Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy
Abstract
1. Introduction
2. Diabetes Mellitus
3. Current Treatment of Canine Type 1 Diabetes
4. Current Treatment Failures
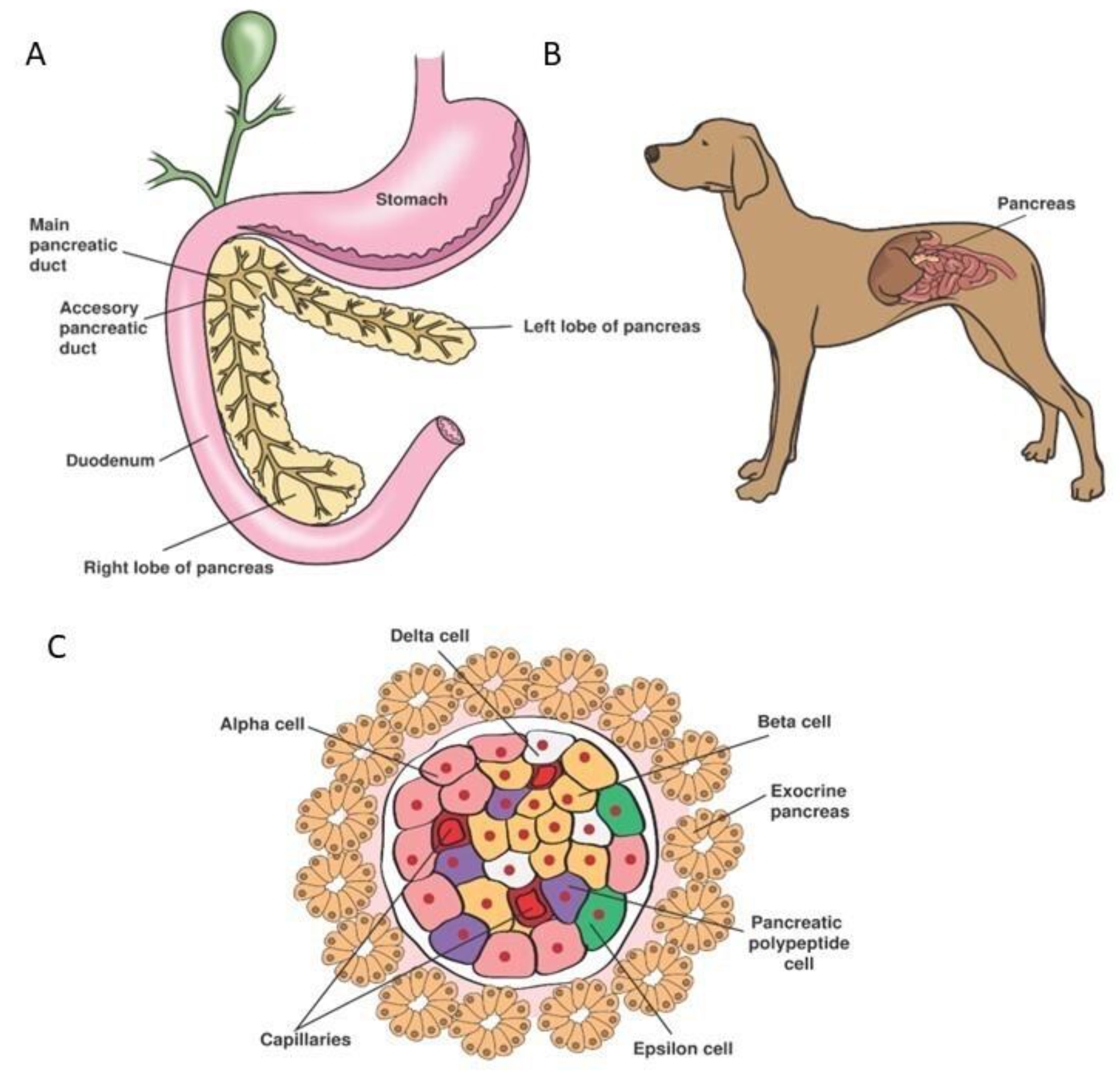
5. Organogenesis and Physiology of the Endocrine Pancreas
6. Future Treatment of Canine Type 1 Diabetes
6.1. Insulin Implantable Pumps and Continuous Glucose Monitoring Systems
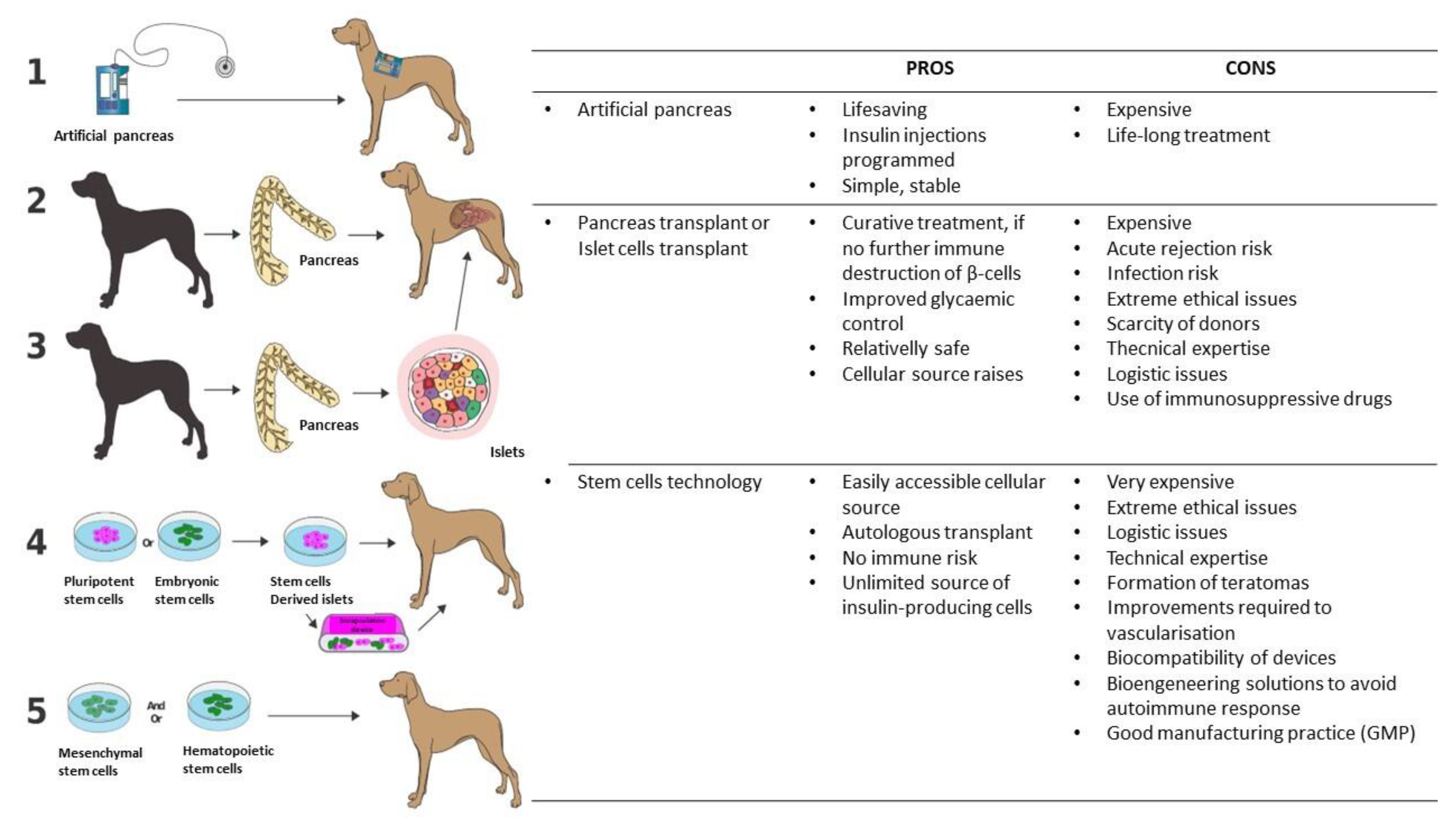
6.2. The Artificial Pancreas
6.3. Pancreatic or Islet Allotransplantations
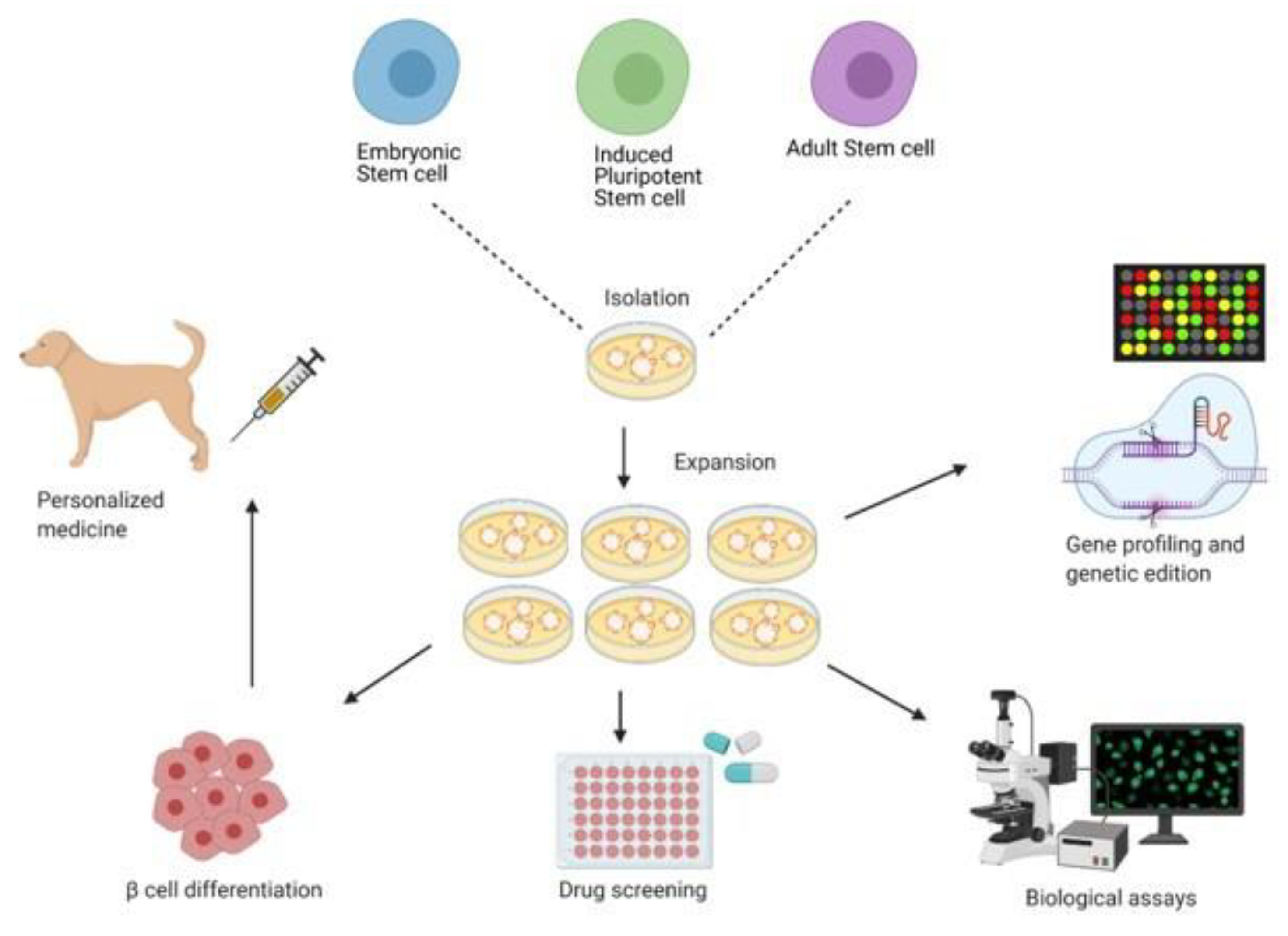
6.4. Stem Cell Approach to the Treatment and Cure of T1DM
6.4.1. Embryonic Stem Cell and Induced Pluripotent Stem Cell
6.4.2. Adult Stem Cells
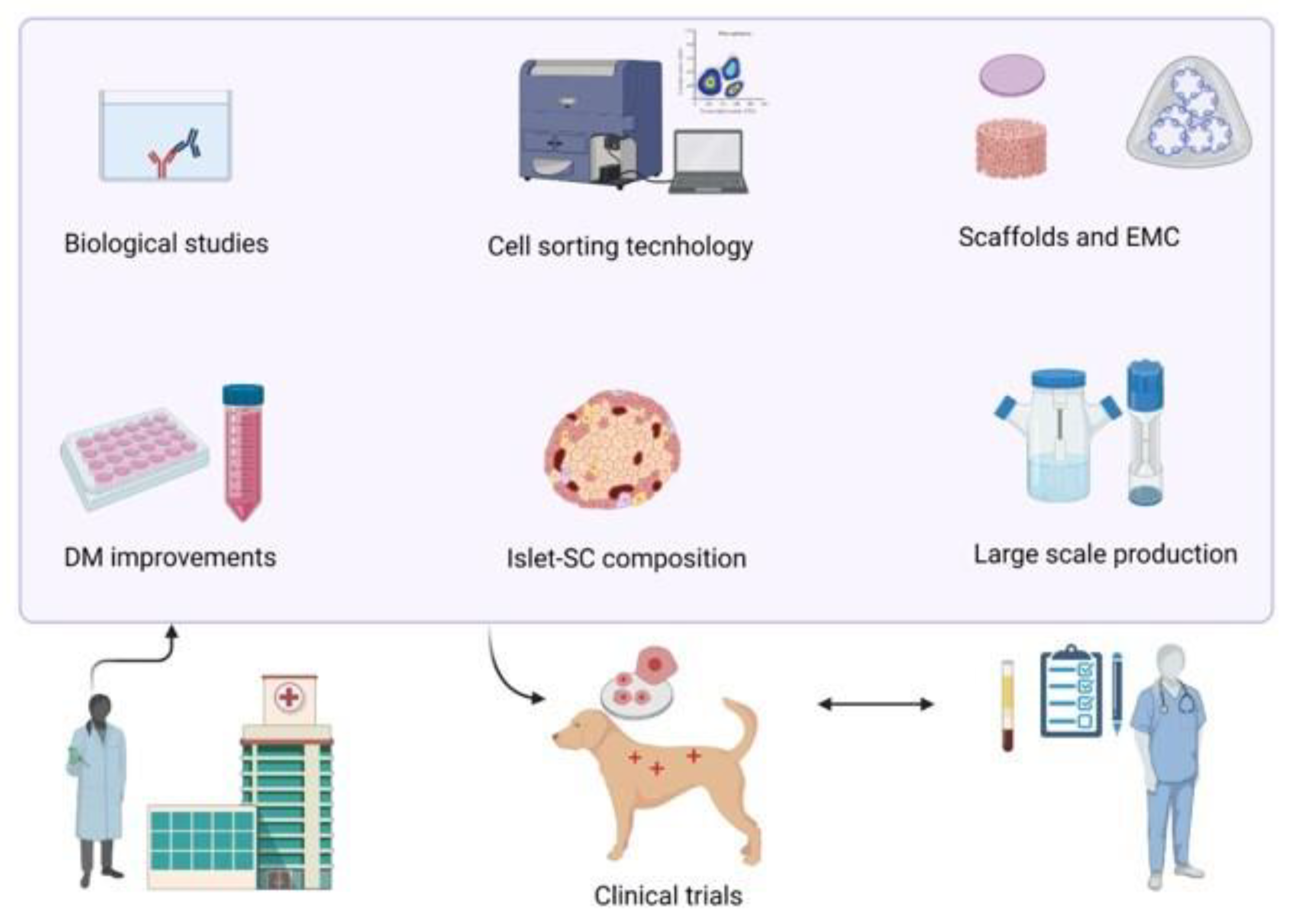
7. Canine Culture of β-Cells: Where Are We Now?
8. Discussion and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakula, A. Paul Langerhans (1847–1888): A centenary tribute. J. R. Soc. Med. 1988, 81, 414–415. [Google Scholar] [CrossRef]
- Luft, R. Oskar Minkowski: Discovery of the pancreatic origin of diabetes, 1889. Diabetologia 1989, 32, 399–401. [Google Scholar] [CrossRef]
- Tan, S.Y.; Merchant, J. Frederick Banting (1891–1941): Discoverer of insulin. Singap. Med. J. 2017, 58, 2–3. [Google Scholar] [CrossRef]
- Denyer, A.L.; Catchpole, B.; Davison, L.J. Genetics of canine diabetes mellitus part 2: Current understanding and future directions. Vet. J. 2021, 270, 105612. [Google Scholar] [CrossRef] [PubMed]
- Denyer, A.L.; Catchpole, B.; Davison, L.J. Genetics of canine diabetes mellitus part 1: Phenotypes of disease. Vet. J. 2021, 270, 105611. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization, Ed.; WHO: Geneva, Switzerland, 2019; Available online: http://apps.who.int/bookorders (accessed on 13 February 2024).
- Ahlgren, K.M.; Fall, T.; Landegren, N.; Grimelius, L.; von Euler, H.; Sundberg, K.; Lindblad-Toh, K.; Lobell, A.; Hedhammar, Å.; Andersson, G.; et al. Lack of evidence for a role of islet autoimmunity in the aetiology of canine diabetes mellitus. PLoS ONE 2014, 9, e105473. [Google Scholar] [CrossRef] [PubMed]
- Shields, E.J.; Lam, C.J.; Cox, A.R.; Rankin, M.M.; Van Winkle, T.J.; Hess, R.S.; Kushner, J.A. Extreme beta-cell deficiency in pancreata of dogs with canine diabetes. PLoS ONE 2015, 10, e0129809. [Google Scholar] [CrossRef] [PubMed]
- Behrend, E.; Holford, A.; Lathan, P.; Rucinsky, R.; Schulman, R. 2018 AAHA Diabetes Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2018, 54, 1–21. [Google Scholar] [CrossRef]
- Hess, R.S.; Kass, P.H.; Ward, C.R. Breed distribution of dogs with diabetes mellitus admitted to a tertiary care facility. J. Am. Vet. Med. Assoc. 2000, 216, 1414–1417. [Google Scholar] [CrossRef]
- Guptill, L.; Glickman, L.; Glickman, N. Time trends and risk factors for diabetes mellitus in dogs: Analysis of Veterinary Medical Data Base records (1970–1999). Vet. J. 2003, 165, 240–247. [Google Scholar] [CrossRef]
- Fall, T.; Hansson Hamlin, H.; Hedhammar, A.; Kämpe, O.; Egenvall, A. Diabetes Mellitus in a Population of 180,000 Insured Dogs: Incidence, Survival, and Breed Distribution. J. Vet. Intern. Med. 2007, 21, 1209–1216. [Google Scholar] [CrossRef]
- Nelson, R.W.; Reusch, C.E. Animal models of disease: Classification and etiology of diabetes in dogs and cats. J. Endocrinol. 2014, 222, T1–T9. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.S. Diagnosis of Diabetes Mellitus in Cats and Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.K. Monitoring Methods for Dogs and Cats with Diabetes Mellitus. J. Diabetes Sci. Technol. 2012, 6, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.; Christensen, N.J.; Iversen, J.; Ørskov, H. Role of glucagon and other hormones in development of diabetic ketoacidosis. Lancet 1975, 305, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Keller, U.; Chiasson, J.-L.; Liljenquist, J.E.; Cherrington, A.D.; Jennings, A.S.; Crofford, O.B. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes 1977, 26, 1040–1051. [Google Scholar] [CrossRef]
- Niessen, S.J.; Hazuchova, K.; Powney, S.L.; Guitian, J.; Niessen, A.P.; Pion, P.D.; Shaw, J.A.; Church, D.B. The big pet diabetes survey: Perceived frequency and triggers for euthanasia. Vet. Sci. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Reusch, C.E.; Liehs, M.R.; Hoyer, M.; Vochezer, R. Fructosamine. A new parameter for diagnosis and metabolic control in diabetic dogs and cats. J. Vet. Intern. Med. 1993, 7, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Sako, T.; Mori, A.; Lee, P.; Sato, T.; Mizutani, H.; Takahashi, T.; Kiyosawa, Y.; Tazaki, H.; Arai, T. Serum glycated albumin: Potential use as an index of glycemic control in diabetic dogs. Vet. Res. Commun. 2009, 33, 473–479. [Google Scholar] [CrossRef]
- Loste, A.; Marca, M.C. Fructosamine and glycated hemoglobin in the assessment of glycaemic control in dogs. Vet. Res. 2001, 32, 55–62. [Google Scholar] [CrossRef]
- Blaxter, A.C.; Cripps, P.J.; Gruffydd-Jones, T.J. Dietary fibre and post prandial hyperglycaemia in normal and diabetic dogs. J. Small Anim. Pract. 1990, 31, 229–233. [Google Scholar] [CrossRef]
- Graham, P.A.; Maskell, I.E.; Rawlings, J.M.; Nash, A.S.; Markwell, P.J. Influence of a high fibre diet on glycaemic control and quality of life in dogs with diabetes mellitus. J. Small Anim. Pract. 2002, 43, 67–73. [Google Scholar] [CrossRef]
- Cohn, L.A.; McCaw, D.L.; Tate, D.J.; Johnson, J.C. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J. Am. Vet. Med. Assoc. 2000, 216, 198–202. [Google Scholar] [CrossRef] [PubMed]
- van de Maele, I.; Rogier, N.; Daminet, S. Retrospective study of owners’ perception on home monitoring of blood glucose in diabetic dogs and cats. Can. Vet. J. 2005, 46, 718. [Google Scholar] [PubMed]
- Keenan, D.B.; Mastrototaro, J.J.; Voskanyan, G.; Steil, G.M. Delays in Minimally Invasive Continuous Glucose Monitoring Devices: A Review of Current Technology. J. Diabetes Sci. Technol. 2009, 3, 1207–1214. [Google Scholar] [CrossRef]
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia 2007, 50, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Heading, K. Hypoglycemia in Dogs: Causes, Management, and Diagnosis. Can. Vet. J. 2018, 59, 642–649. [Google Scholar] [PubMed]
- Shih, H.P.; Wang, A.; Sander, M. Pancreas organogenesis: From lineage determination to morphogenesis. Annu. Rev. Cell Dev. Biol. 2013, 29, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.A.; Swift, G.H.; Hoang, C.Q.; Deering, T.G.; Masui, T.; Lee, Y.-K.; Xue, J.; MacDonald, R.J. The nuclear hormone receptor family member NR5A2 controls aspects of multipotent progenitor cell formation and acinar differentiation during pancreatic organogenesis. Development 2014, 141, 3123–3133. [Google Scholar] [CrossRef]
- Nissim, S.; Weeks, O.; Talbot, J.C.; Hedgepeth, J.W.; Wucherpfennig, J.; Schatzman-Bone, S.; Swinburne, I.; Cortes, M.; Alexa, K.; Megason, S.; et al. Iterative use of nuclear receptor Nr5a2 regulates multiple stages of liver and pancreas development. Dev. Biol. 2016, 418, 108–123. [Google Scholar] [CrossRef]
- Grapin-Botton, A.; Majithia, A.R.; Melton, D.A. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001, 15, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.E.; Berry, A.A.; Kirkwood-Wilson, R.; Roberts, N.A.; Hearn, T.; Salisbury, R.J.; Blaylock, J.; Hanley, K.P.; Hanley, N.A. Development of the human pancreas from foregut to endocrine commitment. Diabetes 2013, 62, 3514–3522. [Google Scholar] [CrossRef] [PubMed]
- Bricout-Neveu, E.; Pechberty, S.; Reynaud, K.; Maenhoudt, C.; José Lecomte, M.; Ravassard, P.; Czernichow, P. Development of the Endocrine Pancreas in the Beagle Dog: From Fetal to Adult Life. Anat. Rec. 2017, 300, 1429–1438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gepts, W.; Veld, P.A. Morphology of the normal and diabetic endocrine pancreas. In Clinical Diabetes. An Illustrated Text; Besser, G.M., Bodansky, H.J., Cudworth, A.G., Eds.; London Gower Medical Publishing: London, UK, 1988; pp. 1.1–1.14. [Google Scholar] [CrossRef]
- Johnston, C.F.; Shaw, C.; O’Hare, M.M.T.; Buchanan, K.D. Anatomy and Physiology of the Pancreatic Islets. In Clinical Diabetes. An Illustrated Text; Besser, G.M., Bodansky, H.J., Cudworth, A.G., Eds.; London Gower Medical Publishing: London, UK, 1988; pp. 1.1–1.14. [Google Scholar]
- da Silva Xavier, G. The Cells of the Islets of Langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.J.; Kim, A.; Miller, K.; Hara, M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2010, 2, 135–145. [Google Scholar] [CrossRef]
- Brissova, M.; Fowler, M.J.; Nicholson, W.E.; Chu, A.; Hirshberg, B.; Harlan, D.M.; Powers, A.C. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 2005, 53, 1087–1097. [Google Scholar] [CrossRef]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.-O.; Caicedo, A. The Unique Cytoarchitecture of Human Pancreatic Islets Has Implications for Islet Cell Function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2349. [Google Scholar] [CrossRef] [PubMed]
- Muraro, M.J.; Dharmadhikari, G.; Grün, D.; Groen, N.; Dielen, T.; Jansen, E.; van Gurp, L.; Engelse, M.A.; Carlotti, F.; de Koning, E.J.; et al. A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst. 2016, 3, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.S.; Barve, A.; Muraro, M.J.; van der Linden, R.; Dharmadhikari, G.; Lyubimova, A.; de Koning, E.J.; van Oudenaarden, A. Cell Type Purification by Single-Cell Transcriptome-Trained Sorting. Cell 2019, 179, 527–542. [Google Scholar] [CrossRef]
- van Gurp, L.; Muraro, M.J.; Dielen, T.; Seneby, L.; Dharmadhikari, G.; Gradwohl, G.; van Oudenaarden, A.; de Koning, E.J.P. A transcriptomic roadmap to α- and β-cell differentiation in the embryonic pancreas. Development 2019, 146, dev173716. [Google Scholar] [CrossRef]
- Elrick, H.; Hlad, C.J.; Arai, Y.; Smith, A. The interaction of glucagon and insulin on blood glucose. J. Clin. Investig. 1956, 35, 757–762. [Google Scholar] [CrossRef]
- Yang, L.; Yang, D.; de Graaf, C.; Moeller, A.; West, G.M.; Dharmarajan, V.; Wang, C.; Siu, F.Y.; Song, G.; Reedtz-Runge, S.; et al. Conformational states of the full-length glucagon receptor. Nat. Commun. 2015, 6, 7859. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Laschke, M.W. Regulatory mechanisms of somatostatin expression. Int. J. Mol. Sci. 2020, 21, 4170. [Google Scholar] [CrossRef]
- Aragón, F.; Karaca, M.; Novials, A.; Maldonado, R.; Maechler, P.; Rubí, B. Pancreatic polypeptide regulates glucagon release through PPYR1 receptors expressed in mouse and human alpha-cells. Biochim. Biophys. Acta 2015, 1850, 343–351. [Google Scholar] [CrossRef]
- Sakata, N.; Yoshimatsu, G.; Kodama, S. Development and characteristics of pancreatic epsilon cells. Int. J. Mol. Sci. 2019, 20, 1867. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 636–676. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Fieten, H.; Penning, L.C.; Leegwater, P.A.J.; Rothuizen, J. New canine models of copper toxicosis: Diagnosis, treatment, and genetics. Ann. N. Y. Acad. Sci. 2014, 1314, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Nantasanti, S.; Spee, B.; Kruitwagen, H.S.; Chen, C.; Geijsen, N.; Oosterhoff, L.A.; van Wolferen, M.E.; Pelaez, N.; Fieten, H.; Wubbolts, R.W.; et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Rep. 2015, 5, 895–907. [Google Scholar] [CrossRef]
- Kruitwagen, H.S.; Oosterhoff, L.A.; Vernooij, I.G.; Schrall, I.M.; van Wolferen, M.E.; Bannink, F.; Roesch, C.; van Uden, L.; Molenaar, M.R.; Helms, J.B.; et al. Long-Term Adult Feline Liver Organoid Cultures for Disease Modeling of Hepatic Steatosis. Stem Cell Rep. 2017, 8, 822–830. [Google Scholar] [CrossRef]
- Kruitwagen, H.S.; Fieten, H.; Penning, L.C. Towards bioengineered liver stem cell transplantation studies in a preclinical dog model for inherited copper toxicosis. Bioengineering 2019, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biol. 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Pratscher, B.; Meneses, A.M.C.; Tschulenk, W.; Walter, I.; Swoboda, A.; Kruitwagen, H.S.; Schneeberger, K.; Penning, L.C.; Spee, B.; et al. Generation of Differentiating and Long-Living Intestinal Organoids Reflecting the Cellular Diversity of Canine Intestine. Cells 2020, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- Tekes, G.; Ehmann, R.; Boulant, S.; Stanifer, M.L. Development of Feline Ileum- and Colon-Derived Organoids and Their Potential Use to Support Feline Coronavirus Infection. Cells 2020, 9, 2085. [Google Scholar] [CrossRef] [PubMed]
- Wiener, D.J.; Studer, I.C.; Brunner, M.A.; Hermann, A.; Vincenti, S.; Zhang, M.; Groch, K.R.; Welle, M.M. Characterization of canine epidermal organoid cultures by immunohistochemical analysis and quantitative PCR. Vet. Dermatol. 2021, 32, 179-e44. [Google Scholar] [CrossRef]
- van Beers, C.A.J.; Devries, J.H. Continuous Glucose Monitoring: Impact on Hypoglycemia. J. Diabetes Sci. Technol. 2016, 10, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Davison, L.J.; Slater, L.A.; Herrtage, M.E.; Church, D.B.; Judge, S.; Ristic, J.M.E.; Catchpole, B. Evaluation of a continuous glucose monitoring system in diabetic dogs. J. Small Anim. Pract. 2003, 44, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wiedmeyer, C.E.; Johnson, P.J.; Cohn, L.A.; Meadows, R.L. Evaluation of a continuous glucose monitoring system for use in dogs, cats, and horses. J. Am. Vet. Med. Assoc. 2003, 223, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Pilosio, B.; Dondi, F.; Linari, G.; Testa, S.; Brugnoli, F.; Gianella, P.; Pietra, M.; Fracassi, F. Accuracy of a flash glucose monitoring system in diabetic dogs. J. Vet. Intern. Med. 2016, 30, 983–988. [Google Scholar] [CrossRef]
- Scavini, M.; Day, P.W.; Eaton, R.P. Long-Term Implantation of a New Implantable Programmable Insulin Pump in Two Diabetic Dogs. Artif. Organs 1992, 16, 518–522. [Google Scholar] [CrossRef]
- Kropff, J.; DeVries, J.H. Continuous glucose monitoring, future products, and update on worldwide artificial pancreas projects. Diabetes Technol. Ther. 2016, 18, S2–S53. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Yamasaki, Y.; Kawamori, R.; Hakui, N.; Abe, H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet 1982, 320, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Shimoda, S.; Sakakida, M.; Nishida, K.; Sekigami, T.; Ichimori, S.; Ichinose, K.; Shichiri, M.; Araki, E. Strict glycemic control in diabetic dogs with closed-loop intraperitoneal insulin infusion algorithm designed for an artificial endocrine pancreas. J. Artif. Organs 2003, 6, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Hanazaki, K.; Yazawa, K.; Ashizawa, S.; Fisher, W.E.; Wang, X.-P.; Nose, Y.; Brunicardi, F.C. Pancreatic polypeptide administration reduces insulin requirements of artificial pancreas in pancreatectomized dogs. Artif. Organs 2005, 29, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Lee, P.; Yokoyama, T.; Oda, H.; Saeki, K.; Miki, Y.; Nozawa, S.; Azakami, D.; Momota, Y.; Makino, Y.; et al. Evaluation of artificial pancreas technology for continuous blood glucose monitoring in dogs. J. Artif. Organs 2011, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Munekage, M.; Yatabe, T.; Kitagawa, H.; Takezaki, Y.; Tamura, T.; Namikawa, T.; Hanazaki, K. An artificial pancreas provided a novel model of blood glucose level variability in beagles. J. Artif. Organs 2015, 18, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Domínguez, M. Historical background of pancreatic islet isolation. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 938. [Google Scholar] [CrossRef]
- Casanova, D. Pancreas transplantation: 50 years of experience. Cirugía Española 2017, 95, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Gayet, R.; Guillaumie, M. La regulation de la secretion interne pancreatique par un processus humoral, demontree par des transplantations de pancreas. CR Soc. Biol. 1927, 97, 1613. [Google Scholar]
- Lichtenstein, I.L.; Barschak, R.M. Experimental transplantation of the pancreas in dogs. J. Int. Coll. Surg. 1957, 28, 1–6. [Google Scholar]
- Reemtsma, K.; Lucas, J.F., Jr.; Rogers, R.E.; Schmidt, F.E.; Davis, F.H., Jr. Islet cell function of the transplanted canine pancreas. Ann. Surg. 1963, 158, 645–654. [Google Scholar] [CrossRef]
- Bergan, J.J.; Hoehn, J.G.; Porter, N.; Dry, L. Total pancreatic allografts in pancreatectomized dogs. Arch. Surg. 1965, 90, 521–526. [Google Scholar] [CrossRef]
- Ota, K.; Mori, S.; Nobori, M.; Inou, T. Allotransplantation of the pancreas in dogs. J. Surg. Res. 1967, 7, 207–214. [Google Scholar] [CrossRef]
- Meyer, W.; Castelfranchi, P.L.; Ruiz, O.J.; Aquino, C.J.; Lillehei, R.C. Pancreas allotransplantation without duodenum. J. Surg. Res. 1972, 12, 128–137. [Google Scholar] [CrossRef]
- Shyr, Y.-M.; Su, C.-H.; Li, A.F.; Wu, C.-W.; Lui, W.-Y. Canine pancreas allotransplantation with enteric drainage. Chin. Med. J. Free. China Ed. 2002, 65, 483–488. [Google Scholar]
- Stucker, F.; Ackermann, D. Immunosuppressive drugs-how they work, their side effects and interactions. Ther. Umsch. Rev. Ther. 2011, 68, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.B.; Knight, M.J.; Scharp, D.W.; Ballinger, W.F.; Lacy, P.E. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia 1973, 9, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Alderson, D.; Farndon, J.R. The metabolic effects of islet transplantation in the diabetic dog. Transplant. Proc. 1984, 16, 831–833. [Google Scholar] [PubMed]
- Evans, M.G.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Reversal of diabetes by transplantation of pure cryopreserved canine islets. Transplant. Proc. 1990, 22, 543–544. [Google Scholar] [CrossRef]
- Brons, I.G.; Davies, H.S.; Makisalo, H.; Rasmussen, A.; Cobbold, S.P.; Waldmann, H.; Calne, R.Y. Transplantation of immunomodulated dog islets. Transplant. Proc. 1994, 26, 754. [Google Scholar]
- van der Burg, M.P.M.; van Suylichem, P.T.R.; Guicherit, O.R.; Frolich, M.; Lemkes, H.H.P.J.; Gooszen, H.G. Glucoregulation after Canine Islet Transplantation: Contribution of Insulin Secretory Capacity, Insulin Action, and the Entero-Insular Axis. Cell Transplant. 1997, 6, 497–503. [Google Scholar] [CrossRef]
- Yang, H.K.; Ham, D.-S.; Park, H.-S.; Rhee, M.; You, Y.H.; Kim, M.J.; Shin, J.; Kim, O.-Y.; Khang, G.; Hong, T.H.; et al. Long-term efficacy and biocompatibility of encapsulated islet transplantation with chitosan-coated alginate capsules in mice and canine models of diabetes. Transplantation 2016, 100, 334–343. [Google Scholar] [CrossRef]
- Alejandro, R.; Cutfield, R.; Shienvold, F.L.; Latif, Z.; Mintz, D.H. Successful Long-Term Survival of Pancreatic Islet Allografts in Spontaneous or Pancreatectomy-induced Diabetes in Dogs: Cyclosporine-induced Immune Unresponsiveness. Diabetes 1985, 34, 825–828. [Google Scholar] [CrossRef][Green Version]
- Alejandro, R.; Feldman, E.C.; Shienvold, F.L.; Mintz, D.H. Advances in canine diabetes mellitus research: Etiopathology and results of islet transplantation. J. Am. Vet. Med. Assoc. 1988, 193, 1050–1055. [Google Scholar]
- Brunetti, P.; Basta, G.; Faloerni, A.; Calcinaro, F.; Pietropaolo, M.; Calafiore, R. Immunoprotection of Pancreatic Islet Grafts within Artificial Microcapsules. Int. J. Artif. Organs 1991, 14, 789–791. [Google Scholar] [CrossRef]
- Lanza, R.P.; Borland, K.M.; Staruk, J.E.; Appel, M.C.; Solomon, B.A.; Chick, W.L. Transplantation of encapsulated canine islets into spontaneously diabetic BB/Wor rats without immunosuppression. Endocrinology 1992, 131, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Kuhtreiber, W.M.; Chick, W.L. Encapsulation Technologies. Tissue Eng. 1995, 1, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Soon-Shiong, P.; Feldman, E.; Nelson, R.; Komtebedde, J.; Smidsrod, O.; Skjak-Braek, G.; Espevik, T.; Heintz, R.; Lee, M. Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation 1992, 54, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Ecker, D.M.; Kühtreiber, W.M.; Marsh, J.P.; Ringeling, J.; Chick, W.L. Transplantation of Islets Using Microencapsulation: Studies in Diabetic Rodents and Dogs. J. Mol. Med. 1999, 77, 206–210. [Google Scholar] [CrossRef]
- Abalovich, A.G.; Bacqué, M.C.; Grana, D.; Milei, J. Pig Pancreatic Islet Transplantation Into Spontaneously Diabetic Dogs. Transplant. Proc. 2009, 41, 328–330. [Google Scholar] [CrossRef]
- Paterson, Y.Z.; Kafarnik, C.; Guest, D.J. Characterization of companion animal pluripotent stem cells. Cytom. Part A 2018, 93, 137–148. [Google Scholar] [CrossRef]
- Harrington, S.; Williams, S.J.; Otte, V.; Barchman, S.; Jones, C.; Ramachandran, K.; Stehno-Bittel, L. Improved yield of canine islet isolation from deceased donors. BMC Vet. Res. 2017, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, Z.; Kao, D.-I.; Amin, S.; Cook, B.; Rao, S.; Zhou, T.; Zhang, T.; Xiang, Z.; Kenyon, R.; Kaymakcalan, O.; et al. ROCKII inhibition promotes the maturation of human pancreatic beta-like cells. Nat. Commun. 2017, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Trott, J.; Tan, E.K.; Ong, S.; Titmarsh, D.M.; Denil, S.L.; Giam, M.; Wong, C.K.; Wang, J.; Shboul, M.; Eio, M.; et al. Long-Term Culture of Self-renewing Pancreatic Progenitors Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Hogrebe, N.J.; Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020, 38, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.B.K.; Gonçalves, C.A.C.; Kim, Y.H.; Grapin-Botton, A. Recapitulating and Deciphering Human Pancreas Development From Human Pluripotent Stem Cells in a Dish. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 129, pp. 143–190. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic β cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Guo, M.; Matsuoka, T.-A.; Hagman, D.K.; Parazzoli, S.D.; Poitout, V.; Stein, R. The islet β cell-enriched MafA activator is a key regulator of insulin gene transcription. J. Biol. Chem. 2005, 280, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Q.; Zhou, Z.; Ikeda, Y. PDX1, Neurogenin-3, and MAFA: Critical transcription regulators for beta cell development and regeneration. Stem Cell Res. Ther. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Takemitsu, H.; Zhao, D.; Ishikawa, S.; Michishita, M.; Arai, T.; Yamamoto, I. Mechanism of insulin production in canine bone marrow derived mesenchymal stem cells. Gen. Comp. Endocrinol. 2013, 189, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef]
- Kunisada, Y.; Tsubooka-Yamazoe, N.; Shoji, M.; Hosoya, M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012, 8, 274–284. [Google Scholar] [CrossRef]
- Russ, H.A.; Parent, A.V.; Ringler, J.J.; Hennings, T.G.; Nair, G.G.; Shveygert, M.; Guo, T.; Puri, S.; Haataja, L.; Cirulli, V.; et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015, 34, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Ko, U.H.; Oh, Y.; Lim, A.; Sohn, J.-W.; Shin, J.H.; Kim, H.; Han, Y.-M. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci. Rep. 2016, 6, 35145. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.G.; Fukuda, S.; Takeda, F.; Nashiro, K.; Shimoda, M.; Okochi, H. Efficient generation of functional pancreatic β-cells from human induced pluripotent stem cells. J. Diabetes 2017, 9, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Velazco-Cruz, L.; Song, J.; Maxwell, K.G.; Goedegebuure, M.M.; Augsornworawat, P.; Hogrebe, N.J.; Millman, J.R. Acquisition of Dynamic Function in Human Stem Cell-Derived β Cells. Stem Cell Rep. 2019, 12, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Riedel, M.J.; Mojibian, M.; Asadi, A.; Xu, J.; Gauvin, R.; Narayan, K.; Karanu, F.; O’neil, J.J.; et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012, 61, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Agulnick, A.D.; Ambruzs, D.M.; Moorman, M.A.; Bhoumik, A.; Cesario, R.M.; Payne, J.K.; Kelly, J.R.; Haakmeester, C.; Srijemac, R.; Wilson, A.Z.; et al. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl. Med. 2015, 4, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Pettus, J.; Reeds, D.; Cavaiola, T.S.; Boeder, S.; Levin, M.; Tobin, G.; Cava, E.; Thai, D.; Shi, J.; Yan, H.; et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2018, 20, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Cantarelli, E.; Sordi, V.; Nano, R.; Piemonti, L. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol. 2016, 53, 683–691. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.-S. Usage of human mesenchymal stem cells in cell-based therapy: Advantages and disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Bonner-Weir, S. Islet growth and development in the adult. J. Mol. Endocrinol. 2000, 24, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Montanya, E.; Nacher, V.; Biarnés, M.; Soler, J. Linear Correlation Between-Cell Mass and Body Weight Throughout the Lifespan in Lewis Rats Role of-Cell Hyperplasia and Hypertrophy. Diabetes 2000, 49, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Tyrberg, B.; Itkin-Ansari, P.; Lakey, J.R.T.; Geron, I.; Monosov, E.Z.; Barcova, M.; Mercola, M.; Levine, F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat. Med. 2006, 12, 310–316. [Google Scholar] [CrossRef]
- Seeberger, K.L.; Dufour, J.M.; Shapiro, A.M.J.; Lakey, J.R.T.; Rajotte, R.V.; Korbutt, G.S. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab. Investig. 2006, 86, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Solar, M.; Cardalda, C.; Houbracken, I.; Martín, M.; Maestro, M.A.; De Medts, N.; Xu, X.; Grau, V.; Heimberg, H.; Bouwens, L.; et al. Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Dev. Cell 2009, 17, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zulewski, H.; Abraham, E.J.; Gerlach, M.J.; Daniel, P.B.; Moritz, W.; Müller, B.; Vallejo, M.; Thomas, M.K.; Habener, J.F. Multipotential Nestin-Positive Stem Cells Isolated From Adult Pancreatic Islets Differentiate Ex Vivo Into Pancreatic Endocrine, Exocrine, and Hepatic Phenotypes. Diabetes 2001, 50, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.J.; Leech, C.A.; Lin, J.C.; Zulewski, H.; Habener, J.F. Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology 2002, 143, 3152–3161. [Google Scholar] [CrossRef]
- Georgia, S.; Bhushan, A. β cell replication is the primary mechanism for maintaining postnatal β cell mass. J. Clin. Investig. 2004, 114, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Habener, J.F.; Stanojevic, V. α-cell role in β-cell generation and regeneration. Islets 2012, 4, 188–198. [Google Scholar] [CrossRef]
- Huch, M.; Bonfanti, P.; Boj, S.F.; Sato, T.; Loomans, C.J.; Van De Wetering, M.; Sojoodi, M.; Li, V.S.; Schuijers, J.; Gracanin, A.; et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013, 32, 2708–2721. [Google Scholar] [CrossRef]
- Huch, M.; Bonfanti, P.; Boj, S.F.; Sato, T.; Loomans, C.J.M.; Van De Wetering, M.; Sojoodi, M.; Li, V.S.W.; Schuijers, J.; Gracanin, A.; et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 2013, 2013, e00940. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Loomans, C.J.; Giuliani, N.W.; Balak, J.; Ringnalda, F.; van Gurp, L.; Huch, M.; Boj, S.F.; Sato, T.; Kester, L.; Lopes, S.M.C.d.S.; et al. Expansion of Adult Human Pancreatic Tissue Yields Organoids Harboring Progenitor Cells with Endocrine Differentiation Potential. Stem Cell Rep. 2018, 10, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Adler, H.; Braun, J.; Kienzle, B.; Wolf, E.; Kolb, H.-J. Canine Embryo-Derived Stem Cells-Toward Clinically Relevant Animal Models for Evaluating Efficacy and Safety of Cell Therapies. Stem Cells 2007, 25, 1850–1851. [Google Scholar] [CrossRef]
- Hayes, B.; Fagerlie, S.R.; Ramakrishnan, A.; Baran, S.; Harkey, M.; Graf, L.; Bar, M.; Bendoraite, A.; Tewari, M.; Torok-Storb, B. Derivation, Characterization, and In Vitro Differentiation of Canine Embryonic Stem Cells. Stem Cells 2008, 26, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nakada, A.; Hashimoto, Y.; Shigeno, K.; Shionoya, Y.; Nakamura, T. Generation of canine induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol. Reprod. Dev. 2010, 77, 2. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, D.J.; Ovchinnikov, D.A.; Wolvetang, E.J. Generation and characterization of LIF-dependent canine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev. 2012, 21, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Thomas, R.; Tsai, S.; Bischoff, S.; Lim, J.-H.; Breen, M.; Olby, N.J.; Piedrahita, J.A. Growth Requirements and Chromosomal Instability of Induced Pluripotent Stem Cells Generated from Adult Canine Fibroblasts. Stem Cells Dev. 2013, 22, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.; Barsby, T.; Guest, D. Derivation of Canine Induced Pluripotent Stem Cells. Reprod. Domest. Anim. 2015, 50, 69–679. [Google Scholar] [CrossRef]
- Aguiar, B.A.; Orechio, D.; Fratini, P.; Carreira, A.C.O.; Castelucci, P.; Miglino, M.A. Isolation and Characterization of Pancreatic Canine Fetal Cells at the Final Stage of Gestation. Anat. Rec. 2019, 302, 1409–1418. [Google Scholar] [CrossRef]
- Hellerström, C.; Lewis, N.J.; Borg, H.; Johnson, R.; Freinkel, N. Method for large-scale isolation of pancreatic islets by tissue culture of fetal rat pancreas. Diabetes 1979, 28, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, P.; Reynaud, K.; Kerr-Conte, J.; Furthner, E.; Ravassard, P. Production, Characterization, and Function of Pseudoislets from Perinatal Canine Pancreas. Cell Transplant. 2019, 28, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Gooch, A.; Zhang, P.; Hu, Z.; Son, N.L.; Avila, N.; Fischer, J.; Roberts, G.; Sellon, R.; Westenfelder, C. Interim report on the effective intraperitoneal therapy of insulin-dependent diabetes mellitus in pet dogs using “Neo-Islets,” aggregates of adipose stem and pancreatic islet cells (INAD 012-776). PLoS ONE 2019, 14, e0218688. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Velazco-Cruz, L.; Goedegebuure, M.M.; Millman, J.R. Advances Toward Engineering Functionally Mature Human Pluripotent Stem Cell-Derived β Cells. Front. Bioeng. Biotechnol. 2020, 8, 786. [Google Scholar] [CrossRef]
| Source | Year | Procedure | Encapsulation Method | Outcome |
|---|---|---|---|---|
| Alejandro et al. [86] | 1985 | Packed islet cell volume of 1.5 mL or less. Immunosuppressive agent: cyclosporin. | Implantation in the liver. | Normoglycaemia for at least 30 days. In the absence of cyclosporin: allograft rejection within a range of 4–10 days. |
| Alejandro et al. [87] | 1988 | Multi-donor intrahepatic islet allografts. Immunosuppressive agent: cyclosporin. | Implantation in the liver. | Six dogs maintained normoglycaemia between 253 and 716 days. |
| Brunetti et al. [88] | 1991 | Enveloped porcine islets. No immunosuppression. | Microspheres in an artificial vascular prosthesis. | For 60 days, one dog reached complete insulin-independence. The others presented blood glucose levels near normal range. |
| Lanza et al. [89] | 1992 | Encapsulated canine islets. | Intraperitoneal implantation in rats. | Normoglycaemia for 1 month. |
| Lanza et al. [90] | 1995 | Encapsulated systems. | Intraperitoneal implantation. | Normoglycaemia mantained from 90–365 days. |
| Soon-Shiong et al. [91] | 1992 | Microencapsulated islets. Cyclosporin in subtherapeutic dose. | Intraperitoneal injection. | All dogs maintained normoglycaemia for 63 to 172 days. |
| Lanza et al. [92] | 1999 | Alginate gel microencapsulated islets. Low dose of cyclosporin. | Implantation into the peritoneum. | All dogs maintained normoglycaemia for 60 to 175 days. |
| Abalovich et al. [93] | 2009 | Polylysin-alginate microencapsulated porcine islets. No immunosuppression. | Implantation in the abdominal cavity. | Decrease in the need for exogenous insulin and higher plasma insulin levels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, F.C.M.; Voorbij, A.W.Y.; Pereira, E.C.; Alves e Almeida, L.M.M.; Moraes, G.R.; De Oliveira, J.T.; Gouw, B.H.T.; Legatti, S.A.M.; Kooistra, H.S.; Spee, B.; et al. Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy. Organoids 2024, 3, 67-82. https://doi.org/10.3390/organoids3020006
Oliveira FCM, Voorbij AWY, Pereira EC, Alves e Almeida LMM, Moraes GR, De Oliveira JT, Gouw BHT, Legatti SAM, Kooistra HS, Spee B, et al. Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy. Organoids. 2024; 3(2):67-82. https://doi.org/10.3390/organoids3020006
Chicago/Turabian StyleOliveira, Flavia C. M., Annemarie W. Y. Voorbij, Elisa C. Pereira, Leonor M. M. Alves e Almeida, Geanne R. Moraes, Joana T. De Oliveira, Boyd H. T. Gouw, Sabrina A. M. Legatti, Hans S. Kooistra, Bart Spee, and et al. 2024. "Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy" Organoids 3, no. 2: 67-82. https://doi.org/10.3390/organoids3020006
APA StyleOliveira, F. C. M., Voorbij, A. W. Y., Pereira, E. C., Alves e Almeida, L. M. M., Moraes, G. R., De Oliveira, J. T., Gouw, B. H. T., Legatti, S. A. M., Kooistra, H. S., Spee, B., Meneses, A. M. C., & Penning, L. C. (2024). Treatment of Canine Type 1 Diabetes Mellitus: The Long Road from Twice Daily Insulin Injection towards Long-Lasting Cell-Based Therapy. Organoids, 3(2), 67-82. https://doi.org/10.3390/organoids3020006








