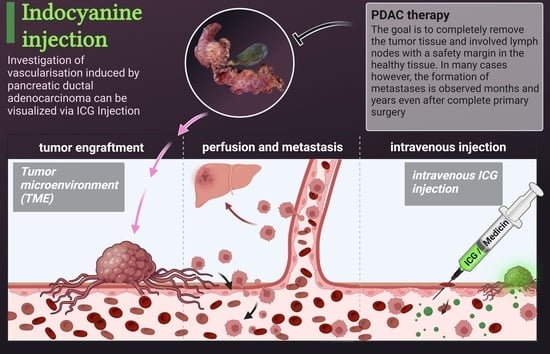
Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
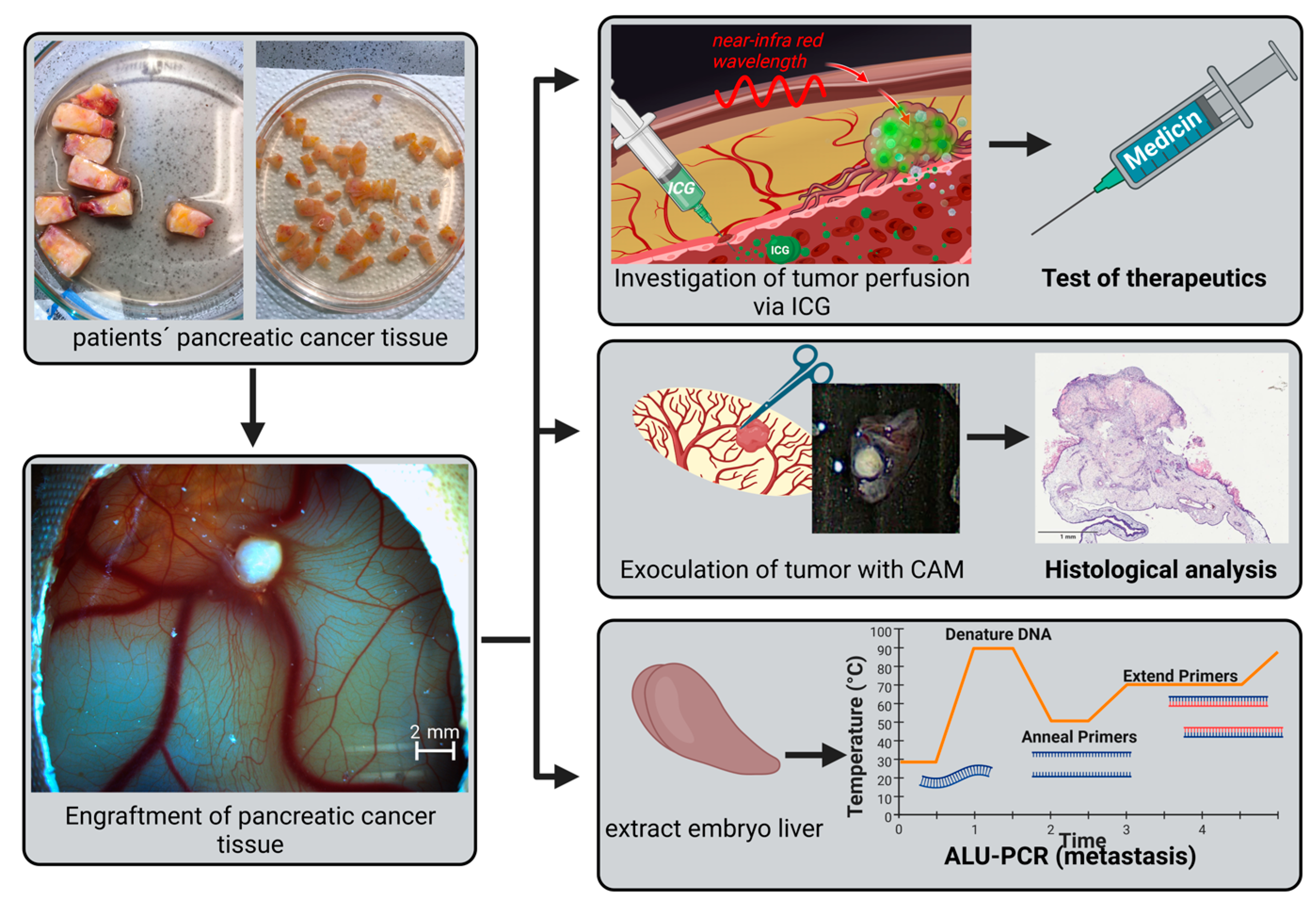
2.2. CAM model
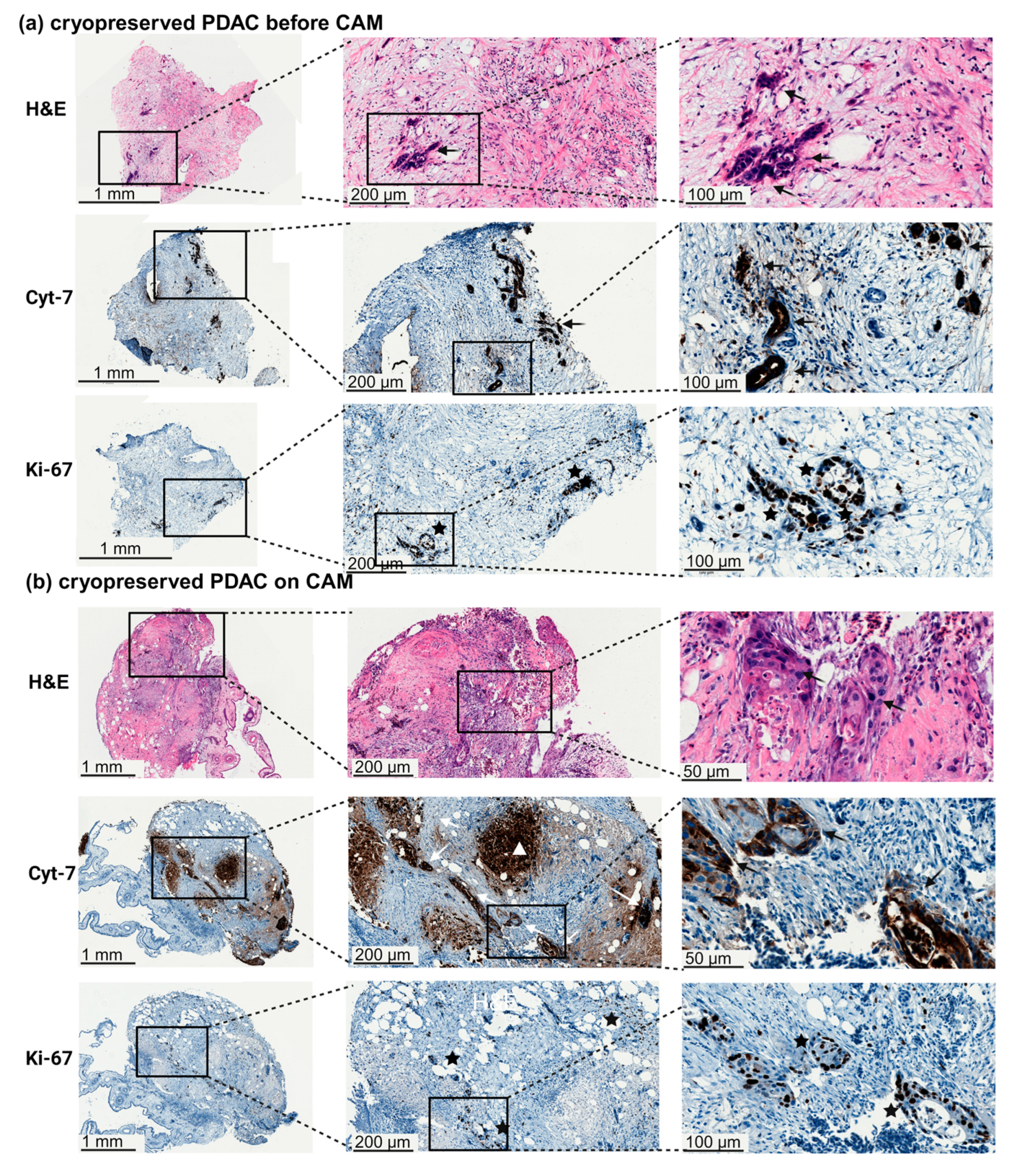
2.3. Cryopreservation of Tumor Tissue
2.4. Intravenous Indocyanine Injection
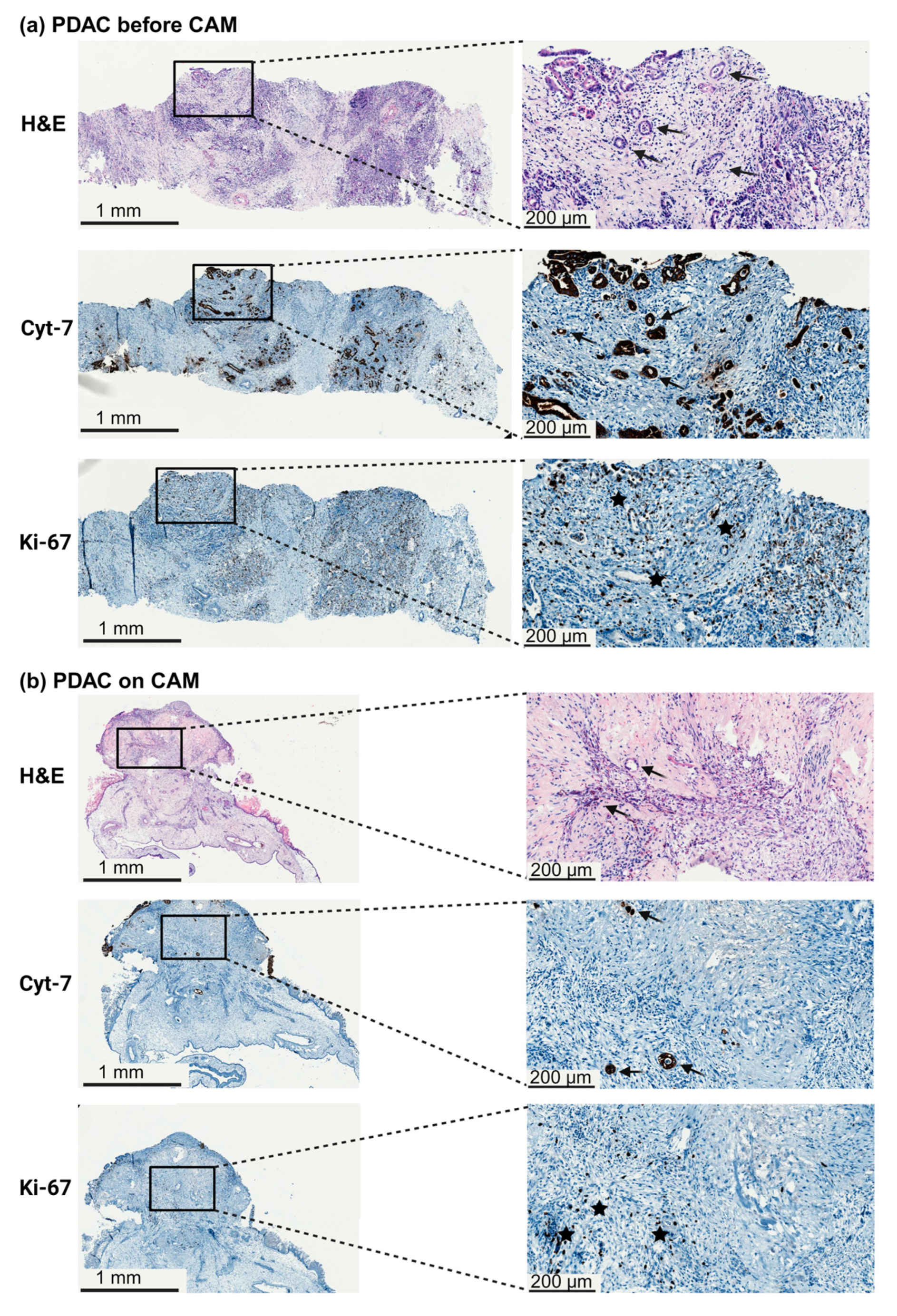
2.5. Histology and Immunohistochemistry
2.6. Polymerase Chain Reaction (PCR)
3. Results
3.1. Engraftment of Pancreatic Tumor Tissue on the CAM
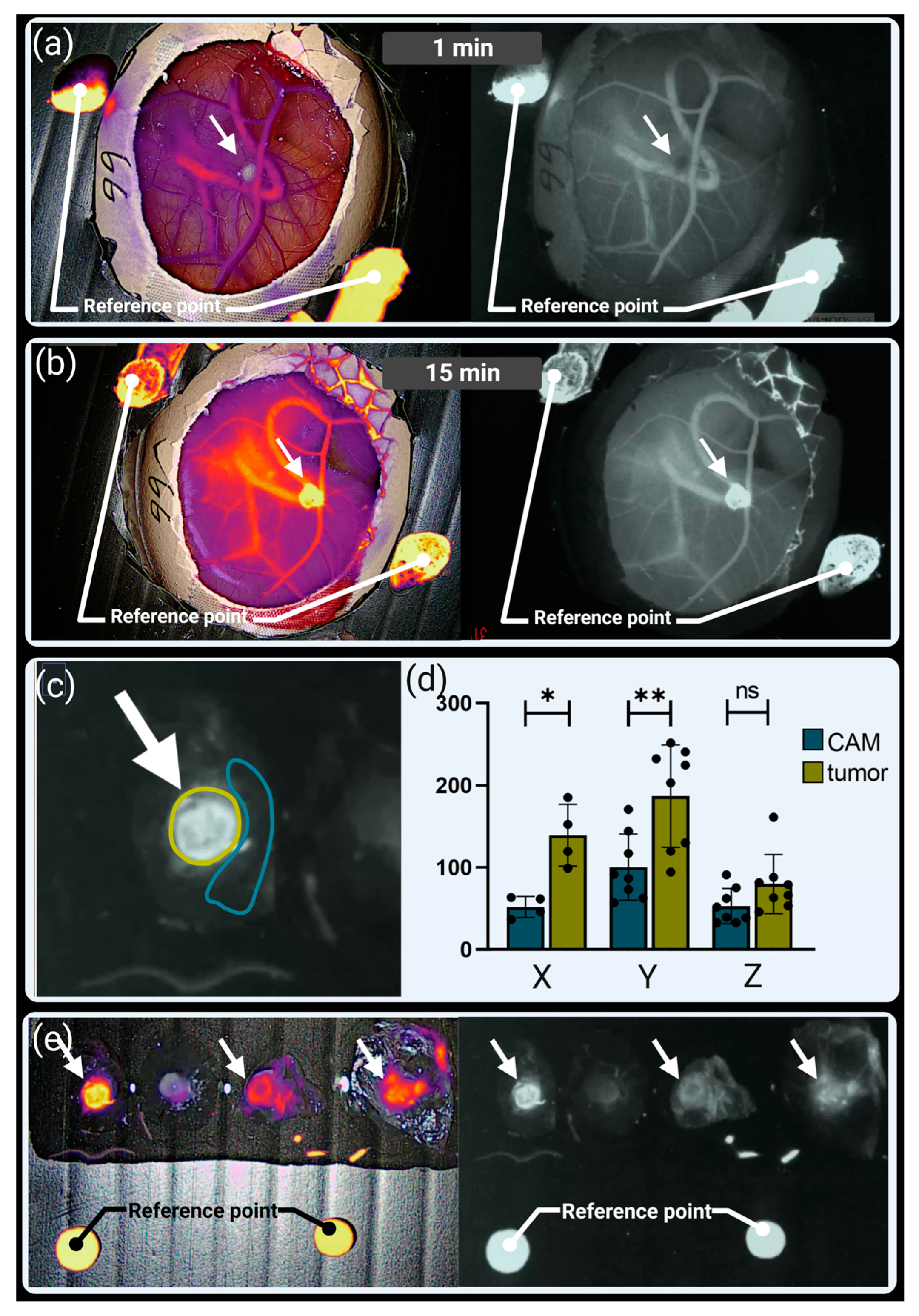
3.2. Visualization of Tumor Perfusion by Intravenous ICG Injection
3.3. Detection of Metastasis Using Alu PCR
4. Discussion
4.1. Intratumoral Perfusion of Pancreatic Cancer Tissue Cultivated on CAM and Future Implications
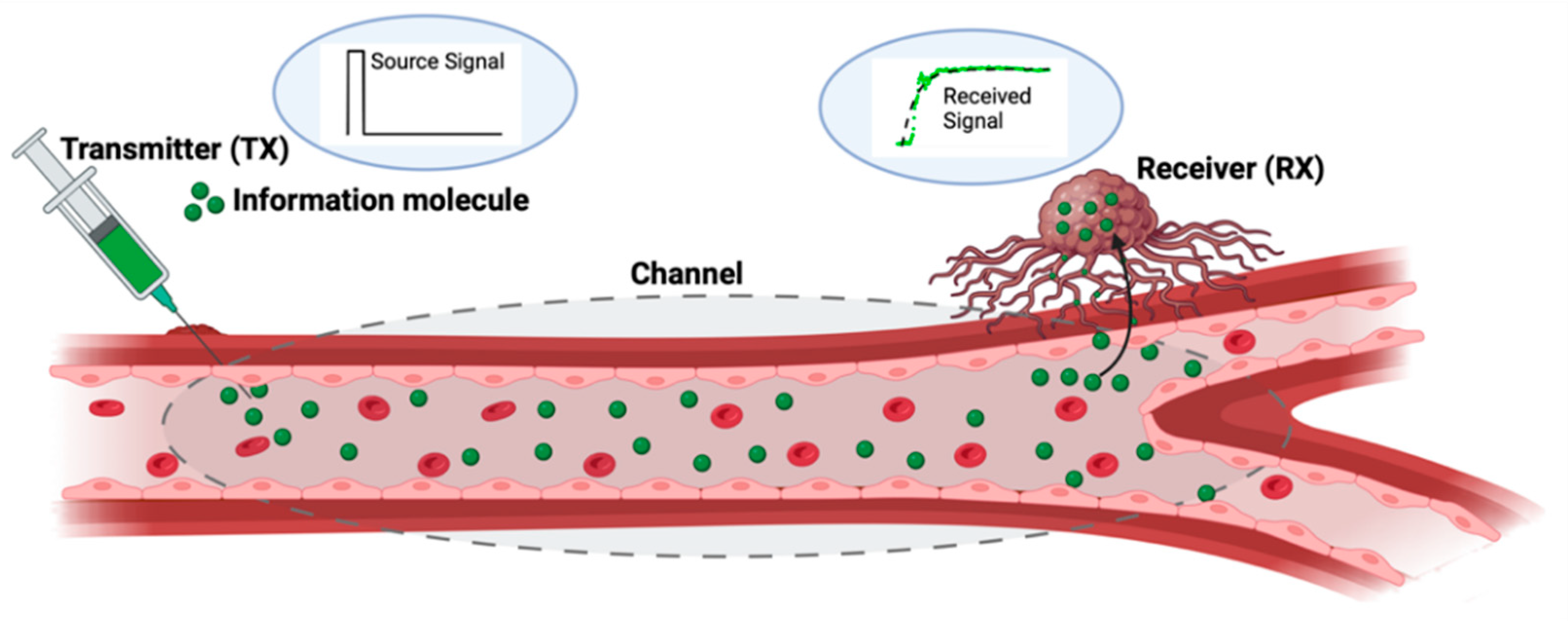
4.2. Characterization and Optimization Employing the Molecular Communications Paradigm
4.3. Organoids and CAM Model
4.4. Impact of Cryopreservation on Personalized Therapy Approaches Recommended by the Molecular Tumor Board
4.5. Advantages of the CAM Model as a Drug-Testing Platform
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schima, W.; Ba-Ssalamah, A.; Kölblinger, C.; Kulinna-Cosentini, C.; Puespoek, A.; Götzinger, P. Pancreatic adenocarcinoma. Eur. Radiol. 2007, 17, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2017, 67, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef]
- Chuong, M.D.; Herrera, R.; Ucar, A.; Aparo, S.; De Zarraga, F.; Asbun, H.; Jimenez, R.; Asbun, D.; Narayanan, G.; Joseph, S.; et al. Causes of Death Among Patients with Initially Inoperable Pancreas Cancer After Induction Chemotherapy and Ablative 5-fraction Stereotactic Magnetic Resonance Image Guided Adaptive Radiation Therapy. Adv. Radiat. Oncol. 2023, 8, 101084. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Bone marrow as a reservoir for disseminated tumor cells: A special source for liquid biopsy in cancer patients. Bonekey Rep. 2014, 3, 584. [Google Scholar] [CrossRef]
- Effenberger, K.E.; Schroeder, C.; Hanssen, A.; Wolter, S.; Eulenburg, C.; Tachezy, M.; Gebauer, F.; Izbicki, J.R.; Pantel, K.; Bockhorn, M. Improved Risk Stratification by Circulating Tumor Cell Counts in Pancreatic Cancer. Clin. Cancer Res. 2018, 24, 2844–2850. [Google Scholar] [CrossRef]
- Vanharanta, S.; Massagué, J. Origins of metastatic traits. Cancer Cell 2013, 24, 410–421. [Google Scholar] [CrossRef]
- Agudo, J.; Aguirre-Ghiso, J.A.; Bhatia, M.; Chodosh, L.A.; Correia, A.L.; Klein, C.A. Targeting cancer cell dormancy. Nat. Rev. Cancer 2023. [Google Scholar] [CrossRef]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Jentzsch, V.; Davis, J.A.A.; Djamgoz, M.B.A. Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers 2020, 12, 3096. [Google Scholar] [CrossRef]
- Jiang, S.; Fagman, J.B.; Ma, Y.; Liu, J.; Vihav, C.; Engstrom, C.; Liu, B.; Chen, C. A comprehensive review of pancreatic cancer and its therapeutic challenges. Aging 2022, 14, 7635–7649. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef]
- Sommers, S.C.; Sullivan, B.A.; Warren, S. Heterotransplantation of human cancer. III. Chorioallantoic membranes of embryonated eggs. Cancer Res. 1952, 12, 915–917. [Google Scholar] [PubMed]
- Kunzi-Rapp, K.; Genze, F.; Küfer, R.; Reich, E.; Hautmann, R.E.; Gschwend, J.E. Chorioallantoic membrane assay: Vascularized 3-dimensional cell culture system for human prostate cancer cells as an animal substitute model. J. Urol. 2001, 166, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Che, P.P.; Sarogni, P.; Sminia, P.; Giovannetti, E.; Voliani, V. Tumor grafted—Chick chorioallantoic membrane as an alternative model for biological cancer research and conventional/nanomaterial-based theranostics evaluation. Expert Opin. Drug Metab. Toxicol. 2021, 17, 947–968. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.-C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar]
- Ribatti, D. The chick embryo chorioallantoic membrane patient-derived xenograft (PDX) model. Pathol. Res. Pract. 2023, 243, 154367. [Google Scholar] [CrossRef]
- Majumder, S.; Chari, S.T.; Ahlquist, D.A. Molecular detection of pancreatic neoplasia: Current status and future promise. World J. Gastroenterol. 2015, 21, 11387–11395. [Google Scholar] [CrossRef]
- Zhen, D.B.; Safyan, R.A.; Konick, E.Q.; Nguyen, R.; Prichard, C.C.; Chiorean, E.G. The role of molecular testing in pancreatic cancer. Therap. Adv. Gastroenterol. 2023, 16, 17562848231171456. [Google Scholar] [CrossRef]
- Behel, V.; Noronha, V.; Choughule, A.; Shetty, O.; Chandrani, P.; Kapoor, A.; Bondili, S.K.; Bajpai, J.; Kumar, R.; Pai, T.; et al. Impact of Molecular Tumor Board on the Clinical Management of Patients with Cancer. JCO Glob. Oncol. 2022, 8, e2200030. [Google Scholar] [CrossRef]
- Idrisova, K.F.; Simon, H.-U.; Gomzikova, M.O. Role of Patient-Derived Models of Cancer in Translational Oncology. Cancers 2022, 15, 139. [Google Scholar] [CrossRef]
- Nakaseko, Y.; Ishizawa, T.; Saiura, A. Fluorescence-guided surgery for liver tumors. J. Surg. Oncol. 2018, 118, 324–331. [Google Scholar] [CrossRef]
- Soltesz, E.G.; Kim, S.; Kim, S.-W.; Laurence, R.G.; De Grand, A.M.; Parungo, C.P.; Cohn, L.H.; Bawendi, M.G.; Frangioni, J.V. Sentinel lymph node mapping of the gastrointestinal tract by using invisible light. Ann. Surg. Oncol. 2006, 13, 386–396. [Google Scholar] [CrossRef]
- Weixler, B.; Lobbes, L.A.; Scheiner, L.; Lauscher, J.C.; Staubli, S.M.; Zuber, M.; Raptis, D.A. The Value of Indocyanine Green Image-Guided Surgery in Patients with Primary Liver Tumors and Liver Metastases. Life 2023, 13, 1290. [Google Scholar] [CrossRef]
- Chauhan, N.; Cabrera, M.; Chowdhury, P.; Nagesh, P.K.B.; Dhasmana, A.; Pranav; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Indocyanine Green-based Glow Nanoparticles Probe for Cancer Imaging. Nanotheranostics 2023, 7, 353–367. [Google Scholar] [CrossRef]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Chude-Okonkwo, U.A.K.; Malekian, R.; Maharaj, B.T.; Vasilakos, A.V. Molecular Communication and Nanonetwork for Targeted Drug Delivery: A Survey. IEEE Commun. Surv. Tutor. 2017, 19, 3046–3096. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N. Peptide and protein drug delivery to and into tumors: Challenges and solutions. Drug Discov. Today 2003, 8, 259–266. [Google Scholar] [CrossRef]
- Salehi, S.; Moayedian, N.S.; Haghjooy Javanmard, S.; Alarcon, E. Lifetime Improvement of a Multiple Transmitter Local Drug Delivery System Based on Diffusive Molecular Communication. IEEE Trans. Nanobiosci. 2018, 17, 352–360. [Google Scholar] [CrossRef]
- Femminella, M.; Reali, G.; Vasilakos, A.V. A Molecular Communications Model for Drug Delivery. IEEE Trans. Nanobiosci. 2015, 14, 935–945. [Google Scholar] [CrossRef]
- Chahibi, Y.; Pierobon, M.; Song, S.O.; Akyildiz, I.F. A Molecular Communication System Model for Particulate Drug Delivery Systems. IEEE Trans. Biomed. Eng. 2013, 60, 3468–3483. [Google Scholar] [CrossRef]
- Ribatti, D. Two new applications in the study of angiogenesis the CAM assay: Acellular scaffolds and organoids. Microvasc. Res. 2022, 140, 104304. [Google Scholar] [CrossRef]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019, 9, 15663. [Google Scholar] [CrossRef]
- Hussein, N.A.; Mohamed, S.N.; Ahmed, M.A. Plasma ALU-247, ALU-115, and cfDNA Integrity as Diagnostic and Prognostic Biomarkers for Breast Cancer. Appl. Biochem. Biotechnol. 2019, 187, 1028–1045. [Google Scholar] [CrossRef]
- Szmulewicz, M.N.; Novick, G.E.; Herrera, R.J. Effects of Alu insertions on gene function. Electrophoresis 1998, 19, 1260–1264. [Google Scholar] [CrossRef]
- Wipper, S. Validierung der Fluoreszenzangiographie für die Intraoperative Beurteilung und Quantifizierung der Myokardperfusion; Ludwig-Maximilians-Universität München: München, Germany, 2006. [Google Scholar]
- Umetani, N.; Giuliano, A.E.; Hiramatsu, S.H.; Amersi, F.; Nakagawa, T.; Martino, S.; Hoon, D.S.B. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J. Clin. Oncol. 2006, 24, 4270–4276. [Google Scholar] [CrossRef]
- Samorani, D.; Fogacci, T.; Panzini, I.; Frisoni, G.; Accardi, F.G.; Ricci, M.; Fabbri, E.; Nicoletti, S.; Flenghi, L.; Tamburini, E.; et al. The use of indocyanine green to detect sentinel nodes in breast cancer: A prospective study. Eur. J. Surg. Oncol. 2015, 41, 64–70. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse reactions due to indocyanine green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Majlesara, A.; Golriz, M.; Hafezi, M.; Saffari, A.; Stenau, E.; Maier-Hein, L.; Müller-Stich, B.P.; Mehrabi, A. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagn. Photodyn. Ther. 2017, 17, 208–215. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Diana, M.; Boni, L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J. Gastroenterol. 2018, 24, 2921–2930. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Kyriakou, T.-C.; Papageorgis, P. Mechanisms of Metastatic Tumor Dormancy and Implications for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 6158. [Google Scholar] [CrossRef] [PubMed]
- Rovithi, M.; Avan, A.; Funel, N.; Leon, L.G.; Gomez, V.E.; Wurdinger, T.; Griffioen, A.W.; Verheul, H.M.W.; Giovannetti, E. Development of bioluminescent chick chorioallantoic membrane (CAM) models for primary pancreatic cancer cells: A platform for drug testing. Sci. Rep. 2017, 7, 44686. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.C.; Mönkemüller, K.; Malfertheiner, P. Molecular markers of pancreatic cancer: Development and clinical relevance. Langenbecks. Arch. Surg. 2008, 393, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.M.; Ribatti, D.; Raica, M. The chick embryo chorioallantoic membrane as a model to study tumor metastasis. Angiogenesis 2008, 11, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Feder, A.-L.; Pion, E.; Troebs, J.; Lenze, U.; Prantl, L.; Htwe, M.M.; Phyo, A.; Haerteis, S.; Aung, T. Extended analysis of intratumoral heterogeneity of primary osteosarcoma tissue using 3D-in-vivo-tumor-model. Clin. Hemorheol. Microcirc. 2020, 76, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Eckford, A.; Haraguchi, T. Molecular Communication; Cambridge University Press: Cambridge, UK, 2013; ISBN 978-1-107-02308-6. [Google Scholar]
- Kuscu, M.; Unluturk, B.D. Internet of Bio-Nano Things: A review of applications, enabling technologies and key challenges. ITU J. Future Evol. Technol. 2021, 2, 1–24. [Google Scholar] [CrossRef]
- Chahibi, Y. Molecular communication for drug delivery systems: A survey. Nano Commun. Netw. 2017, 11, 90–102. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.H.; Park, K. Smart Nanoparticles for Drug Delivery: Boundaries and Opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef]
- Schafer, M.; Salinas, Y.; Ruderer, A.; Enzenhofer, F.; Bruggemann, O.; Martinez-Manez, R.; Rabenstein, R.; Schober, R.; Haselmayr, W. Channel Responses for the Molecule Release from Spherical Homogeneous Matrix Carriers. IEEE Trans. Mol. Biol. Multi-Scale Commun. 2022, 8, 212–228. [Google Scholar] [CrossRef]
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.-S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials 2019, 192, 537–550. [Google Scholar] [CrossRef]
- Schmidt, S.; Alt, Y.; Deoghare, N.; Krüger, S.; Kern, A.; Rockel, A.F.; Wagner, N.; Ergün, S.; Wörsdörfer, P. A Blood Vessel Organoid Model Recapitulating Aspects of Vasculogenesis, Angiogenesis and Vessel Wall Maturation. Organoids 2022, 1, 41–53. [Google Scholar] [CrossRef]
- Kaisto, S.; Saarela, U.; Dönges, L.; Raykhel, I.; Skovorodkin, I.; Vainio, S.J. Optimization of Renal Organoid and Organotypic Culture for Vascularization, Extended Development, and Improved Microscopy Imaging. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Lim, J.; Ching, H.; Yoon, J.-K.; Jeon, N.L.; Kim, Y. Microvascularized tumor organoids-on-chips: Advancing preclinical drug screening with pathophysiological relevance. Nano Converg. 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Fluegen, G.; Garcia, P.; Ghaffari-Tabrizi-Wizsy, N.; Gribaldo, L.; Huang, R.Y.-J.; Rasche, V.; Ribatti, D.; Rousset, X.; Pinto, M.T.; et al. The CAM Model-Q&A with Experts. Cancers 2022, 15, 191. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettner-Sitter, A.; Montagner, A.; Kuenzel, J.; Brackmann, K.; Schäfer, M.; Schober, R.; Weber, F.; Aung, T.; Hackl, C.; Haerteis, S. Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing. Organoids 2024, 3, 1-17. https://doi.org/10.3390/organoids3010001
Ettner-Sitter A, Montagner A, Kuenzel J, Brackmann K, Schäfer M, Schober R, Weber F, Aung T, Hackl C, Haerteis S. Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing. Organoids. 2024; 3(1):1-17. https://doi.org/10.3390/organoids3010001
Chicago/Turabian StyleEttner-Sitter, Andreas, Agata Montagner, Jonas Kuenzel, Kathrin Brackmann, Maximilian Schäfer, Robert Schober, Florian Weber, Thiha Aung, Christina Hackl, and Silke Haerteis. 2024. "Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing" Organoids 3, no. 1: 1-17. https://doi.org/10.3390/organoids3010001
APA StyleEttner-Sitter, A., Montagner, A., Kuenzel, J., Brackmann, K., Schäfer, M., Schober, R., Weber, F., Aung, T., Hackl, C., & Haerteis, S. (2024). Visualization of Vascular Perfusion of Human Pancreatic Cancer Tissue in the CAM Model and Its Impact on Future Personalized Drug Testing. Organoids, 3(1), 1-17. https://doi.org/10.3390/organoids3010001