Expression of Immune Genes and Leukocyte Population in the Conjunctiva, Harderian Gland and Trachea of Chickens Inoculated with a Live Vaccine and a Field Strain Infectious Laryngotracheitis Virus
Abstract
:1. Introduction
2. Materials and Methods
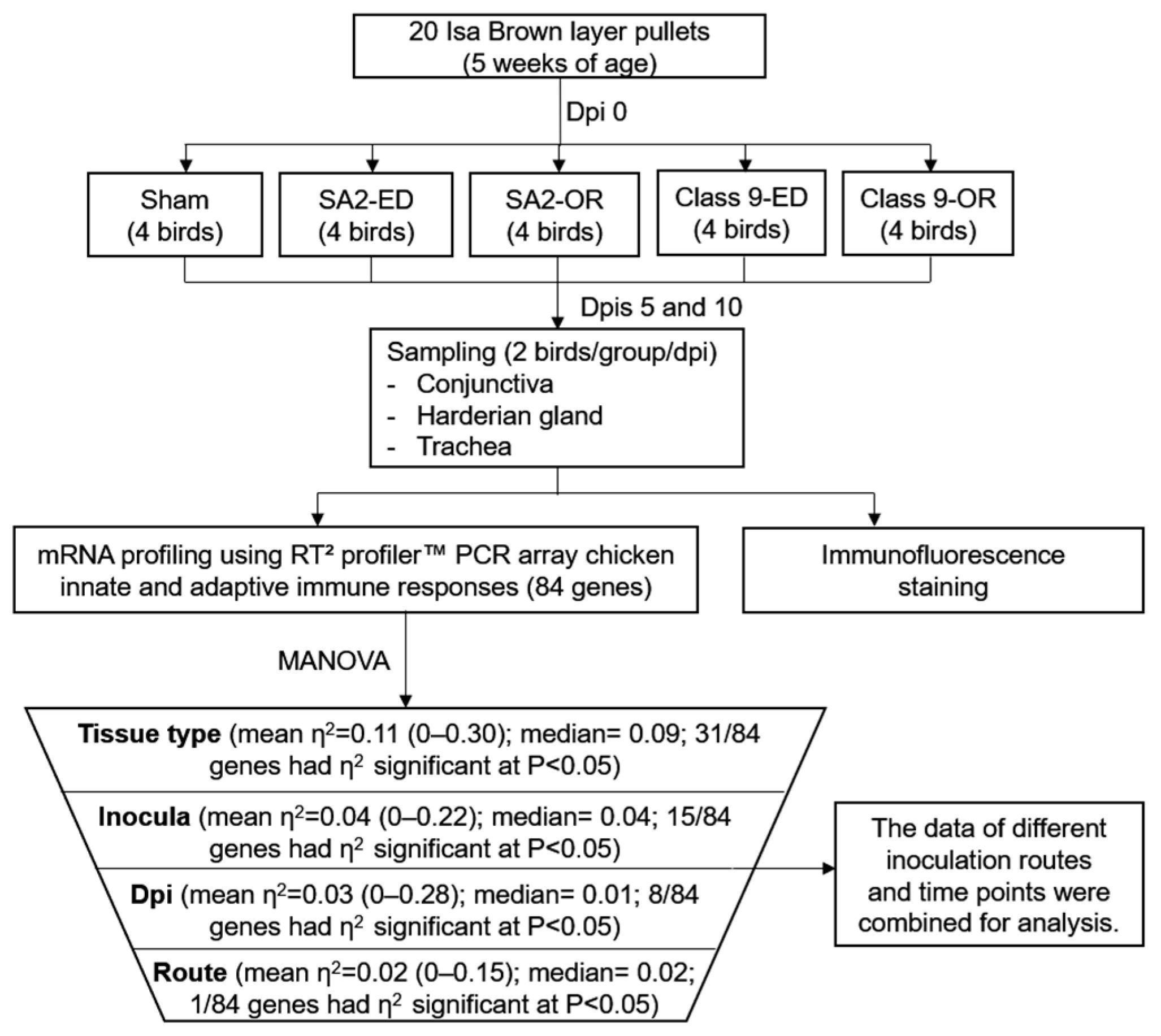
2.1. Experimental Design, Clinical Sign Scoring and Sample Collection
2.2. ILTV DNA Extraction and Quantification
2.3. Total RNA Extraction and Quality Control
2.4. RT2 Profiler PCR Array
2.5. Fluorescence Microscopy
2.6. Histological Tissue Image Analysis
2.7. Statistical Analysis
3. Results
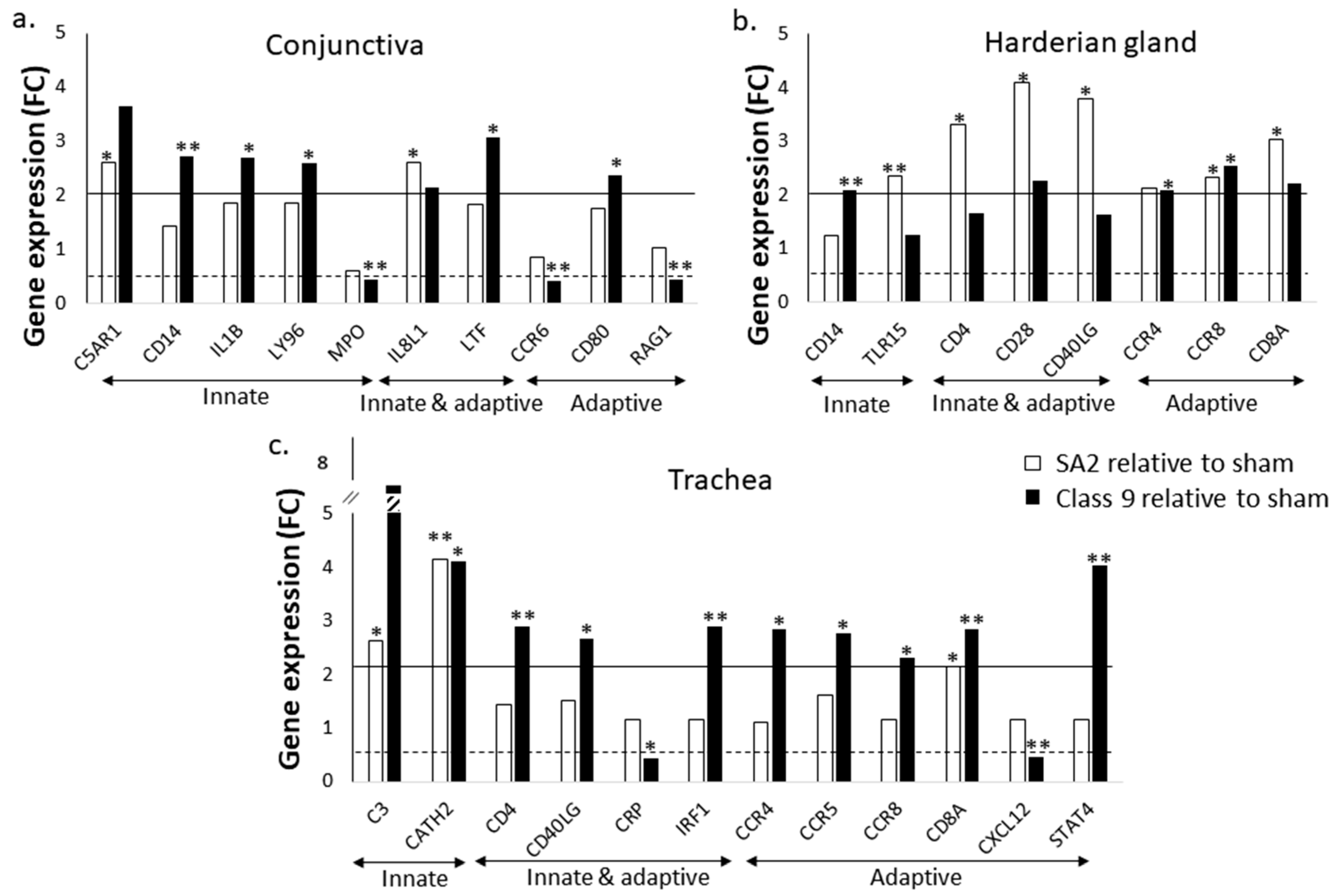
3.1. Virulent ILTV Inoculation Elicited Greater Innate and Adaptive Immune Gene Expression Response in the Trachea and Conjunctiva than ILTV Vaccination but Lower Cell Infiltration
3.2. The Correlations of Total Clinical Score and Choanal ILTV GC with Gene Expression Level Varied with the Virus Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García, M.; Spatz, S. Infectious Laryngotracheitis. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; Volume 1, pp. 189–209. [Google Scholar] [CrossRef]
- Garcia, M. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet. Microbiol. 2017, 206, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, N.C.; Mahmoudian, A.; O’Rourke, D.; Noormohammadi, A.H. Differentiation of infectious laryngotracheitis virus isolates by restriction fragment length polymorphic analysis of polymerase chain reaction products amplified from multiple genes. Avian Dis. 2006, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Neff, C.; Sudler, C.; Hoop, R.K. Characterization of Western European field isolates and vaccine strains of avian infectious laryngotracheitis virus by restriction fragment length polymorphism and sequence analysis. Avian Dis. 2008, 52, 278–283. [Google Scholar] [CrossRef]
- Ojkic, D.; Swinton, J.; Vallieres, M.; Martin, E.; Shapiro, J.; Sanei, B.; Binnington, B. Characterization of field isolates of infectious laryngotracheitis virus from Ontario. Avian Dis. 2006, 35, 286–292. [Google Scholar] [CrossRef]
- Chen, H.Y.; Cui, P.; Cui, B.A.; Li, H.P.; Jiao, X.Q.; Zheng, L.L.; Cheng, G.; Chao, A.J. Immune responses of chickens inoculated with a recombinant fowlpox vaccine coexpressing glycoprotein B of infectious laryngotracheitis virus and chicken IL-18. FEMS Immunol. Med. Microbiol. 2011, 63, 289–295. [Google Scholar] [CrossRef]
- Fahey, K.J.; York, J.J.; Bagust, T.J. Laryngotracheitis herpesvirus infection in the chicken. II. The adoptive transfer of resistance with immune spleen cells. Avian Pathol. 1984, 13, 265–275. [Google Scholar] [CrossRef]
- Honda, T.; Okamura, H.; Taneno, A.; Yamada, S.; Takahashi, E. The role of cell-mediated immunity in chickens inoculated with the cell-associated vaccine of attenuated infectious laryngotracheitis virus. J. Vet. Med. Sci. 1994, 56, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Fahey, K.J.; Bagust, T.J.; York, J.J. Laryngotracheitis herpesvirus infection in the chicken: The role of humoral antibody in immunity to a graded challenge infection. Avian Pathol. 1983, 12, 505–514. [Google Scholar] [CrossRef]
- Honda, T.; Taneno, A.; Sakai, E.; Yamada, S.; Takahashi, E. Immune response and in vivo distribution of the virus in chickens inoculated with the cell-associated vaccine of attenuated infectious laryngotracheitis (ILT) virus. J. Vet. Med. Sci. 1994, 56, 691–695. [Google Scholar] [CrossRef]
- Maekawa, D.; Riblet, S.M.; Whang, P.; Hurley, D.J.; Garcia, M. Activation of cytotoxic lymphocytes and presence of regulatory T cells in the trachea of non-vaccinated and vaccinated chickens as a recall to an infectious laryngotracheitis virus (ILTV) challenge. Vaccines 2021, 9, 865. [Google Scholar] [CrossRef]
- Maekawa, D.; Whang, P.; Riblet, S.M.; Hurley, D.J.; Guy, J.S.; García, M. Assessing the infiltration of immune cells in the upper trachea mucosa after infectious laryngotracheitis virus (ILTV) vaccination and challenge. Avian Pathol. 2021, 50, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Hurley, D.J.; Gogal, R.M., Jr.; Sharif, S.; Read, L.R.; Williams, S.M.; Jerry, C.F.; Maekawa, D.A.; García, M. Immune responses in the eye-associated lymphoid tissues of chickens after ocular inoculation with vaccine and virulent strains of the respiratory infectious laryngotracheitis virus (ILTV). Viruses 2019, 11, 635. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, J.J.; Wooming, A.; Li, X.; Zhou, H.; Bottje, W.G.; Kong, B. Transcriptional profiling of host gene expression in chicken embryo lung cells infected with laryngotracheitis virus. BMC Genom. 2010, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Coppo, M.J.C.; Devlin, J.M.; Legione, A.R.; Vaz, P.K.; Lee, S.W.; Quinteros, J.A.; Gilkerson, J.R.; Ficorilli, N.; Reading, P.C.; Noormohammadi, A.H.; et al. Infectious laryngotracheitis virus viral chemokine-binding protein glycoprotein G alters transcription of key inflammatory mediators in vitro and in vivo. J. Virol. 2017, e01534-17. [Google Scholar] [CrossRef]
- Vagnozzi, A.; Riblet, S.; Zavala, G.; Ecco, R.; Afonso, C.L.; García, M. Evaluation of the transcriptional status of host cytokines and viral genes in the trachea of vaccinated and non-vaccinated chickens after challenge with the infectious laryngotracheitis virus. Avian Pathol. 2016, 45, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vagnozzi, A.E.; Beltran, G.; Zavala, G.; Read, L.; Sharif, S.; Garcia, M. Cytokine gene transcription in the trachea, Harderian gland, and trigeminal ganglia of chickens inoculated with virulent infectious laryngotracheitis virus (ILTV) strain. Avian Pathol. 2018, 47, 497–508. [Google Scholar] [CrossRef]
- Thilakarathne, D.S.; Noormohammadi, A.H.; Browning, G.F.; Quinteros, J.A.; Underwood, G.J.; Hartley, C.A.; Coppo, M.J.; Devlin, J.M.; Diaz-Méndez, A. Pathogenesis and tissue tropism of natural field recombinants of infectious laryngotracheitis virus. Vet. Microbiol. 2020, 243, 108635. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Williams, S.M.; Zavala, G.; Guy, J.S.; García, M. The route of inoculation dictates the replication patterns of the infectious laryngotracheitis virus (ILTV) pathogenic strain and chicken embryo origin (CEO) vaccine. Avian Pathol. 2017, 46, 585–593. [Google Scholar] [CrossRef]
- Kirkpatrick, N.C.; Mahmoudian, A.; Colson, C.A.; Devlin, J.M.; Noormohammadi, A.H. Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis. Avian Pathol. 2006, 35, 449–453. [Google Scholar] [CrossRef]
- Nazir, S.; Yegoraw, A.A.; Charlesworth, R.P.G.; Williamson, S.; Sharpe, S.; Walkden-Brown, S.W.; Gerber, P.F. Marked differences in virulence of three Australian field isolates of infectious laryngotracheitis virus in meat and layer chickens. Avian Pathol. 2020, 49, 600–661. [Google Scholar] [CrossRef]
- Yegoraw, A.A.; Assen, A.M.; Gerber, P.F.; Walkden-Brown, S.W. Transmission of infectious laryngotracheitis virus vaccine and field strains: The role of degree of contact and transmission by whole blood, plasma and poultry dust. Vet. Res. 2021, 52, 91. [Google Scholar] [CrossRef] [PubMed]
- Callison, S.A.; Riblet, S.M.; Oldoni, I.; Sun, S.; Zavala, G.; Williams, S.; Resurreccion, R.S.; Spackman, E.; García, M. Development and validation of a real-time Taqman PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry. J. Virol. Methods 2007, 139, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lillehoj, H.; Min, W.; Kim, C.H.; Park, M.S.; Hong, Y.H.; Lillehoj, E.P. Comparative microarray analysis of intestinal lymphocytes following Eimeria acervulina, E. maxima, or E. tenella infection in the chicken. PLoS ONE 2011, 6, e27712. [Google Scholar] [CrossRef]
- Yang, F.; Lei, X.; Rodriguez-Palacios, A.; Tang, C.; Yue, H. Selection of reference genes for quantitative real-time PCR analysis in chicken embryo fibroblasts infected with avian leukosis virus subgroup J. BMC Res. Notes. 2013, 6, 402. [Google Scholar] [CrossRef]
- Nazir, S.; Charlesworth, R.P.G.; Moens, P.; Gerber, P.F. Evaluation of autofluorescence quenching techniques on formalin- fixed chicken tissues. J. Immunol. Methods 2021, 496, 113097. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.M.; Baskin, D.G.; Frevert, C.W.; Stahl, W.L.; Rosa-Molinar, E. Controls for immunohistochemistry: The Histochemical Society’s standards of practice for validation of immunohistochemical assays. J. Histochem. Cytochem. 2014, 62, 693–697. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Halama, N.; Zoernig, I.; Spille, A.; Westphal, K.; Schirmacher, P.; Jaeger, D.; Grabe, N. Estimation of immune cell densities in immune cell conglomerates: An approach for high-throughput quantification. PLoS ONE 2009, 4, e7847. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.; Andronicos, N.; Gerber, P.F. Expression of Immune Genes and Leukocyte Population in the Conjunctiva, Harderian Gland and Trachea of Chickens Inoculated with a Live Vaccine and a Field Strain Infectious Laryngotracheitis Virus. Poultry 2024, 3, 399-408. https://doi.org/10.3390/poultry3040030
Tran TT, Andronicos N, Gerber PF. Expression of Immune Genes and Leukocyte Population in the Conjunctiva, Harderian Gland and Trachea of Chickens Inoculated with a Live Vaccine and a Field Strain Infectious Laryngotracheitis Virus. Poultry. 2024; 3(4):399-408. https://doi.org/10.3390/poultry3040030
Chicago/Turabian StyleTran, Thanh Tien, Nicholas Andronicos, and Priscilla F. Gerber. 2024. "Expression of Immune Genes and Leukocyte Population in the Conjunctiva, Harderian Gland and Trachea of Chickens Inoculated with a Live Vaccine and a Field Strain Infectious Laryngotracheitis Virus" Poultry 3, no. 4: 399-408. https://doi.org/10.3390/poultry3040030
APA StyleTran, T. T., Andronicos, N., & Gerber, P. F. (2024). Expression of Immune Genes and Leukocyte Population in the Conjunctiva, Harderian Gland and Trachea of Chickens Inoculated with a Live Vaccine and a Field Strain Infectious Laryngotracheitis Virus. Poultry, 3(4), 399-408. https://doi.org/10.3390/poultry3040030






