The Impact of Fucoidan Extracts on Heat-Stress-Induced Loss of In Vitro Fast-Twitch Muscle Function in Mice
Abstract
1. Introduction
2. Results
2.1. Heat-Stress-Induced Loss of EDL Function
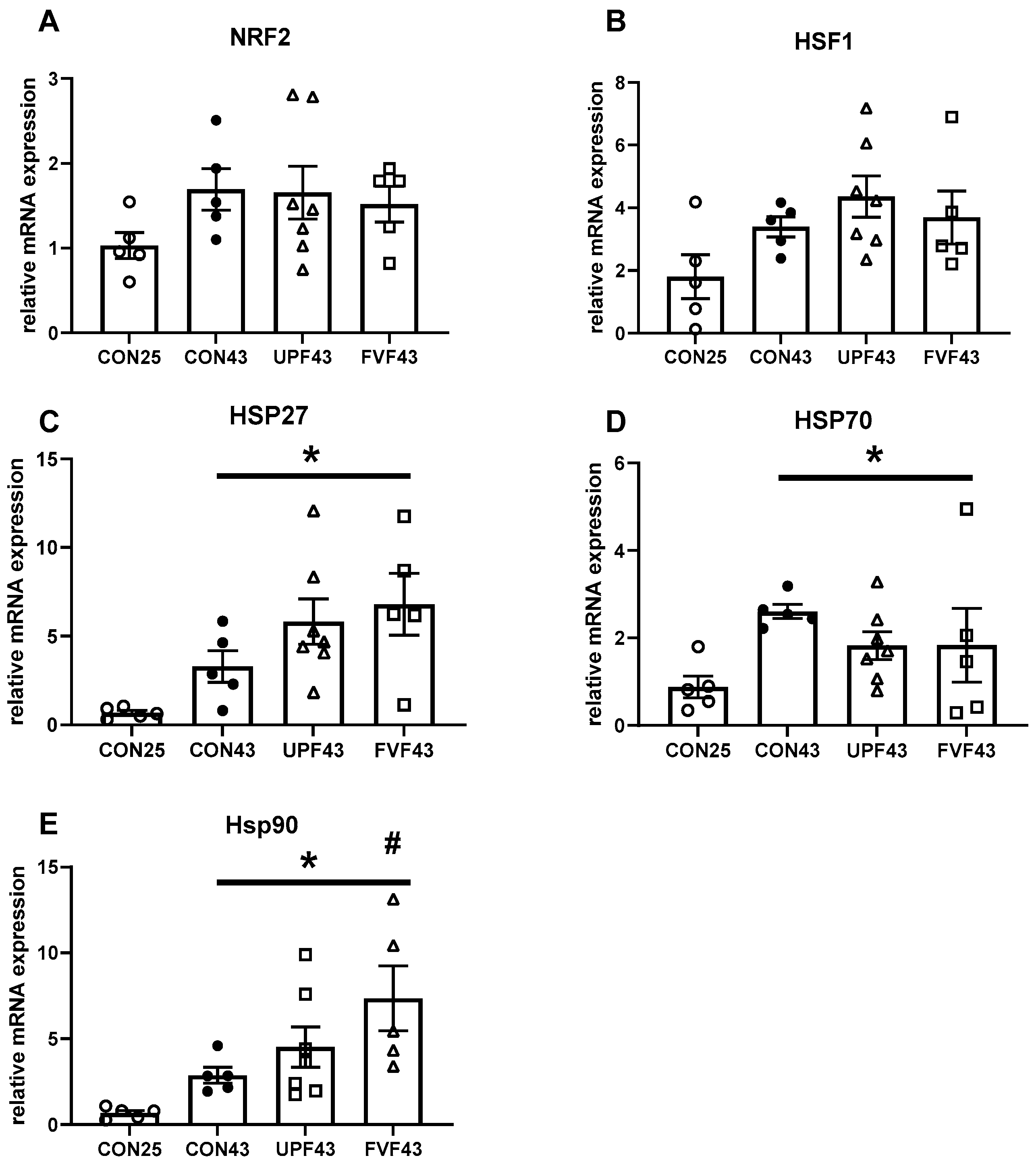
2.2. Heat Shock Protein Expression Following Heat Stress
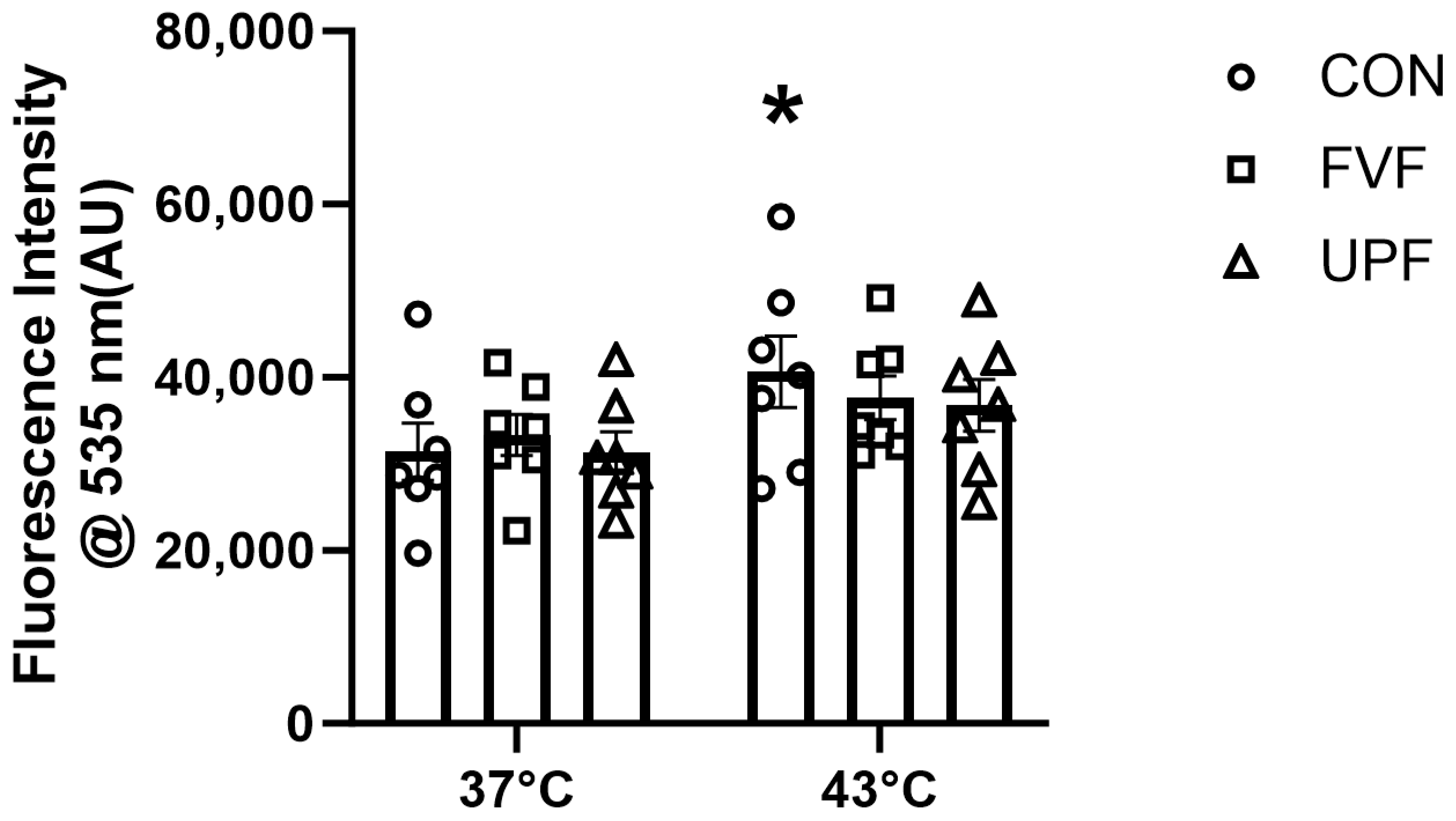
2.3. Heat-Induced ROS Production in C2C12 Myoblasts
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. C57BL/6 Mouse Treatment with Fucoidan
4.3. In Vitro Muscle Function Testing
4.4. Gene Transcription Analysis—Real-Time Quantitative PCR (qRT-PCR)
4.5. C2C12 Tissue Culture—Temperature-Induced ROS Assay
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANGPT | Angiopoietins |
| ANOVA | Analysis of variance |
| CCL2 | C-C motif ligand 2 |
| °C | Degrees celsius |
| EDL | Extensor digitorum longus |
| EMG | Electromyography |
| FOXO | Forkhead box family |
| FVF | Fucus vesiculosus |
| H2DCFDA | 2′,7′-dichlorofluorescein |
| HBSS | Hanks’ balanced salt solution |
| HMWF | High-molecular-weight fucoidan |
| HSF | Heat shock factor |
| HSP | Heat shock protein |
| LMWF | Low-molecular-weight fucoidan |
| MMWF | Medium-molecular-weight fucoidan |
| MRT | Mean residence time |
| NAC | N-acetylcysteine |
| NRF | Nuclear factor erythroid 2-related fact |
| qRT-PCR | Real-time quantitative reverse-transcription PCR |
| ROS | Reactive oxygen species |
| SIRT | Sirtuin |
| O2− | Superoxide |
| UPF | Undaria pinnatifida |
| VEGF | Vascular endothelial growth factor |
References
- Malak, B.; Celichowski, J.; Drzymała-Celichowska, H. The influence of temperature on contractile properties of motor units in rat medial gastrocnemius. J. Electromyogr. Kinesiol. 2023, 68, 102738. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; Lind, A.R. The influence of temperature on the isometric characteristics of fast and slow muscle in the cat. Pflugers Arch. 1981, 389, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ranatunga, K.W. The force-velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J. Physiol. 1984, 351, 517–529. [Google Scholar] [CrossRef]
- Segal, S.S.; Faulkner, J.A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am. J. Physiol. 1985, 248 Pt 1, C265–C270. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Larsson, L.; Edström, L. Contractile properties, fatiguability and glycolytic metabolism in fast- and slow-twitch rat skeletal muscles of various temperatures. Acta Physiol. Scand. 1985, 125, 235–243. [Google Scholar] [CrossRef]
- Lännergren, J.; Westerblad, H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J. Physiol. 1987, 390, 285–293. [Google Scholar] [CrossRef]
- Place, N.; Yamada, T.; Zhang, S.J.; Westerblad, H.; Bruton, J.D. High temperature does not alter fatigability in intact mouse skeletal muscle fibres. J. Physiol. 2009, 587 Pt 19, 4717–4724. [Google Scholar] [CrossRef]
- Drinkwater, E. Effects of peripheral cooling on characteristics of local muscle. Med. Sport. Sci. 2008, 53, 74–88. [Google Scholar]
- Giesbrecht, G.G.; Wu, M.P.; White, M.D.; Johnston, C.E.; Bristow, G.K. Isolated effects of peripheral arm and central body cooling on arm performance. Aviat. Space Environ. Med. 1995, 66, 968–975. [Google Scholar]
- Mallette, M.M.; Green, L.A.; Gabriel, D.A.; Cheung, S.S. The effects of local forearm muscle cooling on motor unit properties. Eur. J. Appl. Physiol. 2018, 118, 401–410. [Google Scholar] [CrossRef]
- Sargeant, A.J. Effect of muscle temperature on leg extension force and short-term power output in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 693–698. [Google Scholar] [CrossRef]
- Hedley, A.M.; Climstein, M.; Hansen, R. The effects of acute heat exposure on muscular strength, muscular endurance, and muscular power in the euhydrated athlete. J. Strength Cond. Res. 2002, 16, 353–358. [Google Scholar] [PubMed]
- De Ruiter, C.J.; De Haan, A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflugers Arch. 2000, 440, 163–170. [Google Scholar] [CrossRef]
- Cornwall, M.W. Effect of temperature on muscle force and rate of muscle force production in men and women. J. Orthop. Sports Phys. Ther. 1994, 20, 74–80. [Google Scholar] [CrossRef]
- Ota, K.; Sasaki, K. Influence of temperature on twitch potentiation following submaximal voluntary contractions in human plantar flexor muscles. Physiol. Rep. 2023, 11, e15802. [Google Scholar] [CrossRef]
- Ball, D. Contrasting effects of heat stress on neuromuscular performance. Exp. Physiol. 2021, 106, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Locke, M.; Celotti, C. The effect of heat stress on skeletal muscle contractile properties. Cell Stress Chaperones 2014, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Carey, M.F.; Snow, R.J.; Stathis, C.G.; Hargreaves, M. Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. Am. J. Physiol. 1996, 271 Pt 2, R1251–R1255. [Google Scholar] [CrossRef]
- van der Poel, C.; Stephenson, D.G. Reversible changes in Ca(2+)-activation properties of rat skeletal muscle exposed to elevated physiological temperatures. J. Physiol. 2002, 544, 765–776. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]
- Kolbeck, R.C.; She, Z.W.; Callahan, L.A.; Nosek, T.M. Increased superoxide production during fatigue in the perfused rat diaphragm. Am. J. Respir. Crit. Care Med. 1997, 156, 140–145. [Google Scholar] [CrossRef]
- Nethery, D.; Stofan, D.; Callahan, L.; DiMarco, A.; Supinski, G. Formation of reactive oxygen species by the contracting diaphragm is PLA(2) dependent. J. Appl. Physiol. 1999, 87, 792–800. [Google Scholar] [CrossRef]
- Reid, M.B.; Shoji, T.; Moody, M.R.; Entman, M.L. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J. Appl. Physiol. 1992, 73, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Christofi, F.L.; Wright, V.P.; Liu, C.Y.; Merola, A.J.; Berliner, L.J.; Clanton, T.L. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am. J. Physiol.-Cell Physiol. 2000, 279, C1058–C1066. [Google Scholar] [CrossRef]
- Moopanar, T.R.; Allen, D.G. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37 degrees C. J. Physiol. 2005, 564 Pt 1, 189–199. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Diaz, P.T.; Brownstein, E.; Clanton, T.L. Effects of N-acetylcysteine on in vitro diaphragm function are temperature dependent. J. Appl. Physiol. 1994, 77, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, P.; Ding, X.; Li, H. N-acetylcysteine stimulates the proliferation and differentiation in heat-stressed skeletal muscle cells. J. Therm. Biol. 2024, 124, 103958. [Google Scholar] [CrossRef]
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Jin, D.; Qi, J.; Wang, X.; Zhang, C.; An, P.; Luo, Y.; Luo, J. Fucoidan Protects against Doxorubicin-Induced Cardiotoxicity by Reducing Oxidative Stress and Preventing Mitochondrial Function Injury. Int. J. Mol. Sci. 2022, 23, 10685. [Google Scholar] [CrossRef]
- Ajisaka, K.; Yokoyama, T.; Matsu, K. Structural Characteristics and Antioxidant Activities of Fucoidans from Five Brown Seaweeds. J. Appl. Glycosci. 2016, 63, 31–37. [Google Scholar] [CrossRef]
- Dörschmann, P.; Apitz, S.; Hellige, I.; Neupane, S.; Alban, S.; Kopplin, G.; Ptak, S.; Fretté, X.; Roider, J.; Zille, M.; et al. Evaluation of the Effects of Fucoidans from Fucus Species and Laminaria hyperborea against Oxidative Stress and Iron-Dependent Cell Death. Mar. Drugs 2021, 19, 557. [Google Scholar] [CrossRef]
- Edwards, J.N.; Macdonald, W.A.; van der Poel, C.; Stephenson, D.G. O2(*-) production at 37 degrees C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am. J. Physiol.-Cell Physiol. 2007, 293, C650–C660. [Google Scholar] [CrossRef]
- Yu, T.; Deuster, P.; Chen, Y. Role of dynamin-related protein 1-mediated mitochondrial fission in resistance of mouse C2C12 myoblasts to heat injury. J. Physiol. 2016, 594, 7419–7433. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dohl, J.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury. J. Cell. Physiol. 2019, 234, 13292–13302. [Google Scholar] [CrossRef] [PubMed]
- Elmubarak, M.H.; Ranatunga, K.W. Temperature sensitivity of tension development in a fast-twitch muscle of the rat. Muscle Nerve 1984, 7, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Segal, S.S.; Faulkner, J.A.; White, T.P. Skeletal muscle fatigue in vitro is temperature dependent. J. Appl. Physiol. 1986, 61, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Naito, H.; Kakigi, R.; Ichinoseki-Sekine, N.; Ogura, Y.; Sugiura, T.; Katamoto, S. Heat stress activates the Akt/mTOR signalling pathway in rat skeletal muscle. Acta Physiol. 2013, 207, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Xiang, X.; Dai, Z.; Chang, J.; Zhang, M.; Cai, H.; Zhang, H.; Zhang, M.; Guo, Y.; et al. Ursolic acid prevents endoplasmic reticulum stress-mediated apoptosis induced by heat stress in mouse cardiac myocytes. J. Mol. Cell. Cardiol. 2014, 67, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Lian, R.; Li, Z.; Liu, Y.; Yang, S.; Huang, Z.; Zhao, Z.; Li, Y.; Sun, C.; Lin, S.; et al. Tea Polyphenols Enhanced the Antioxidant Capacity and Induced Hsps to Relieve Heat Stress Injury. Oxid. Med. Cell. Longev. 2021, 2021, 9615429. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Fahri, F.T.; Leury, B.J.; Dunshea, F.R. Dietary antioxidants at supranutritional doses modulate skeletal muscle heat shock protein and inflammatory gene expression in sheep exposed to heat stress. J. Anim. Sci. 2014, 92, 4897–4908. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, M.C.; Kim, D.W.; Kim, H.; Song, M.; Lee, T.K.; Lee, J.C.; Kim, H.; Cho, J.H.; Kim, Y.M.; et al. Antioxidant Properties of Fucoidan Alleviate Acceleration and Exacerbation of Hippocampal Neuronal Death Following Transient Global Cerebral Ischemia in High-Fat Diet-Induced Obese Gerbils. Int. J. Mol. Sci. 2019, 20, 554. [Google Scholar] [CrossRef]
- Ahmad, T.; Ishaq, M.; Karpiniec, S.; Park, A.; Stringer, D.; Singh, N.; Ratanpaul, V.; Wolfswinkel, K.; Fitton, H.; Caruso, V.; et al. Oral Macrocystis pyrifera Fucoidan Administration Exhibits Anti-Inflammatory and Antioxidant Properties and Improves DSS-Induced Colitis in C57BL/6J Mice. Pharmaceutics 2022, 14, 2383. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data Across the Phaeophyceae. Front. Plant Sci. 2020, 11, 556312. [Google Scholar] [CrossRef] [PubMed]
- Husni, A.; Izmi, N.; Ayunani, F.Z.; Kartini, A.; Husnayain, N.; Isnansetyo, A. Characteristics and Antioxidant Activity of Fucoidan from Sargassum hystrix: Effect of Extraction Method. Int. J. Food Sci. 2022, 2022, 3689724. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.-R.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Mol. Med. 2005, 15, 695–699. [Google Scholar] [CrossRef]
- Tan, J.; Song, Y.; Wang, J.; Wu, N.; Yue, Y. Pharmacokinetics of fucoidan and low molecular weight fucoidan from Saccharina japonica after oral administration to mice. J. Oceanol. Limnol. 2023, 41, 1900–1909. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef]
- Kuhlenhoelter, A.M.; Kim, K.; Neff, D.; Nie, Y.; Blaize, A.N.; Wong, B.J.; Kuang, S.; Stout, J.; Song, Q.; Gavin, T.P.; et al. Heat therapy promotes the expression of angiogenic regulators in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R377–R391. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, I.J.; McMillan, D.R. Stress (heat shock) proteins: Molecular chaperones in cardiovascular biology and disease. Circ. Res. 1998, 83, 117–132. [Google Scholar] [CrossRef]
- Garcia de la Serrana, D.; Johnston, I.A. Expression of heat shock protein (Hsp90) paralogues is regulated by amino acids in skeletal muscle of Atlantic salmon. PLoS ONE 2013, 8, e74295. [Google Scholar]
- Ojima, K.; Ichimura, E.; Suzuki, T.; Oe, M.; Muroya, S.; Nishimura, T. HSP90 modulates the myosin replacement rate in myofibrils. Am. J. Physiol.-Cell Physiol. 2018, 315, C104–C114. [Google Scholar] [CrossRef]
- Švec, X.; Štorkánová, H.; Špiritović, M.; Slabý, K.; Oreská, S.; Pekáčová, A.; Heřmánková, B.; Bubová, K.; Česák, P.; Khouri, H.; et al. Hsp90 as a Myokine: Its Association with Systemic Inflammation after Exercise Interventions in Patients with Myositis and Healthy Subjects. Int. J. Mol. Sci. 2022, 23, 11451. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Somogyvári, M.; Sőti, C. Hsp90 Stabilizes SIRT1 Orthologs in Mammalian Cells and C. elegans. Int. J. Mol. Sci. 2018, 19, 3661. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; Reizis, B.; Robbins, P.D. SIRT1 associates with eIF2-alpha and regulates the cellular stress response. Sci. Rep. 2011, 1, 150. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Bialopiotrowicz, E.; Sewastianik, T.; Noyszewska-Kania, M.M.; Szydlowski, M.; Jablonska, E.; Gorniak, P.; Polak, A.; Kiliszek, P.; Warzocha, K.; Juszczynski, P. Functional Link Between Heat Shock Protein HSP90alpha and Sirtuin 1 (SIRT1) in the Pathogenesis of Diffuse Large B Cell Lymphoma. Blood 2016, 128, 4120. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Che, Y.; Qu, W.; Li, J. Fucoidan inhibits apoptosis and improves cardiac remodeling by inhibiting p53 transcriptional activation through USP22/Sirt 1. Front. Pharmacol. 2023, 14, 1164333. [Google Scholar] [CrossRef]
- Bouchama, A.; Abuyassin, B.; Lehe, C.; Laitano, O.; Jay, O.; O’Connor, F.G. Classic and exertional heatstroke. Nat. Rev. Dis. Primers 2022, 8, 8. [Google Scholar] [CrossRef]
- Racinais, S.; Cocking, S.; Périard, J.D. Sports and environmental temperature: From warming-up to heating-up. Temperature 2017, 4, 227–257. [Google Scholar] [CrossRef]
- Murray, K.O.; Brant, J.O.; Spradlin, R.A.; Thome, T.; Laitano, O.; Ryan, T.E.; Riva, A.; Kladde, M.P.; Clanton, T.L. Exertional heat stroke causes long-term skeletal muscle epigenetic reprogramming, altered gene expression, and impaired satellite cell function in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 326, R160–R175. [Google Scholar] [CrossRef]
- Alkassas, W.; Rajab, A.M.; Alrashood, S.T.; Khan, M.A.; Dibas, M.; Zaman, M. Heat-related illnesses in a mass gathering event and the necessity for newer diagnostic criteria: A field study. Environ. Sci. Pollut. Res. Int. 2021, 28, 16682–16689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Tsai, Y.-H.; Tsai, T.-Y.; Chiu, Y.-S.; Wei, L.; Chen, W.-C.; Huang, C.-C. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 2014, 7, 239–252. [Google Scholar] [CrossRef]
- McBean, S.E.; Church, J.E.; Thompson, B.K.; Taylor, C.J.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y.; Poel, C. Oral fucoidan improves muscle size and strength in mice. Physiol. Rep. 2021, 9, e14730. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef]
- Park, A.Y.; Nafia, I.; Stringer, D.N.; Karpiniec, S.S.; Fitton, J.H. Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab. Mar. Drugs 2021, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.M.; Barker, R.G.; Horvath, D.; van der Poel, C. Benefits of Prenatal Taurine Supplementation in Preventing the Onset of Acute Damage in the Mdx Mouse. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Schertzer, J.D.; Ryall, J.G.; Lynch, G.S. Systemic administration of IGF-I enhances oxidative status and reduces contraction-induced injury in skeletal muscles of mdx dystrophic mice. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E499–E505. [Google Scholar] [CrossRef] [PubMed]
- Hakim, C.H.; Wasala, N.B.; Duan, D. Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J. Vis. Exp. 2013, 9, 50183. [Google Scholar] [CrossRef]
- Kucewicz, S.T.C.; Borkowski, M.; Church, J.E.; van der Poel, C. Measurement of Murine Neuromuscular Function Using the In Situ Preparation. Methods Mol. Biol. 2024, 2746, 147–154. [Google Scholar] [PubMed]

| Fucoidan Extract | Neutral Carbohydrates (%) | Sulfate (%) | Fucoidan (%) | Polyphenols (%) | Peak Molecular Weight (kDa) |
|---|---|---|---|---|---|
| FVF | 62.7 | 25 | 92.9 | 3.3 | 49.6 |
| UPF | 43.5 | 25.9 | 86 | <2 | 46.8 |
| Carbohydrate breakdown (mass %) of neutral carbohydrates | |||||
| Fucoidan Extract | Fucose (%) | Xylose (%) | Galactose (%) | Arabinose (%) | Rhamnose (%) |
| FVF | 46 | 7 | 4 | 1 | 0 |
| UPF | 21 | 1 | 18 | 1 | 0 |
| Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | |
|---|---|---|
| β2M | GTATGCTATCCAGAAAACCC | CTGAAGGACATATCTGACATC |
| HSF1 | AGAGAAAGATCCCTCTGATG | AGTGATATCGGAGATTTATGGG |
| NRF2 | CTAGCCTTTTCTCCGCCTTT | GAGGCTACTTGCAGCAGAGG |
| HSP27 | CTTCACCCGGAAATACAC | CGAAAGTAACCGGAATGG |
| HSP70 | AGTTCTTTGTGTTTGGACTC | TAACAGTCAACGCAATTACC |
| HSP90 | GCGGCAAAGACAAGAAAAG | CAAGTGGTCCTCCCAGTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucewicz, S.T.C.; Piantella, S.; Church, J.E.; Taylor, C.J.; van der Poel, C. The Impact of Fucoidan Extracts on Heat-Stress-Induced Loss of In Vitro Fast-Twitch Muscle Function in Mice. Muscles 2025, 4, 6. https://doi.org/10.3390/muscles4010006
Kucewicz STC, Piantella S, Church JE, Taylor CJ, van der Poel C. The Impact of Fucoidan Extracts on Heat-Stress-Induced Loss of In Vitro Fast-Twitch Muscle Function in Mice. Muscles. 2025; 4(1):6. https://doi.org/10.3390/muscles4010006
Chicago/Turabian StyleKucewicz, Samantha T. C., Stefan Piantella, Jarrod E. Church, Caroline J. Taylor, and Chris van der Poel. 2025. "The Impact of Fucoidan Extracts on Heat-Stress-Induced Loss of In Vitro Fast-Twitch Muscle Function in Mice" Muscles 4, no. 1: 6. https://doi.org/10.3390/muscles4010006
APA StyleKucewicz, S. T. C., Piantella, S., Church, J. E., Taylor, C. J., & van der Poel, C. (2025). The Impact of Fucoidan Extracts on Heat-Stress-Induced Loss of In Vitro Fast-Twitch Muscle Function in Mice. Muscles, 4(1), 6. https://doi.org/10.3390/muscles4010006






