Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India
Abstract
:1. Introduction
2. Results
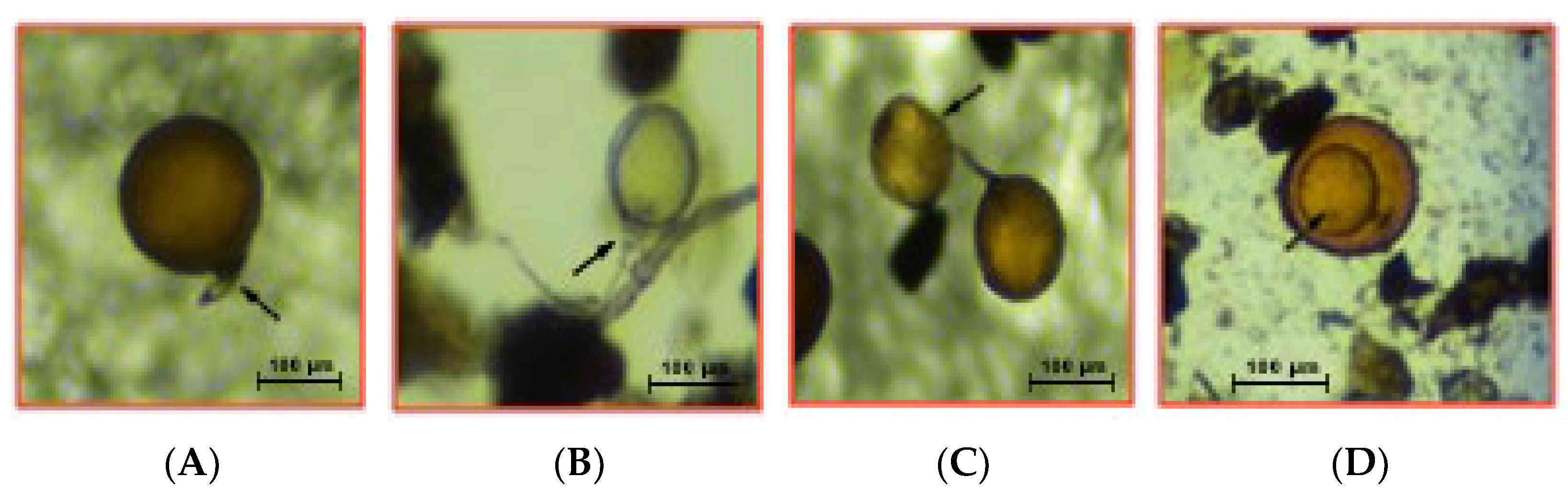
2.1. Quantification of AM Fungal Spores
2.2. Estimation of Available Phosphorus in Soil
3. Materials and Methods
3.1. Establishment of Permanent Preservation Plots (PPPs)
3.2. Location of the Plots
3.2.1. TWH PPP
3.2.2. BGKL PPP
3.2.3. DSP PPP
3.3. Sample Collection
3.4. Quantification of AM Fungal Spores
3.5. Estimation of Available Phosphorus in Soil
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rausch, C.; Daram, P.; Brunner, S.; Jansa, J.; Lalol, M.; Leggewele, G.; Amrhein, N.; Bucher, M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 2001, 414, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Bagyaraj, D.J. VA Mycorrhiza; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Pate, J.S. The Mycorrhizal association: Just one of many nutrient acquiring specialization in natural ecosystems. In Management of Mycorrhizas in Agriculture and Forestry; Robson, A., Abbott, L., Malajezuk, N., Eds.; Kluwer Academic Publishers: London, UK, 1994; pp. 1–10. [Google Scholar]
- Janos, D.P. Mycorrhizae influence tropical succession. Biotropica 1980, 12, 56–64. [Google Scholar] [CrossRef]
- Loyanachan, T.E. Mycorrhizae and their role towards natural resource management. In Proceedings of the Extended Summary, International Conference on Managing Natural Resources for Sustainable Agriculture Production in the 21st Century, New Delhi, India, 14–18 February 2000; pp. 14–18. [Google Scholar]
- Ravarkar, K.P.; Juma, N.G.; Mc Gill, W.B. Vesicular-arbuscular mycorrhiza hyphal bridges and N-transfer from legume to non-legume plant. In Proceedings of the Extended Summary International Conference on Managing Natural Resources for Sustainable Agriculture Production in the 21st Century, New Delhi, India, 14–18 February 2000; Volume 1, pp. 657–658. [Google Scholar]
- Zhang, J.; Quan, C.; Ma, L.; Chu, G.; Liu, Z.; Tang, X. Plant community and soil properties drive arbuscular mycorrhizal fungal diversity: A case study in tropical forests. Soil. Ecol. Lett. 2021, 3, 52–62. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Sansom, C.E.; Jones, E.E.; Perry, N.B.; Monk, J.; Ridgway, H.J. Arbuscular mycorrhizal fungi associated with Leptospermum scoparium (manuka): Effects on plant growth and essential oil content. Symbiosis 2018, 75, 39–50. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.J.; Zhang, K.; Tian, C.Y.; Guo, J.X. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crop. Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Santana, S.R.; Voltolini, T.V.; Antunes, G.D.; da Silva, V.M.; Simões, W.L.; Morgante, C.V.; de Freitas, A.D.; Chaves, A.R.; Aidar, S.D.; Fernandes-Júnior, P.I. Inoculation of plant growth-promoting bacteria attenuates the negative effects of drought on sorghum. Arch. Microbiol. 2020, 202, 1015–1024. [Google Scholar] [CrossRef]
- Kakkar, R.; Kumar, K.V.; Remadevi, O.K.; Manjunatha, M.; Saritha, B.; Sharma, B.; Kiranraddi, M.; Dattaraja, H.S.; Suresh, H.S. Establishing permanent preservation plots in Bannerghatta National Park for long-term ecological studies to monitor climate change. My For. 2018, 54, 19–34. [Google Scholar]
- Bever, J.D.; Schultz, P.A.; Pringle, A.; Morton, H.B. Arbuscular mycorrhizal fungi: More diverse than meets the eye, and the ecological tale of why. Bioscience 2001, 51, 923–932. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Su, Y.; Zhang, Y.; Wu, M.; Zhang, Z.; Pei, K.; Sun, L.; Wan, S.; Liang, Y. Diversity of arbuscular mycorrhizal fungal spore communities and its relations to plants under increased temperature and precipitation in a natural grassland. Chi Sci Bull. 2013, 58, 4109–4119. [Google Scholar] [CrossRef] [Green Version]
- Hiiesalu, I.; Pärtel, M.; Davison, J.; Gerhold, P.; Metsis, M.; Moora, M.; Öpik, M.; Vasar, M.; Zobel, M.; Wilson, S.D. Species richness of arbuscular mycorrhizal fungi: Associations with grassland plant richness and biomass. New Phytol. 2014, 203, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.D.; Dai, M.; Gomez, E.F.; Torres-Arias, Y.; Bainard, J.D.; Sheng, M.; Eilers, W.; Hamel, C. Arbuscular mycorrhizal fungal communities are influenced by agricultural land use and not soil type among the Chernozem great groups of the Canadian Prairies. Plant Soil 2015, 387, 351–362. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, Y.; Björn, L.O.; Rosendahl, S.; Olsson, P.A.; Li, S.; Fu, X. Community dynamics of arbuscular mycorrhizal fungi in high-input and intensively irrigated rice cultivation systems. Appl. Environ. Microbiol. 2015, 1, 2958–2965. [Google Scholar] [CrossRef] [Green Version]
- Joner, E.J. The effect of long-term fertilization with organic or inorganic fertilizers on mycorrhiza-mediated phosphorus uptake in subterranean clover. Biol. Fert. Soils. 2000, 32, 435–440. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Vestberg, M.; Walker, C.; Hurme, T.; Zhang, X.; Lindström, K. Diversity and infectivity of arbuscular mycorrhizal fungi in agricultural soils of the Sichuan Province of mainland China. Mycorrhiza 2008, 18, 59–68. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Teixeira, H.; Correia, M.; Timóteo, S.; Heleno, R.; Öpik, M.; Moora, M. Arbuscular mycorrhizal fungi communities from tropical Africa reveal strong ecological structure. New Phytol 2017, 213, 380–390. [Google Scholar] [CrossRef]
- Freitas, R.O.; Buscardo, E.; Nagy, L.; Maciel, A.B.S.; Carrenho, R.; Luizão, R.C.C. Erratum to: Arbuscular mycorrhizal fungal communities along a pedo-hydrological gradient in a Central Amazonian terra firme forest. Mycorrhiza 2014, 24, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.M.R.; Silva, D.K.A.D.; Goto, B.T.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in Atlantic forest areas under different land uses. Agric. Ecosyst. Environ. 2014, 185, 245–252. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, J.; Sánchez-Gallen, I.; Hernández-Cuevas, L.; Hernández, L.; Cruz, C. What can the arbuscular mycorrhizal fungi community tell us about plant biodiversity loss? In Recent Advances on Mycorrhizal Fungi, Fungi Biology; Pagano, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 23–33. [Google Scholar]
- Álvarez-Sánchez, J.J.; Antoninka, N.C.; Chaudhary, A.; Lau, V.B.; Owen, M.K.; Sánchez-Gallen, S.M.; Guadarrama, I.; Castillo, P.S. Large-scale diversity patterns in spore communities of arbuscular mycorrhizal fungi. In Mycorrhiza Ocurrence in Natural and Restored Environments; Pagano, M., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 29–47. [Google Scholar]
- Senapati, M.; Das, A.B.; Das, P. Association of vesicular arbuscular mycorrhizal fungi with 21 forest tree species. Indian J. For. 2000, 23, 326–331. [Google Scholar]
- Singh, S.S.; Tiwari, S.C.; Dkhar, M.S. Species Diversity of Vesicular-Arbuscular Mycorrhizal (VAM) Fungi in Jhum Fallow and Natural Forest Soils of Arunachal Pradesh, North Eastern India. Trop. Ecol. 2003, 44, 207–215. [Google Scholar]
- Dhar, P.P.; Mridha, M.A.U. Arbuscular mycorrhizal associations in different forest tree species of Hazarikhil forest of Chittagong, Bangladesh. J. For. Res. 2012, 23, 115–122. [Google Scholar] [CrossRef]
- Wubet, T.; Weiss, M.; Kottke, I.; Teketay, D.; Ober Winkler, F. Molecular diversity of arbuscular mycorrhizal fungi in Prunus africana, an endangered medicinal tree species in dry Afromontane forests of Ethiopia. New Phytol. 2004, 161, 517–528. [Google Scholar] [CrossRef]
- Cong, L.C.; Ye, Z.S.; Lei, L.; Huang, J.S. Arbuscular mycorrhizal fungi associated with common tree species in a tropical rain forest in Bawangling of Hainan Island, China. Chin. J. Ecol. 2010, 29, 269–273. [Google Scholar]
- Mosse, B.; Bowen, G.D. A key to the recognition, of some Endogone spore types. Trans. Br. Mycol. Soc. 1968, 51, 485–492. [Google Scholar] [CrossRef]
- Hayman, D.S. Endogone spore numbers in soil and vesicular-arbuscular mycorrhiza in wheat as influenced by season and soil treatment. Trans. Br. Mycol. Soc. 1970, 54, 53–63. [Google Scholar] [CrossRef]
- Sutton, J.C.; Baron, G.L. Population dynamics of Endogone spores in soil. Can. J. Bot. 1972, 50, 1909–1914. [Google Scholar] [CrossRef]
- Hayman, D.S.; Johnson, A.M.; Ruddlesd, I. The influence of phosphate and crop species on Endogone spores and vesicular-arbuscular mycorrhiza under field conditions. Plant Soil. 1975, 43, 489–495. [Google Scholar] [CrossRef]
- Black, R.; Tinker, P. Interaction between effects of vesicular-arbuscular mycorrhiza and fertilizer phosphorus on yield of potatoes in the field. Nature 1977, 267, 510–511. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Schenk, N.C.; Perez, Y. Manual for the Identification of VA Mycorrhizal Fungi; Synergetics Publication: Gainesville, FL, USA, 1990. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total Organic and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Jakobsen, I. Transport of Phosphorus and Carbon in VA mycorrhizas. In Mycorrhiza; Varma, A., Hock, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 297–324. [Google Scholar]
- Sarkar, A.; Asaeda, T.; Wang, Q.; Kaneko, Y.; Rashid, M.H. Response of Miscanthus sacchariflorus to zinc stress mediated by arbuscular mycorrhizal fungi. Flora 2017, 234, 60–68. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Sharma, G.D.; Mishra, R.R. Status of mycorrhizae in sub-tropical forest ecosystem of Meghalaya. Acta. Bot. Indica 1986, 14, 87–92. [Google Scholar]
- Sieverding, E. Vesicular Arbuscular Mycorrhizal Management in Tropical Agrosystems; Deutsche Gesellschaft fur TechnischeZesammenarbeit (GTZ): Eschborn, Germany, 1991. [Google Scholar]
- Mohan Kumar, V.; Sivaswamy, S.N. Vesicular abuscularmycorrhizae. Adv. Pl. Sci. 1992, 50, 17–28. [Google Scholar]
- Byra Reddy, M.S.; Krishna, L.; NaikBhaskar, V.; Bagyaraj, D.J. Status of mycorrhizal associations in some tropical tree species. J. Soil Biol. Ecol. 1994, 14, 51–57. [Google Scholar]
- Uniyal, K.; Uniyal, D.P. Population dynamics of arbuscular mycorrhizal fungi in Dalbergiasisso. Roxb. Indian For. 2000, 126, 782–787. [Google Scholar]
- Muthukumar, T.; Udaiyan, K. Arbuscular Mycorrhizas of plants growing in western ghats, South India. Mycorrhiza 2000, 9, 297–313. [Google Scholar] [CrossRef]
- Onguene, N.A.; Kuyper, T.W. Mycorrizal association in the rain forest of South Cameroon. For. Ecol. Mang. 2001, 140, 277–287. [Google Scholar] [CrossRef]
- Tamuli, P.; Boruah, P. AM association of Agar wood tree in Jorhat district of the Brahmaputra Valley. Indian For. 2002, 128, 991–994. [Google Scholar]
- Bhattacharya, S.; Bagyaraj, D.J. Arbuscular mycorrhizal fungi associated with Arabica coffea. Geobios 2002, 29, 93–96. [Google Scholar]
- Leroy, C.; Maes, A.Q.; Louisanna, E.; Schimann, H.; Séjalon-Delmas, N. Taxonomic, phylogenetic and functional diversity of root-associated fungi in bromeliads: Effects of host identity, life forms and nutritional modes. New Phytol. 2021, 231, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.M.; Feiler, H.P.; Lacerda-Júnior, G.V.; Fernandes-Júnior, P.I.; Aidar, S.T.; Araújo, V.A.V.P.; Matteoli, F.P.; Pereira, A.P.A.; Melo, I.S.; Cardoso, E.J.B.N. Unravelling the Community of Arbuscular Mycorrhizal Fungi Associated with Endemic Plants from a Neotropical Dry Forest. 2022. Available online: https://assets.researchsquare.com/files/rs-2066211/v1/41c5bbbd-2428-4d4a-8ea5-fe284919b1bf.pdf?c=1665854066 (accessed on 30 December 2021).
- Sparling, G.P.; Tinker, P.B. Mycorrhizas in Pennie grassland.I. Level of Infection in the field. Appl. Ecol. 1978, 15, 943–950. [Google Scholar] [CrossRef]
- Schenk, N.C.; Kinloch, R.A. Incidence of mycorrhizal fungi on six field crops in monoculture on a newly cleared woodland site. Mycologia 1980, 72, 445–455. [Google Scholar] [CrossRef]
- Thapar, G.R.; Dunisway, J.M. Evaluation of plant response to colonization by vesicular arbuscular mycorrhizal fungi. In Methods and principles of Mycorrhizal Research; Schenck, N.C., Ed.; APS: St. Paul, MI, USA, 1992; pp. 77–80. [Google Scholar]
- Kalita, R.K.; Dutta, D.; Borah, D.P. Association of arbuscular mycorrhizas with twenty biomass species in Assam. Indian For. 2004, 130, 699–703. [Google Scholar]
- De Freitas, M.A.; Vieira, R.S.; Entiauspe-Neto, O.M.; e Sousa, S.O.; Farias, T.; Sousa, A.G.; de Moura, G.J. Herpetofauna of the Northwest Amazon forest in the state of Maranhão, Brazil, with remarks on the Gurupi Biological Reserve. ZooKeys 2017, 643, 141. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.W.; Wang, G.H.; Yang, L. Biodiversity of arbuscular mycorrhizal fungi in a tropical rainforest of Xishuangbanna, southwest China. Fungal Divers 2003, 13, 233–242. [Google Scholar]
- Yang, A.N.; Lu, L.; Zhang, N. The diversity of arbuscular mycorrhizal fungi in the subtropical forest of Huangshan (Yellow Mountain), East-Central China. World J. Microb. Biot. 2011, 27, 2351–2358. [Google Scholar] [CrossRef]
- Abbot, L.K.; Robson, A.D. The distribution and abundance of vesicular-arbuscular endophytes in some Western Australian soils. Aus. J. Bot. 1977, 25, 515–522. [Google Scholar] [CrossRef]
- Baylis, G.T.S. Host treatment and spore production by Endogone. N. Z. J. Bot. 1969, 7, 173–174. [Google Scholar] [CrossRef]
- Ahmad, N. An Assessment and Enumerations of Vesicular-Arbuscular Mycorrhiza Propagules in Some forest Sites of Jengka. J. Trop. For. Sci. 1996, 9, 137–146. [Google Scholar]
- Gong, M.G.; Ming, T.; Zhang, Q.M.; Feng, X.X. Effects of climatic and edaphic factors on arbuscular mycorrhizal fungi in the rhizosphere of Hippo phaerhamnoides in the Loess Plateau, China. Acta Ecol. Sin. 2012, 32, 62–67. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.G.; Zhang, B.; Yuan, Z.; Fu, Z.; Yuan, Y.; Zhu, L.; Ma, S.; Zhang, J. Arbuscular mycorrhizal fungi associated with tree species in a planted forest of Eastern China. Forests 2019, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Keresztúri, P.; Sándor, Z.; Peles, F.; Iglói, A.; Szabó, A. Influence of soil phosphorus forms on the AM fungi sporulation in the case of five different soil types. Cereal Res. Commun. 2006, 34, 219–222. [Google Scholar] [CrossRef]
- Jeffries, Use of mycorrhiza in agriculture. Cri. Rev. Biotech. 1987, 5, 319–357. [CrossRef]
- Jacobsen, I.; Abbott, L.K.; Robson, A. External hyphae of vesicular arbuscular mycorrhizal fungi associated with Trofoluimsubterraneum L. I. spread of hyphae and phosphorus inflow into roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- George, E.; Marschner, H.; Jakobsen, I. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit. Rev. Biotechnol. 1995, 15, 257–270. [Google Scholar] [CrossRef]
- Ortas, I.; Harris, P.J.; Rowell, D.L. Enhanced uptake of phosphorus by mycorrhizal sorghum plants as influenced by forms of nitrogen. Plant Soil 1996, 184, 255–264. [Google Scholar] [CrossRef]



| PPP | AMF Spore Number Per Gram of Soil | |||||
|---|---|---|---|---|---|---|
| ThalewoodHouse Plot (TWH) | Bugarikallu Plot (BGKL) | Doresanipalya Plot (DSP) | ||||
| DS | WS | DS | WS | DS | WS | |
| Sub-Plot 1 | 14.1 | 18.86 | 21.15 | 10.20 | 12.96 | 21.12 |
| Sub-Plot 2 | 5.92 | 11.08 | 28.60 | 25.22 | 7.52 | 21.00 |
| Sub-Plot 3 | 15.40 | 31.00 | 24.40 | 5.47 | 6.72 | 14.08 |
| Sub-Plot 4 | 7.20 | 10.14 | 28.40 | 6.48 | 9.72 | 8.70 |
| Sub-Plot 5 | 12.22 | 16.94 | 18.00 | 15.40 | 9.90 | 16.32 |
| Sub-Plot 6 | 11.88 | 4.80 | 15.40 | 1.44 | 8.48 | 27.44 |
| Sub-Plot 7 | 5.64 | 9.84 | 35.56 | 14.52 | 4.00 | 24.12 |
| Sub-Plot 8 | 4.44 | 5.46 | 26.88 | 7.52 | 6.08 | 12.22 |
| Sub-Plot 9 | 12.04 | 20.02 | 11.52 | 10.36 | 5.76 | 15.04 |
| Sub-Plot 10 | 12.32 | 14.70 | 9.12 | 20.88 | 18.54 | 11.84 |
| Sub-Plot 11 | 4.48 | - | 21.44 | 20.46 | 15.84 | 11.70 |
| Sub-Plot 12 | 7.68 | 5.20 | 8.26 | 20.88 | 19.62 | 11.84 |
| Sub-Plot 13 | 8.32 | 6.24 | 13.58 | 8.46 | 16.74 | 12.96 |
| Sub-Plot 14 | 12.76 | 1.56 | 11.62 | 15.54 | 23.20 | 27.36 |
| Sub-Plot 15 | 11.96 | 4.40 | 13.44 | 24.48 | 24.30 | 22.80 |
| Sub-Plot 16 | 6.76 | 14.7 | 13.92 | 1.25 | 28.16 | 27.00 |
| Sub-Plot 17 | 6.72 | 9.90 | 15.40 | 19.96 | 24.32 | 16.56 |
| Sub-Plot 18 | 19.44 | 6.82 | 8.12 | 21.11 | 18.72 | 25.92 |
| Sub-Plot 19 | 5.04 | 5.46 | 16.40 | 1.29 | 33.12 | 34.00 |
| Sub-Plot 20 | 8.64 | 5.88 | 15.82 | 21.84 | 20.70 | 22.50 |
| Sub-Plot 21 | 18.72 | 6.33 | 11.64 | 10.62 | 23.20 | 25.50 |
| Sub-Plot 22 | 13.60 | 14.72 | 6.08 | 14.80 | 22.32 | 23.36 |
| Sub-Plot 23 | 12.60 | 6.16 | 8.90 | 15.60 | 31.20 | 30.72 |
| Sub-Plot 24 | 13.12 | 12.74 | 9.00 | 25.22 | 31.02 | 25.68 |
| Sub-Plot 25 | 16.56 | 6.24 | 8.96 | 26.60 | 34.56 | 30.48 |
| SD | 4.38 | 6.57 | 7.73 | 7.97 | 9.33 | 7.15 |
| t-test Values for the Three Plots during Dry and Wet Seasons | ||||||
| TWH | BGKL | DSP | ||||
| t-test value | 0.3188 | 0.6483 | −1.0804 | |||
| p = 0.05 | 0.7512 | 0.5199 | 0.2854 | |||
| PPP | Dry Season | Wet Season | ||
|---|---|---|---|---|
| AMF Spore Number Per Gram of Soil | Available P(mg/kg) | AMF Spore Number Per Gram of Soil | Available P (mg/kg) | |
| TWH | 10.70 | 5.82 | 10.20 | 6.04 |
| BGKL | 16.06 | 4.97 | 14.62 | 3.28 |
| DSP | 18.27 | 6.92 | 20.81 | 7.20 |
| SD | 3.89 | 0.97 | 5.33 | 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boya, S.; Puttaswamy, P.; Mahadevappa, N.; Sharma, B.; Othumbamkat, R. Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India. Bacteria 2023, 2, 70-80. https://doi.org/10.3390/bacteria2010006
Boya S, Puttaswamy P, Mahadevappa N, Sharma B, Othumbamkat R. Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India. Bacteria. 2023; 2(1):70-80. https://doi.org/10.3390/bacteria2010006
Chicago/Turabian StyleBoya, Saritha, Poorvashree Puttaswamy, Nethravathi Mahadevappa, Balasubramanya Sharma, and Remadevi Othumbamkat. 2023. "Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India" Bacteria 2, no. 1: 70-80. https://doi.org/10.3390/bacteria2010006
APA StyleBoya, S., Puttaswamy, P., Mahadevappa, N., Sharma, B., & Othumbamkat, R. (2023). Enumerating Indigenous Arbuscular Mycorrhizal Fungi (AMF) Associated with Three Permanent Preservation Plots of Tropical Forests in Bangalore, Karnataka, India. Bacteria, 2(1), 70-80. https://doi.org/10.3390/bacteria2010006









