Livestock Reservoir Hosts: An Obscured Threat to Control of Human Schistosomiasis in Nigeria
Abstract
Simple Summary
Abstract
1. Introduction
2. Life Cycle of Schistosoma haematobium
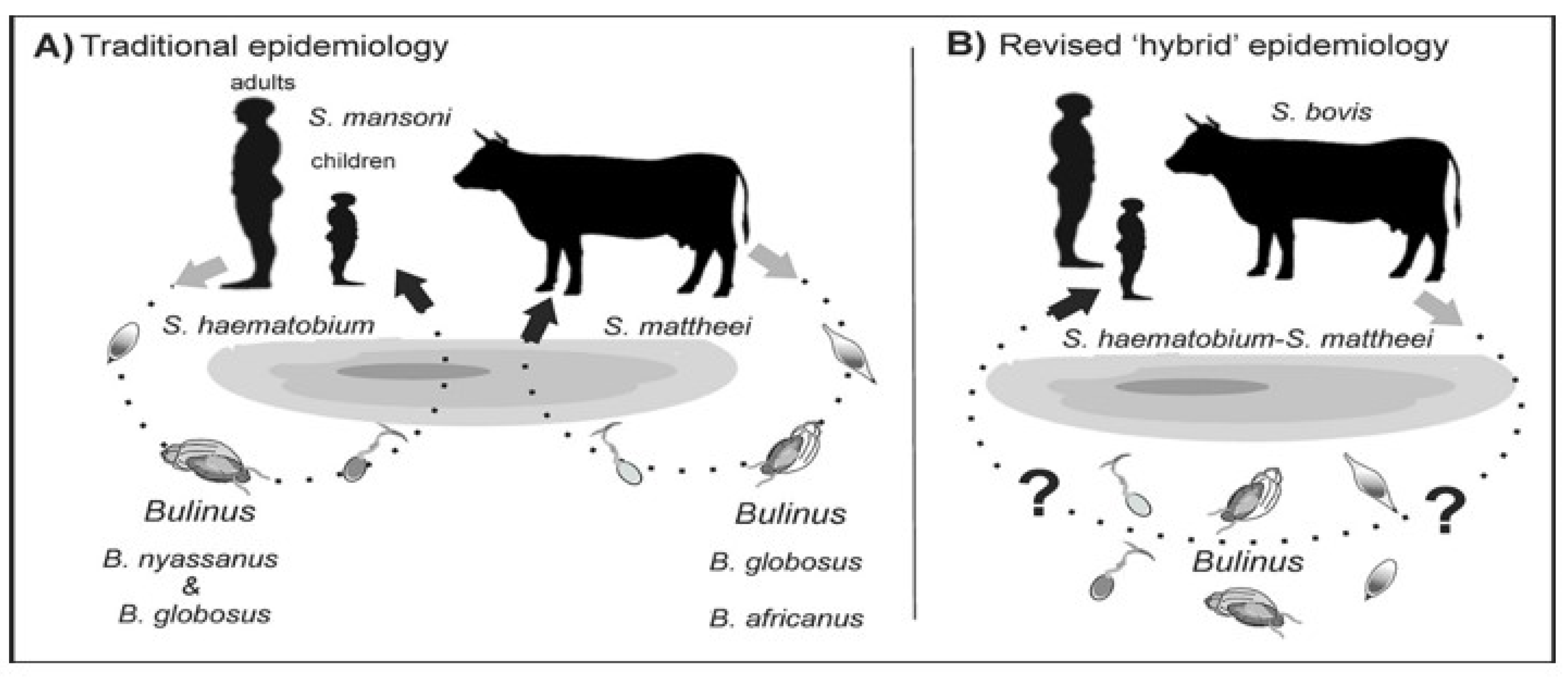
3. Zoonotic Schistosomiasis
4. Schistosomiasis Control Programme in Nigeria
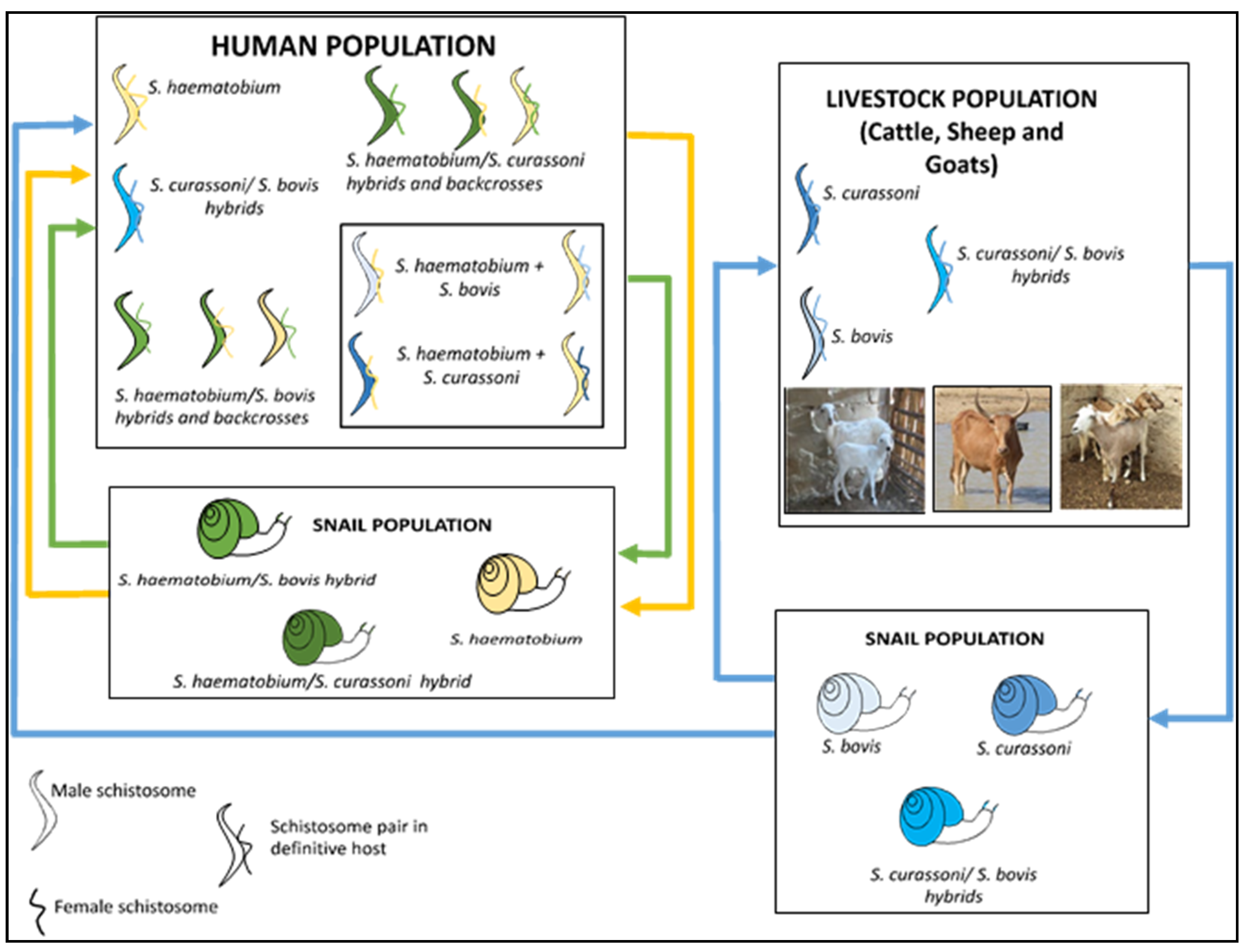
5. Implication of Hybridization for Schistosomiasis Control Efforts
6. Factors Promoting Hybridization of Schistosome Species
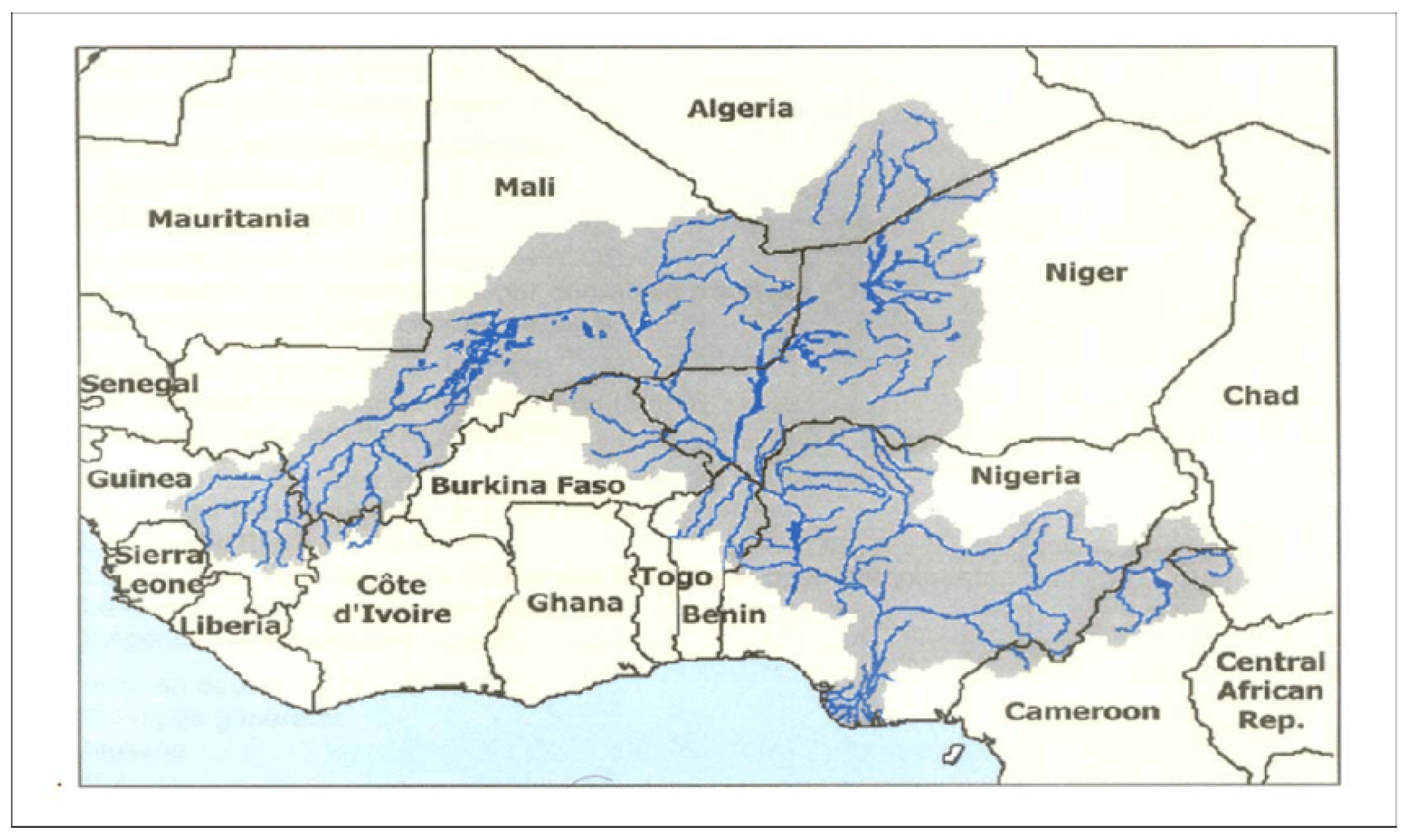
6.1. Availability of Shared Freshwater Bodies and Natural Events
6.2. Developmental Projects and Flooding
6.3. In-Country and Trans-Border Migration of Livestock
6.4. Livestock Schistosomiasis Diagnostic Testing Methods
6.5. Poor Access to Water, Sanitation, and Hygiene (WASH) Facilities
7. Future Research Agenda
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hotez, P.J.; Fenwick, A. Schistosomiasis in Africa: An emerging tragedy in our new global health decade. PLoS Negl. Trop Dis. 2009, 3, e485. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009, 3, e412. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Schistosomiasis. 8 January 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 30 January 2023).
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Stothard, J.R.; Kayuni, S.A.; Al-Harbi, M.H.; Musaya, J.; Webster, B.L. Future schistosome hybridizations: Will all Schistosoma haematobium hybrids please stand-up! PLoS Negl. Trop. Dis. 2020, 14, e0008201. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Chitsulo, L.; Montresor, A.; Savioli, L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002, 82, 139–146. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. DPDx—Laboratory Identification of Parasites of Public Health Concern. Schistosomiasis. Available online: https://www.cdc.gov/dpdx/schistosomiasis/index.html (accessed on 30 January 2023).
- Ekpo, U.F.; Hürlimann, E.; Schur, N.; Oluwole, A.S.; Abe, E.M.; Mafe, M.A.; Nebe, O.J.; Isiyaku, S.; Olamiju, F.; Kadiri, M.; et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat. Health 2013, 7, 355–366. [Google Scholar] [CrossRef]
- World Health Organization. Helminth Control in School-Age Children: A Guide for Managers of Control Programmes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44671/9789241548267_eng.pdf (accessed on 30 January 2023).
- Rollinson, D. A wake-up call for urinary schistosomiasis: Reconciling research effort with public health importance. Parasitology 2009, 136, 1593–1610. [Google Scholar] [CrossRef]
- Webster, B.L.; Southgate, V.R. Mating interactions of Schistosoma haematobium and S. intercalatum with their hybrid offspring. Parasitology 2003, 126, 327–338. [Google Scholar] [CrossRef]
- Léger, E.; Garba, A.; Hamidou, A.A.; Webster, B.L.; Pennance, T.; Rollinson, D.; Webster, J.P. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg. Infect. Dis. 2016, 22, 2212–2214. [Google Scholar] [CrossRef]
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridisation between cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571. [Google Scholar] [CrossRef]
- Soentjens, P.; Cnops, L.; Huyse, T.; Yansouni, C.; Vos, D.D.; Bottieau, E.; Clerinx, J.; Esbroeck, M.V. Diagnosis and clinical management of Schistosoma haematobium–Schistosoma bovis hybrid infection in a cluster of travelers returning from Mali. Clin. Infect. Dis. 2016, 63, 1626–1629. [Google Scholar] [CrossRef]
- Savassi, B.A.E.S.; Mouahid, G.; Lasica, C.; Mahaman, S.-D.K.; Garcia, A.; Courtin, D.; Allienne, J.-F.; Ibikounlé, M.; Moné, H. Cattle as natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 interactions, with new cercarial emergence and genetic patterns. Parasitol. Res. 2020, 119, 2189–2205. [Google Scholar] [CrossRef] [PubMed]
- Savassi, B.A.E.S.; Dobigny, G.; Etougbétché, J.R.; Avocegan, T.T.; Quinsou, F.T.; Gauthier, P.; Ibikounlé, M.; Moné, H.; Mouahid, G. Mastomys natalensis (Smith, 1834) as a natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 x Schistosoma bovis Sonsino, 1876 introgressive hybrids. Parasitol. Res. 2021, 120, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Tchuenté, L.; Southgate, V.; Njiokou, F.; Njiné, T.; Kouemeni, L.; Jourdane, J. The evolution of schistosomiasis at Loum, Cameroon: Replacement of Schistosoma intercalatum by S. haematobium through introgressive hybridisation. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 664–665. [Google Scholar] [CrossRef]
- Webster, B.L.; Tchuenté, L.T.; Jourdane, J.; Southgate, V.R. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J. Helminthol. 2005, 79, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasit Vectors 2015, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Nelwan, M.L. Schistosomiasis: Life cycle, diagnosis, and control. Curr. Ther. Res. Clin. Exp. 2019, 91, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Mafiana, C.F.; Ekpo, U.F.; Ojo, D.A. Urinary schistosomiasis in preschool children in settlements around Oyan Reservoir in Ogun State, Nigeria: Implications for control. Trop. Med. Int. Health 2003, 8, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Curwen, R.S.; Wilson, R.A. Invasion of skin by schistosome cercariae: Some neglected facts. Trends Parasitol. 2003, 19, 63–66. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Salafsky, B.; Ramaswany, K. Comparison of skin invasion among three major species of Schistosoma. Trends Parasitol. 2005, 21, 201–203. [Google Scholar] [CrossRef]
- Nation, C.S.; Da’dara, A.A.; Marchant, J.K.; Skelly, P.J. Schistosome migration in the definitive host. PLoS Negl. Trop. Dis. 2020, 14, e0007951. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Parasites—Schistosomiasis. In Biology; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/parasites/schistosomiasis/biology.html (accessed on 30 January 2023).
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int. J. Parasitol. 2008, 38, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Huyse, T.; Van Den Broeck, F.; Hellemans, B.; Volckaert, F.A.M.; Polman, K. Hybridisation between the two major African schistosome species of humans. Int. J. Parasitol. 2013, 43, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Standley, C.J.; Mugisha, L.; Dobson, A.P.; Stothard, J.R. Zoonotic schistosomiasis in non-human primates: Past, present and future activities at the human–wildlife interface in Africa. J. Helminthol. 2012, 86, 131–140. [Google Scholar] [CrossRef]
- Léger, E.; Webster, J.P. Hybridizations within the Genus Schistosoma: Implications for evolution, epidemiology and control. Parasitology 2017, 144, 65–80. [Google Scholar] [CrossRef]
- Fan, P.C.; Lin, L.H. Hybridization of Schistosoma mansoni and Schistosoma japonicum in mice. Southeast Asian J. Trop. Med. Public Health 2005, 36, 89–96. [Google Scholar]
- Boissier, J.; Moné, H.; Mitta, G.; Bargues, M.D.; Molyneux, D.; Mas-Coma, S. Schistosomiasis reaches Europe. Lancet Infect. Dis. 2015, 15, 757–758. [Google Scholar] [CrossRef]
- Steinauer, M.L.; Hanelt, B.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Kinuthia, J.M.; Mutuku, M.W.; Mungai, B.N.; Wilson, W.D.; Mkoji, G.M.; et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol. Ecol. 2008, 17, 5062–5074. [Google Scholar] [CrossRef]
- Borlase, A.; Webster, J.P.; Rudge, J.W. Opportunities and challenges for modelling epidemiological and evolutionary dynamics in a multihost, multiparasite system: Zoonotic hybrid schistosomiasis in West Africa. Evol. Appl. 2017, 11, 501–515. [Google Scholar] [CrossRef]
- The Carter Center. Schistosomiasis Control Program. 2021. Available online: https://www.cartercenter.org/resources/pdfs/factsheets/schistosomiasis-facts.pdf (accessed on 30 January 2023).
- Mogaji, H.O.; Dedeke, G.A.; Bada, B.S.; Bankole, S.; Adeniji, A.; Fagbenro, M.T.; Omitola, O.O.; Oluwole, A.S.; Odoemene, N.S.; Abe, E.M.; et al. Distribution of ascariasis, trichuriasis and hookworm infections in Ogun State, Southwestern Nigeria. PLoS ONE 2020, 15, e0233423. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Africa. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Optimizing Schistosomiasis MDA Implementation in Countries. Data Analysis Tool, June–July 2019. Available online: https://espen.afro.who.int/system/files/content/resources/Schistosomiasis%20Data%20analysis%20tool%20-%20Presentation%20%2820190724_English%29.pdf (accessed on 30 January 2023).
- WHO. Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/rest/bitstreams/1410449/retrieve (accessed on 30 January 2023).
- World Health Organization; Regional Office for Africa. Expanded Special Project for Elimination of Neglected Tropical Diseases (ESPEN). Nigeria Overview. Available online: https://espen.afro.who.int/countries/nigeria (accessed on 30 January 2023).
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: Progress report, 2021. Wkly. Epidemiol. Rec. 2022, 97, 621–632. Available online: https://www.who.int/publications/i/item/who-wer9748-621-632 (accessed on 30 January 2023).
- Krentel, A.; Gyapong, M.; Mallya, S.; Boadu, N.Y.; Amuyunzu-Nyamongo, M.; Stephens, M.; McFarland, D.A. Review of the factors influencing the motivation of community drug distributors towards the control and elimination of neglected tropical diseases (NTDs). PLoS Negl. Trop. Dis. 2017, 11, e0006065. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, M.; Abd Elaziz, K.M.; Helmy, H.; Farid, H.A.; Kamal, H.A.; Ramzy, R.M.R.; Shannon, W.D.; Weil, G.J. The effect of compliance on the impact of mass drug administration for elimination of lymphatic filariasis in Egypt. Am. J. Trop. Med. Hyg. 2007, 77, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Olamiju, O.J.; Olamiju, F.O.; Adeniran, A.A.; Mba, I.C.; Ukwunna, C.C.; Okoronkwo, C.; Ekpo, U.F. Public awareness and knowledge of neglected tropical diseases (NTDs) control activities in Abuja, Nigeria. PLoS Negl. Trop. Dis. 2014, 8, e3209. [Google Scholar] [CrossRef] [PubMed]
- Assaré, R.K.; N’Tamon, R.N.; Bellai, L.G.; Koffi, J.A.; Mathieu, T.-B.I.; Ouattara, M.; Hürlimann, E.; Coulibaly, J.T.; Diabaté, S.; N’Goran, E.K.; et al. Characteristics of persistent hotspots of Schistosoma mansoni in western Côte d’Ivoire. Parasit Vectors 2020, 13, 337. [Google Scholar] [CrossRef]
- Mogaji, H.O.; Odoh, I.M.; Iyeh, C.I.; Adeniran, A.A.; Oyedeji, S.I.; Okoh, H.I.; Bayegun, A.A.; Omitola, O.O.; Umunnakwe, C.U.; Olamiju, F.O.; et al. Attendee’s awareness about preventive chemotherapy neglected tropical diseases (PC-NTD) control during the first world neglected tropical diseases day in Ekiti State, Nigeria. PLoS Negl. Trop. Dis. 2021, 15, e0009315. [Google Scholar] [CrossRef]
- King, K.C.; Stelkens, R.B.; Webster, J.P.; Smith, D.F.; Brockhurst, M.A. Hybridization in parasites: Consequences for adaptive evolution, pathogenesis, and public health in a changing world. PLoS Pathog. 2015, 11, e1005098. [Google Scholar] [CrossRef]
- Moné, H.; Minguez, S.; Ibikounlé, M.; Allienne, J.-F.; Massougbodji, A.; Mouahid, G. Natural interactions between S. haematobium and S. guineensis in the Republic of Benin. Sci. World J. 2012, 2012, 793420. [Google Scholar] [CrossRef]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Webster, J.P.; Rollinson, D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: Species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013, 7, e2110. [Google Scholar] [CrossRef]
- Webster, J.P.; Molyneux, D.H.; Hotez, P.J.; Fenwick, A. The contribution of mass drug administration to global health: Past, present and future. Philos. Trans./R Soc. Lond. B—Biol. Sci. 2014, 369, 20130434. [Google Scholar] [CrossRef]
- Lamberton, P.H.L.; Hogan, S.C.; Kabatereine, N.B.; Fenwick, A.; Webster, J.P. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am. J. Trop. Med. Hyg. 2010, 83, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Valentim, C.L.L.; Cioli, D.; Chevalier, F.D.; Cao, X.; Taylor, A.B.; Holloway, S.P.; Pica-Mattoccia, L.; Guidi, A.; Basso, A.; Tsai, I.J.; et al. Genetic and molecular basis of drug resistance and species-specific drug action in schistosome parasites. Science 2013, 342, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Faye, D.S.; Stothard, J.R.; Sousa-Figueiredo, J.C.; Rollinson, D. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: Monitoring treatment success and re-infection patterns. Acta Trop. 2013, 128, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Grigg, M.E.; Bonnefoy, S.; Hehl, A.B.; Suzuki, Y.; Boothroyd, J.C. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science 2001, 294, 161–165. [Google Scholar] [CrossRef]
- Schelkle, B.; Faria, P.J.; Johnson, M.B.; van Oosterhout, C.; Cable, J. Mixed infections and hybridisation in monogenean parasites. PLoS ONE 2012, 7, e39506. [Google Scholar] [CrossRef]
- Hanelt, B.; Mwangi, I.N.; Kinuthia, J.M.; Maina, G.M.; Agola, L.E.; Mutuku, M.W.; Steinauer, M.L.; Agwanda, B.R.; Kigo, L.; Mungai, B.N.; et al. Schistosomes of small mammals from the Lake Victoria Basin, Kenya: New species, familiar species, and implications for schistosomiasis control. Parasitology 2010, 137, 1109–1118. [Google Scholar] [CrossRef]
- Akinwale, O.; Ajayi, M.; Akande, D.; Gyang, P.; Adeleke, M.; Adeneye, A.; Adebayo, M.; Dike, A. Urinary schistosomiasis around Oyan Reservoir, Nigeria: Twenty years after the first outbreak. Iran J. Public Health 2010, 39, 92–95. [Google Scholar]
- Dida, G.O.; Gelder, F.B.; Anyona, D.N.; Matano, A.S.; Abuom, P.O.; Adoka, S.O.; Ouma, C.; Kanangire, C.K.; Owuor, P.O.; Ofulla, A.V. Distribution and abundance of schistosomiasis and fascioliasis host snails along the Mara River in Kenya and Tanzania. Infect. Ecol. Epidemiol. 2014, 4, 24281. [Google Scholar] [CrossRef]
- Zhou, X.N.; Yang, G.J.; Yang, K.; Wang, X.H.; Hong, Q.B.; Sun, L.P.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R.; Utzinger, J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008, 78, 188–194. [Google Scholar] [CrossRef]
- John, R.; Ezekiel, M.; Philbert, C.; Andrew, A. Schistosomiasis transmission at high altitude crater lakes in western Uganda. BMC Infect. Dis. 2008, 8, 110. [Google Scholar] [CrossRef]
- McCreesh, N.; Nikulin, G.; Booth, M. Predicting the effects of climate change on Schistosoma mansoni transmission in eastern Africa. Parasit Vectors 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Zhang, S.Q.; Xu, X.J.; Huang, Y.X.; Steinmann, P.; Utzinger, J.; Wang, T.P.; Xu, J.; Zheng, J.; Zhou, X.N. Effect of floods on the transmission of schistosomiasis in the Yangtze River valley, People’s Republic of China. Parasitol. Int. 2008, 57, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mari, L.; Ciddio, M.; Casagrandi, R.; Perez-Saez, J.; Bertuzzo, E.; Rinaldo, A.; Sokolow, S.H.; De Leo, G.A.; Gatto, M. Heterogeneity in schistosomiasis transmission dynamics. J. Theor. Biol. 2017, 432, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.E.; Egwunyenga, A.O. Schistosomiasis: The aftermath of 2012 floods in Delta State, Southern Nigeria. Int. Med. J. 2015, 22, 218–223. [Google Scholar]
- Oladejo, S.O.; Ofoezie, I.E. Unabated schistosomiasis transmission in Erinle River Dam, Osun State, Nigeria: Evidence of neglect of environmental effects of development projects. Trop. Med. Int. Health 2006, 11, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Abe, E.M.; Guo, Y.H.; Shen, H.; Mutsaka-Makuvaza, M.J.; Habib, M.R.; Xue, J.B.; Midzi, N.; Xu, J.; Li, S.Z.; Zhou, X.N. Phylogeography of Bulinus truncates (Audouin, 1827) (Gastropoda: Planorbidae) in selected African countries. Trop. Med. Infect. Dis. 2018, 3, 127. [Google Scholar] [CrossRef]
- Al-Gamal, A.S.; Sokona, Y.; Dodo, A. Climatic changes and groundwater resources in Africa. Int. J. Clim. Chang. Strat. Manag. 2009, 1, 133–145. [Google Scholar] [CrossRef]
- Federal Ministry of Agriculture and Rural Development. Agricultural Sector Food Security and Nutrition Strategy 2016–2025. Available online: https://ngfrepository.org.ng:8443/jspui/bitstream/123456789/5377/1/Agriculture-FSN-Strategy-2016-25_Printed-Version_1562696265%20%281%29.pdf (accessed on 30 January 2023).
- Food and Agriculture Organization of the United Nations. The Future of Livestock in Nigeria. Opportunities and Challenges in the Face of Uncertainty; FAO: Rome, Italy, 2019; Available online: https://www.fao.org/3/ca5464en/ca5464en.pdf (accessed on 30 January 2023).
- Léger, E.; Borlase, A.; Fall, C.B.; Diouf, N.D.; Diop, S.D.; Yasenev, L.; Catalano, S.; Thiam, C.T.; Ndiaye, A.; Emery, A.; et al. Prevalence and distribution of schistosomiasis in human, livestock, and snail populations in northern Senegal: A One Health epidemiological study of a multi-host system. Lancet Planet. Health 2020, 4, e330–e342. [Google Scholar] [CrossRef]
- Southgate, V. Schistosomiasis in the Senegal River Basin: Before and after the construction of the dams at Diama, Senegal and Manantali, Mali and future prospects. J. Helminthol. 1997, 71, 125–132. [Google Scholar] [CrossRef]
- Duplantier, J.M.; Sène, M. Rodents as reservoir hosts in the transmission of Schistosoma mansoni in Richard-Toll, Senegal, West Africa. J. Helminthol. 2000, 74, 129–135. [Google Scholar] [CrossRef]
- Ofoezie, I.E.; Imevbore, A.M.; Balogun, M.O.; Ogunkoya, O.O.; Asaolu, S.O. A study of an outbreak of schistosomiasis in two resettlement villages near Abeokuta, Ogun State, Nigeria. J. Helminthol. 1991, 65, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Olamiju, F.; Nebe, O.J.; Mogaji, H.; Marcus, A.; Amodu–Agbi, P.; Urude, R.O.; Apake, E.; Olamiju, O.; Okoronkwo, C.; Achu, I.; et al. Schistosomiasis outbreak during COVID-19 pandemic in Takum, Northeast Nigeria: Analysis of infection status and associated risk factors. PLoS ONE 2022, 17, e0262524. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P.; Gower, C.M.; Knowles, S.C.L.; Molyneux, D.H.; Fenton, A. One health–An ecological and evolutionary framework for tackling Neglected Zoonotic Diseases. Evol. Appl. 2016, 9, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, A.O. Farming households’ environment, nutrition and health interplay in southwest Nigeria. Int. J. Sci. Res. Agric. Sci. 2016, 3, 84–98. [Google Scholar] [CrossRef]
- Blench, R. Pastoral Cross-Border Movement. In Natural Resource Conflicts in North-Central Nigeria: A Handbook and Case Studies; Mandaras Publishing: London, UK, 2004; pp. 133–137. [Google Scholar]
- Umoh, N.R. Pastoralism in Nigeria’s middle-belt region: A resource or a curse? Int. J. Dev. Econ. Sustain. 2017, 5, 11–30. [Google Scholar]
- Rass, N. Policies and Strategies to Address the Vulnerability of Pastoralists in Sub-Saharan Africa. In Pro-Poor Livestock Policy Initiative; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Enwezor, F.; Umoh, J.U.; Esievo, K.; Anere, J.I. Transhumance pastoralism as risk factor in the trypanosome infections in cattle. Bull. Anim. Health Prod. Afr. 2009, 57, 44–48. [Google Scholar] [CrossRef]
- Aruwayo, A.; Adeola, S.S.; Ibrahim, U. Assessment of the challenges of nomadic farming activities in Daura agricultural zone of Katsina State, Nigeria. Niger. J. Anim. Prod. 2021, 48, 200–209. [Google Scholar] [CrossRef]
- Kamani, J.; Apanaskevich, D.A.; Gutiérrez, R.; Nachum-Biala, Y.; Baneth, G.; Harrus, S. Morphological and molecular identification of Rhipicephalus (Boophilus) microplus in Nigeria, West Africa: A threat to livestock health. Exp. Appl. Acarol. 2017, 73, 283–296. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Dai, S.-M.; Xue, J.-B.; Li, Y.-L.; Lv, S.; Xu, J.; Li, S.-Z.; Guo, J.-G.; Zhou, X.-N. The epidemiological status of schistosomiasis in P. R. China after the World Bank Loan Project, 2002–2017. Acta Trop. 2019, 195, 135–141. [Google Scholar] [CrossRef]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in China: Current status and future prospects. PloS Negl. Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef]
- Gower, C.M.; Vince, L.; Webster, J.P. Should we be treating animal schistosomiasis in Africa? The need for a One Health economic evaluation of schistosomiasis control in people and their livestock. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, P.; Léger, E.; Hollenberg, E.; Diouf, N.; Sène, M.; Webster, J.P.; Häsler, B. Estimating the financial impact of livestock schistosomiasis on traditional subsistence and transhumance farmers keeping cattle, sheep and goats in northern Senegal. Parasites Vectors 2022, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.V.; Lambert, S.; Neves, M.I.; Borlase, A.; Léger, E.; Diouf, N.D.; Sène, M.; Webster, J.P.; Walker, M. Modelling livestock test-and-treat: A novel One Health strategy to control schistosomiasis and mitigate drug resistance. Front. Trop. Dis. 2022, 3, 893066. [Google Scholar] [CrossRef]
- Colley, D.G.; Binder, S.; Campbell, C.; King, C.H.; Tchuenté, L.A.T.; N'Goran, E.K.; Erko, B.; Karanja, D.M.; Kabatereine, N.B.; van Lieshout, L.; et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 2013, 88, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Urbano, B.; Léger, E.; Gabain, I.; De Dood, C.J.; Diouf, N.D.; Borlase, A.; Rudge, J.W.; Corstjens, P.L.A.M.; Sène, M.; Van Dam, G.J.; et al. Sensitivity and specificity of human point-of-care circulating cathodic antigen (PO-C CCA) test in African livestock for rapid diagnosis of schistosomiasis: A Bayesian latent class analysis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zhou, Y.-B.; Liang, S.; Jiang, Q.-W. Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors 2012, 5, 275. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ponpetch, K.; Zhou, Y.-B.; Guo, J.; Erko, B.; Stothard, J.R.; Murad, M.H.; Zhou, X.-N.; Satrija, F.; Webster, J.P.; et al. Diagnosis of Schistosoma infection in non-human animal hosts: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010389. [Google Scholar] [CrossRef]
- Xu, B.; Gordon, C.A.; Hu, W.; McManus, D.P.; Chen, H.-G.; Gray, D.J.; Ju, C.; Zeng, X.-J.; Gobert, G.N.; Ge, J.; et al. A novel procedure for precise quantification of Schistosoma japonicum eggs in bovine feces. PLoS Negl. Trop. Dis. 2012, 6, e1885. [Google Scholar] [CrossRef]
- Yu, J.M.; de Vlas, S.J.; Jiang, Q.W.; Gryseels, B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol. Int. 2007, 56, 45–49. [Google Scholar] [CrossRef]
- Utzinger, J.; Becker, S.L.; van Lieshout, L.; van Dam, G.J.; Knopp, S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015, 21, 529–542. [Google Scholar] [CrossRef]
- Hotez, P.J.; Asojo, O.A.; Adesina, A.M. Nigeria: “Ground zero” for the high prevalence neglected tropical diseases. PLoS Negl. Trop. Dis. 2012, 6, e1600. [Google Scholar] [CrossRef] [PubMed]
- Global Data Lab. Area Profile Report—Nigeria. Available online: https://globaldatalab.org/areadata/profiles/NGAt/ (accessed on 30 January 2023).
- Food and Agriculture Organization of the United Nations. Nigeria Agriculture at a Glance. 2022. Available online: https://www.fao.org/nigeria/fao-in-nigeria/nigeria-at-a-glance/en/ (accessed on 30 January 2023).
- The World Bank. Third National Urban Water Sector Reform Project for Nigeria. 25 November 2021. Available online: https://projects.worldbank.org/en/projects-operations/project-detail/P123513 (accessed on 30 January 2023).
- United Nations Children’s Fund. Nigeria—Water, Sanitation & Hygiene. Available online: https://www.unicef.org/nigeria/water-sanitation-and-hygiene (accessed on 30 January 2023).
- Mogaji, H.O.; Ekpo, U.F.; Yusuff, Q.A.; Yusuff, H.A.; Adeaga, D.O.; Monday, J.; Adeniran, A.A. Impacts of water, sanitation and hygiene (WASH) interventions on intestinal helminthiasis of school-aged children in Ogun State, South-Western Nigeria. Trop. Med. Int. Health 2015, 20, 233. [Google Scholar]
- Ibok, E.E.; Daniel, E.E. Rural water supply and sustainable development in Nigeria: A case analysis of Akwa Ibom State. Am. J. Rural Dev. 2014, 2, 68–73. [Google Scholar] [CrossRef]
- Ihuah, P.W.; Kakulu, I.I. Rural water supply projects and sustainable development in Nigeria. J. Sustain. Dev. Afr. 2014, 16, 56–68. [Google Scholar]
- Strunz, E.C.; Addiss, D.G.; Stocks, M.E.; Ogden, S.; Utzinger, J.; Freeman, M.C. Water, sanitation, hygiene, and soil-transmitted helminth infection: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001620. [Google Scholar] [CrossRef]
- Grimes, J.E.; Tadesse, G.; Mekete, K.; Wuletaw, Y.; Gebretsadik, A.; French, M.D.; Harrison, W.E.; Drake, L.J.; Gardiner, I.A.; Yard, E.; et al. School water, sanitation, and hygiene, soil-transmitted helminths, and schistosomes: National mapping in Ethiopia. PLoS Negl. Trop. Dis. 2016, 10, e0004515. [Google Scholar] [CrossRef]
- Bayegun, A.A.; Omitola, O.O.; Umunnakwe, C.U.; Akande, F.A.; Akinwale, O.P.; Mogaji, H.O.; Ademolu, K.O.; Gyang, V.P.; Odoemene, S.N.; Stothard, J.R.; et al. Morphometric analysis of schistosome eggs recovered from human urines in communities along the shoreline of Oyan River Dam in Ogun State, Nigeria. J. Helminthol. 2023, 96, e89. [Google Scholar] [CrossRef]
- Onyekwere, A.M.; Rey, O.; Allienne, J.F.; Nwanchor, M.C.; Alo, M.; Uwa, C.; Boissier, J. Population genetic structure and hybridization of Schistosoma haematobium in Nigeria. Pathogens 2022, 11, 425. [Google Scholar] [CrossRef]
- Ndifon, G.T.; Betterton, C.; Rollinson, D. Schistosoma curassoni Brumpt, 1931 and S. bovis (Sonsino, 1876) in cattle in northern Nigeria. J. Helminthol. 1988, 62, 33–34. [Google Scholar] [CrossRef]

| Production Sector | Definition | Cattle Produced in 2019 (of National Total of 18.4 Million) | Percentage Production of Sector (%) |
|---|---|---|---|
| Pastoralism | Extensive livestock production system that involves mobility across a large landscape and continuous utilization of grazing and water system across a given area | 15,088,000 | 82 |
| Agro-pastoralism | A production system that combines raising of crops with pastoralism | 3,128,000 | 17 |
| Commercial | An organized system of livestock rearing usually practised in permanent ranches with 10–500 livestock of the same type | 184,000 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogaji, H.O.; Omitola, O.O.; Bayegun, A.A.; Ekpo, U.F.; Taylor-Robinson, A.W. Livestock Reservoir Hosts: An Obscured Threat to Control of Human Schistosomiasis in Nigeria. Zoonotic Dis. 2023, 3, 52-67. https://doi.org/10.3390/zoonoticdis3010006
Mogaji HO, Omitola OO, Bayegun AA, Ekpo UF, Taylor-Robinson AW. Livestock Reservoir Hosts: An Obscured Threat to Control of Human Schistosomiasis in Nigeria. Zoonotic Diseases. 2023; 3(1):52-67. https://doi.org/10.3390/zoonoticdis3010006
Chicago/Turabian StyleMogaji, Hammed Oladeji, Olaitan Olamide Omitola, Adedotun Ayodeji Bayegun, Uwem Friday Ekpo, and Andrew W. Taylor-Robinson. 2023. "Livestock Reservoir Hosts: An Obscured Threat to Control of Human Schistosomiasis in Nigeria" Zoonotic Diseases 3, no. 1: 52-67. https://doi.org/10.3390/zoonoticdis3010006
APA StyleMogaji, H. O., Omitola, O. O., Bayegun, A. A., Ekpo, U. F., & Taylor-Robinson, A. W. (2023). Livestock Reservoir Hosts: An Obscured Threat to Control of Human Schistosomiasis in Nigeria. Zoonotic Diseases, 3(1), 52-67. https://doi.org/10.3390/zoonoticdis3010006







