Abstract
Adult stem cells play critical roles in the basal maintenance of tissue integrity, also known as homeostasis, and in tissue regeneration following damage. The highly conserved Wnt signalling pathway is a key regulator of stem cell fate. In the gastrointestinal tract, Wnt signalling activation drives homeostasis and damage-induced repair. Additionally, deregulated Wnt signalling is a common hallmark of age-associated tissue dysfunction and cancer. Studies using mouse and fruit fly models have greatly improved our understanding of the functional contribution of the Wnt signalling pathway in adult intestinal biology. Here, we summarize the latest knowledge acquired from mouse and Drosophila research regarding canonical Wnt signalling and its key functions during stem cell driven intestinal homeostasis, regeneration, ageing and cancer.
Keywords:
Wnt signalling; intestine; homeostasis; regeneration; stem cells; Drosophila; mouse models; cancer 1. Introduction
Wingless-related integration site (Wnt) was named after the Drosophila wingless gene and the mouse int1 gene. Int1 was discovered in 1982 as a gene overexpressed in breast cancer [1]. Subsequent studies revealed first the amino acid sequence and next demonstrated that Int1 was a secreted protein with the potential to act as a signalling molecule [2,3]. Five years later, int1 was found to be homolog to the Drosophila gene wingless (wg), which had been previously characterized as a segment polarity gene through seminal work that identified regulators of body axis during embryonic development [4,5]. Thereafter, int/wingless became Wnt, giving also the generic name to the pathway itself, and Int1 became Wnt1 as the first ligand identified. More than three decades later, there are no doubts about the importance of the Wnt pathway as an evolutionarily conserved system, which is broadly implicated in diverse biological processes such as embryonic development, adult tissue homeostasis, regeneration and disease [6,7,8].
The Wnt pathway is divided into β-catenin-dependent (canonical) and independent (non-canonical) signalling. In both cases, the major components of the pathway are the Wnt ligands, which act in an autocrine or paracrine fashion by binding their Frizzled (Fz) receptors. In canonical Wnt signalling, Fz receptors engage with co-receptors Lrp5/6, at the cell surface. Here, we will focus on the canonical pathway, as it is the one mainly studied in stem cell biology and the intestine. Briefly, in steady state conditions, the levels of cytoplasmic β-catenin are kept low through phosphorylation by a complex of proteins, known as the ‘destruction complex’, which includes Axin, Adenomatous polyposis coli (Apc), glycogen synthase kinase 3 (Gsk3) and casein kinase 1α (Ck1α) [9]. This destruction complex promotes the ubiquitination of β-catenin and its degradation by the proteasome. Moreover, the ADP-ribose polymerase Tankyrase (Tnks) has been described to target Axin and stimulate its degradation through proteolysis [10]. Activation of the signalling pathway upon binding of Wnt ligands to receptors initiates a series of signalling events, including the activation by phosphorylation of cytoplasmic Dishevelled (Dsh), which ultimately leads to inactivation of the destruction complex and stabilization of β-catenin, its accumulation in the cytosol and translocation into the nucleus where it forms complexes with Tcf/Lef transcription factors, among others, to regulate target gene expression [8,11,12].
Work in the last two decades has demonstrated a central role of the Wnt pathway in the regulation of adult stem cells and, hence, the maintenance of tissue homeostasis. Stem cells are highly dependent on extrinsic cues derived from their microenvironment, also known as niche. Wnt signals are an essential component of a wide range of stem cell niches, including that of the gastrointestinal epithelium [13,14].
The intestinal epithelium is constantly turned over through the action of dedicated intestinal stem cells (ISCs). This process needs to be sustained and highly regulated as its disruption leads to either tissue wasting or the development of gastrointestinal disorders, including cancer. In this review, we will summarize the latest findings regarding the contribution of canonical Wnt signalling to ISCs and their activity during normal tissue homeostasis, regeneration, ageing and intestinal cancer. We will discuss data derived from studies in mice and the fruit fly Drosophila melanogaster, with an emphasis on the use of the fly as an increasingly valuable model system and powerful genetic tool for studying various aspects of Wnt signalling in intestinal health and pathogenesis.
2. The Adult Mammalian Intestine
The mammalian gut develops from the endoderm. During gastrulation the undifferentiated endoderm is pre-patterned into three regions along the anterior–posterior axis: the foregut, which forms the stomach and other organs; the midgut, which forms the small intestine; and the hindgut, which gives rise to the large intestine [15]. The adult gastrointestinal tract is a tubular structure composed of three layers consisting of smooth muscle, connective tissue and mucosa. The intestinal epithelium is part of the mucosa layer, which is supported by the lamina propia and the muscularis mucosae. The mucosa acts as a barrier, preventing the entry of harmful substances and exhibits both innate and adaptive immune functions. It also acts as a selective filter, which enables the uptake of nutrients, water, and various other beneficial materials from the intestinal lumen. From anterior to posterior the mammalian intestinal tube is comprised of the small intestine, which is divided into duodenum, jejunum, and ileum, and the large intestine, which consists of the cecum, colon and rectum. The architecture of the small intestine is organised in a way that maximises the surface area available for the absorption of nutrients. This is due to the presence of crypts of Lieberkühn and protrusions of the epithelia known as villi (Figure 1i). The large intestine comprises a simple columnar epithelium with crypts of Lieberkühn and a proliferative stem/progenitor zone. However, unlike the small intestine, the large intestine lacks villi [16].
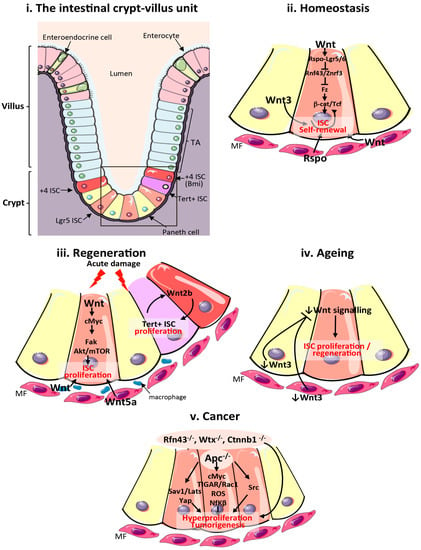
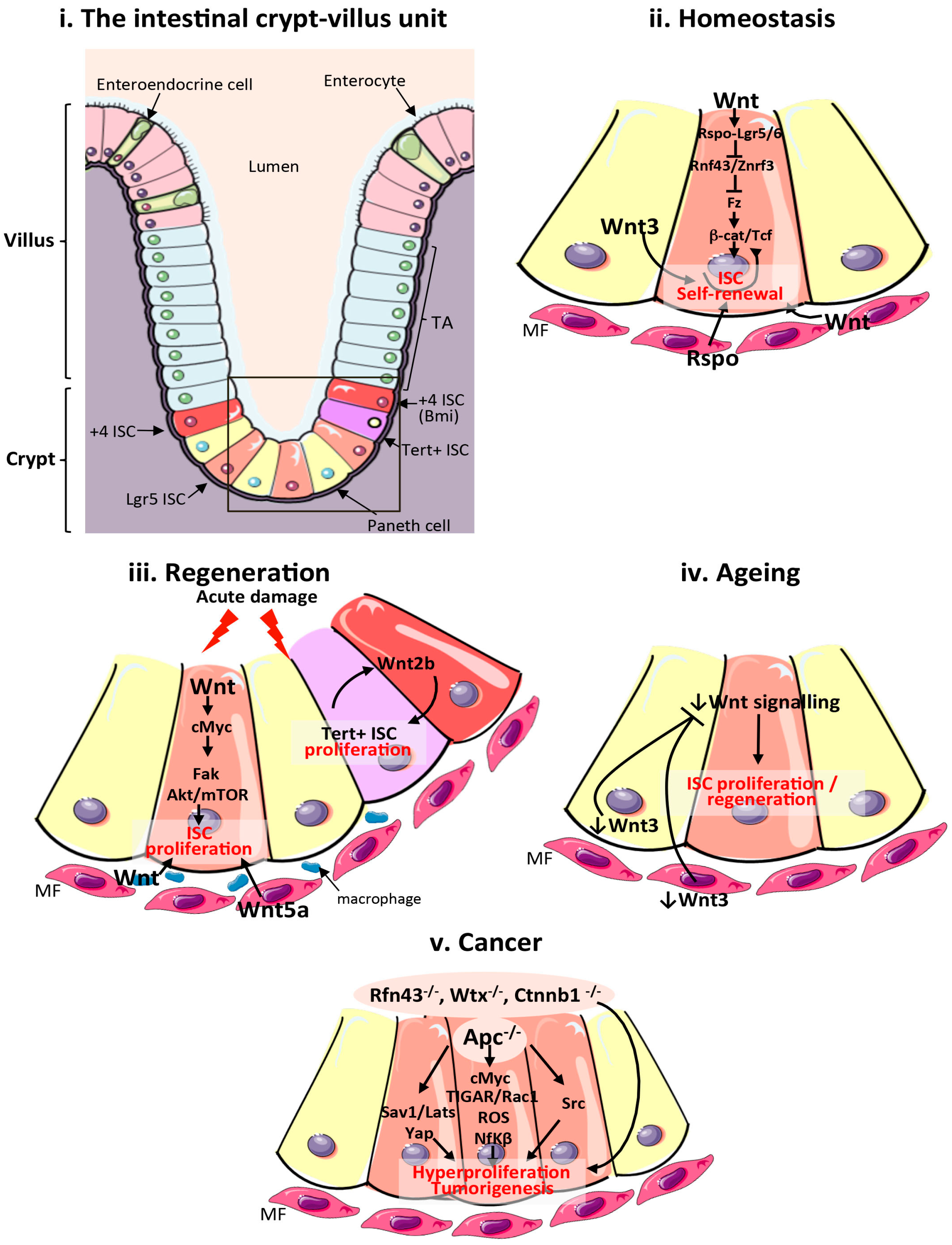
Figure 1.
Wnt signalling in the mammalian intestine during homeostasis, regeneration, ageing and cancer. (i) Schematic of the cellular composition and architecture of a crypt–villi unit in the mammalian intestine. The stroma is depicted in purple and the gut lumen in peach. The boxed area highlights the crypt and stem cell niche, which are magnified in (ii–v); (ii) Main sources of the Wnt stem cell niche and pathway activation during intestinal homeostasis. Wnt3 from Paneth cells, and Wnt and Rspo from mesenchymal and epithelial stem cell niches are important sources of Wnt during homeostasis; (iii) Sources of the Wnt stem cell niche and pathway activation during intestinal regeneration following damage. Wnt2b signalling from intestinal epithelial cells is required to activate proliferation of quiescent Tert + intestinal stem cells (ISCs). Wnt from macrophages and Wnt5a from the mesenchyme are also important sources of Wnt during regeneration. Wnt activation of cMyc is known to target Fak and Akt/mTOR pathways to increase ISC proliferation in response to damage; (iv) Reduced production of mesenchymal and Paneth cell Wnt3 and canonical Wnt pathway activity in the ageing intestinal epithelium impairs ISC proliferation; (v). Wnt pathway activation during intestinal tumourigenesis and functional pathways activated following Apc loss. ISCs: intestinal stem cells; TA: transit amplifying cells; Tert: telomerase reverse transcriptase; MF: mesenchymal fibroblasts; Apc: Adenomatous polyposis coli; Fak: focal adhesion kinase; ROS: reactive oxygen species; TIGAR: TP53-inducible glycolysis and apoptosis regulator; Rspo: R-Spondin; Sav1: Salvador 1; mTOR: mammalian target of rapamycin; Yap1: Yes associated protein 1; Lats: Large tumour suppressor kinase 1; Nf-κβ: Nuclear factor κβ.
The mammalian intestine contains multipotent stem cells, which have the ability to self-renew and generate undifferentiated transit amplifying cells and the various specialized cell types within the intestinal epithelium, such as the absorptive enterocytes, secretory goblet cells, enteroendocrine cells and Paneth cells (Figure 1i). One of the most notable advancements in the field of intestinal biology has been the discovery of various populations of stem cells within the intestine, characterized by the expression of specific markers, such as Lgr5 [17], Bmi1 [18], Musashi1 (Msi1) [19], Ascl2 [20] and Sox9 [21] among others. Lgr5 is perhaps the most common marker used to target, functionally characterize and lineage trace mammalian ISCs [17]. Lgr5 belongs to the G protein coupled receptor family and it is one of 80 known Wnt target genes in the mammalian intestine [22,23]. ISCs were first discovered using labeling with [3H] Thymidine following irradiation-induced damage of the tissue [24,25,26]. Studies using this method were able to define the label retaining +4 position crypt cells as a population of slowly cycling stem cells, which are distinct from the fast-cycling stem cells, also called crypt base columnar cells (CBC) (Figure 1i). Crypt base columnar cells show active proliferation in response to homeostatic niche signals, while label-retaining cells remain quiescent during normal homeostasis and proliferate in response to damage.
Fast-cycling stem cells have generally been distinctly identified by their expression of the lgr5, Olfm4 and Ascl2 genes, while slower cycling stem cells have been characterized by the expression of markers including Bmi1, Lrig1, Hopx and telomerase reverse transcriptase (mTert) [18,27,28,29,30,31,32]. Nevertheless, recent studies using single cell RNA sequencing technology have revealed subpopulations of cells that exhibit properties of both slow and fast cycling stem cells [33,34,35]. A subpopulation of label-retaining cells acts as Lgr5 precursor cells that can regenerate the tissue upon damage [34], while a subpopulation of Lgr5 stem cells co-expressing Mex3a also act as a slowly cycling reserve pool of stem cells able to rapidly divide upon damage [35]. More work is needed to elucidate the role and regulation of these cell populations.
Intestinal stem cells reside at the base of the crypt while most differentiated cells migrate up the crypt–villus axis in the small intestine [36] with the exception of Paneth cells, an essential component of the intestinal stem cell niche [37], which intercalate between ISCs at the crypt base (Figure 1i). The distinct compartmentalization of stem cells from their differentiated linages, especially in the small intestine, has been attributed to gradients of various signalling pathways along the crypt–villus axis, including Wnt and EphB [22,38,39]. Β-catenin and Tcf inversely control the expression of EphB2 and EphB3 receptors and their ligand Ephrin-B1 within the crypt–villus axis, which leads to higher Eph signalling further from the crypt base. Experiments using EphB2/EphB3 null mice showed that these genes are required to restrict cell intermingling and compartmentalize cell populations within the intestinal epithelium [40].
3. The Adult Drosophila Intestine
The adult digestive tract of the fruit fly Drosophila melanogaster is a tubular structure surrounded by visceral muscle, enteric neurons and gut-associated trachea, which are akin to the mammalian vasculature. As it is the case for the mammalian intestine, the fly gut ensures essential physiological functions of the living organism, such as the incorporation and processing of food, nutrient absorption and elimination of solid waste, and displays key endocrine, immune and metabolic roles. The fly intestine consists of a monolayer epithelium, divided into three domains of different developmental origins: the foregut, the midgut, and the hindgut. The foregut and the hindgut epithelium are of ectodermal origin whereas the midgut epithelium originates from the endoderm. The foregut comprises the pharynx, the esophagus and the crop. The midgut extends from the cardia until the junction with the hindgut, where the Malpighian tubules, which display functions similar to the mammalian kidneys, connect with the gut. The Drosophila adult midgut is described to be the structural and functional equivalent of the mammalian small intestine [17,41].
The fly midgut is replenished by ISCs [42,43]. Drosophila ISCs undergo cell division to renew themselves and generate uncommitted enteroblasts (EBs), which are progenitor cells that can further differentiate into either secretory enteroendocrine cells (EEs) or absorptive enterocytes (ECs) [44]. ISCs and EBs are characterized by the expression of the snail family transcription factor escargot (esg) [42] and headcase (hdc) [45]. Drosophila ISCs do not reside within discrete anatomical locations equivalent to the mammalian crypts and are instead scattered along the basal membrane of the intestinal epithelium. However, they are in either direct or close contact with their microenvironment, which includes uncommitted progenitor cells (EBs), differentiated midgut epithelial cells, visceral muscle (VM) and trachea cells, which all constitute niches as they provide factors that regulate ISC self-renewal and differentiation [46,47,48,49,50,51,52] (Figure 2i).
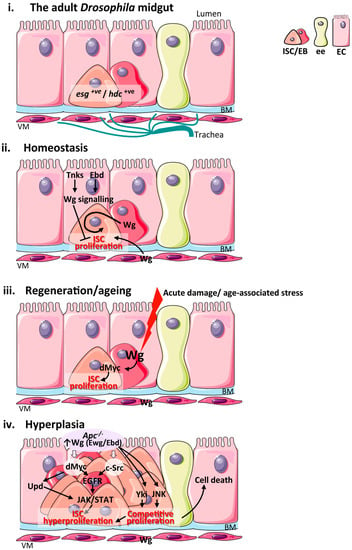
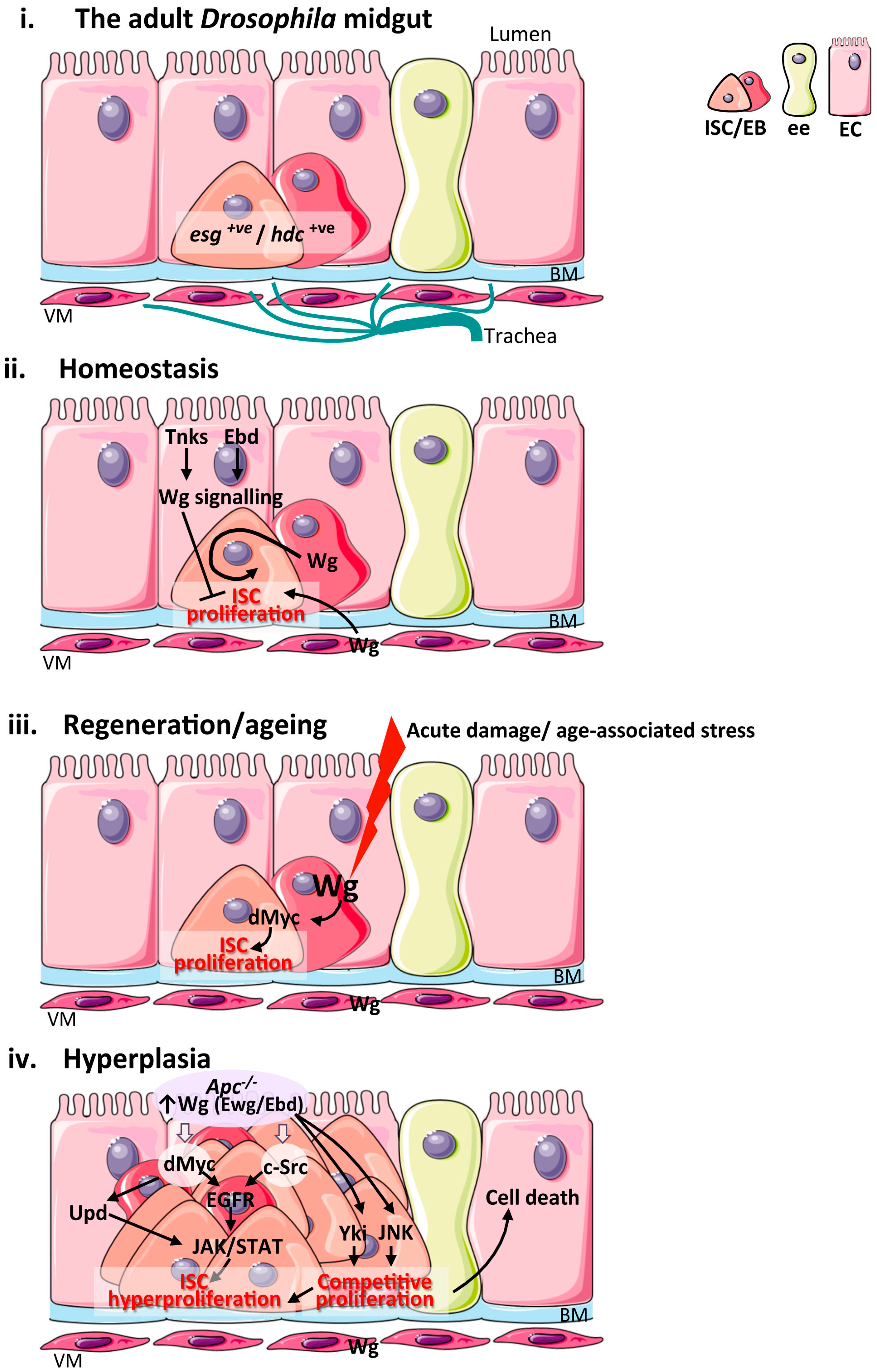
Figure 2.
Wnt signalling in the adult Drosophila midgut during homeostasis, regeneration, ageing and hyperplasia. (i) Schematic of the cellular composition and architecture of the adult Drosophila midgut epithelium and its microenvironment; (ii) Main sources of the Wnt stem cell niche and pathway activation during intestinal homeostasis. The VM and EBs produce Wg ligand to active signalling within ISC and drive their proliferation. Wg signalling activation in ECs by Tnks and Ebd inhibit ISC proliferation non-autonomously; (iii) Sources of the Wg stem cell niche and pathway activation during intestinal regeneration following damage and upon ageing. Up-regulation of Wg from EBs activates Wg signalling and its downstream target dMyc to drive ISC proliferation. The VM niche expresses Wg but is dispensable for ISC proliferation in this context; (iv) Wnt pathway activation during intestinal hyperplasia and functional pathways downstream of Apc. JNK and Yki activation in Apc−/− cells drive ISC proliferation and cell competition leading to apoptosis of neighbouring wild type cells. ISC: intestinal stem cell; EB: enteroblast; EC: enterocyte; ee: enteroendocrine cell; BM: basement membrane; VM: visceral muscle; esg: escargot; hdc: headase; Wg: Wingless; Tnks: Tankyrase; Ebd: Earthbound; Ewg: Erect wing; Yki: Yorkie; JNK: c-Jun N-terminal kinase; Upds: Unpaired cytokines; EGFR: Epithelial growth factor receptor; JAK: Janus kinase.
Due to the similarities between the Drosophila midgut and mammalian intestine and the relative simplicity of the invertebrate model system, the adult fly midgut has become a powerful paradigm for investigating the role of many conserved signalling pathways, including Wnt signalling, in the regulation of ISC activity [51,53].
4. Wnt Signalling in Mammalian Intestinal Homeostasis and Regeneration
Initial evidence towards a central role of the Wnt pathway in intestinal-stem-cell homeostasis came from pioneering studies in mice showing that genetic ablation of canonical Wnt singling transcription factors Tcf-4 and β-catenin or the use of an inhibitor of the Wnt receptor, Dickkopf-related protein 1 (Dkk1), abolished the proliferative capacity of the small intestine and led to severe disruption of intestinal epithelial integrity, including the loss of crypts [54,55,56,57,58,59].
The discovery of Lgr5 as a stem cell marker [17] and the establishment of intestinal organoids from purified crypts [60] represented a turning point in the study of ISC biology as it permitted, amongst other things, targeted manipulation of genes within ISCs and the isolation of the intestinal epithelium for in vitro studies. Single-sorted Lgr5 positive (Lgr5+ve) stem cells were first shown to be able to produce an organised crypt–villus organoid structure in the absence of the epithelial niche [60]. Later studies reported that the efficiency of organoid formation from single Lgr5+ve cells was significantly increased by the addition of Paneth cells due to the provision of a Wnt niche [37]. The importance of an epithelial and mesenchymal Wnt-niche in the maintenance of stem cells in the intestine has been recently challenged by a study reporting that impairment of Wnt secretion from the intestinal epithelium or underlying smooth muscle through conditional knockout of porcupine resulted in no obvious defects on intestinal epithelial structure, Wnt activation or the proliferative rate of ISCs [61]. That study suggested a potential redundant nature of the Wnt stem cell niche in the mammalian intestine, which was later confirmed by a recent report revealing intestinal defects following global prevention of Wnt secretion through ubiquitous knockout of Wntless [62].
Lgr receptors potentiate Wnt signalling within ISCs following binding to the ligand, R-spondin. R-spondin/Lgr binding leads to activation of downstream Wnt signalling through downregulation of Fz specific E3 ubiquitin ligases Rnf43 and Znrf3 [63,64] (Figure 1ii). Impairment of Wnt signalling by Rnf43 and Znrf3 suppresses proliferation in the intestine through ubiquitination and degradation of surface-expressed Fz5 [63]. The receptor, Fz7 is also enriched in and required for ISC function in the mammalian intestine [65]. Recent research has demonstrated the importance of a non-redundant cooperation between R-spondin and Wnt signalling, by showing that Wnt proteins are required to prime ISCs by maintaining R-spondin receptor expression, which then drives the further expansion of stem cells via R-spondin ligands [66].
In addition to its function in intestinal homeostasis, Wnt signalling also plays a crucial role during the regeneration of the mammalian intestine following injury. The intestinal epithelium can robustly regenerate in response to multiple forms of stressors/damaging agents that disrupt the tissue, such as cytotoxic drugs [67], gamma radiation [68], and following surgical resection [69]. Regeneration is characterized by an increase in proliferation within the crypt compartment [70]. Multiple studies show Wnt signalling activation as a key event in the induction of intestinal regeneration (Figure 1iii). The Wnt target c-Myc is upregulated within the crypt compartment of the intestinal epithelium in response to DNA damage and its activation is essential to induce intestinal regeneration through focal adhesion kinase (Fak) and Akt/mTOR signalling [71]. Additionally, Wnt5a is expressed by the stroma in response to intestinal damage and induces crypt regeneration in a TGFβ-dependent manner [72]. Interestingly, Wnt produced by macrophages, a key constituent of the stroma, represents an important component of the stem cell niche, which is required for intestinal regeneration and animal survival following damage by irradiation [73]. More recently, damage-inducible Wnt2b expressed in epithelial cells within the crypt has also been proven essential for the regeneration of the intestine upon irradiation by stimulating proliferation of a subpopulation of quiescent Tert+ve ISCs [74] (Figure 1iii). While conditional ablation of Tert+ve cells by diphtheria toxin A expression did not affect intestinal homeostasis, it impaired tissue regeneration following injury [74]. Altogether, this evidence points to the presence of multiple inducible, non-redundant Wnt ligand sources, which are essential for pathway activation and the execution of intestinal regeneration.
5. Wnt Signalling in Intestinal Homeostasis and Regeneration in Drosophila
In addition to the well-known general benefits offered by their short life cycle and large number of offspring, there are at least two key advantages of using Drosophila for the study of Wnt signalling in the intestine: the presence of genetic tools to individually label every cell types within the midgut, and the low redundancy of Wnt ligands.
Multiple-redundant stem cell populations have been identified in mouse models [18,27,28,29,30,31,32,34,35,75]. Moreover, recent studies have highlighted the great plasticity in the mouse intestinal epithelium as evidenced by the de-differentiation potential of committed lineages, including secretory progenitor enterocytes [76], enteroendocrine cells [77] and Paneth cell precursors [78]. This regain of stemness in order to re-populate the mouse intestine upon damage makes the pool of ‘reserve stem cell potential’ even larger and complicates studies of mammalian ISCs.
Functional studies of ISCs in the adult Drosophila midgut are less complex. The discovery of esg [42] and, more recently, hdc [45] as markers of all stem/progenitor cells (ISCs/EBs), has allowed global targeting of this cell population and unambiguous assessment of their role in intestinal homeostasis and regeneration [45,79]. De-differentiation of committed lineages has not been reported in the adult Drosophila midgut, suggesting a lower degree of plasticity or reserve stem cell potential in the invertebrate tissue when compared to the mammalian intestine. However, recent studies have reported the existence of cell division without mitotic spindle formation in polyploid ECs, also known as amitosis, and plasticity in the rate of turnover of ECs as a means to maintain intestinal epithelial homeostasis in conditions where stem cell pools are compromised [80,81].
There are multiple sources of Wnt ligands in the mammalian intestine (Table 1) [54,82], which has complicated studies on the homeostatic Wnt stem cell niche [61,83,84,85]. However, low redundancy in the function of Wnt ligands is revealed during intestinal regeneration upon damage [54,74,84]. Whether the differences between Wnt signalling activity in regeneration and homeostasis reflect the activation of a distinct, ‘regeneration specific’ Wnt signature or different levels of signalling activity required for each process remains to be addressed.

Table 1.
List of fly and mammalian Wnts and their expression and function within the intestine. Known fly and mammalian Wnt ligand genes and their expression status in the intestine as determined by FlyGut-seq and NCBI, respectively. Reported intestinal function of Wnts is referenced.
The scenario concerning the Wnt stem cell niche in the Drosophila midgut appears simpler, even though it shares similarities with its mammalian counterpart. Only Wingless (Wg) and Wnt4 appear to be expressed in the adult fly midgut (Table 1) and Wg is so far the only Wnt ligand reported to have a functional role in the tissue (Table 1). Pioneering studies identified the visceral muscle (VM), which surrounds the intestinal epithelium, as the main source of the Wg stem cell niche in homeostatic conditions [86] (Figure 2ii). Global knockdown of wg or intestinal epithelial loss of genes encoding for fz and fz2 receptors prevented homeostatic ISC self-renewal [86]. Further studies have described and characterized novel sources of the Wg stem cell niche and additional roles of the pathway in intestinal homeostasis and regeneration [87,95,96]. In addition to the visceral muscle, epithelial Wg is expressed in the midgut–hindgut junction in homeostatic tissues. Here, Wnt pathway activation appears graded along the length of the adult intestine, peaking at compartment boundaries [96]. Interestingly, work by the same group has demonstrated that Wg pathway activation within ECs impairs ISC proliferation non-autonomously during homeostasis [96,97] (Figure 2ii).
Wg expression by progenitor cells (EBs) is upregulated following damage to the intestinal epithelium [87,95] (Figure 2iii). Cell-specific knockdown experiments have demonstrated that Wg from EBs activates Wnt signalling and induction of the conserved pathway target dMyc within ISCs to drive ISC proliferation upon damage (Figure 2iii). Importantly, while this source of the ligand is essential to drive ISC proliferation and tissue regeneration in response to injury, it is dispensable for homeostatic ISC self-renewal [87]. A strikingly similar phenomenon has been recently described for Wnt2 in the mouse intestine [74] (Figure 1iii).
6. Wnt Signalling in Ageing and Tumorigenesis of the Mammalian Intestine
Aging is a complex process, which leads to a decline in tissue integrity and functionality. Ageing affects stem cell function and, therefore, the regenerative capacity of self-renewing tissues. Persistent expression of Wnt1 within the skin epidermis, which also contains Lgr5+ve stem cells, leads to senescence and exhaustion of the stem cell compartment through the sustained activation of mTOR, resulting in a premature ageing phenotype [98]. Conversely, a decrease in canonical Wnt signalling upon ageing results in reduced regenerative potential of the intestine (Figure 1iv). Wnt3 is reduced within stem cells and their niches in the ageing intestine and the addition of the ligand to intestinal organoids of ageing animals can restore ISC function [90]. This reduction of Wnt signalling in the aging intestine has been proposed to represent a protective mechanism to counteract age-associated mutations that could cause intestinal hyperproliferation. However, this also leads to an overall reduced regenerative potential of ISCs upon ageing. Therefore, a full understanding of ageing-specific Wnt signalling events may lead to the design of targeted therapies to prevent age-associated intestinal dysfunction without driving tissue malignancies, such as cancer.
Wnt signalling is perhaps best known for its role as a key driver of intestinal cancer, typically through loss of the negative regulator of the pathway Apc [99,100]. Apc is part of the destruction complex, which counteracts pathway activation by targeting β-catenin for ubiquitination and degradation by the proteasome [101]. Apc and CRC were first linked by the discovery and characterization of familial adenomatous polyposis (FAP) [102,103], an inherited form of CRC characterized by mutations in the Apc gene [104]. Apc is mutated in 80–90% of hereditary and spontaneous forms of colorectal cancer (CRC) [105]. Over 60% of the mutations within Apc occur in the mutation cluster region (MCR) and affect binding to Axin or β-catenin [106], which results in the accumulation of β-catenin and excessive Wnt signalling. Although with lower incidence, CRC can also occur as a result of activating mutations in the Ctnnb1 gene that encodes for β-catenin. Two independent Ctnnb1 mutations have been shown to disrupt specific serine/threonine residues within β-catenin, which are normally subject to Gsk-3β phosphorylation and required for subsequent protein degradation, thereby leading to accumulation of β-catenin [106]. Loss of function mutations in components of the Wnt pathway, beyond Apc, have been associated with CRC. Mutations in Rnf43 were found by whole exome sequencing in 18% of colorectal adenomas, particularly in cases with high microsatellite instability (MSI-H) [107]. The Wilms tumour suppressor (Wtx), a part of the β-catenin destruction complex, [108] is also mutated in colorectal tumours with MSI-H [109]. However, in this article we will focus on CRC driven by Apc loss.
The study of Apc and its role in CRC was pioneered by the generation of genetically engineered mouse models [110,111]. Conditional loss of function experiments showed that Apc is required for cell proliferation and differentiation within the intestinal epithelium, as well as for the migration of cells along the crypt–villus axis [111]. Later work assessing the contribution of ISCs to the generation of intestinal tumours showed that knockdown of Apc within Lgr5+ve stem cells leads to rapid intestinal adenoma formation. This work provided the first demonstration of a role of ISCs as the cells of origin in CRC [112]. Complementarily, in vitro work showed that silencing Lgr5 leads to reduced cell proliferation, migration and the tumourigenic potential of colorectal cancer cell lines [113]. Furthermore, high Lgr5 expression in cells derived from mouse tumours correlates with strong upregulation of Wnt signalling [113]. However, work on genetically engineered mouse models shows that ablation of Lgr5 stem cells within Apc-driven adenomas, is not sufficient to affect tumour burden. Therefore, there may be multiple redundant cell populations, including Lgr5−ve ISCs, which contribute to intestinal tumourigenesis [68].
Extensive work has been carried out to determine the mechanisms by which Apc loss drives proliferation in CRC. For a comprehensive account of the literature on this subject, please see [114,115]. Our goal here is to highlight some key studies on the mechanisms driving intestinal hyperproliferation following Apc loss from ISCs, as this is most directly related to the subject of this review (Figure 1v). One such mechanism is mediated by activation of the Rac1 GTPase. Apc loss leads to myc activation, which is in turn required to activate Rac1, leading to intestinal tumourigenesis via reactive oxygen species (ROS) production and NF-κB signalling [116]. The TP53-inducible glycolysis and apoptosis regulator (TIGAR), a protein involved in glucose metabolism, cooperates with Rac1 to drive proliferation in response to Apc loss in the intestine [117] (Figure 1v).
Activation of the non-receptor tyrosine kinase c-Src is increased by up to 15-fold in human CRC [118]. Functional genetic studies in mice and Drosophila show that Src is required to induce intestinal tumourigenesis and ISC proliferation following Apc loss [119]. Src activation in ISCs is sufficient to drive intestinal hyperplasia, while conditional knock out of Apc and Src within Lgr5+ve stem cells resulted in reduced tumour burden and increased animal survival [119] (Figure 1v).
Another pathway characterized as a downstream effector of Apc loss in the intestine includes the Hippo signalling pathway, a conserved tumour suppressor pathway associated with CRC [120]. The transcription factor Yes-associated protein (Yap), which is normally inactivated by Hippo signalling, is required for the formation of adenomas following loss of Apc. In this context, Apc acts as a scaffold for Hippo pathway kinases Salvador (Sav1) and Large tumour suppressor kinase 1 (Lats) to facilitate phosphorylation and subsequent degradation of Yap [121] (Figure 1v). An accompanying article published in this special issue provides a comprehensive review of Wnt and Hippo signalling interactions in the intestine [122].
The above-described studies support the ‘bottom up’ model of CRC, where loss of Apc in the crypt/stem cell compartment is required to induce intestinal tumourigenesis. An opposing model of CRC is the ‘top down’ model, which postulates that cells from the villi can also drive intestinal transformation. Apc loss from the villi only is not sufficient for the generation of persistent intestinal tumours [112,123]. However, combinations of Apc loss and activation of NF-κB induce de-differentiation and drive tumorigenesis from villi [124]. A similar outcome is observed upon loss of TGFβ, through inactivation of the TGFβ type 1 receptor in animals deficient for Apc and carrying a constitutively active Kras mutation [125]. Further studies to better define the mechanisms through which Wnt signalling drives intestinal tumourigenesis are vital to identify novel therapeutic targets for CRC.
7. Wnt Signalling in Intestinal Hyperplasia and Ageing in Drosophila
Multiple studies have shown that the Drosophila midgut undergoes age-related dysfunction of the intestinal epithelium, which is characterized by excessive ISC proliferation and aberrant differentiation [126,127,128,129]. ISC proliferation dictates the global wellbeing of the organism and animal lifespan [130]. Midgut epithelial Wg expression is induced upon ageing and drives age-dependent ISC hyperproliferation through activation of its target dMyc within ISCs [87] (Figure 2iii). Critically, partial knockdown of Wg or Myc prevents age-dependent intestinal hyperplasia without disrupting ISC homeostasis [87], highlighting the potential benefits to the organism of maintaining controlled levels of Wnt signalling activation in the intestine. Interestingly, a recent report reveals differences in the way Wnt signalling is regulated in the ageing mouse and fly intestine. Unlike in flies, the ageing mouse intestinal epithelium displays a reduced regenerative potential due to a decline in canonical Wnt signalling [90] (Figure 1iv). It therefore appears that the ageing fly intestine is more similar to ageing haematopoietic stem cells and the skin epidermis, which are also characterized by exacerbated Wnt signaling [98,131,132], than to the ageing mouse intestine.
The adult Drosophila midgut has been successfully used to model various aspects of colorectal-cancer-like hyperplasia. Over activation of Wnt signalling through overexpression of wg, activated β-catenin or loss of Drosophila Apc leads to increased ISC proliferation and epithelial hyperplasia [86,133,134,135] (Figure 2iv). Downregulation of dMyc or overexpression of dominant negative Tcf, suppresses intestinal hyperplasia after Apc loss [133,135], suggesting the involvement of the canonical pathway in this process. Recent work has revealed that two conserved suppressors of Drosophila Apc1, earthbound (Ebd) and erect wing (Ewg) cooperate with β-catenin and Tcf to promote target gene activation and intestinal hyperplasia following loss of Apc1 [97] (Figure 2iv). Ebd is known to physically associate with and promote the formation and stability of the β-catenin–Tcf complex and the recruitment of β-catenin to the chromatin [136]. Ewg is a DNA binding transcriptional activator that shares DNA binding specificity with the human nuclear respiratory factor-1 (Nrf-1) [137]. The potential role Nrf1, in mammalian Wnt signalling merits future investigation. Interestingly, Jerky (also known Jrk or Jh8), the human homolog of Ebd is detected at high levels in colon carcinoma and it is associated with increased nuclear β-catenin and the overexpression of Wnt target genes in human colorectal tumors [138].
Pathways mediating intestinal hyperproliferation downstream of Apc loss in Drosophila also include the EGFR/MAPK and JAK/STAT signalling. Wg signalling regulates ISC proliferation by inducing the production of ligands of the EGFR and JAK/STAT pathways in EBs and ECs respectively [135]. This paracrine EGFR and JAK/STAT signalling crosstalk mediates intestinal hyperproliferation following Wg overexpression and Apc1 loss. Moreover, Wnt signalling activates the non-receptor tyrosine kinase c-Src (Src) in vivo [119]. Src drives tumourigenesis upon Apc loss in the adult fly midgut through ISC upregulation of EGFR and JAK/STAT signalling [119] (Figure 2iv). Lastly, as in mammals [120,121], Hippo signaling also mediates tumourigenesis of Apc deficient cells (Apc−/−) in the adult fly midgut [139]. Interestingly, the work in Drosophila reveals tumor–host-cell competition as an important determinant in the expansion of Apc−/− cells, which appears to involve activation of the c-Jun N-terminal Kinase (JNK) and the Hippo signalling transcription factor Yorkie (Yki) within Apc−/− cells, and apoptosis of the surrounding wild type cells [139] (Figure 2iv). Further investigation of the role of Wg signalling in cell competition within the Drosophila midgut could provide a new understanding on the pathology of Apc driven tumourigenesis in the intestine.
Oncogenic cooperation in CRC has also been modeled in Drosophila. Cooperation between loss of Apc and hyperactivation of ras (Apc−/−, rasV12), which characterizes malignant stages of human colorectal tumours [140,141], drives the progression of Apc mutant intestinal tumors and the activation of Apc−/−, rasV12 specific transcriptional targets in the Drosophila adult midgut [142,143]. This paradigm should not only provide an excellent model to analyze the genetic events involved in malignant tumor progression, but may also represent an attractive system to identify processes specific to such genetic combinations and to test therapeutic agents. In fact, chemical compounds have been successfully used in complex Drosophila models bearing combinations of various ‘CRC-like’ oncogenic mutations that generate invasive intestinal tumours derived from differentiated hindgut cells, the functional equivalent of the mammalian colon [144]. Further use of the above-described paradigms is likely to provide new insights into the functional molecular networks driving various stages of CRC, which may contribute to the design of personalized therapeutics for the disease.
8. Conclusions and Perspectives
Many interesting questions remain to be addressed regarding the role of Wnt signalling in the intestine and, in particular, in ISC function. Understanding the role of ‘regeneration specific’ Wnt ligands, which are redundant for basal tissue homeostasis, is one of them. Studies in both mice and Drosophila have evidenced the existence of damage-inducible Wnt stem cell niches that are specifically needed to drive the acute proliferative response of ISCs following injury, but are dispensable for homeostatic tissue self-renewal [71,87]. Intestinal regeneration shares many molecular features of tumorigenesis [116,119]. Identification of the mechanisms activated by damage/stress-inducible sources of Wnt ligands might represent an excellent therapeutic window for the targeting of Wnt-driven intestinal hyperplasia while preserving organismal health.
Another interesting aspect of Wnt signalling in the intestine relates to the role of short range signalling in the system. Pioneer work in Drosophila revealed that restriction of Wg secretion through cell membrane tethering maintains cell growth functions of the ligand in the wing disc [145]. Later work in the mammalian intestine presented evidence for short-range Wg signalling in stem cell proliferation through membrane-tethered Wnt3 [89]. The extent of developmental and adult intestinal-specific functions of such short range signalling remains largely unexplored. Drosophila is likely to provide invaluable answers to this and other unexplored aspects of the regulation of intestinal health and disease by Wnt signalling.
New, sophisticated mammalian CRC models are being successfully created through the use of novel technologies such as CRISPR/Cas9. This includes the generation of mouse models carrying multiple gene mutations [146], metastatic CRC models [147,148,149,150], tools to trace cancer stem cells in vivo [151] and complex gene editing within cultured intestinal organoids [152,153]. It will only be a matter of time before the conservation of intricate molecular networks identified in Drosophila can be assessed in such powerful mammalian paradigms.
Acknowledgments
We thank to all our colleagues, whose work has contributed to this review and apologies to those whose work has not been discussed due to space restrictions. J.B.C. is a Sir Henry Dale Fellow jointly funded by the Wellcome Trust and the Royal Society (Grant Number 104103/Z/14/Z).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nusse, R.; Varmus, H.E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31, 99–109. [Google Scholar] [CrossRef]
- Papkoff, J.; Brown, A.M.; Varmus, H.E. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol. Cell. Biol. 1987, 7, 3978–3984. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.K.; Shackleford, G.M.; Brown, A.M.; Sanders, G.S.; Varmus, H.E. Nucleotide sequence and expression in vitro of cDNA derived from mRNA of int-1, a provirally activated mouse mammary oncogene. Mol. Cell. Biol. 1985, 5, 3337–3344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rijsewijk, F.; Schuermann, M.; Wagenaar, E.; Parren, P.; Weigel, D.; Nusse, R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 1987, 50, 649–657. [Google Scholar] [CrossRef]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Kishida, S.; Yamamoto, H.; Ikeda, S.; Kishida, M.; Sakamoto, I.; Koyama, S.; Kikuchi, A. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J. Biol. Chem. 1998, 273, 10823–10826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, A.; Benchabane, H.; Tacchelly-Benites, O.; Yang, E.; Nojima, H.; Ahmed, Y. The ADP-ribose polymerase Tankyrase regulates adult intestinal stem cell proliferation during homeostasis in Drosophila. Development 2016, 143, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.M.; Szczerkowski, J.L.A.; Habib, S.J. Wnt ligand presentation and reception: From the stem cell niche to tissue engineering. Open Biol 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.K.; Mulberg, A.E.; Grand, R.J. Development of the human gastrointestinal tract: Twenty years of progress. Gastroenterology 1999, 116, 702–731. [Google Scholar] [CrossRef]
- Bowcutt, R.; Forman, R.; Glymenaki, M.; Carding, S.R.; Else, K.J.; Cruickshank, S.M. Heterogeneity across the murine small and large intestine. World J. Gastroenterol. 2014, 20, 15216–15232. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgi, E.; Capecchi, M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008, 40, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Booth, C.; Tudor, G.L.; Booth, D.; Brady, G.; Hurley, P.; Ashton, G.; Clarke, R.; Sakakibara, S.; Okano, H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 2003, 71, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jubb, A.M.; Chalasani, S.; Frantz, G.D.; Smits, R.; Grabsch, H.I.; Kavi, V.; Maughan, N.J.; Hillan, K.J.; Quirke, P.; Koeppen, H. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene 2006, 25, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, K.; Kawaguchi, Y.; Akiyama, H.; Horiguchi, M.; Kodama, S.; Kuhara, T.; Hosokawa, S.; Elbahrawy, A.; Soeda, T.; Koizumi, M.; et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011, 43, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Sancho, E.; Verweij, C.; de Lau, W.; Oving, I.; Hurlstone, A.; van der Horn, K.; Batlle, E.; Coudreuse, D.; Haramis, A.P.; et al. The β-catenin/Tcf-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002, 111, 241–250. [Google Scholar] [CrossRef]
- Van Der Flier, L.; Sabates-Bellver, J.; Oving, I.; Haegebarth, A.; De Palo, M.; Anti, M.; Van Gijn, M.; Suijkerbuijk, S.; Van De Wetering, M.; Marra, G.; et al. The intestinal Wnt/Tcf signature. Gastroenterology 2007, 132, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Kovacs, L.; Hamilton, E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974, 7, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 1977, 269, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Owen, G.; Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002, 115, 2381–2388. [Google Scholar] [PubMed]
- Takeda, N.; Jain, R.; LeBoeuf, M.R.; Wang, Q.; Lu, M.M.; Epstein, J.A. Interconversion between intestinal stem cell populations in distinct niches. Science 2011, 334, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.K.; Carlone, D.L.; Richmond, C.A.; Farilla, L.; Kranendonk, M.E.; Henderson, D.E.; Baffour-Awuah, N.Y.; Ambruzs, D.M.; Fogli, L.K.; Algra, S.; et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.E.; Wang, Y.; Li, Y.; Poulin, E.J.; Means, A.L.; Washington, M.K.; Higginbotham, J.N.; Juchheim, A.; Prasad, N.; Levy, S.E.; et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012, 149, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, L.G.; Haegebarth, A.; Stange, D.E.; van de Wetering, M.; Clevers, H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 2009, 137, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, L.G.; van Gijn, M.E.; Hatzis, P.; Kujala, P.; Haegebarth, A.; Stange, D.E.; Begthel, H.; van den Born, M.; Guryev, V.; Oving, I.; et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 2009, 136, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Schuijers, J.; van der Flier, L.G.; van Es, J.; Clevers, H. Robust cre-mediated recombination in small intestinal stem cells utilizing the OLFM4 locus. Stem Cell Rep. 2014, 3, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Buczacki, S.J.; Zecchini, H.I.; Nicholson, A.M.; Russell, R.; Vermeulen, L.; Kemp, R.; Winton, D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013, 495, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Barriga, F.M.; Montagni, E.; Mana, M.; Mendez-Lago, M.; Hernando-Momblona, X.; Sevillano, M.; Guillaumet-Adkins, A.; Rodriguez-Esteban, G.; Buczacki, S.J.A.; Gut, M.; et al. Mex3a marks a slowly dividing subpopulation of Lgr5+ intestinal stem cells. Cell Stem Cell 2017, 20, 801–816.e7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am. J. Anat. 1974, 141, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Nie, Q.; Holmes, W.R. The interplay between Wnt mediated expansion and negative regulation of growth promotes robust intestinal crypt structure and homeostasis. PLoS Comput. Biol. 2015, 11, e1004285. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Genander, M.; Halford, M.M.; Annerén, C.; Sondell, M.; Chumley, M.J.; Silvany, R.E.; Henkemeyer, M.; Frisén, J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 2006, 125, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Henderson, J.T.; Beghtel, H.; van den Born, M.M.; Sancho, E.; Huls, G.; Meeldijk, J.; Robertson, J.; van de Wetering, M.; Pawson, T.; et al. Β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 2002, 111, 251–263. [Google Scholar] [CrossRef]
- Casali, A.; Batlle, E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell 2009, 4, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Micchelli, C.A.; Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006, 439, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ohlstein, B.; Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 2006, 439, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Boquete, J.P.; Lemaitre, B. A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila. PLoS Genet. 2017, 13, e1006854. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.P.; Truong, M.E.; Gomez, A.; Jones, D.L. Intestinal stem cell ablation reveals differential requirements for survival in response to chemical challenge. Dev. Biol. 2017, 424, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Edgar, B.A. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 2009, 136, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.M.; Chia, L.A.; Kosinski, C.M.; Kuo, C.J. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell. Mol. Life Sci. 2011, 68, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, A.; Cordero, J.B.; Diao, F.; Strathdee, K.; White, B.H.; Sansom, O.J.; Vidal, M. Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr. Biol. 2014, 24, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, S.; Mana-Capelli, S.; Roth Flach, R.J.; Danai, L.V.; Amcheslavsky, A.; Nie, Y.; Kaneko, S.; Yao, X.; Chen, X.; et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev. Cell 2014, 31, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Loza-Coll, M.A.; Southall, T.D.; Sandall, S.L.; Brand, A.H.; Jones, D.L. Regulation of Drosophila intestinal stem cell maintenance and differentiation by the transcription factor Escargot. EMBO J. 2014, 33, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Naszai, M.; Carroll, L.R.; Cordero, J.B. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut. Insect Biochem. Mol. Biol. 2015, 67, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Gerencser, A.A.; Jasper, H. Signal integration by Ca2+ regulates intestinal stem-cell activity. Nature 2015, 528, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.B.; Sansom, O.J. Wnt signalling and its role in stem cell-driven intestinal regeneration and hyperplasia. Acta Physiol. 2012, 204, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Farin, H.F.; Van Es, J.H.; Clevers, H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 2012, 143, 1518–1529.e7. [Google Scholar] [CrossRef] [PubMed]
- Fevr, T.; Robine, S.; Louvard, D.; Huelsken, J. Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 2007, 27, 7551–7559. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Kuhnert, F.; Davis, C.R.; Kuo, C.J. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle 2004, 3, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Moerer, P.; van Donselaar, E.; Huls, G.; Peters, P.J.; Clevers, H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998, 19, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, F.; Davis, C.R.; Wang, H.T.; Chu, P.; Lee, M.; Yuan, J.; Nusse, R.; Kuo, C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA 2004, 101, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Gregorieff, A.; Begthel, H.; Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003, 17, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- San Roman, A.K.; Jayewickreme, C.D.; Murtaugh, L.C.; Shivdasani, R.A. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Rep. 2014, 2, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Degirmenci, B.; Moor, A.E.; Herr, P.; Zimmerli, D.; Moor, M.B.; Hausmann, G.; Cantù, C.; Aguet, M.; Basler, K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016, 15, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Spit, M.; Jordens, I.; Low, T.Y.; Stange, D.E.; van de Wetering, M.; van Es, J.H.; Mohammed, S.; Heck, A.J.; Maurice, M.M.; et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012, 488, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D.J.; Phesse, T.J.; Barker, N.; Schwab, R.H.; Amin, N.; Malaterre, J.; Stange, D.E.; Nowell, C.J.; Currie, S.A.; Saw, J.T.; et al. Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5+ stem cells. Stem Cell Rep. 2015, 4, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Janda, C.Y.; Chang, J.; Zheng, G.X.Y.; Larkin, K.A.; Luca, V.C.; Chia, L.A.; Mah, A.T.; Han, A.; Terry, J.M.; et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 2017, 545, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Konsavage, W.M.; Jin, G.; Yochum, G.S. The Myc 3′ Wnt-responsive element regulates homeostasis and regeneration in the mouse intestinal tract. Mol. Cell. Biol. 2012, 32, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bernal, N.P.; Stehr, W.; Zhang, Y.; Profitt, S.; Erwin, C.R.; Warner, B.W. Evidence for active Wnt signaling during postresection intestinal adaptation. J. Pediatr. Surg. 2005, 40, 1025–1029, discussion 1029. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, K.; Potten, C.S. Radiation-hypersensitive cells in small intestinal crypts; their relationships to clonogenic cells. Br. J. Cancer Suppl. 1986, 7, 20–22. [Google Scholar] [PubMed]
- Ashton, G.H.; Morton, J.P.; Myant, K.; Phesse, T.J.; Ridgway, R.A.; Marsh, V.; Wilkins, J.A.; Athineos, D.; Muncan, V.; Kemp, R.; et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 2010, 19, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Ajima, R.; Luo, C.T.; Yamaguchi, T.P.; Stappenbeck, T.S. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012, 338, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Aranda, E.; Hayakawa, Y.; Bhanja, P.; Atay, S.; Brodin, N.P.; Li, J.; Asfaha, S.; Liu, L.; Tailor, Y.; et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.N.; Kim, M.J.; Jung, Y.S.; Lien, E.M.; Jun, S.; Park, J.I. Quiescence exit of tert+ stem cells by Wnt/β-catenin is indispensable for intestinal regeneration. Cell Rep. 2017, 21, 2571–2584. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Chia, L.A.; Li, X.; Ootani, A.; Su, J.; Lee, J.Y.; Su, N.; Luo, Y.; Heilshorn, S.C.; Amieva, M.R.; et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. USA 2012, 109, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, P.W.; Basak, O.; Farin, H.F.; Wiebrands, K.; Kretzschmar, K.; Begthel, H.; van den Born, M.; Korving, J.; de Sauvage, F.; van Es, J.H.; et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell 2016, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Gevaert, O.; Zheng, G.X.Y.; Anchang, B.; Probert, C.S.; Larkin, K.A.; Davies, P.S.; Cheng, Z.F.; Kaddis, J.S.; Han, A.; et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 2017, 21, 78–90.e76. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, U.; Saxena, M.; O′Neill, N.K.; Saadatpour, A.; Yuan, G.C.; Herbert, Z.; Murata, K.; Shivdasani, R.A. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell 2017, 21, 65–77.e65. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Z. No intestinal stem cell regeneration after complete progenitor ablation in Drosophila adult midgut. J. Genet. Genom. 2015, 42, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Patel, P.H.; Kohlmaier, A.; Pavlovic, B.; Zhang, C.; Edgar, B.A. Intestinal Stem Cell Pool Regulation in Drosophila. Stem Cell Rep. 2017, 8, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Lucchetta, E.M.; Ohlstein, B. Amitosis of polyploid cells regenerates functional stem cells in the Drosophila intestine. Cell Stem Cell 2017, 20, 609–620.e606. [Google Scholar] [CrossRef] [PubMed]
- Gregorieff, A.; Pinto, D.; Begthel, H.; Destree, O.; Kielman, M.; Clevers, H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 2005, 129, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Escudero, S.; Shivdasani, R.A. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc. Natl. Acad. Sci. USA 2012, 109, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Donahue, B.; Peignon, G.; Letourneur, F.; Cagnard, N.; Slomianny, C.; Perret, C.; Shroyer, N.F.; Romagnolo, B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc. Natl. Acad. Sci. USA 2012, 109, 8965–8970. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.E.; Farin, H.F.; Macůrková, M.; van Es, J.H.; Clevers, H.C.; Korswagen, H.C. Retromer dependent recycling of the Wnt secretion factor Wls is dispensable for stem cell maintenance in the mammalian intestinal epithelium. PLoS ONE 2013, 8, e76971. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Xu, N.; Xi, R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 2008, 455, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.B.; Stefanatos, R.K.; Scopelliti, A.; Vidal, M.; Sansom, O.J. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 2012, 31, 3901–3917. [Google Scholar] [CrossRef] [PubMed]
- Lickert, H.; Kispert, A.; Kutsch, S.; Kemler, R. Expression patterns of Wnt genes in mouse gut development. Mech. Dev. 2001, 105, 181–184. [Google Scholar] [CrossRef]
- Farin, H.F.; Jordens, I.; Mosa, M.H.; Basak, O.; Korving, J.; Tauriello, D.V.; de Punder, K.; Angers, S.; Peters, P.J.; Maurice, M.M.; et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016, 530, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Nalapareddy, K.; Nattamai, K.J.; Kumar, R.S.; Karns, R.; Wikenheiser-Brokamp, K.A.; Sampson, L.L.; Mahe, M.M.; Sundaram, N.; Yacyshyn, M.B.; Yacyshyn, B.; et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017, 18, 2608–2621. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, S.; Yamaguchi, T.P.; Hebrok, M. Wnt5a is essential for intestinal elongation in mice. Dev. Biol. 2009, 326, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.S.; Dismuke, A.D.; Powell, A.E.; Carroll, K.H.; Wong, M.H. Wnt-reporter expression pattern in the mouse intestine during homeostasis. BMC Gastroenterol. 2008, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Medegan, B.; Braun, D.P. Wnt9A induction linked to suppression of human colorectal cancer cell proliferation. Int. J. Mol. Sci. 2016, 17, 495. [Google Scholar] [CrossRef] [PubMed]
- Ouko, L.; Ziegler, T.R.; Gu, L.H.; Eisenberg, L.M.; Yang, V.W. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J. Biol. Chem. 2004, 279, 26707–26715. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Osman, D.; David, F.P.; Fang, H.Y.; Boquete, J.P.; Deplancke, B.; Lemaitre, B. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013, 3, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Benchabane, H.; Wang, Z.; Ahmed, Y. Regulation of stem cell proliferation and cell fate specification by Wingless/Wnt signaling gradients enriched at adult intestinal compartment boundaries. PLoS Genet. 2016, 12, e1005822. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Benchabane, H.; Wang, Z.; Zimmerman, C.; Xin, N.; Perochon, J.; Kalna, G.; Sansom, O.J.; Cheng, C.; Cordero, J.B.; et al. Intestinal stem cell overproliferation resulting from inactivation of the APC tumor suppressor requires the transcription cofactors Earthbound and Erect wing. PLoS Genet. 2017, 13, e1006870. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Chodosh, L.A.; Williams, B.O.; Gutkind, J.S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 2009, 5, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ichii, S.; Horii, A.; Nakatsuru, S.; Furuyama, J.; Utsunomiya, J.; Nakamura, Y. Inactivation of both APC alleles in an early stage of colon adenomas in a patient with familial adenomatous polyposis (FAP). Hum. Mol. Genet. 1992, 1, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.B.; Smith, K.J.; Beazer-Barclay, Y.; Hamilton, S.R.; Vogelstein, B.; Kinzler, K.W. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994, 54, 5953–5958. [Google Scholar] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Bussey, H.J.R. Familial Polyposis coli: Family Studies, Histopathology, Differential Diagnosis, and Results of Treatment; Johns Hopkins University Press: Baltimore, MD, USA, 1975. [Google Scholar]
- Bisgaard, M.L.; Fenger, K.; Bülow, S.; Niebuhr, E.; Mohr, J. Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Hum. Mutat. 1994, 3, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The ABC of APC. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Nagase, H.; Ando, H.; Horii, A.; Ichii, S.; Nakatsuru, S.; Aoki, T.; Miki, Y.; Mori, T.; Nakamura, Y. Somatic mutations of the APC gene in colorectal tumors: Mutation cluster region in the APC gene. Hum. Mol. Genet. 1992, 1, 229–233. [Google Scholar] [PubMed]
- Giannakis, M.; Hodis, E.; Jasmine Mu, X.; Yamauchi, M.; Rosenbluh, J.; Cibulskis, K.; Saksena, G.; Lawrence, M.S.; Qian, Z.R.; Nishihara, R.; et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014, 46, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Major, M.B.; Camp, N.D.; Berndt, J.D.; Yi, X.; Goldenberg, S.J.; Hubbert, C.; Biechele, T.L.; Gingras, A.C.; Zheng, N.; Maccoss, M.J.; et al. Wilms tumor suppressor WTX negatively regulates Wnt/β-catenin signaling. Science 2007, 316, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Network, C.G.A. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Shibata, H.; Toyama, K.; Shioya, H.; Ito, M.; Hirota, M.; Hasegawa, S.; Matsumoto, H.; Takano, H.; Akiyama, T.; Toyoshima, K.; et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997, 278, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Sansom, O.J.; Reed, K.R.; Hayes, A.J.; Ireland, H.; Brinkmann, H.; Newton, I.P.; Batlle, E.; Simon-Assmann, P.; Clevers, H.; Nathke, I.S.; et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004, 18, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Barker, N.; McNeil, N.; Hu, Y.; Camps, J.; McKinnon, K.; Clevers, H.; Ried, T.; Gaiser, T. LGR5 positivity defines stem-like cells in colorectal cancer. Carcinogenesis 2014, 35, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple roles of APC and its therapeutic implications in colorectal cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Jackstadt, R.; Sansom, O.J. Mouse models of intestinal cancer. J. Pathol. 2016, 238, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Myant, K.B.; Cammareri, P.; McGhee, E.J.; Ridgway, R.A.; Huels, D.J.; Cordero, J.B.; Schwitalla, S.; Kalna, G.; Ogg, E.L.; Athineos, D.; et al. ROS production and NF-κB activation triggered by RAC1 facilitate Wnt-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell 2013, 12, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Lee, P.; Ceteci, F.; Nixon, C.; Blyth, K.; Sansom, O.J.; Vousden, K.H. Opposing effects of TIGAR- and RAC1-derived ROS on Wnt-driven proliferation in the mouse intestine. Genes Dev. 2016, 30, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Talamonti, M.S.; Roh, M.S.; Curley, S.A.; Gallick, G.E. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J. Clin. Investig. 1993, 91, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.B.; Ridgway, R.A.; Valeri, N.; Nixon, C.; Frame, M.C.; Muller, W.J.; Vidal, M.; Sansom, O.J. c-Src drives intestinal regeneration and transformation. EMBO J. 2014, 33, 1474–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Willis, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 2011, 108, E1312–E1320. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Maitra, A.; Anders, R.A.; Taketo, M.M.; Pan, D. Β-catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015, 29, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Kriz, V.; Korinek, V. Wnt, RSPO and Hippo signalling in the intestine and intestinal stem cells. Genes 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, P.W.; Kretzschmar, K.; Begthel, H.; van den Born, M.; Korving, J.; Morsink, F.; Farin, H.; van Es, J.H.; Offerhaus, G.J.; Clevers, H. Generation of an inducible colon-specific Cre enzyme mouse line for colon cancer research. Proc. Natl. Acad. Sci. USA 2016, 113, 11859–11864. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, S.; Fingerle, A.A.; Cammareri, P.; Nebelsiek, T.; Göktuna, S.I.; Ziegler, P.K.; Canli, O.; Heijmans, J.; Huels, D.J.; Moreaux, G.; et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013, 152, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Cammareri, P.; Vincent, D.F.; Hodder, M.C.; Ridgway, R.A.; Murgia, C.; Nobis, M.; Campbell, A.D.; Varga, J.; Huels, D.J.; Subramani, C.; et al. TGFβ pathway limits dedifferentiation following Wnt and MAPK pathway activation to suppress intestinal tumourigenesis. Cell Death Differ. 2017, 24, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Hochmuth, C.E.; Jasper, H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 2008, 3, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Jasper, H. Intestinal inflammation and stem cell homeostasis in aging Drosophila melanogaster. Front. Cell. Infect. Microbiol. 2013, 3, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, Y.; Jasper, H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe 2016, 19, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jasper, H. Gastrointestinal stem cells in health and disease: From flies to humans. Dis. Model Mech. 2016, 9, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Karpac, J.; Supoyo, S.; Degennaro, M.; Lehmann, R.; Jasper, H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010, 6, e1001159. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.C.; Nattamai, K.J.; Dorr, K.; Marka, G.; Uberle, B.; Vas, V.; Eckl, C.; Andra, I.; Schiemann, M.; Oostendorp, R.A.; et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 2013, 503, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Beebe, K.; Sudmeier, L.; Micchelli, C.A. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 2009, 136, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.; Vidal, M.; Sansom, O. APC as a master regulator of intestinal homeostasis and transformation: From flies to vertebrates. Cell Cycle 2009, 8, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.B.; Stefanatos, R.K.; Myant, K.; Vidal, M.; Sansom, O.J. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development 2012, 139, 4524–4535. [Google Scholar] [CrossRef] [PubMed]
- Benchabane, H.; Xin, N.; Tian, A.; Hafler, B.P.; Nguyen, K.; Ahmed, A.; Ahmed, Y. Jerky/Earthbound facilitates cell-specific Wnt/Wingless signalling by modulating β-catenin-TCF activity. EMBO J. 2011, 30, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Benchabane, H.; Tian, A.; Nguyen, K.; Klofas, L.; Ahmed, Y. Erect Wing facilitates context-dependent Wnt/Wingless signaling by recruiting the cell-specific Armadillo-TCF adaptor Earthbound to chromatin. Development 2011, 138, 4955–4967. [Google Scholar] [CrossRef] [PubMed]
- Pangon, L.; Ng, I.; Giry-Laterriere, M.; Currey, N.; Morgan, A.; Benthani, F.; Tran, P.N.; Al-Sohaily, S.; Segelov, E.; Parker, B.L.; et al. JRK is a positive regulator of β-catenin transcriptional activity commonly overexpressed in colon, breast and ovarian cancer. Oncogene 2016, 35, 2834–2841. [Google Scholar] [CrossRef] [PubMed]
- Suijkerbuijk, S.J.; Kolahgar, G.; Kucinski, I.; Piddini, E. Cell Competition drives the growth of intestinal adenomas in Drosophila. Curr Biol. 2016, 26, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, R.; Huang, P.; Yang, F.; Quan, Z.; Xu, N.; Xi, R. APC loss-induced intestinal tumorigenesis in Drosophila: Roles of Ras in Wnt signaling activation and tumor progression. Dev. Biol. 2013, 378, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Martorell, Ò.; Merlos-Suárez, A.; Campbell, K.; Barriga, F.M.; Christov, C.P.; Miguel-Aliaga, I.; Batlle, E.; Casanova, J.; Casali, A. Conserved mechanisms of tumorigenesis in the Drosophila adult midgut. PLoS ONE 2014, 9, e88413. [Google Scholar] [CrossRef] [PubMed]
- Bangi, E.; Murgia, C.; Teague, A.G.; Sansom, O.J.; Cagan, R.L. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, C.; Baena-Lopez, A.; Vincent, J.P. Patterning and growth control by membrane-tethered Wingless. Nature 2014, 505, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; van Jaarsveld, R.H.; Ponsioen, B.; Zimberlin, C.; van Boxtel, R.; Buijs, A.; Sachs, N.; Overmeer, R.M.; Offerhaus, G.J.; Begthel, H.; et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015, 521, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, A.; Drost, J.; Suijkerbuijk, S.J.; van Boxtel, R.; de Ligt, J.; Offerhaus, G.J.; Begthel, H.; Beerling, E.; Tan, E.H.; Sansom, O.J.; et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl. Acad. Sci. USA 2017, 114, E2357–E2364. [Google Scholar] [CrossRef] [PubMed]
- De Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef] [PubMed]
- O′Rourke, K.P.; Loizou, E.; Livshits, G.; Schatoff, E.M.; Baslan, T.; Manchado, E.; Simon, J.; Romesser, P.B.; Leach, B.; Han, T.; et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 2017, 35, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Roper, J.; Tammela, T.; Cetinbas, N.M.; Akkad, A.; Roghanian, A.; Rickelt, S.; Almeqdadi, M.; Wu, K.; Oberli, M.A.; Sánchez-Rivera, F.J.; et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 2017, 35, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, M.; Ohta, Y.; Nishikori, S.; Matano, M.; Takano, A.; Fujii, M.; Date, S.; Sugimoto, S.; Kanai, T.; Sato, T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 2017, 545, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Shimokawa, M.; Date, S.; Takano, A.; Matano, M.; Nanki, K.; Ohta, Y.; Toshimitsu, K.; Nakazato, Y.; Kawasaki, K.; et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 2016, 18, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).