The Effect of Cell-Free Nontuberculous Mycobacterium Supernatants on Antibiotic Resistance and Biofilm Formation of Opportunistic Pathogens
Abstract
:1. Introduction
2. Results
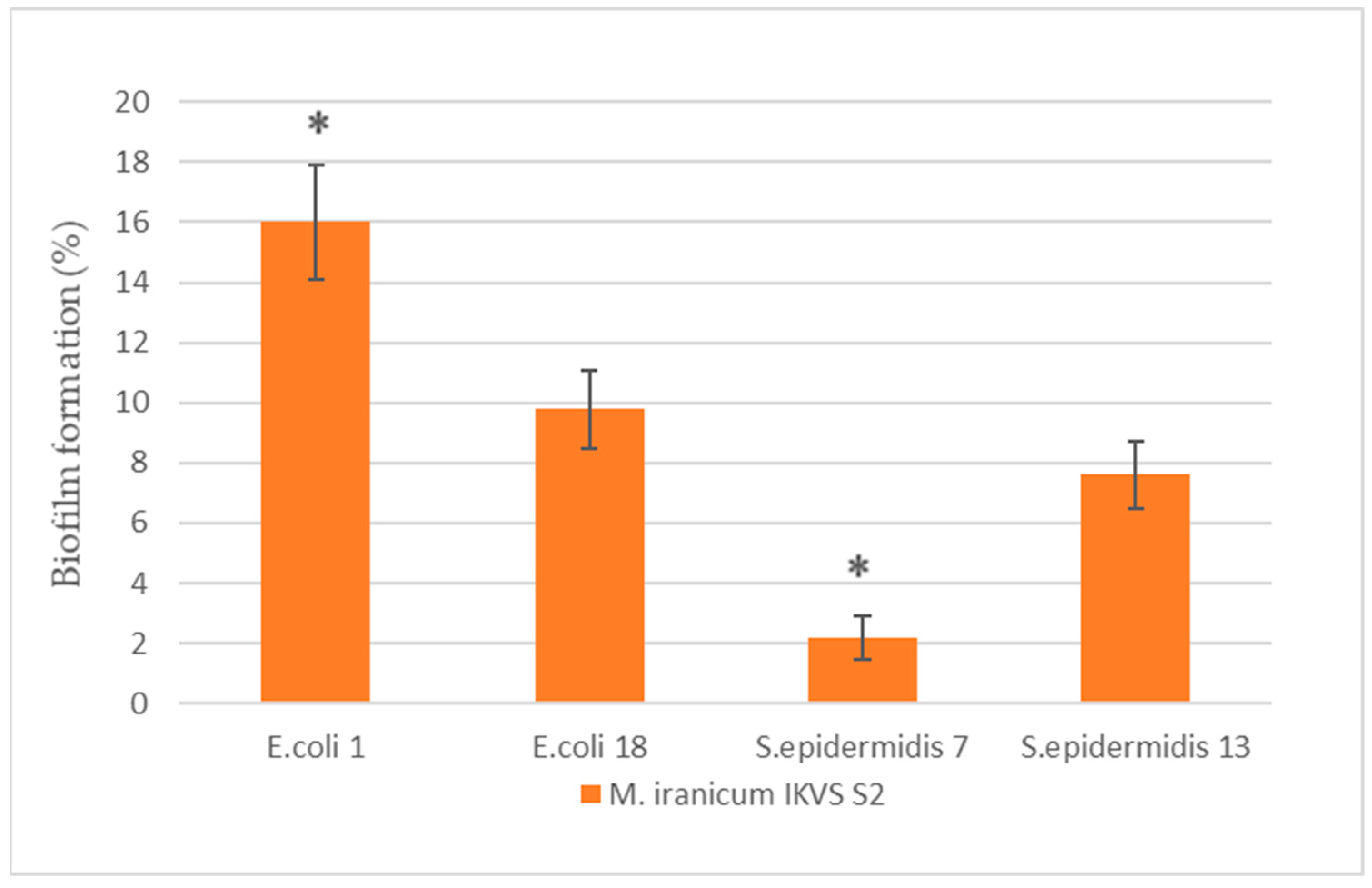
2.1. The Effect of Cell-Free M. iranicum Supernatants on Biofilm Formation of E. coli and S. epidermidis
2.2. The Effect of the Cell-Free M. iranicum Supernatants on Antibiotic Susceptibility of the Strains of E. coli IKVS 1 and 18
2.3. The Effect of the Cell-Free M. iranicum Supernatants on Antibiotic Susceptibility of the Strains of S. epidermidis IKVS 7 and 13
3. Discussion
4. Materials and Methods
4.1. Biophysiochemical and Cultural–Morphological Properties of NTM Strains
4.2. Characteristics of Opportunistic Pathogens
4.3. Receiving the Cell-Free M. iranicum Supernatant
4.4. Determination of Bacteria Antibiotic Susceptibility
4.5. Determination of the Biofilm Formation Intensity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimina, V.N.; Degtyareva, S.Y.; Beloborodova, E.N.; Kulabukhova, E.I.; Rusakova, L.I.; Fesenko, O.V. Mycobacterioses: The current state of the problem. CMAC 2017, 19, 276–282. [Google Scholar]
- Delghandi, M.R.; El-Matbouli, M.; Menanteau-Ledouble, S. Mycobacteriosis and infections with non-tuberculous mycobacteria in aquatic organisms: A Review. Microorganisms 2020, 8, 1368. [Google Scholar] [CrossRef] [PubMed]
- Morgado, S.; Ramos, N.V.; Pereira, B.B.D.N.; Freitas, F.; da Fonseca, E.L.; Vicente, A.C. Multidrug-resistant Mycolicibacterium fortuitum infection in a companion cat (Felis silvestris catus) in Brazil. Access Microbiol. 2022, 4, 000317. [Google Scholar] [CrossRef] [PubMed]
- Lopeman, R.C.; Harrison, J.; Desai, M.; Cox, J.A.G. Mycobacterium abscessus: Environmental bacterium turned clinical nightmare. Microorganisms 2019, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, K.; Strachan, T.; Matej, R.; Ricna, D.; Bloomfield, M. Manifestations of cutaneous mycobacterial infections in patients with inborn errors of IL-12/IL-23-IFNγ immunity. Eur. J. Dermatol. 2022, 32, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.; Nunes, D.H.; Fernandes, C.; Funchal, G.D.G. Skin infection by Mycobacterium marinum—Diagnostic and therapeutic challenge. An. Bras. Dermatol. 2022, 97, 366–368. [Google Scholar] [CrossRef]
- Pampaloni, A.; Tosto, S.; Locatelli, M.E.; Gentile, A.; Scuderi, D.; Marino, A.; Cosentino, F.; Moscatt, V.; Nunnari, G.; Cacopardo, B. Skin and soft tissue infection by Mycobacterium intracellulare in an immunocompetent patient. IDCases 2020, 19, e00720. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Marcos, L.A.; Henao-Martínez, A.F.; Rodríguez-Morales, A.J.; Villamil-Gómez, W.E.; Gotuzzo, E.; Bonifaz, A. Cutaneous mycobacterial infections. Clin. Microbiol. Rev. 2018, 32, e00069-18. [Google Scholar] [CrossRef]
- Máiz Carro, L.; Barbero Herranz, E.; Nieto Royo, R. Respiratory infections due to nontuberculous mycobacterias. Med. Clin. 2018, 150, 191–197. [Google Scholar] [CrossRef]
- Acharya, S.; Anwar, S.; Medina, Y.; Thapa, S.; Glaser, A. Mycobacterium abscessus pneumonia in severe alcoholism. Cureus 2022, 14, e26251. [Google Scholar] [CrossRef]
- Ranson, E.L.; Tsevat, R.K.; von Bredow, B.; Kamau, E.; Yang, S.; Prabaker, K.K. Catheter-Related bloodstream infection caused by Mycolicibacterium iranicum, California, USA. Emerg. Infect Dis. 2023, 29, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Senozan, E.A.; Adams, D.J.; Giamanco, N.M.; Warwick, A.B.; Eberly, M.D. A catheter-related bloodstream infection with Mycobacterium frederiksbergense in an immunocompromised child. Pediatr. Infect Dis. J. 2015, 34, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, Y.; Kozai, Y.; Yamasa, H.; Sakurada, M.; Kashiyama, T.; Honda, H. A cluster of central line-associated bloodstream infections due to rapidly growing nontuberculous mycobacteria in patients with hematologic disorders at a Japanese tertiary care center: An outbreak investigation and review of the literature. Infect. Control Hosp. Epidemiol. 2015, 36, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, J.; Gao, Y.; Sun, Y.; Dai, J.; Wu, Y.; Qu, D.; Ma, G.; Fang, X. Staphylococcus epidermidis small basic protein (Sbp) forms amyloid fibrils, consistent with its function as a scaffolding protein in biofilms. J. Biol. Chem. 2018, 293, 14296–14311. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Dhawan, B.; Garg, B.; Shankar, V.; Chandra Nag, T. A Comparison of bacterial adhesion and biofilm formation on commonly used orthopaedic metal implant materials: An in vitro study. Indian J. Orthop. 2019, 53, 148–153. [Google Scholar] [CrossRef]
- Gogoleva, O.A.; Shchuplova, E.A. The influence of non-tuberculous mycobacteria on the interaction of opportunistic microorganisms with erythrocytes. Folia Microbiol. 2020, 65, 417–421. [Google Scholar] [CrossRef]
- Pashkova, T.M.; Morozova, N.V.; Kuzmin, M.D.; Kartashova, O.L.; Popova, L.P. Characteristics of the pathogenic potential of Escherichia coli isolated from patients with calculous pyelonephritis. Urology 2021, 4, 19–24. [Google Scholar] [CrossRef]
- Shchuplova, E.A.; Herzen, N.V.; Fadeev, S.B.; Valyshev, A.V. Antihemoglobin activity of enterococci. Bull. OSU 2014, 13, 139–142. [Google Scholar]
- Mack, D.; Davies, A.P.; Harris, L.G.; Knobloch, J.K.; Rohde, H. Staphylococcus epidermidis biofilms: Functional molecules, relation to virulence, and vaccine potential. Top. Curr. Chem. 2009, 288, 157–182. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Dheilly, A.; Soum-Soutera, E.; Klein, G.L.; Bazire, A.; Compere, C.; Haras, D.; Dufour, A. Antibiofilm activity of the marine bacterium Pseudoalteromonas sp. strain 3J6. Appl. Environ. Microbiol. 2010, 76, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Bacterial landlines: Contact-dependent signaling in bacterial populations. Curr. Opin. Microbiol. 2009, 12, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.C.; Simões, M.; Vieira, M.J. The effects of metabolite molecules produced by drinking water-isolated bacteria on their single and multispecies biofilms. Biofouling 2011, 27, 685–699. [Google Scholar] [CrossRef]
- Vacheva, A.; Ivanova, R.; Paunova-Krasteva, T.; Stoitsova, S. Released products of pathogenic bacteria stimulate biofilm formation by Escherichia coli K-12 strains. Antonie Van Leeuwenhoek 2012, 102, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Xavier, K.B.; Bassler, B.L. LuxS quorum sensing: More than just a numbers game. Curr. Opin. Microbiol. 2003, 6, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, M.H.; Schaaf, S.; Koester, W.; Krooneman, J.; van der Meer, W.; Harmsen, H.; Landini, P. Biofilm formation by Escherichia coli is stimulated by synergistic interactions and co-adhesion mechanisms with adherence-proficient bacteria. Res. Microbiol. 2006, 157, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013, 29, 13. [Google Scholar] [CrossRef]
- Cardiliya, A.P.; Chandrasekar, M.J.N.; Nanjan, M.J. Incidence of biofilms among the multidrug resistant E. coli, isolated from urinary tract infections in the Nilgiris district, South India. Braz. J. Microbiol. 2023, 54, 1809–1818. [Google Scholar] [CrossRef]
- Shaikhrazieva, N.D.; Bulycheva, I.A.; Lopushov, D.V.; Sabayeva, F.N. Etiological structure and antibiotic resistance of hospital strains of microorganisms in the department of anesthesiology and intensive care. Medical Almanac 2019, 1, 32–34. [Google Scholar] [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef]
- Lynch, S.V.; Dixon, L.; Benoit, M.R.; Brodie, E.L.; Keyhan, M.; Hu, P.; Ackerley, D.F.; Andersen, G.L.; Matin, A. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob. Agents Chemother. 2007, 51, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.S.; Giraud, C.; Spagnolo, J.; de Bentzmann, S. Biofilms: The secret story of microbial communities. In Bacterial Pathogenesis: Molecular and Cellular Mechanisms; Locht, C., Simonet, M., Eds.; Caister Academic Press: Norfolk, UK, 2012; pp. 129–168. [Google Scholar]
- Zakirov, I.I.; Kadyrova, E.R.; Safina, A.I.; Kayumov, A.R. Antibiotic resistance of Staphylococcus aureus and Pseudomonas aeruginosa on the model of cystic fibrosis as a chronic disease of the bronchopulmonary system. Pediatrics 2018, 97, 176–186. [Google Scholar] [CrossRef]
- Carcione, D.; Leccese, G.; Conte, G.; Rossi, E.; Intra, J.; Bonomi, A.; Sabella, S.; Moreo, M.; Landini, P.; Brilli, M.; et al. Lack of direct correlation between biofilm formation and antimicrobial resistance in clinical Staphylococcus epidermidis isolates from an italian hospital. Microorganisms 2022, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Belkova, N.L.; Andreeva, A.M. Introduction to the Molecular Ecology of Microorganisms; Printhouse: Yaroslavl, Russia, 2009; p. 91. [Google Scholar]
- Gupta, R.S.; Lo, B.; Son, J. Phylogenomics and comparative genomic studies robustly support division of the genus mycobacterium into an emended genus mycobacterium and four novel genera. Front. Microbiol. 2018, 13, 9–67. [Google Scholar] [CrossRef]
- Bukharin, O.V.; Usvyatsov, B.Y.; Khanina, E.A. Determination of the Antihemoglobin Activity of Microorganisms. Patent of Russia No. 2262705.2005, 4 August 2005. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing, EUCAST. Available online: http://www.eucast.org (accessed on 18 October 2023).
- Gladysheva, I.V.; Cherkasov, S.V. Antibiofilm activity of cell-free supernatants of vaginal isolates of Corynebacterium amycolatum against Pseudomonas aeruginosa and Klebsiella pneumoniae. Arch. Microbiol. 2023, 205, 158. [Google Scholar] [CrossRef]

| Antibiotic/Strain | E. coli IKVS 1 Control, mm | Strain Growth Inhibition under the Influence of Supernatant M. iranicum, mm | |
|---|---|---|---|
| IKVS S1 | IKVS S2 | ||
| ceftriaxone | 21.3 ± 2,4 | 21.6 ± 1.4 | 23 ± 1.4 |
| cefepime | 22 ± 2.4 | 18 ± 2.1 | 17 ± 1.4 * |
| gentamicin | 23 ± 2.4 | 19 ± 1.4 | 11 ± 1.4 * |
| vancomycin | 11.3 ± 1.7 | 7 ± 1.4 * | 9 ± 1.3 |
| clindamycin | 8.6 ± 1.6 | 20.3 ± 1.7 * | 13 ± 1.4 * |
| cefotaxime | 26 ± 2.1 | 21 ± 1.4 * | 20 ± 1.4 * |
| meropenem | 23.3 ± 2.4 | 24 ± 1.4 | 20.3 ± 1.4 |
| rifampicin | 0 | 0 | 0 |
| benzylpenicillin | 2.6 ± 0.5 | 2.6 ± 1.3 | 2.6 ± 0.5 |
| azithromycin | 14.3 ± 1.9 | 11.6 ± 1.7 | 14.3 ± 1.9 |
| ciprofloxacin | 25.3 ± 2.1 | 24 ± 1.4 | 25.3 ± 2.1 |
| clarithromycin | 8.3 ± 1.6 | 7 ± 1.4 | 8.3 ± 1.6 |
| Antibiotic/Strain | E. coli IKVS 18 Control, mm | Strain Growth Inhibition under the Influence of Supernatant M. iranicum, mm | |
|---|---|---|---|
| IKVS S1 | IKVS S2 | ||
| ceftriaxone | 27 ± 1.4 | 17 ± 1.4 * | 21 ± 1.4 * |
| cefepime | 22.6 ± 2.1 | 17 ± 1.4 * | 17 ± 1.4 * |
| gentamicin | 20.6 ± 2.1 | 15 ± 1.4 * | 13 ± 1.4 * |
| vancomycin | 14.6 ± 1.8 | 15 ± 1.4 | 14.6 ± 1.04 |
| clindamycin | 0 | 0 | 0 |
| cefotaxime | 27 ± 1.4 | 18 ± 1.4 * | 21.6 ± 1.4 * |
| meropenem | 27 ± 1.4 | 23 ± 1.4 | 27 ± 1.4 |
| rifampicin | 0 | 0 | 0 |
| benzylpenicillin | 9.3 ± 1.6 | 8.3 ± 1.6 | 11.6 ± 1.8 |
| azithromycin | 16 ± 1.4 | 10.6 ± 1.9 * | 12.3 ± 1.8 |
| ciprofloxacin | 29 ± 1.4 | 20.3 ± 1.04 * | 23 ± 1.4 * |
| clarithromycin | 0 | 0 | 0 |
| Antibiotic/Strain | S. epidermidis IKVS 7 Control, mm | Strain Growth Inhibition under the Influence of Supernatant M. iranicum, mm | |
|---|---|---|---|
| IKVS S1 | IKVS S2 | ||
| ceftriaxone | 21 ± 1.4 | 15.3 ± 1.4 * | 17.3 ± 1.7 |
| cefepime | 0 | 0 | 0 |
| gentamicin | 20.3 ± 1.7 | 15 ± 1.4 * | 16.3 ± 1.4 |
| vancomycin | 16 ± 1.4 | 11 ± 1.4 * | 13.6 ± 1.2 |
| clindamycin | 25 ± 1.4 | 20.3 ± 1.4 * | 16.3 ± 1.3 * |
| cefotaxime | 25 ± 1.4 | 20.6 ± 2.08 * | 19.6 ± 2.08 * |
| meropenem | 32.3 ± 1.7 | 27.6 ± 1.7 * | 28.3 ± 2.4 |
| rifampicin | 26.6 ± 1.04 | 20 ± 1.4 * | 20.6 ± 2.08 * |
| benzylpenicillin | 10 ± 1.4 | 9.3 ± 1.04 | 13.6 ± 2.08 |
| azithromycin | 22.6 ± 2.08 | 15.3 ± 1.4 * | 16.3 ± 1.7 * |
| ciprofloxacin | 23 ± 1.4 | 20.3 ± 1.7 | 17.6 ± 2.08 * |
| clarithromycin | 21.6 ± 1.4 | 15.3 ± 1.7 * | 17.6 ± 2.08 |
| Antibiotic/Strain | S. epidermidis IKVS 13 Control, mm | Strain Growth Inhibition under the Influence of Supernatant M. iranicum, mm | |
|---|---|---|---|
| IKVS S1 | IKVS S2 | ||
| ceftriaxone | 23 ± 1.4 | 15.6 ± 1.04 * | 20.6 ± 2.08 |
| cefepime | 0 | 0 | 0 |
| gentamicin | 20 ± 1.4 | 15.3 ± 1.7 * | 20.6 ± 1.4 |
| vancomycin | 13 ± 1.2 | 13 ± 1.2 | 13.6 ± 1.2 |
| clindamycin | 26 ± 1.4 | 19.6 ± 1.04 * | 20.3 ± 1.7 * |
| cefotaxime | 23.3 ± 1.7 | 17.3 ± 1.7 * | 18.3 ± 1.7 * |
| meropenem | 32 ± 2.08 | 27 ± 2.08 * | 24 ± 2.08 * |
| rifampicin | 24.6 ± 1.5 | 19.3 ± 1.7 * | 22.6 ± 2.08 |
| benzylpenicillin | 11.6 ± 1.04 | 10.6 ± 1.7 | 16 ± 2.08 |
| azithromycin | 22 ± 1.4 | 16.3 ± 1.7 * | 14.3 ± 1.2 * |
| ciprofloxacin | 25.3 ± 1.7 | 20.3 ± 1.7 * | 25.3 ± 1.7 |
| clarithromycin | 23.3 ± 1.7 | 16.3 ± 1.7 * | 16 ± 2.05 * |
| Nº | Typical Strain | Similarity, % |
|---|---|---|
| 1 | MK493230.1 Mycolicibacterium iranicum | 407/407(100%) |
| 2 | MH542421.1 Mycolicibacterium iranicum | 407/407(100%) |
| 3 | MK890472.1 Mycolicibacterium sp. | 407/407(100%) |
| 4 | MK890466.1 Mycolicibacterium sp. | 407/407(100%) |
| 5 | LC458852.1 Mycolicibacterium iranicum | 407/407(100%) |
| 6 | MH581232.1 Mycolicibacterium sp. | 407/407(100%) |
| 7 | MK811419.1 Mycolicibacterium iranicum | 407/407(100%) |
| 8 | KX390634.1 Mycobacterium iranicum strain H39 | 407/407(100%) |
| 9 | HQ009482.1 Mycobacterium iranicum strain M05 | 407/407(100%) |
| 10 | KU861842.1 Mycobacterium iranicum strain Y31 | 407/407(100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchuplova, E.A.; Gogoleva, O.A. The Effect of Cell-Free Nontuberculous Mycobacterium Supernatants on Antibiotic Resistance and Biofilm Formation of Opportunistic Pathogens. Bacteria 2023, 2, 174-184. https://doi.org/10.3390/bacteria2040013
Shchuplova EA, Gogoleva OA. The Effect of Cell-Free Nontuberculous Mycobacterium Supernatants on Antibiotic Resistance and Biofilm Formation of Opportunistic Pathogens. Bacteria. 2023; 2(4):174-184. https://doi.org/10.3390/bacteria2040013
Chicago/Turabian StyleShchuplova, Elena A., and Olga A. Gogoleva. 2023. "The Effect of Cell-Free Nontuberculous Mycobacterium Supernatants on Antibiotic Resistance and Biofilm Formation of Opportunistic Pathogens" Bacteria 2, no. 4: 174-184. https://doi.org/10.3390/bacteria2040013
APA StyleShchuplova, E. A., & Gogoleva, O. A. (2023). The Effect of Cell-Free Nontuberculous Mycobacterium Supernatants on Antibiotic Resistance and Biofilm Formation of Opportunistic Pathogens. Bacteria, 2(4), 174-184. https://doi.org/10.3390/bacteria2040013






