, 11, 3165. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, T.T. The Laetiporus sulphureus fermented product enhances the antioxidant status, intestinal tight junction, and morphology of broiler chickens. Animals 2021, 11, 149. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Șoica, C.; Iuga, M.; Mironeasa, S. Effects of Supplementing Grape Pomace to Broilers Fed Polyunsaturated Fatty Acids Enriched Diets on Meat Quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, P.J.A.; Morrissey, P.A.; Flynn, A. Increased storage stability of chicken muscle by dietary a-tocopherol supplementation. Ir. J. Agric. Food Res. 1993, 32, 67–73. [Google Scholar]
- Brandon, S.; Morrissey, P.A.; Buckley, D.J.; Frigg, M. Influence of dietary a-tocopheryl acetate on the oxidative stability of chicken tissues. In Proceedings of the 11th European Symposium on The Quality of Poultry Meat, Tours, France, 4–8 October 1993; pp. 397–403. [Google Scholar]
- Morrissey, P.; Buckley, D.; Sheehy, P.; Monahan, F. Vitamin E and meat quality. Proc. Nutr. Soc. 1994, 53, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.Y.; Min, Y.N.; Liu, F.Z. Dietary vitamin E improves meat quality and antioxidant capacity in broilers by upregulating the expression of antioxidant enzyme genes. J. Appl. Anim. Res. 2018, 46, 397–401. [Google Scholar] [CrossRef]
- Dev, K.; Mir, N.A.; Biswas, A.; Kannoujia, J.; Begum, J.; Kant, R.; Mandal, A. Dietary synbiotic supplementation improves the growth performance, body antioxidant pool, serum biochemistry, meat quality, and lipid oxidative stability in broiler chickens. Anim. Nutr. 2020, 6, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Leskovec, J.; Levart, A.; Perić, L.; Stojčić, M.Đ.; Tomović, V.; Pirman, T.; Salobir, J.; Rezar, V. Antioxidative effects of supplementing linseed oil-enriched diets with α-tocopherol, ascorbic acid, selenium, or their combination on carcass and meat quality in broilers. Poult. Sci. 2019, 98, 6733–6741. [Google Scholar] [CrossRef]
- Cai, S.J.; Wu, C.X.; Gong, L.M.; Song, T.; Wu, H.; Zhang, L.Y. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012, 91, 2532–2539. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J. Sci. Food Agric. 2018, 99, 1066–1072. [Google Scholar] [CrossRef]
- Adeyemi, K.D. Comparative effect of dietary Morinda lucida leaf and Butylated hydroxyanisole (BHA) on carcass traits, meat quality, and oxidative stability of broiler chickens. J. Food Sci. Technol. 2021, 58, 4359–4369. [Google Scholar] [CrossRef]
- Lu, L.; Luo, X.G.; Ji, C.; Liu, B.; Yu, S.X. Effect of manganese supplementation and source on carcass traits, meat quality, and lipid oxidation in broilers1. J. Anim. Sci. 2007, 85, 812–822. [Google Scholar] [CrossRef]

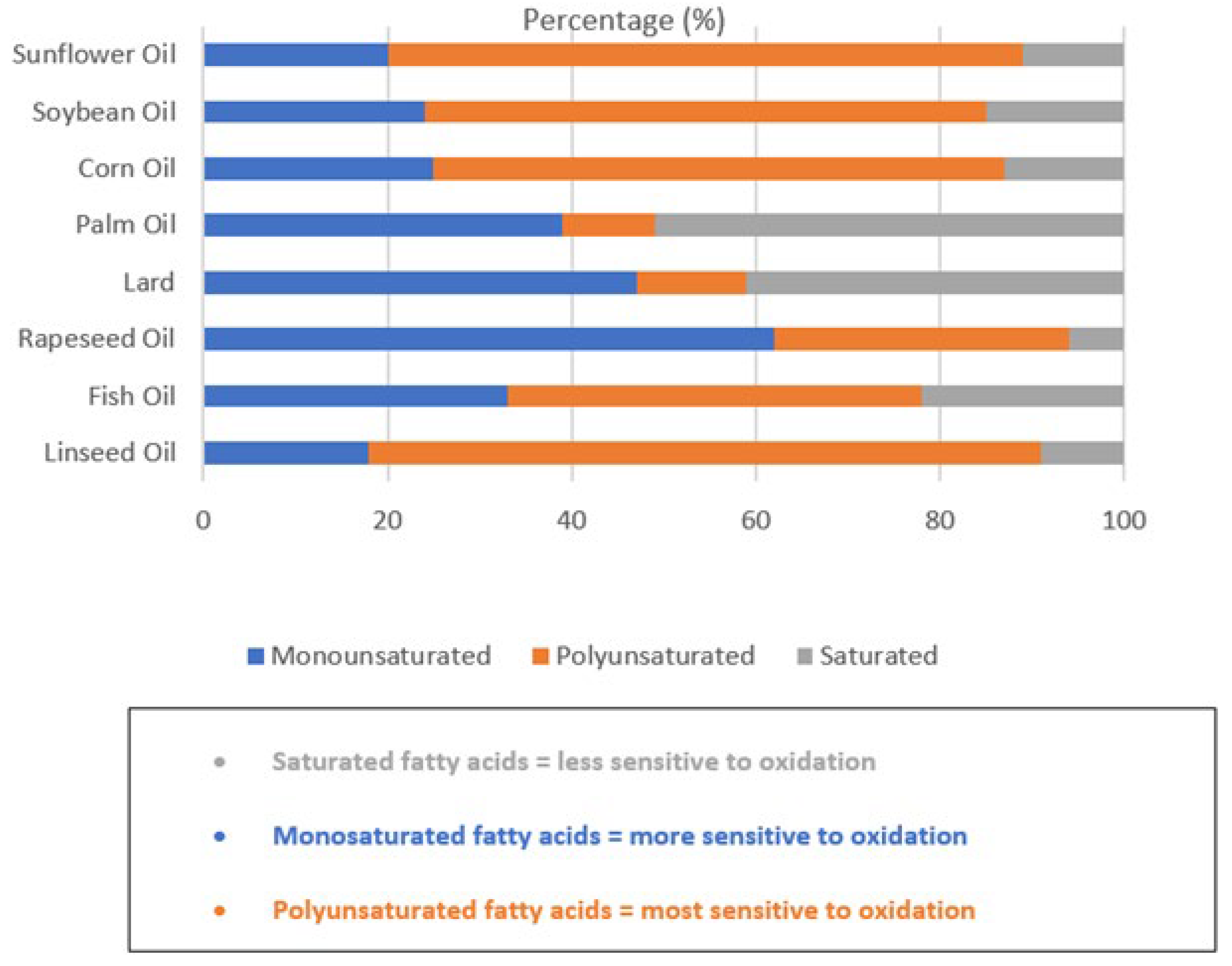

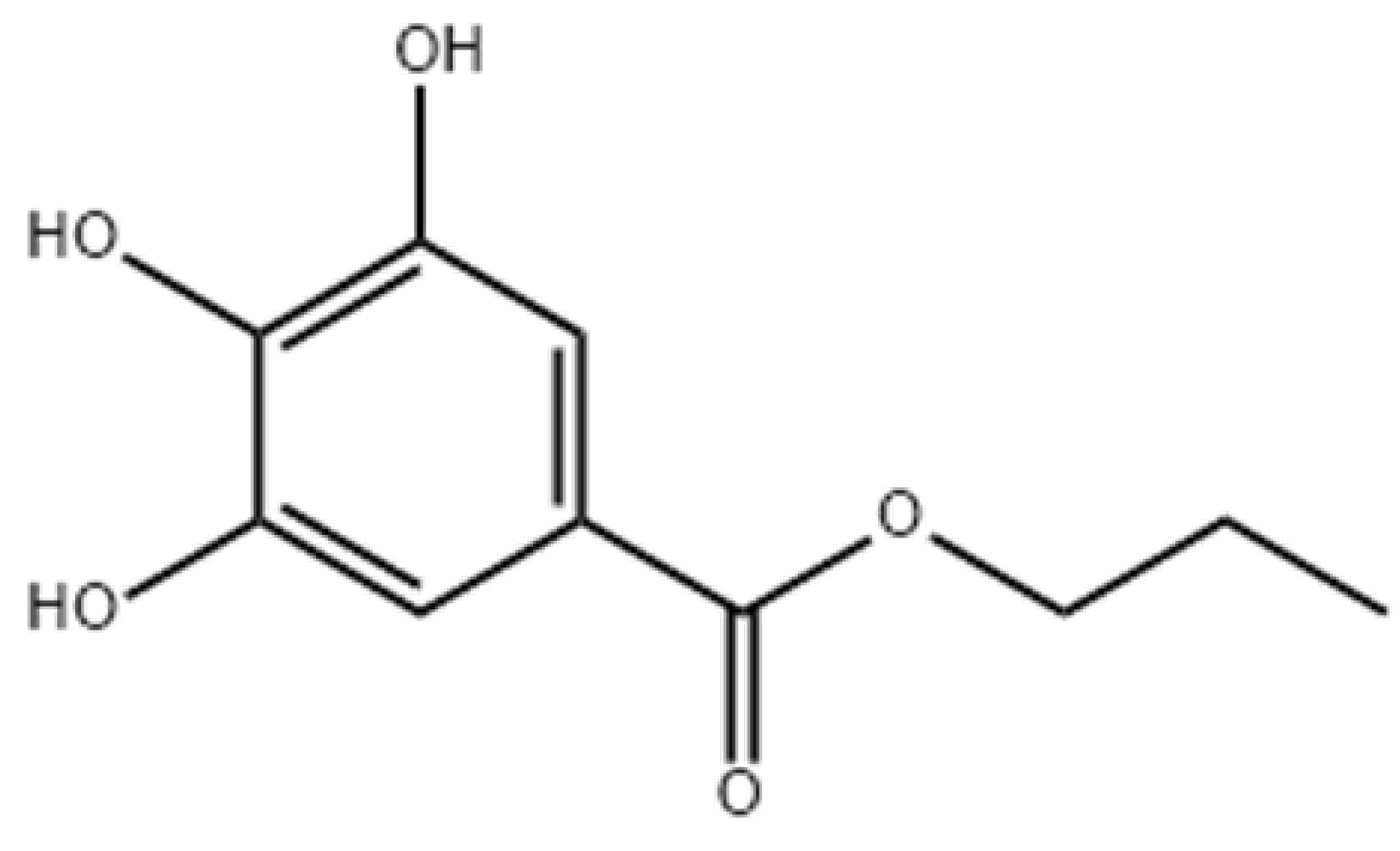
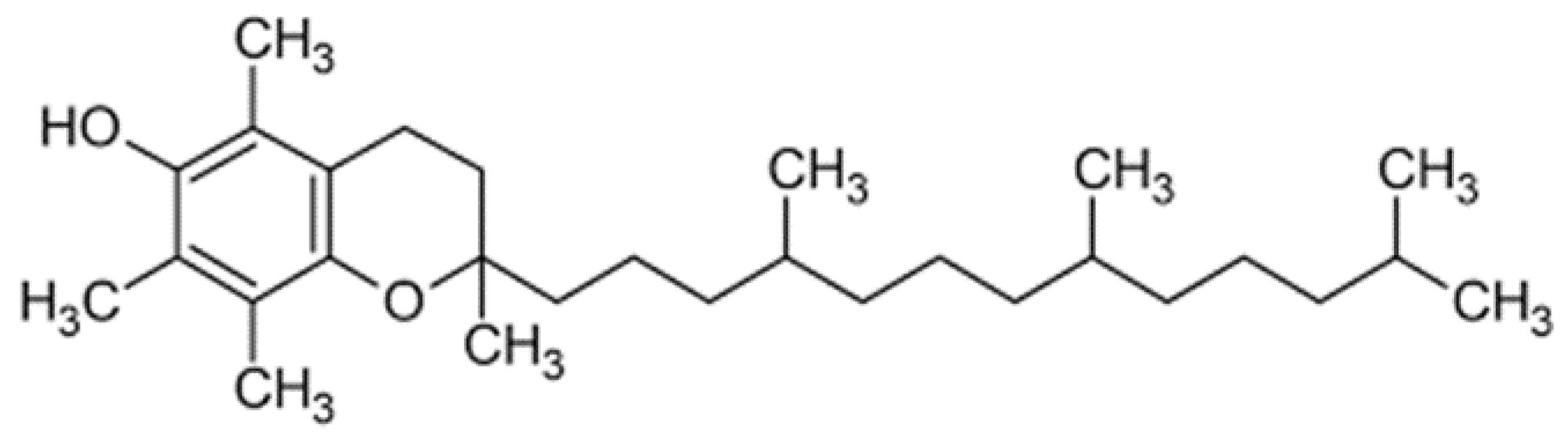


| Authors | Source of Oxidative Stress | Oxidative Stress Parameter Measured | Meat Quality Effect |
|---|---|---|---|
| Zhang et al. (2011) [32] | 5% Oxidized animal/vegetable fat | Thiobarbituric acid, pH, Dinitrophenylhydrazine drip loss, sarcoplasmic reticulum Ca (SERCA) | Significant reduction in SERCA activity, Increased drip loss, reduced pH |
| Griffin et al. (2017) [33] | Normal commercial conditions | HIF-1a | Incidence of white stripe and woody breast on day 16 |
| Gao et al. (2009) [34] | Dexamethasone 2 mg/kg of body weight | Thiobarbituric acid, lipid peroxidation in meat, Superoxide dismutase, fatty acid composition of meat | Significant increase in Thiobarbituric acid in muscle, lipid peroxidation significantly increased with DEX |
| Voljc et al. (2013) [35] | Linseed oil inclusion compared to palm oil | Malondialdehyde | Linseed oil significantly increased the malondialdehyde levels in breast meat, very significant increase during cooking |
| Zhang et al. (2011) [32] | Corticosterone (4 mg/kg of body weight) | pH, malondialdehyde | Higher pH, malondialdehyde levels significantly higher in meat |
| Pozzo et al. (2013) [36] | Ochratoxin | Thiobarbituric acid | Increased thiobarbituric acid in breast and thigh |
| Tang et al. (2021) [37] | Typical commercial metabolic levels of thioredoxin peroxiredoxins sulfoxide Reductase effect when Se supplemented | T-AOC | Se reduced drip loss, increased pH and reduced sheer force |
| Tavarez et al. (2011) [14] | Oxidized soybean oil | Thiobarbituric acid | Antioxidant inclusion (ethoxyquin, propyl gallate) reduced thiobarbituric acid in breast meat and increased serum concentration of vitamins A and E |
| Cui et al. (2018) [38] | Normal commercial conditions | Malondialdehyde, Plasma T-AOC | Inclusion of antioxidant (Moringa oleifera) reduced MDA in breast meat and significantly increase Plasma T-AOC |
| Nasr et al. (2021) [39] | Reduced stocking density | Cooking loss %, drip loss %, and bacterial count of breast meat | The highest stocking density (20 birds/M2) significantly increased cooking and drip loss % and significantly increased the total bacterial count of the breast meat. |
| Zhao et al. (2019) [40] | Heat stress | Drip loss %, Cooking loss %, MDA, and shear force in breast meat | Heat stress significantly increased MDA, drip loss %, Cooking loss % in meat. No significant difference in shear force. Inclusion of Eucommia ulmoides leaf significantly reduced cooking and drip loss % and MDA in the meat. |
| Xiao et al. (2011) [41] | Oxidized oil | TBARS, Carbonyls (protein oxidation), Hexanol, Pentanal | Dietary inclusion of vitamin E (500 IU) significantly reduced lipid and protein oxidation of breast meat. Vacuum packaging delayed the onset of oxidation in the meat |
| Xiao et al. (2011) [42] |
| Authors | Source of Oxidative Stress | Oxidative Stress Parameter Measured | Performance Effect |
|---|---|---|---|
| Lin et al. (2004) [93] | Corticosterone 30 mg/kg feed | significantly reduced Superoxide dismutase, significantly increased Thiobarbituric acid | significantly reduced BWG, FI, significantly increased FCR |
| Erdogan et al. (2005) [71] | Cadmium 25 mg/L via drinking line | significantly increased malondialdehyde | Significantly reduced BWG, FI, and increased FCR |
| Tawfeek et al. (2014) [91] | Heat stress | significantly reduced Total antioxidant capacity | Significantly reduced BWG, FI, and increased FCR |
| Liang et al. (2015) [94] | Consumption of oxidized oil | Total antioxidant capacity | Significantly reduced BWG, FI, and increased FCR |
| Li et al. (2019) [92] | High stocking density | significantly increased malondialdehyde | Significantly reduced BWG, FI, and increased FCR |
| Wang et al. (2019) [95] | Heat stress | significantly increased malondialdehyde, reduced Superoxide dismutase, and Total antioxidant capacity | Significantly reduced BWG, FI, and increased FCR |
| Ahmad et al. (2020) [96] | Heat stress | significantly increased malondialdehyde | Significantly reduced BWG, FI, and increased FCR |
| Authors | Source of Oxidative Stress | Oxidative Stress Parameter Measured | Gut Health Effect |
|---|---|---|---|
| Deng et al. (2012) [70] | Vanadium supplementation (5, 15, 30, 45, and 60 mg/kg) | Malondialdehyde significantly increased at 30, 45, and 60 mg/kg | Significantly reduced levels of AOX enzymes in the intestinal tract |
| Osselaere et al. (2013) [101] | Deoxynivalenol | Real-time-PCR of genes of interest significant upregulation of xanthine oxidoreductase | Impaired tight junction integrity |
| Gou et al. (2018) [69] | Iron ingestion (245, 908 and 1651 mg/kg) | Malondialdehyde significantly increased | Reduced villi height, significant change in cecal microbiota (more potentially pathogenic varieties) |
| De Souza et al. (2020) [102] | Deoxynivalenol | Significantly reduced Glutathione levels | Reduced villi height, increased crypt depth, reduced goblet cells |
| Panaite et al. (2020) [103] | Normal conditions compared to an AOX treatment | Control birds had increased malondialdehyde and reduced glutathione levels | Lower levels of lactobacilli in the intestine |
| Sun et al. (2020) [104] | Lipopolysaccharides | malondialdehyde significantly increased | Reduced villi height, increased crypt depth |
| Author | Antioxidant | Effect |
|---|---|---|
| Wen et al. (2020) [149] | Ginger extract 1000 mg/kg 1 | Significantly increased BWG and reduced FCR |
| Hosseini-Vashan et al. (2016) [148] | Tomato pomace 3 and 5% 2 | Increased BWG |
| De Grande et al. (2020) [147] | 60 ppm zinc | Significantly reduced FCR |
| Rajani et al. (2011) [150] | Synthetic AOX (BHT, propyl gallate and ethoxyquin) | Significantly increased BWG and reduced FCR |
| Alian et al. (2020) [151] | 0.3 mg/kg nano selenium | Significantly increased BWG and reduced FCR |
| Boostani et al. (2015) [152] | 0.3 mg/kg organic or nano-selenium | 3.5% increase in BWG |
| Shen et al. (2019) [153] | Bamboo leaf extract 1, 2, 3, 4, 5 g/kg feed | 1 and 2 g/kg significantly improved ADG and FCR |
| Zhao et al., (2019) [40] | Eucommia ulmoides leaf 500 and 100 mg/kg feed in birds subjected to heat stress | Significant improvement in ADG, FI, and FCR compared to heat-stressed control |
| Cheng et al. (2018) [154] | Mannan oligosaccharides 1 g/kg feed in birds subjected to heat stress | Significantly increased BWG, FI compared to heat-stressed control |
| Chang et al. (2020) [155] | Chitosan Oligosaccharide 200 mg/kg feed in birds subjected to heat stress | Significantly improved ADG, FI, and FCR compared to heat-stressed control |
| Hussan et al. (2022) [156] | Nano Zinc Oxide, 0, 2.5, 5, 10, 20 ppm | 2.5 ppm significantly improved BWG, FI, and FCR |
| Author | Antioxidant | Effect |
|---|---|---|
| Cheng et al. (2017) [158] | 20 IU alpha tocopherol acetate or 20 IU dietary natural alpha tocopherols with and without immunosuppression | Significantly increased total antioxidant capacity for both forms of tocopherols |
| Karadas et al. (2016) [159] | 200 mg/kg vitamin E (Natural Alpha tocopherol), 0.5 mg/kg selenium, 100 mg/kg of 5% lutein, 100 mg/kg of 5% lycopene, and 25 mg/kg of 10% canthaxanthin compared to a control. | significantly improved the total antioxidant capacity vitamin E and selenium supplementation resulted in a significant increase in the total carotenoid concentration in the plasma. |
| De Grande et al. (2020) [147] | 60 ppm zinc sulphate or the zinc amino acid complex | zinc amino acid complex resulted in reduced plasma malondialdehyde and glutathione peroxidase activity |
| Akhavast and Daneshyar, (2017) [160] | Rosemary extract 1.5, 3, and 6 mL/L of water | 3 or 6 mL resulted in significant improvements in total antioxidant capacity |
| Yildirim et al. (2017) [161] | 100 or 200 mg/kg Rosemary ethanol extract | Significant antioxidant enzyme levels in blood |
| Liu et al. (2020) [162] | control (corn/soy diet), a control + antibiotic (chlortetracycline 75 mg/kg), and control+ chestnut wood extract (1000 mg/kg) | Significant improvement in total antioxidant capacity, glutathione peroxidase and superoxide dismutase in breast meat and improved growth performance |
| Author | Stress Source | Antioxidant | Effect |
|---|---|---|---|
| Sahin et al. (2017) [168] | Heat stress | 200 µg/kg Chromium picolinate or chromium histidine | Mitigation of negative effects of heat stress on performance and improved oxidative status |
| Tang et al. (2018) [37] | Heat stress | 3% rosemary extract 6 | Reduced circulating levels of malondialdehyde and heat shock proteins in the cardiac muscles |
| Cheng et al. (2018) [154] | Heat stress | 250 mg/kg mannan oligosaccharides + cyclic heat stress | Improved performance of supplemented birds in heat stress. Significantly reduced jejunal malondialdehyde and superoxide dismutase |
| Pan et al. (2018) [169] | Transport stress | 100 mg/kg Forsythia suspensa with transport stress (3 h transport at 27 degrees 7 | Reduced corticosterone and malondialdehyde levels in serum compared to control. Improved ability to scavenge reactive oxygen species |
| Gao et al. (2010) [34] | Dexamethasone to induce Oxidative stress | Vitamin E 20 and 200 mg/kg diet | Significant reduction in lipid peroxidation in plasma and meat, Superoxide dismutase activity increased with Vit E |
| Zhao et al., (2019) [41] | Heat stress | Eucommia ulmoides leaf 500 and 100 mg/kg feed in birds subjected to heat stress | Significant improvement in ADG, FI and FCR compared to heat-stressed control |
| Chang et al. (2020) [155] | Heat Stress | Chitosan Oligosaccharide 200 mg/kg feed in birds subjected to heat stress | Significantly improved ADG, FI and FCR compared to heat-stressed control |
| Author | Stress Source | Antioxidant | Effect |
|---|---|---|---|
| Sarsour and Persia (2022) [170] | Heat stress | Sulfur amino acids 30% additional | Improved antioxidant status and reduced intestinal permeability |
| Chen et al. (2022) [171] | Fluorine-induced chronic oxidative stress | selenium methionine 0.203 mg/kg selenium selenite 0.198 mg/kg | Improved antioxidant status, reduced malondialdehyde. Reduced intestinal permeability |
| Burin (2019) [172] | Heat stress | Zinc amino acid complex 0, 20, 40, 60 mg/kg | Significantly reduced oxidation products in blood and liver and improved gut integrity markers |
| Sarker et al. (2021) [173] | Aflatoxin B1 | Lycopene 200 mg/kg | Improved intestinal integrity and oxidative status |
| Lin and Lee (2021) [174] | Normal commercial environment | Laetiporus sulphureus fermented product (5%, 10%) | Significantly improved oxidative status and tight junction integrity. Increased villus height in ileum and jejunum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desbruslais, A.; Wealleans, A.L. Oxidation in Poultry Feed: Impact on the Bird and the Efficacy of Dietary Antioxidant Mitigation Strategies. Poultry 2022, 1, 246-277. https://doi.org/10.3390/poultry1040022
Desbruslais A, Wealleans AL. Oxidation in Poultry Feed: Impact on the Bird and the Efficacy of Dietary Antioxidant Mitigation Strategies. Poultry. 2022; 1(4):246-277. https://doi.org/10.3390/poultry1040022
Chicago/Turabian StyleDesbruslais, Alexandra, and Alexandra L. Wealleans. 2022. "Oxidation in Poultry Feed: Impact on the Bird and the Efficacy of Dietary Antioxidant Mitigation Strategies" Poultry 1, no. 4: 246-277. https://doi.org/10.3390/poultry1040022
APA StyleDesbruslais, A., & Wealleans, A. L. (2022). Oxidation in Poultry Feed: Impact on the Bird and the Efficacy of Dietary Antioxidant Mitigation Strategies. Poultry, 1(4), 246-277. https://doi.org/10.3390/poultry1040022






