Assessing the Effects of Phytogenic Feed Additives on Broilers during a Necrotic Enteritis Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Management and Diets
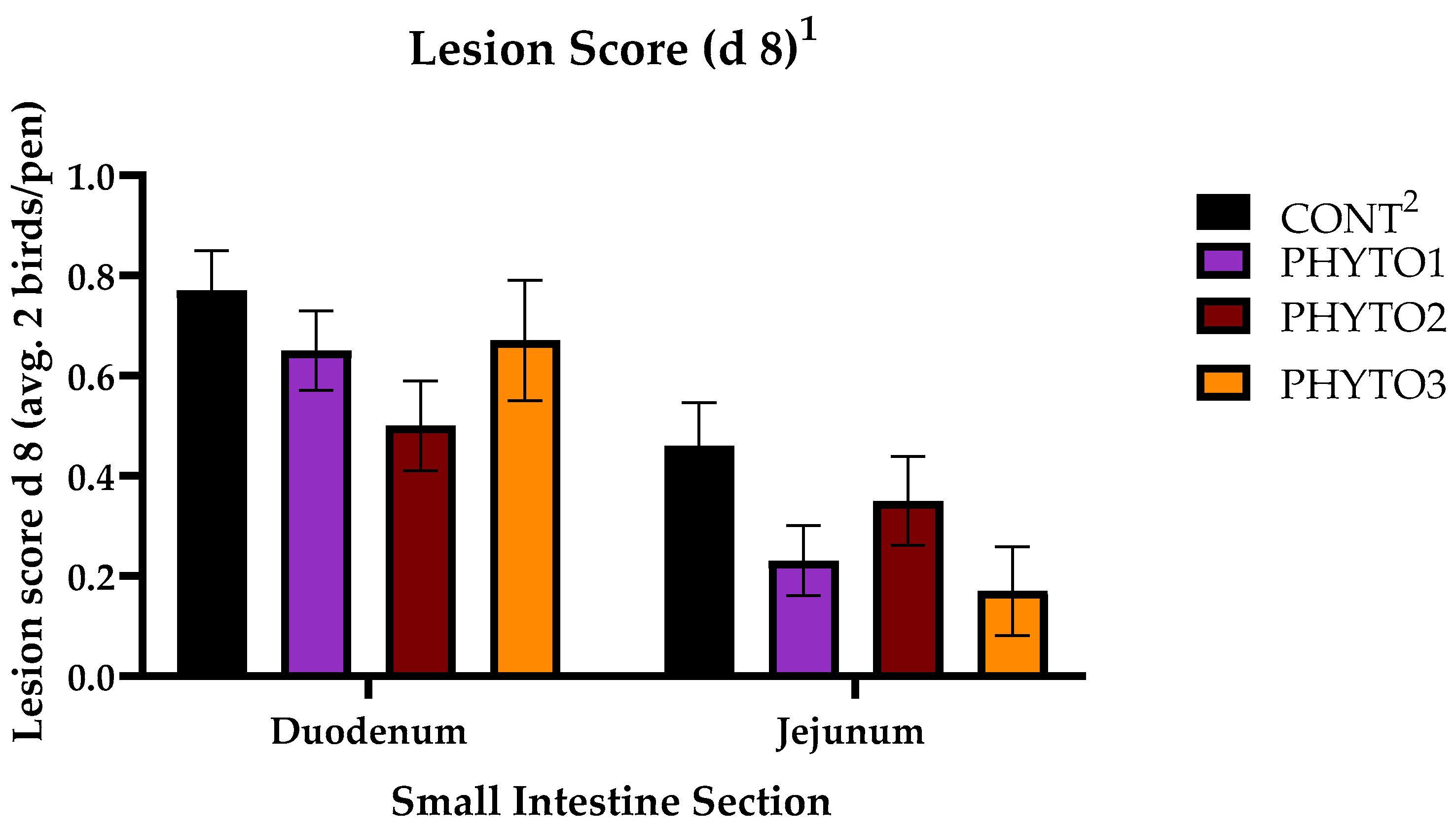
2.2. Necrotic Enteritis Challenge and Lesion Scoring
2.3. Performance
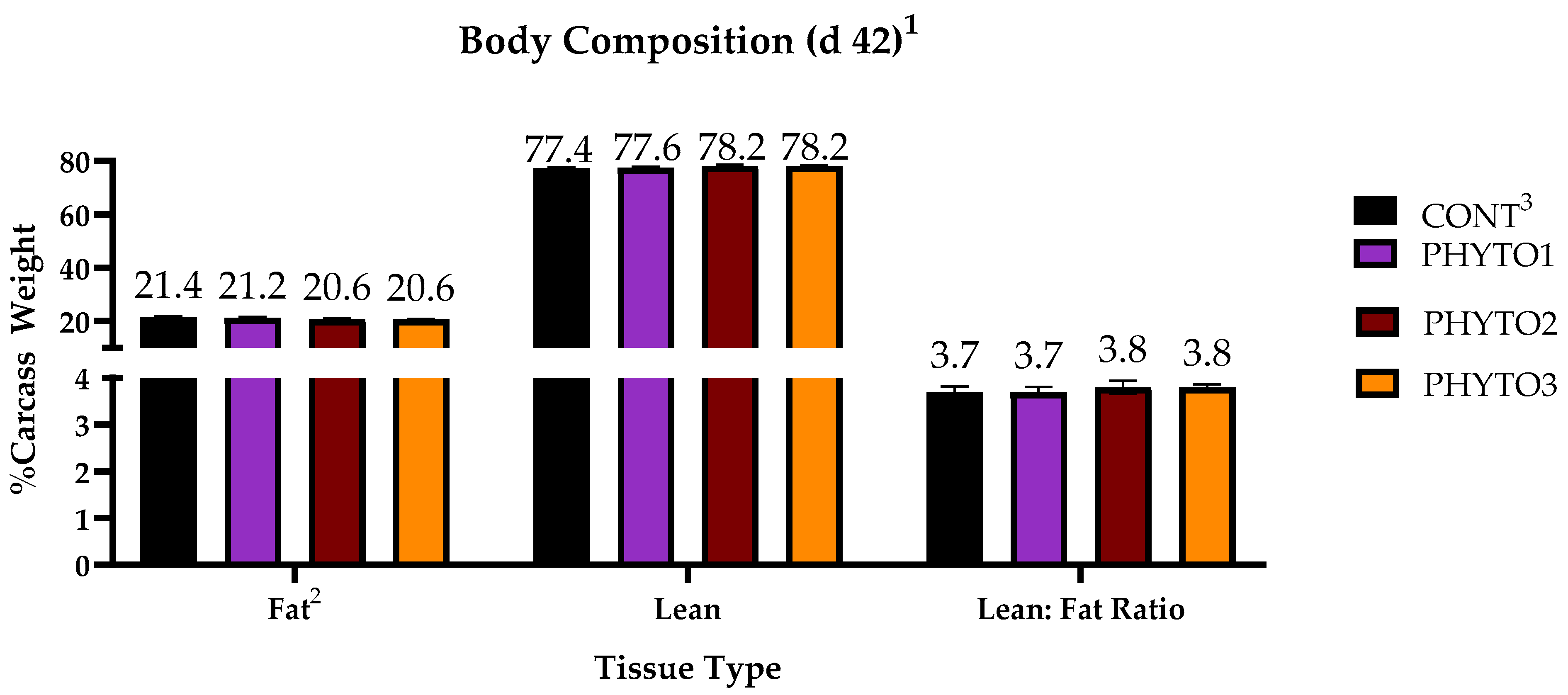
2.4. Carcass and Body Composition
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emami, N.K.; Dalloul, R.A. Centennial review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021, 100, 101330. [Google Scholar] [CrossRef] [PubMed]
- Wade, B.; Keyburn, A. The true cost of necrotic enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic enteritis in broiler chickens: A review on the pathogen, pathogenesis, and prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef]
- Bueno, I.; Ricke, I.; Hwang, H.; Smith, E.; Nault, A.; Johnson, T.J.; Singer, R.S. Efficacy of antibiotic and non-antibiotic interventions in preventing and treating necrotic enteritis in broiler chickens: A systematic review. Avian Dis. 2023, 67, 20–32. [Google Scholar] [CrossRef]
- Wati, T.; Ghosh, T.K.; Syed, B.; Haldar, S. Comparative efficacy of a phytogenic feed additive and an antibiotic growth promoter on production performance, caecal microbial population and humoral immune response of broiler chickens inoculated with enteric pathogens. Anim. Nutr. 2015, 1, 213–219. [Google Scholar] [CrossRef]
- Granstad, S.; Kristoffersen, A.B.; Benestad, S.L.; Sjurseth, S.K.; David, B.; Sørensen, L.; Fjermedal, A.; Edvardsen, D.H.; Sanson, G.; Løvland, A.; et al. Effect of feed additives as alternatives to in-feed antimicrobials on production performance and intestinal Clostridium perfringens counts in broiler chickens. Animals 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, N.; Sola-Oriol, D.; Perez, J.F. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Flees, J.J.; Emami, N.K.; Greene, E.; Ganguly, B.; Dridi, S. Phytogenic water additives improve broiler growth performance via modulation of intermediary metabolism-related signaling pathways. Animals 2021, 11, 750. [Google Scholar] [CrossRef]
- Yadav, A.S.; Gautham Kolluri, G.K.; Marappan Gopi, M.G.; Kumaragurubaran Karthik, K.K.; Malik, Y.S.; Kuldeep Dhama, K.D. Exploring alternatives to antibiotics as health promoting agents in poultry-a review. J. Exp. Biol. Agric. Sci. 2016, 4, 368–383. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Ozdogan, M.; Topal, E.; Paksuz, E.P.; Kirkan, S. Effect of different levels of crude glycerol on the morphology and some pathogenic bacteria of the small intestine in male broilers. Animal 2014, 8, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar] [CrossRef]
- Blue, C.E.C.; Emami, N.K.; White, M.B.; Cantley, S.; Dalloul, R.A. Inclusion of Quillaja saponin Clarity Q manages growth performance, immune response, and nutrient transport of broilers during subclinical necrotic enteritis. Microorganisms 2023, 11, 1894. [Google Scholar] [CrossRef]
- Calik, A.; Omara, I.I.; White, M.B.; Evans, N.P.; Karnezos, T.P.; Dalloul, R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms 2019, 7, 257. [Google Scholar] [CrossRef]
- Prescott, J.F.; Sivendra, R.; Barnum, D.A. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can. Vet. J. 1978, 19, 181–183. [Google Scholar]
- Emami, N.K.; Calik, A.; White, M.B.; Kimminau, E.A.; Dalloul, R.A. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 2020, 7, 572142. [Google Scholar] [CrossRef]
- Saiyed, M.A.; Joshi, R.S.; Savaliya, F.P.; Patel, A.B.; Mishra, R.K.; Bhagora, N.J. Study on inclusion of probiotic, prebiotic and its combination in broiler diet and their effect on carcass characteristics and economics of commercial broilers. Vet. World 2015, 8, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- El-Ashram, S.; Abdelhafez, G.A. Effects of phytogenic supplementation on productive performance of broiler chickens. J. Appl. Poult. Res. 2020, 29, 852–862. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Mansbridge, S.C.; Rose, S.P.; Lillehoj, H.S.; Bravo, D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 2019, 98, 3443–3449. [Google Scholar] [CrossRef] [PubMed]
- Gharaibeh, M.H.; Khalifeh, M.S.; Nawasreh, A.N.; Hananeh, W.M.; Awawdeh, M.S. Assessment of immune response and efficacy of essential oils application on controlling necrotic enteritis induced by Clostridium perfringens in broiler chickens. Molecules 2021, 26, 4527. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Paraskevas, V.; Tsirtsikos, P.; Palamidi, I.; Steiner, T.; Schatzmayr, G.; Fegeros, K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. J. Anim. Feed Sci. 2011, 168, 223–231. [Google Scholar] [CrossRef]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Tierernahr. 2003, 57, 99–106. [Google Scholar]
- Ott, C.P.; Omara, I.I.; Persia, M.E.; Dalloul, R.A. The impact of β-glucans on performance and response of broiler chickens during a coccidiosis challenge. Poult. Sci. 2018, 97, 2713–2721. [Google Scholar] [CrossRef]
- Mitchell, A.D.; Rosebrough, R.W.; Conway, J.M. Body composition analysis of chickens by dual energy x-ray absorptiometry. Poult. Sci. 1997, 76, 1746–1752. [Google Scholar] [CrossRef]
- Mitsch, P.; Zitterl-Eglseer, K.; Köhler, B.; Gabler, C.; Losa, R.; Zimpernik, I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004, 83, 669–675. [Google Scholar] [CrossRef]
- Martinez, D.A.; Weil, J.T.; Suesuttajit, N.; Umberson, C.; Scott, A.; Coon, C.N. The relationship between performance, body composition, and processing yield in broilers: A systematic review and meta-regression. Animals 2022, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, D.; Wertelecki, T.; Houszka, M.; Kamel, C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 2006, 90, 255–268. [Google Scholar] [CrossRef]
- McReynolds, J. Efficacy of multistrain direct-fed microbial and phytogenetic products in reducing necrotic enteritis in commercial broilers. Poult. Sci. 2009, 88, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, N.K.; Kheravii, S.K.; Keerqin, C.; Ionescu, C.; Blanchard, A.; Wu, S.-B. Potential of a mixture of eugenol and garlic tincture to improve performance and intestinal health in broilers under necrotic enteritis challenge. Anim. Nutr. 2021, 8, 26–37. [Google Scholar] [CrossRef] [PubMed]

| Feeding Phase (Days) a | |||
|---|---|---|---|
| Ingredients (%) | Starter (1–14) | Grower (14–28) | Finisher (28–42) |
| Corn (7.81% CP) | 59.53 | 64.12 | 65.70 |
| Soybean meal (48% CP) | 33.5 | 28.80 | 26.86 |
| Soybean oil (9000 kcal/kg) | 2.18 | 2.60 | 3.50 |
| Dicalcium phosphate (18.5% P, 22% Ca) | 2.05 | 1.92 | 1.70 |
| Calcium carbonate (37% Calcium) | 1.11 | 1.00 | 0.90 |
| Sodium chloride | 0.3 | 0.3 | 0.3 |
| Sodium bicarbonate | 0.07 | 0.07 | 0.05 |
| DL-methionine (990 g/kg) b | 0.38 | 0.34 | 0.29 |
| L-lysine hydrochloride (788 g L-Lysine/kg) c | 0.37 | 0.35 | 0.24 |
| L-threonine (985 g/kg) d | 0.15 | 0.14 | 0.10 |
| Vitamin/trace mineral premix e | 0.36 | 0.36 | 0.36 |
| Calculated analysis (% unless specified) | |||
| ME (kCal/kg) | 3007 | 3087 | 3168 |
| Crude protein | 21.81 | 19.90 | 18.94 |
| Total phosphorus | 0.76 | 0.71 | 0.66 |
| Available phosphorus | 0.45 | 0.42 | 0.38 |
| Calcium | 0.90 | 0.84 | 0.76 |
| Chlorine | 0.33 | 0.33 | 0.29 |
| Sodium | 0.16 | 0.16 | 0.15 |
| Potassium | 0.85 | 0.77 | 0.73 |
| Methionine | 0.67 | 0.61 | 0.55 |
| Methionine + cysteine | 0.98 | 0.89 | 0.82 |
| Lysine | 1.32 | 1.19 | 1.05 |
| Threonine | 0.86 | 0.78 | 0.71 |
| Linoleic acid | 1.44 | 1.52 | 1.55 |
| Dietary cation–anion balance (mEq) | 194 | 174 | 170 |
| Dietary Treatments 1 | Statistics | |||||
|---|---|---|---|---|---|---|
| CONT | PHYTO1 | PHYTO2 | PHYTO3 | SEM | p-Value | |
| Days 0 to 8 | ||||||
| ADG | 25.12 | 25.14 | 24.43 | 24.68 | 0.31 | 0.2959 |
| ADFI | 27.63 | 27.65 | 27.71 | 27.43 | 0.32 | 0.9308 |
| FCR | 1.10 | 1.10 | 1.13 | 1.11 | 0.01 | 0.1535 |
| Days 9 to 14 | ||||||
| ADG | 34.40 | 37.77 | 35.84 | 37.97 | 1.04 | 0.0617 |
| ADFI | 51.58 | 52.47 | 51.85 | 52.40 | 0.97 | 0.8958 |
| FCR | 1.52 a | 1.39 b | 1.45 ab | 1.38 b | 0.03 | 0.0123 |
| Days 0 to 14 | ||||||
| ADG | 33.51 | 34.89 | 33.33 | 34.82 | 0.55 | 0.0879 |
| ADFI | 43.06 | 43.23 | 42.88 | 43.33 | 0.57 | 0.9464 |
| FCR | 1.29 a | 1.24 b | 1.29 a | 1.25 b | 0.01 | 0.0088 |
| Days 15 to 28 | ||||||
| ADG | 79.41 | 77.55 | 77.67 | 81.84 | 1.33 | 0.1222 |
| ADFI | 120.81 | 122.59 | 123.16 | 126.96 | 1.74 | 0.1123 |
| FCR | 1.53 | 1.58 | 1.59 | 1.55 | 0.02 | 0.1225 |
| Days 0 to 28 | ||||||
| ADG | 59.05 | 59.29 | 57.48 | 60.61 | 0.92 | 0.1334 |
| ADFI | 87.00 | 88.37 | 87.13 | 90.15 | 1.11 | 0.1731 |
| FCR | 1.48 | 1.49 | 1.52 | 1.49 | 0.01 | 0.3093 |
| Days 29 to 42 | ||||||
| ADG | 116.71 | 126.82 | 127.38 | 123.78 | 3.19 | 0.0824 |
| ADFI | 199.96 | 206.31 | 204.94 | 206.41 | 2.23 | 0.1488 |
| FCR | 1.74 | 1.63 | 1.62 | 1.67 | 0.04 | 0.1249 |
| Days 0 to 42 | ||||||
| ADG | 60.61 | 62.97 | 63.77 | 63.66 | 1.28 | 0.2748 |
| ADFI | 97.32 | 99.73 | 101.13 | 100.52 | 2.09 | 0.5923 |
| FCR | 1.61 a | 1.58 b | 1.59 b | 1.58 b | 0.01 | 0.0241 |
| % Mortality | 7.67 | 7.69 | 6.92 | 6.54 | 1.42 | 0.9213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blue, C.E.C.; White, M.B.; Dalloul, R.A. Assessing the Effects of Phytogenic Feed Additives on Broilers during a Necrotic Enteritis Challenge. Poultry 2024, 3, 346-353. https://doi.org/10.3390/poultry3040026
Blue CEC, White MB, Dalloul RA. Assessing the Effects of Phytogenic Feed Additives on Broilers during a Necrotic Enteritis Challenge. Poultry. 2024; 3(4):346-353. https://doi.org/10.3390/poultry3040026
Chicago/Turabian StyleBlue, Candice E. C., Mallory B. White, and Rami A. Dalloul. 2024. "Assessing the Effects of Phytogenic Feed Additives on Broilers during a Necrotic Enteritis Challenge" Poultry 3, no. 4: 346-353. https://doi.org/10.3390/poultry3040026
APA StyleBlue, C. E. C., White, M. B., & Dalloul, R. A. (2024). Assessing the Effects of Phytogenic Feed Additives on Broilers during a Necrotic Enteritis Challenge. Poultry, 3(4), 346-353. https://doi.org/10.3390/poultry3040026






