A Bibliographic Exploration of Bacterial Houses: Biofilm Matrix Research and Future Frontiers
Abstract
1. Introduction
2. Materials and Methods
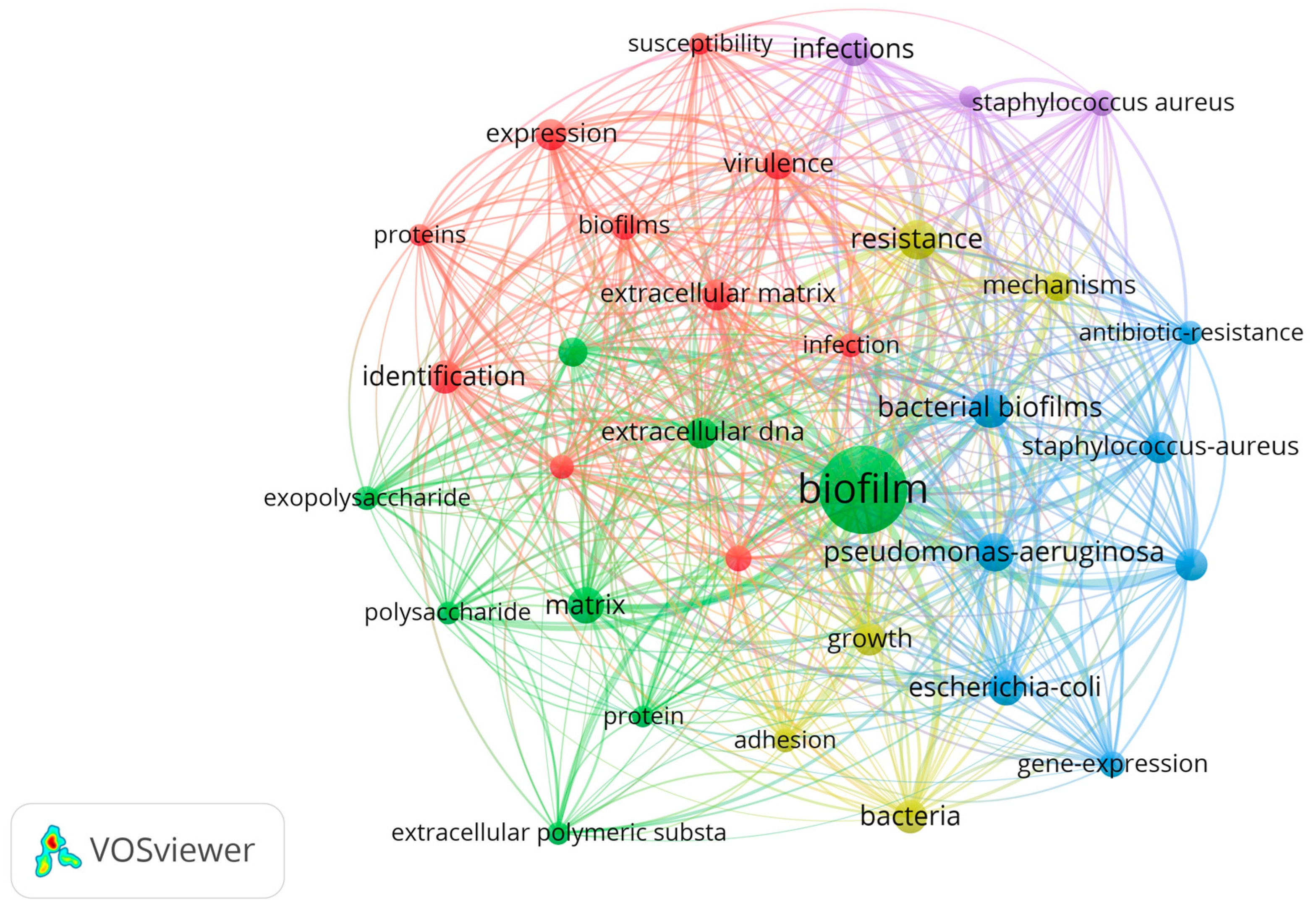
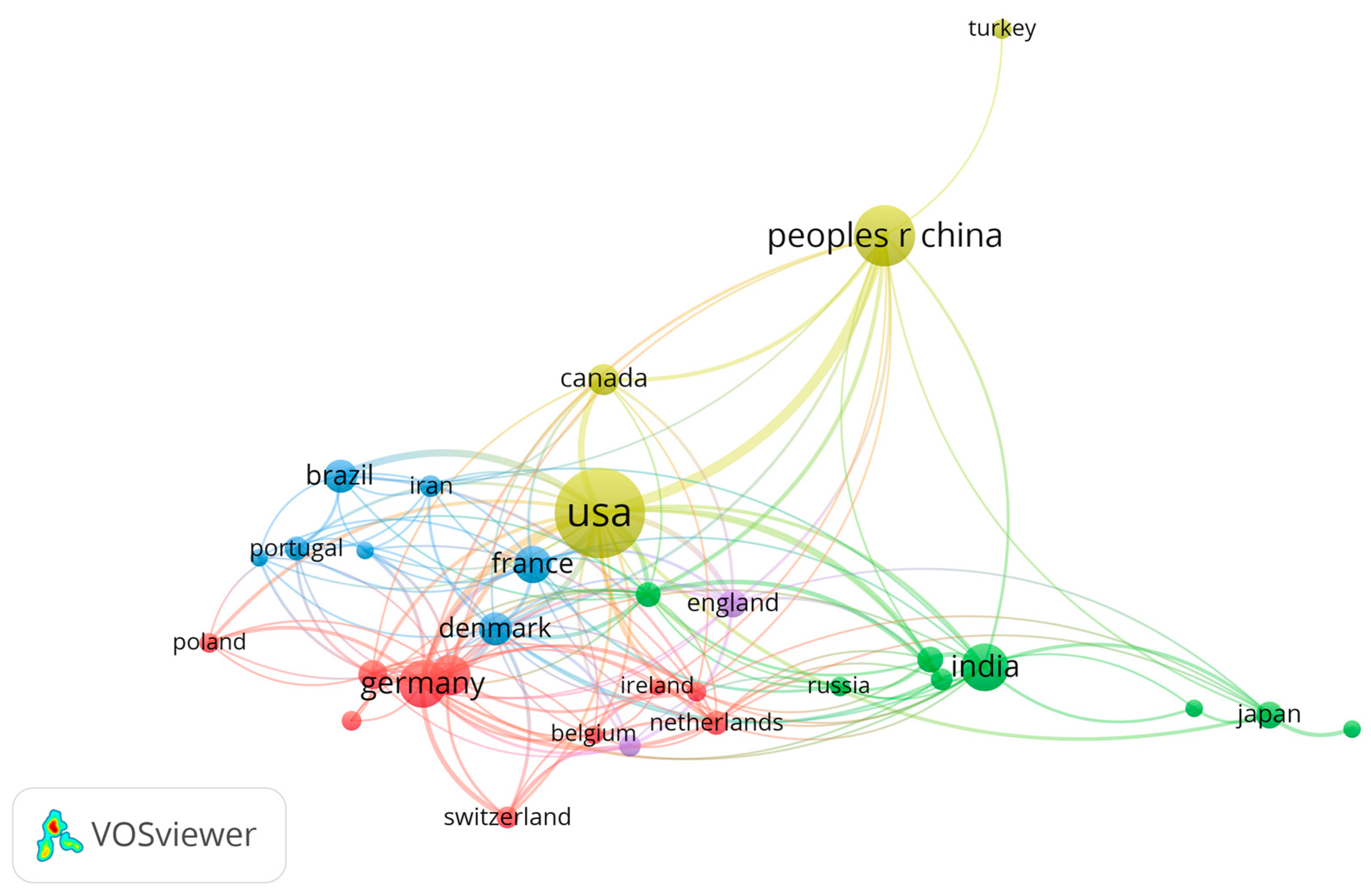
3. Results
4. Discussion
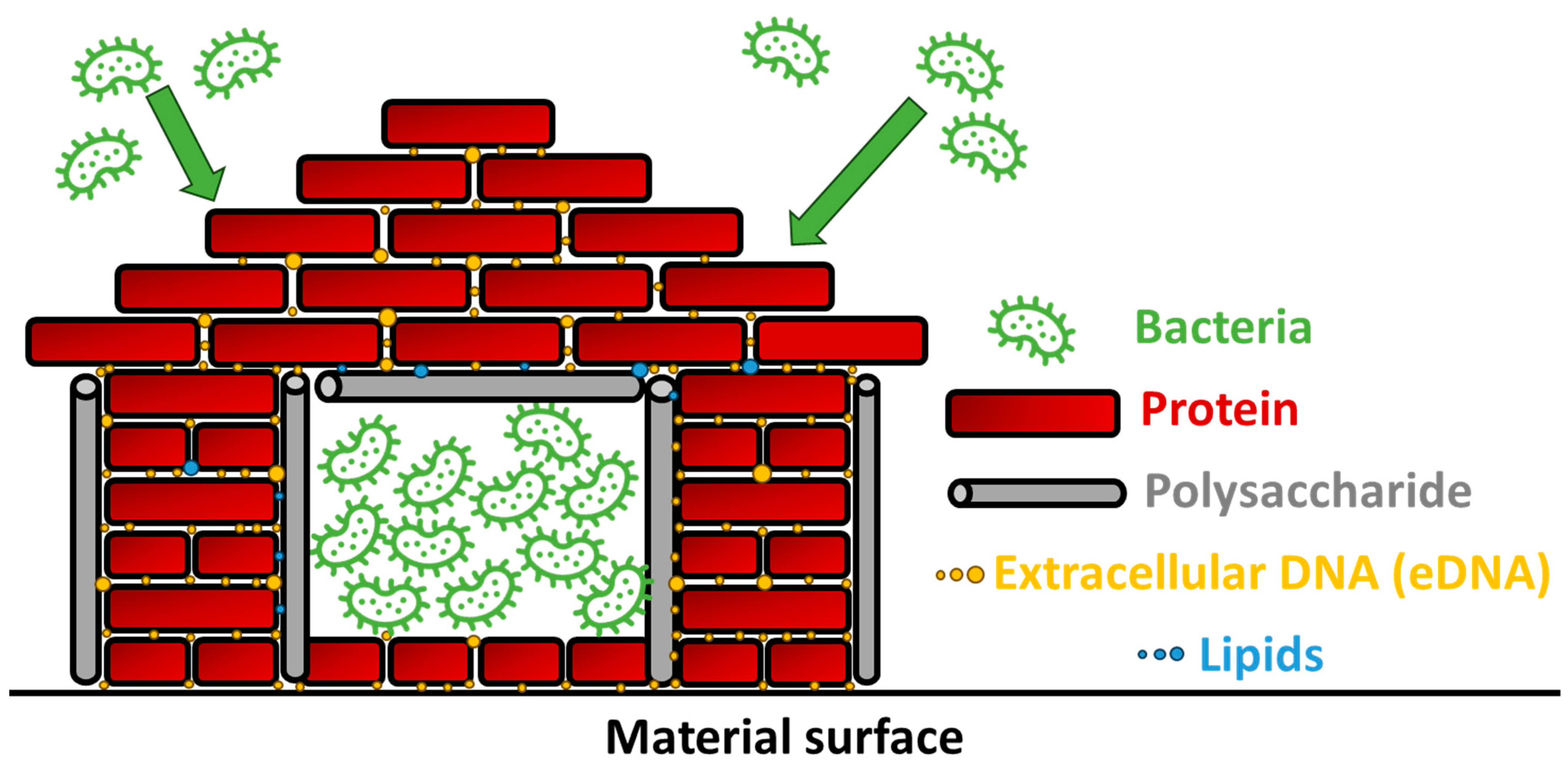
4.1. Bacterial Architects: Crafting Biofilm Habitats for Life’s Processes
| Components | Key Findings | Reference |
|---|---|---|
| Protein | Microorganisms primarily exist as biofilms, surface-attached microbial communities with diverse compositions, where proteinaceous elements, including adhesins and flagella subunits, are crucial. | [18] |
| Protein | Protein involvement varies in different stages of Staphylococci biofilm formation. | [19] |
| Protein | This study explores Staphylococcus aureus biofilm dynamics on clinically relevant materials, revealing surface-dependent variations in formation efficiency and the evolving roles of poly-N-acetyl-β-(1-6)-glucosamine (PNAG) and proteins in early and later stages, respectively. | [20] |
| Polysaccharide | The study unveils the crucial role of the Psl exopolysaccharide in biofilm formation by mucoid Pseudomonas aeruginosa, indicating that Psl is a vital matrix component for both nonmucoid and mucoid biofilms, with potential implications for designing therapies for Pseudomonas aeruginosa infections in cystic fibrosis patients. | [21] |
| Polysaccharide | Pel, one of the extracellular polysaccharides produced by Pseudomonas aeruginosa, serves a dual function in biofilms by acting as a crucial structural scaffold for cell-to-cell interactions and enhancing resistance to aminoglycoside antibiotics, with its impact being strain-specific in biofilm development. | [22] |
| Polysaccharide | Extracellular polysaccharides, including alginate, Pel, and Psl in Pseudomonas aeruginosa, contribute to biofilm matrix formation, with strain-specific variations in Pel and Psl functions, suggesting redundancy as a mechanism for biofilm stability and adaptability. | [23] |
| Extracellular DNA | The research developed a novel approach with mass spectrometry, identifying previously unrecognized DNA-binding lipoproteins in Staphylococcus aureus biofilms that enhance biofilm formation, contribute to structure, and are linked to nuclease production, extending the electrostatic net model to include these proteins as anchor points between extracellular DNA and the bacterial cell surface. | [24] |
| Extracellular DNA | Extracellular DNA in biofilms, initially overlooked but now recognized for its pivotal role, influences bacterial adhesion, biofilm structure, and antimicrobial resistance through active secretion or controlled cell lysis, acid–base interactions, the chelation of cations, and triggering genetic responses, highlighting its potential as a target for biofilm sensitization and novel antimicrobial strategies. | [25] |
| Lipid | This paper highlights that while rhamnolipids have been previously considered crucial for hydrocarbon uptake in bacterial cells, recent evidence indicates their primary role in surface-associated motility and biofilm development, providing insights into their environmental impact in microbial ecosystems. | [26] |
4.2. Exploring the Biofilm Matrix Landscape
| Category | Model Species | Main Findings | Reference |
|---|---|---|---|
| Engineering | Shewanella sp. HRCR-1 | U(VI) was immobilized by the Shewanella sp. HRCR-1 biofilms | [27] |
| Engineering | Shewanella oneidensis | Cr(VI) was immobilized by the Shewanella oneidensis MR-1 biofilm | [4] |
| Engineering | Comamonas testosteroni | Biodegradation of 3-chloroaniline by Comamonas testosteroni biofilm and c-di-GMP | [28] |
| Engineering | Shewanella oneidensis/Escherichia coli | Electricity genernation and high-performance microbial fuel cells by biofilm matrix | [29] |
| Engineering | Bacillus halodurans | Biofilm matrix enhanced the self-repairing process in concrete | [30] |
| Engineering | Pseudomonas | Biofilm matrix formed a ba permeable reactive barrier (PRB) working with zerovalent iron to stop the organic pollutant | [36,37,38] |
| Medicine | Pseudomonas aeruginosa | Biofilm matrix led to infection in a patient with COVID-19 | [31] |
| Medicine | Pseudomonas aeruginosa | Biofilm matrix led to chronic lung infection | [32] |
| Medicine | Staphylococcus aureus | Staphylococcus aureus biofilm formation causing infection can be reduced by linezolid or vancomycin | [33] |
| Medicine | Staphylococcus | The study found that various environmental factors, including temperature, pH, salt, glucose concentration, and oxygen levels, significantly influence the biofilm formation of Staphylococcus | [35] |
| Chemical characterization | Shewanella oneidensis | Molecular ion signal intensity for in situ biofilm matrix SIMS analysis was improved | [34] |
| Chemical characterization | Shewanella oneidensis | In situ molecular imaging of the biofilm matrix was achieved by SIMS | [39,40] |
| Chemical characterization | Pseudomonas aeruginosa | The biofilm matrix is identified by MALDI-TOF MS | [41] |
4.3. Biofilm Matrix in the Era of Big Data and Machine Learning
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyon, P. The cognitive cell: Bacterial behavior reconsidered. Front. Microbiol. 2015, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Peng, N.; Du, Y.; Ji, L.; Cao, B. Disruption of putrescine biosynthesis in Shewanella oneidensis enhances biofilm cohesiveness and performance in Cr (VI) immobilization. Appl. Environ. Microbiol. 2014, 80, 1498–1506. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wu, H.; Song, Z.; Høiby, N.; Molin, S.; Givskov, M. Combating biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Cai, Z.; Yang, L. Recent progress in experimental and human disease-associated multi-species biofilms. Comput. Struct. Biotechnol. J. 2019, 17, 1234–1244. [Google Scholar] [CrossRef]
- Lee, J.-W.; Nam, J.-H.; Kim, Y.-H.; Lee, K.-H.; Lee, D.-H. Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J. Microbiol. 2008, 46, 174–182. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. Apmis 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y. A bibliography study of Shewanella oneidensis biofilm. FEMS Microbiol. Ecol. 2023, 99, fiad124. [Google Scholar] [CrossRef]
- Pranckutė, R. Web of Science (WoS) and Scopus: The titans of bibliographic information in today’s academic world. Publications 2021, 9, 12. [Google Scholar] [CrossRef]
- Huang, T.; Zhong, W.; Lu, C.; Zhang, C.; Deng, Z.; Zhou, R.; Zhao, Z.; Luo, X. Visualized analysis of global studies on cervical spondylosis surgery: A bibliometric study based on web of science database and VOSviewer. Indian. J. Orthop. 2022, 56, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Tamala, J.K.; Maramag, E.I.; Simeon, K.A.; Ignacio, J.J. A bibliometric analysis of sustainable oil and gas production research using VOSviewer. Clean. Eng. Technol. 2022, 7, 100437. [Google Scholar] [CrossRef]
- Cavalcante, W.Q.d.F.; Coelho, A.; Bairrada, C.M. Sustainability and tourism marketing: A bibliometric analysis of publications between 1997 and 2020 using vosviewer software. Sustainability 2021, 13, 4987. [Google Scholar] [CrossRef]
- Maier, D.; Maier, A.; Așchilean, I.; Anastasiu, L.; Gavriș, O. The relationship between innovation and sustainability: A bibliometric review of the literature. Sustainability 2020, 12, 4083. [Google Scholar] [CrossRef]
- Rice, S.A.; Tan, C.H.; Mikkelsen, P.J.; Kung, V.; Woo, J.; Tay, M.; Hauser, A.; McDougald, D.; Webb, J.S.; Kjelleberg, S. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J. 2009, 3, 271–282. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Fong, J.N.C.; Yildiz, F.H. Biofilm matrix proteins. Microb. Biofilms 2015, 10, 201–222. [Google Scholar]
- Speziale, P.; Pietrocola, G.; Foster, T.J.; Geoghegan, J.A. Protein-based biofilm matrices in Staphylococci. Front. Cell. Infect. Microbiol. 2014, 4, 171. [Google Scholar] [CrossRef]
- Hiltunen, A.K.; Savijoki, K.; Nyman, T.A.; Miettinen, I.; Ihalainen, P.; Peltonen, J.; Fallarero, A. Structural and functional dynamics of Staphylococcus aureus biofilms and biofilm matrix proteins on different clinical materials. Microorganisms 2019, 7, 584. [Google Scholar] [CrossRef]
- Ma, L.; Wang, S.; Wang, D.; Parsek, M.R.; Wozniak, D.J. The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.L.; Parsek, M.R. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012, 14, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, J.S.; Flack, C.E.; Lister, J.; Ricker, E.B.; Ibberson, C.B.; Jenul, C.; Moormeier, D.E.; Delmain, E.A.; Bayles, K.W.; Horswill, A.R. Identification of extracellular DNA-binding proteins in the biofilm matrix. MBio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, Ł.; Ławniczak, Ł.; Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Ahmed, B.; Kennedy, D.W.; Wang, Z.; Shi, L.; Marshall, M.J.; Fredrickson, J.K.; Isern, N.G.; Majors, P.D.; Beyenal, H. Contribution of extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms to U (VI) immobilization. Environ. Sci. Technol. 2011, 45, 5483–5490. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Cohen, Y.; Cao, B. Elevated level of the second messenger c-di-GMP in Comamonas testosteroni enhances biofilm formation and biofilm-based biodegradation of 3-chloroaniline. Appl. Microbiol. Biotechnol. 2015, 99, 1967–1976. [Google Scholar] [CrossRef]
- Zhao, C.e.; Wu, J.; Ding, Y.; Wang, V.B.; Zhang, Y.; Kjelleberg, S.; Loo, J.S.C.; Cao, B.; Zhang, Q. Hybrid conducting biofilm with built-in bacteria for high-performance microbial fuel cells. ChemElectroChem 2015, 2, 654–658. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Y.; Qian, S. Influence of bacterial incorporation on mechanical properties of engineered cementitious composites (ECC). Constr. Build. Mater. 2019, 196, 195–203. [Google Scholar] [CrossRef]
- Qu, J.; Cai, Z.; Liu, Y.; Duan, X.; Han, S.; Liu, J.; Zhu, Y.; Jiang, Z.; Zhang, Y.; Zhuo, C. Persistent bacterial coinfection of a COVID-19 patient caused by a genetically adapted Pseudomonas aeruginosa chronic colonizer. Front. Cell. Infect. Microbiol. 2021, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hengzhuang, W.; Wu, H.; Damkiær, S.; Jochumsen, N.; Song, Z.; Givskov, M.; Høiby, N.; Molin, S. Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2012, 65, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.A.G.; Afonina, I.; Kline, K.A. Eradicating biofilm infections: An update on current and prospective approaches. Curr. Opin. Microbiol. 2021, 63, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, J.; Ding, Y.; Yu, J.; Hua, X.; Evans, J.E.; Yu, X.; Lao, D.B.; Heldebrant, D.J.; Nune, S.K. Improving the molecular ion signal intensity for in situ liquid SIMS analysis. J. Am. Soc. Mass. Spectrom. 2016, 27, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Pereira, J.E.; Maltez, L.; Poeta, P.; Igrejas, G. Influence of environmental factors on biofilm formation of Staphylococci isolated from wastewater and surface water. Pathogens 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.B.; Hiebert, D.R. Subsurface biofilm barriers for contaminated ground water containment. Ground Water Curr. 2000, 36, 3–4. [Google Scholar]
- Cajthaml, T.; Bhatt, M.; Šašek, V.; Matějů, V. Bioremediation of PAH-contaminated soil by composting: A case study. Folia Microbiol. 2002, 47, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.K.; Omar, S.H.; Nour Eldin, H.M.; Chekroud, Z.A. Bioremediation of kerosene II: A case study in contaminated clay (Laboratory and field: Scale microcosms). World J. Microbiol. Biotechnol. 2008, 24, 1451–1460. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Yao, J.; Szymanski, C.; Fredrickson, J.; Shi, L.; Cao, B.; Zhu, Z.; Yu, X.-Y. In situ molecular imaging of the biofilm and its matrix. Anal. Chem. 2016, 88, 11244–11252. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Yao, J.; Xiong, Y.; Zhu, Z.; Yu, X.-Y. Molecular evidence of a toxic effect on a biofilm and its matrix. Analyst 2019, 144, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Asghari, E.; Kiel, A.; Kaltschmidt, B.P.; Wortmann, M.; Schmidt, N.; Hüsgen, B.; Hütten, A.; Knabbe, C.; Kaltschmidt, C.; Kaltschmidt, B. Identification of microorganisms from several surfaces by MALDI-TOF MS: P. aeruginosa is leading in biofilm formation. Microorganisms 2021, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Crisci, C.; Ghattas, B.; Perera, G. A review of supervised machine learning algorithms and their applications to ecological data. Ecol. Model. 2012, 240, 113–122. [Google Scholar] [CrossRef]
- Kopaczka, M.; Nestler, J.; Merhof, D. Face detection in thermal infrared images: A comparison of algorithm-and machine-learning-based approaches. In Proceedings of the Advanced Concepts for Intelligent Vision Systems: 18th International Conference, ACIVS 2017, Antwerp, Belgium, 18–21 September; 2017; pp. 518–529. [Google Scholar]
- Stilgoe, J. Machine learning, social learning and the governance of self-driving cars. Soc. Stud. Sci. 2018, 48, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.N.; Malwe, A.S.; Sharma, A.K.; Shastri, V.; Hibare, K.; Sharma, V.K. Molib: A machine learning based classification tool for the prediction of biofilm inhibitory molecules. Genomics 2020, 112, 2823–2832. [Google Scholar] [CrossRef]
- Alpkvist, E.; Picioreanu, C.; Van Loosdrecht, M.C.M.; Heyden, A. Three-dimensional biofilm model with individual cells and continuum EPS matrix. Biotechnol. Bioeng. 2006, 94, 961–979. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y. A Bibliographic Exploration of Bacterial Houses: Biofilm Matrix Research and Future Frontiers. Bacteria 2024, 3, 183-193. https://doi.org/10.3390/bacteria3030013
Ding Y. A Bibliographic Exploration of Bacterial Houses: Biofilm Matrix Research and Future Frontiers. Bacteria. 2024; 3(3):183-193. https://doi.org/10.3390/bacteria3030013
Chicago/Turabian StyleDing, Yuanzhao. 2024. "A Bibliographic Exploration of Bacterial Houses: Biofilm Matrix Research and Future Frontiers" Bacteria 3, no. 3: 183-193. https://doi.org/10.3390/bacteria3030013
APA StyleDing, Y. (2024). A Bibliographic Exploration of Bacterial Houses: Biofilm Matrix Research and Future Frontiers. Bacteria, 3(3), 183-193. https://doi.org/10.3390/bacteria3030013






