Refractance Window Drying as an Alternative Method for Brewer’s Spent Grain Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
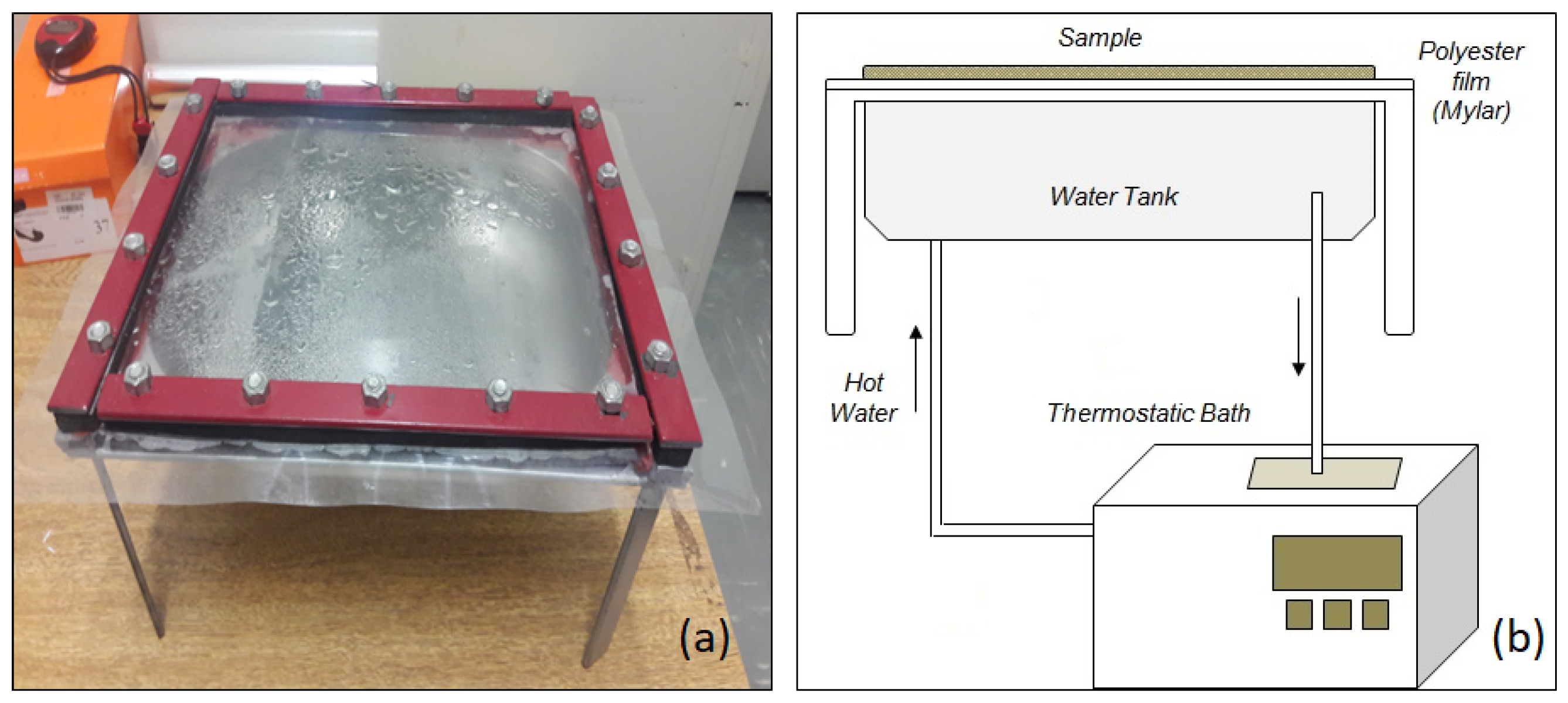
2.2. Experimental Apparatus
2.3. Experimental Design
2.4. Moisture and Water Activity (aw) Analysis
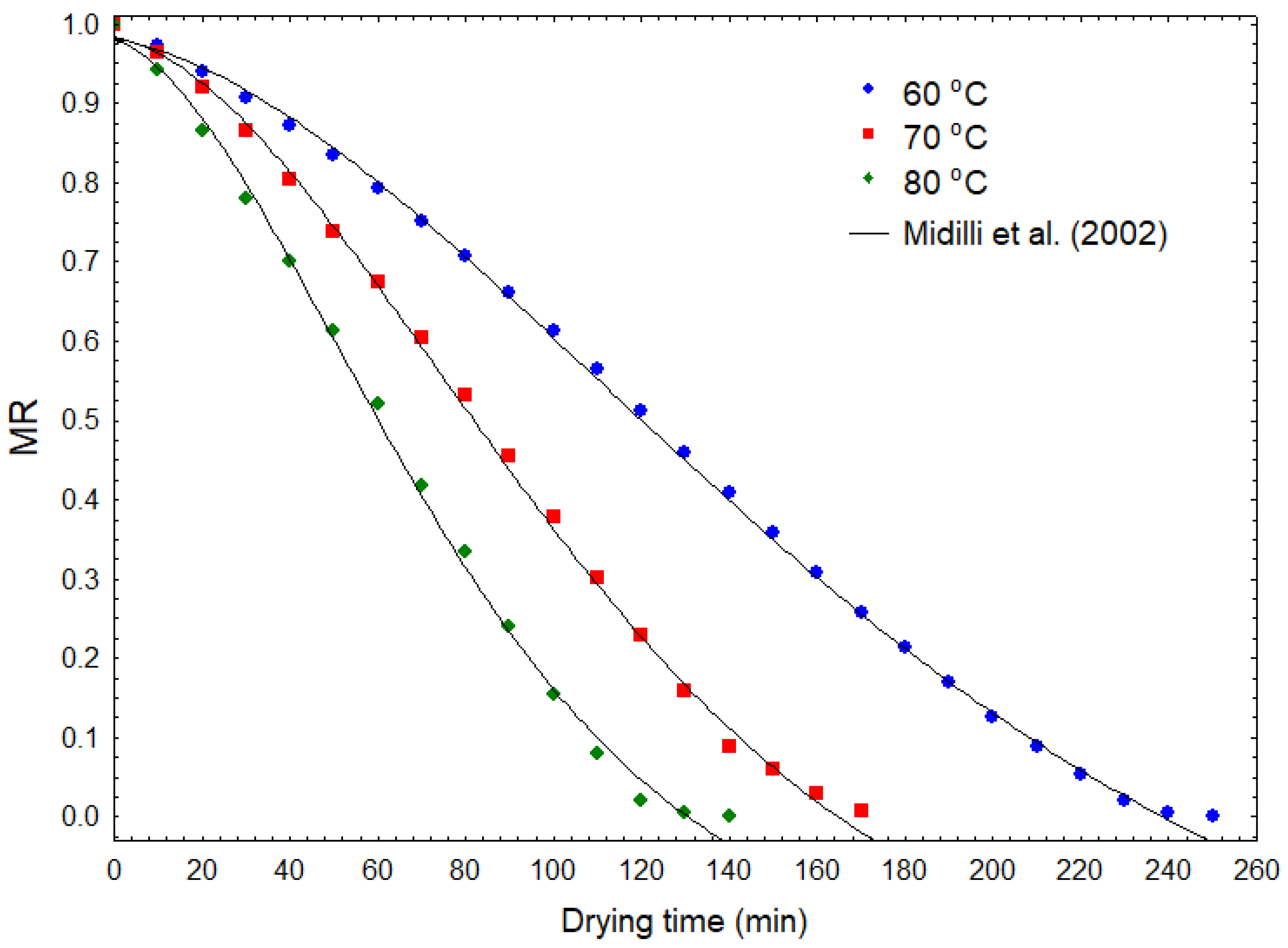
2.5. Drying Kinetics
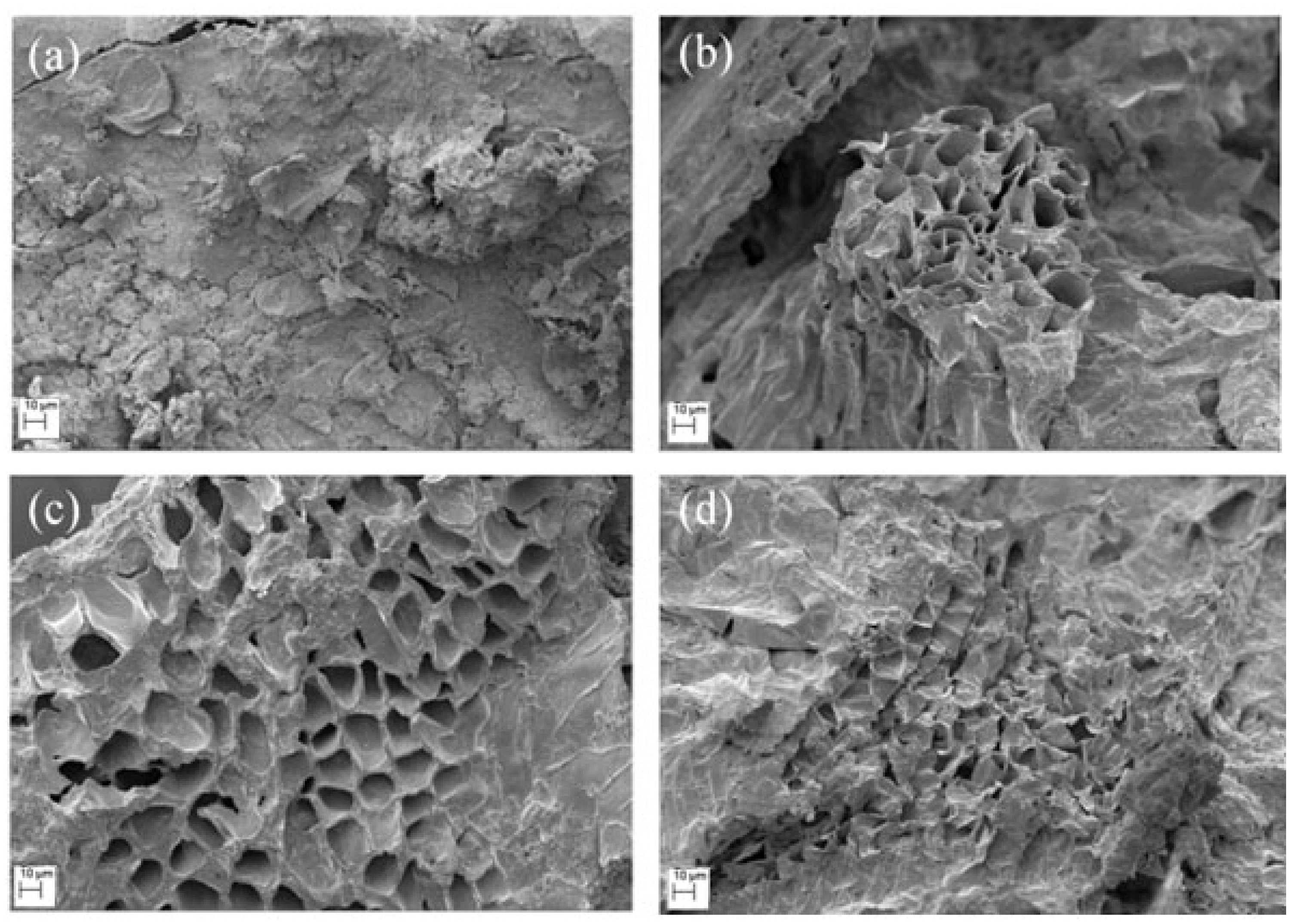
2.6. Scanning Electron Microscopy (SEM)
2.7. Analysis of Bioactive Compounds
2.7.1. Total Phenolic Content (TPC)
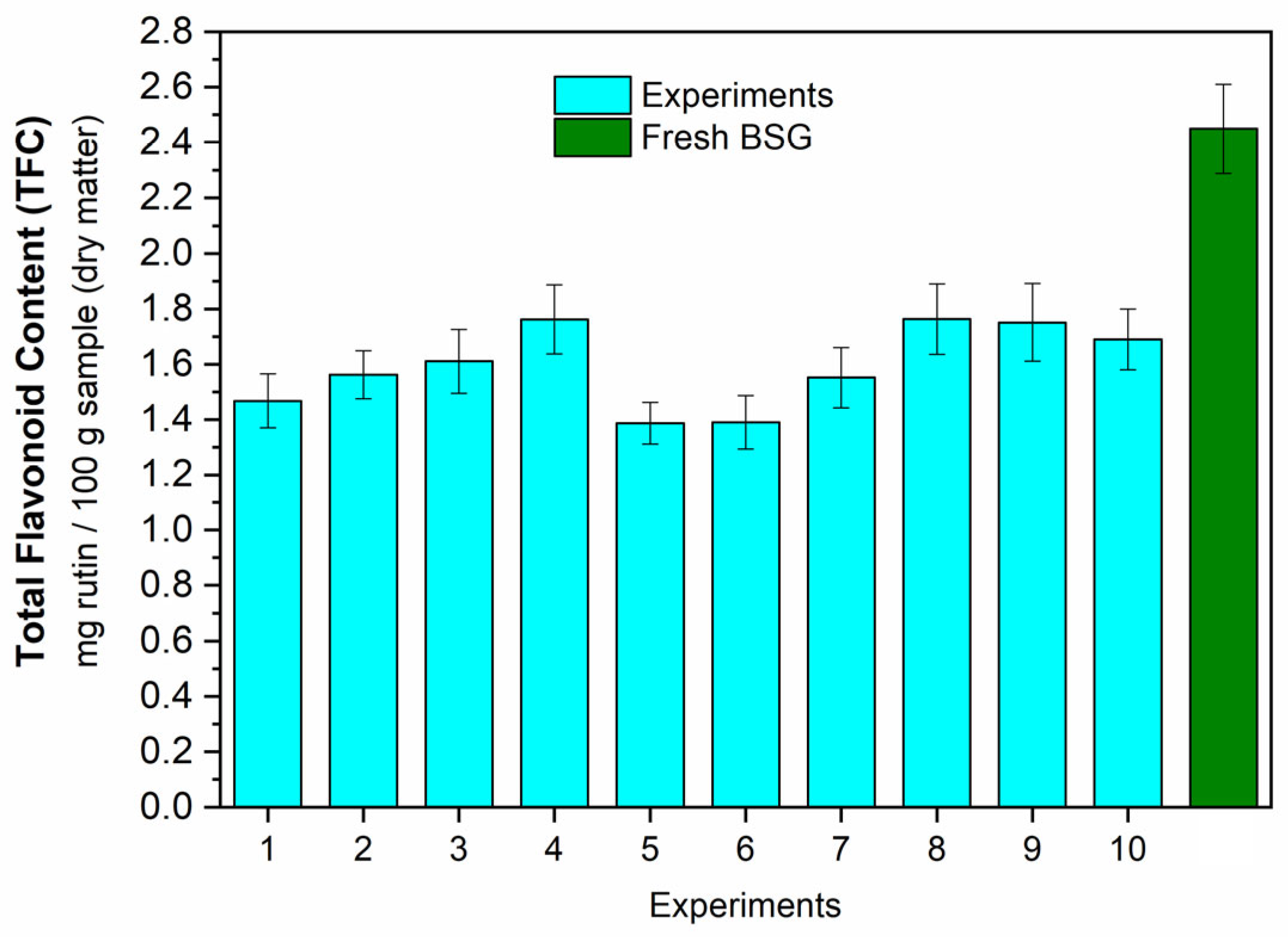
2.7.2. Total Flavonoid Content (TFC)
2.7.3. Acidity or Citric Acid Content (CA)
2.7.4. Ascorbic Acid Content (AA)
3. Results and Discussion
3.1. General Considerations
3.2. Initial Tests
3.3. Experimental Design Results
3.3.1. Moisture and Water Activity (aw)
3.3.2. Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunze, W. Technology Brewing & Malting, 4th ed.; International Edition; VLB: Berlin, Germany, 2010; 1057p. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewer’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Lamas, D.L.; Gende, L.B. Valorization of brewer’s spent grain for the development of novel beverage and food products. Appl. Food Res. 2023, 3, 100314. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Nigam, P.S. An overview: Recycling of solid barley waste generated as a by-product in distillery and brewery. Waste Manag. 2017, 62, 255–261. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Ganesan, A.R.; Mariniello, L.; Conterno, L.; Kumaravel, V. Valorization of brewer’s spent grain for sustainable food packaging. J. Clean. Prod. 2023, 385, 135726. [Google Scholar] [CrossRef]
- Marcus, A.; Fox, G. Fungal Biovalorization of a Brewing Industry Byproduct, Brewer’s Spent Grain: A Review. Foods 2021, 10, 2159. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Guarda, E.C.; Oliveira, A.C.; Antunes, S.; Freitas, F.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation. Appl. Sci. 2021, 11, 3222. [Google Scholar] [CrossRef]
- Gutiérrez-Barrutia, M.B.; del Castillo, M.D.; Arcia, P.; Cozzano, S. Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods 2022, 11, 1403. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Bazsefidpar, N.; Yazdi, A.P.G.; Karimi, A.; Yahyavi, M.; Amini, M.; Gavlighi, H.A.; Simal-Gandara, J. Brewers spent grain protein hydrolysate as a functional ingredient for muffins: Antioxidant, antidiabetic, and sensory evaluation. Food Chem. 2024, 435, 137565. [Google Scholar] [CrossRef]
- Becker, D.; Bakuradze, T.; Hensel, M.; Beller, S.; Yélamos, C.C.; Richling, E. Influence of Brewer’s Spent Grain Compounds on Glucose Metabolism Enzymes. Nutrients 2021, 13, 2696. [Google Scholar] [CrossRef]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- Birsan, R.I.; Wilde, P.; Waldron, K.W.; Rai, D.K. Anticholinesterase Activities of Different Solvent Extracts of Brewer’s Spent Grain. Foods 2021, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovéz, C.; Del Nozal, M.J. Variability of brewers’ spent grain with in a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Stroem, L.K.; Desai, D.K.; Hoadley, A.F.A. Superheated steam drying of Brewer’s spent grain in a rotary drym. Adv. Powder Technol. 2009, 20, 240–244. [Google Scholar] [CrossRef]
- Clarke, P.T. Refractance WindowTM–“Down under”. In Proceedings of the International Drying Symposium (IDS 2004) B, São Paulo, Brazil, 22–25 August 2004; pp. 813–820. [Google Scholar]
- Nindo, C.I.; Tang, J. Refractance Window dehydration technology: A novel contact drying method. Dry. Technol. 2007, 25, 37–48. [Google Scholar] [CrossRef]
- Raghavi, L.M.; Moses, J.A.; Anandharamakrishnan, C. Refractance window drying of foods: A review. J. Food Eng. 2018, 222, 267–275. [Google Scholar] [CrossRef]
- Múnera-Tangarife, R.D.; Solarte-Rodríguez, E.; Vélez-Pasos, C.; Ochoa-Martínez, C.I. Factors Affecting the Time and Process of CMC Drying Using Refractance Window or Conductive Hydro-Drying. Gels 2021, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, M.; Zotarelli, M.F.; Durigon, A.; Carciofi, B.A.M.; Laurindo, J.B. Production of tomato powder by refractance window drying. Dry. Technol. 2015, 33, 1463–1473. [Google Scholar] [CrossRef]
- Ortiz-Jerez, M.J.; Gulati, T.; Datta, A.K.; Ochoa-Martínez, C.I. Quantitative understanding of Refractance WindowTM Drying. Food Bioprod. Process. 2015, 95, 237–253. [Google Scholar] [CrossRef]
- Abonyi, B.I.; Feng, H.; Tang, J.; Edwards, C.G.; Chew, B.P.; Mattinson, D.S.; Fellman, J.K. Quality Retention in Strawberry and Carrot Purees Dried with Refractance WindowTM System. J. Food Sci. 2002, 67, 1051–1056. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ Vr.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Pavan, M.A.; Schmidt, S.J.; Feng, H. Water sorption behavior and thermal analysis of freeze-dried, Refractance Window-dried and hot-air dried açaí (Euterpe oleracea Martius) juice. LWT–Food Sci. Technol. 2012, 48, 75–81. [Google Scholar] [CrossRef]
- Frabetti, A.C.C.; Durigon, A.; Laurindo, J.B. Effect of process variables on the drying of guava pulp by cast-tape drying. LWT–Food Sci. Technol. 2018, 96, 620–626. [Google Scholar] [CrossRef]
- Asiimwe, A.; Kigozi, J.B.; Baidhe, E.; Muyonga, J.H. Optimization of refractance window drying conditions for passion fruit puree. LWT–Food Sci. Technol. 2022, 154, 112742. [Google Scholar] [CrossRef]
- Dadhaneeya, H.; Nayak, P.K.; Saikia, D.; Kondareddy, R.; Ray, S.; Kesavan, R.K. The impact of refractance window drying on the physicochemical properties and bioactive compounds of malbhog banana slice and pulp. Appl. Food Res. 2023, 3, 100279. [Google Scholar] [CrossRef]
- Zalpouri, R.; Singh, M.; Kaur, P.; Kaur, A.; Gaikwad, K.K.; Singh, A. Drying Kinetics, Physicochemical and Thermal Analysis of Onion Puree Dried Using a Refractance Window Dryer. Processes 2023, 11, 700. [Google Scholar] [CrossRef]
- Núñez, H.; Jaques, A.; Belmonte, K.; Córdova, A.; Lafuente, G.; Ramírez, C. Effect of CO2 Laser Microperforation Pretreatment on the Dehydration of Apple Slices during Refractive Window Drying. Foods 2023, 12, 2187. [Google Scholar] [CrossRef] [PubMed]
- Tontul, I.; Ergin, F.; Eroglu, E.; Kuçukçetin, K.; Topuz, A. Physical and microbiological properties of yogurt powder produced by refractance window drying. Int. Dairy J. 2018, 85, 169–176. [Google Scholar] [CrossRef]
- Silva, N.C.; Freitas, L.V.D.; Silva, T.C.; Duarte, C.R.; Barrozo, M.A.S. Use of Refractance Window Drying as an Alternative Method for Processing the Microalga Spirulina platensis. Molecules 2023, 28, 720. [Google Scholar] [CrossRef] [PubMed]
- AOAC, Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC: Gaithersburg, MD, USA, 1995; Volume 1. [Google Scholar]
- Tang, Z.; Cenkowski, S.; Muir, W.E. Modelling the Superheated-steam Drying of a Fixed Bed of Brewer’s Spent Grain. Biosyst. Eng. 2004, 87, 67–77. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Adhikari, B.P. Water activity in food processing and preservation. In Drying Technologies in Food Processing; Chen, X.D., Mujumdar, A.S., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA; John Wiley & Sons: West Sussex, UK, 2008; p. 55. [Google Scholar]
- Chen, X.D.; Patel, K.C. Biological changes during food drying processes. In Drying Technologies in Food Processing; Chen, X.D., Mujumdar, A.S., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA; John Wiley & Sons: West Sussex, UK, 2008; p. 90. [Google Scholar]
- Barrozo, M.A.S.; Sartori, D.J.M.; Freire, J.T. A Study of the Statistical Discrimination of the Drying Kinetics Equation. Food Bioprod. Process. 2004, 82, 219–225. [Google Scholar] [CrossRef]
- Freire, F.B.; Barrozo, M.A.S.; Sartori, D.J.M.; Freire, J.T. Study of the drying kinetics in thin layer: Fixed and moving bed. Dry. Technol. 2007, 23, 1451–1464. [Google Scholar] [CrossRef]
- Barrozo, M.A.S.; Sartori, D.J.M.; Freire, J.T.; Achcar, J.A. Discrimination of Equilibrium Moisture Equations For Soybean Using Nonlinearity Measures. Dry. Technol. 2007, 14, 1779–1794. [Google Scholar] [CrossRef]
- Felipe, C.A.S.; Barrozo, M.A.S. Drying of soybean seeds in a concurrent moving bed: Heat and mass transfer and quality analysis. Dry. Technol. 2003, 21, 439–456. [Google Scholar] [CrossRef]
- Lewis, W.K. The rate of drying of solid materials. Indian Chem. Eng. 1921, 13, 427. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin-Layer; Purdue University: West Lafayette, IN, USA, 1949. [Google Scholar]
- Overhults, D.G.; White, G.M.; Hamilton, H.E.; Ross, I.J. Drying soybeans with heated air. Trans. ASAE 1973, 16, 112–113. [Google Scholar] [CrossRef]
- Brooker, D.B.; Bakker-Arkema, F.W.; Hall, C.W. Drying Cereal Grains; Avi: Westport, CT, USA, 1974. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin–ciocalteu reagent. Methods Enzymol. 1999, 299, 52–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Silva, N.C.; Duarte, C.R.; Barrozo, M.A.S. Effects of dehydration methods on quality characteristics of yellow passion fruit coproducts. J. Sci. Food Agric. 2017, 97, 4750–4759. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s spent grain from different types of malt: Evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Wu, J. Food-based uses of brewers spent grains: Current applications and future possibilities. Food Biosci. 2023, 54, 102774. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Nobile, M.A.D. Microencapsulation of extracted bioactive compounds from brewer’s spent grain to enrich fish burguers. Food Bioprod. Process. 2016, 100 Pt A, 450–456. [Google Scholar] [CrossRef]
- Shon, Y.J.; Kim, W.C.; Lee, S.-H.; Hong, S.; Kim, S.-Y.; Park, M.H.; Lee, P.; Lee, J.; Park, K.H.; Lim, W.; et al. Antimelanogenic potential of brewer’s spent grain extract through modulation of the MAPK/MITF axis. Sustain. Mater. Technol. 2023, 38, e00721. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidant and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Hansuld, M.K.; Briant, A.M. The effect of citric acid on selected edible starches and flours. J. Food Sci. 1954, 19, 581–589. [Google Scholar] [CrossRef]
- Cooray, S.T.; Chen, W.N. Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. J. Funct. Foods 2018, 42, 85–94. [Google Scholar] [CrossRef]
- Shetty, R.; Petersen, F.R.; Podduturi, R.; Molina, G.E.S.; Wätjen, A.P.; Madsen, S.K.; Zioga, E.; Øzmerih, S.; Hobley, T.J.; Bang-Berthelsen, C.H. Fermentation of brewer’s spent grain liquids to increase shelf life and give an organic acid enhanced ingredient. LWT-Food Sci. Technol. 2023, 182, 114911. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Sablani, S.S.; Rahman, M.S. Fundamentals of Food Dehydration. In Food Drying Science and Technology; Hui, Y.H., Clary, C., Farid, M.M., Fasina, O.O., Noomhorm, A., Welti-Chanes, J., Eds.; DEStech Publication, Inc.: Lancaster, PA, USA, 2008; Chapter 1; pp. 1–42. [Google Scholar]





| Independent Variables | −1.414 | −1 | 0 | +1 | +1.414 |
|---|---|---|---|---|---|
| Temperature (°C) | 55.9 | 60.0 | 70.0 | 80.0 | 84.1 |
| Time (h) | 1.6 | 2.0 | 3.0 | 4.0 | 4.4 |
| Equation in the Literature | Reference |
|---|---|
| Lewis [42] | |
| Page [43] | |
| Overhults et al. [44] | |
| Brooker et al. [45] | |
| Midilli et al. [46] |
| Exp. | Temperature | Moisture (%) | Water Activity (aw) | Drying Time (min) |
|---|---|---|---|---|
| 1 | 60 °C | 8.36 ± 0.18 | 0.527 | 250 |
| 2 | 70 °C | 5.85 ± 0.46 | 0.425 | 170 |
| 3 | 80 °C | 5.10 ± 0.22 | 0.352 | 140 |
| Fresh BSG | 75.89 ± 1.31% | 0.977 | - | |
| Model | Exp. | Parameters of the Models | Statistical Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| K | n | A | B | R2 | SSE | RMSE | ||
| Lewis [38] | 60 °C | 6.99 × 10−3 | - | - | - | 0.8857 | 0.3276 | 0.1122 |
| 70 °C | 1.02 × 10−2 | - | - | - | 0.8866 | 0.2309 | 0.1133 | |
| 80 °C | 1.42 × 10−2 | - | - | - | 0.9037 | 0.1720 | 0.1071 | |
| Page [39] | 60 °C | 6.40 × 10−5 | 1.95 | - | - | 0.9907 | 0.0266 | 0.0320 |
| 70 °C | 1.19 × 10−4 | 1.97 | - | - | 0.9924 | 0.0155 | 0.0293 | |
| 80 °C | 3.36 × 10−4 | 1.87 | - | - | 0.9911 | 0.0159 | 0.0326 | |
| Overhults et al. [40] | 60 °C | 7.06 × 10−3 | 1.95 | - | - | 0.9907 | 0.0266 | 0.0320 |
| 70 °C | 1.03 × 10−2 | 1.97 | - | - | 0.9924 | 0.0155 | 0.0293 | |
| 80 °C | 1.39 × 10−2 | 1.87 | - | - | 0.9911 | 0.0159 | 0.0326 | |
| Brooker et al. [41] | 60 °C | 8.18 × 10−3 | - | 1.1566 | - | 0.9176 | 0.2363 | 0.0953 |
| 70 °C | 1.19 × 10−2 | - | 1.1508 | - | 0.9172 | 0.1686 | 0.0968 | |
| 80 °C | 1.62 × 10−2 | - | 1.1350 | - | 0.9265 | 0.1313 | 0.0936 | |
| Midilli et al. [42] | 60 °C | 1.07 × 10−4 | 1.77 | 0.9808 | −7.29 × 10−4 | 0.9991 | 0.0024 | 0.0097 |
| 70 °C | 1.80 × 10−4 | 1.82 | 0.9832 | −8.50 × 10−4 | 0.9986 | 0.0028 | 0.0125 | |
| 80 °C | 4.81 × 10−4 | 1.73 | 0.9787 | −8.28 × 10−4 | 0.9975 | 0.0045 | 0.0172 | |
| Exp. | Temp. (T) °C | Time (t) h | Moisture (%) | Water Activity (aw) |
|---|---|---|---|---|
| 1 | 60.0 | 2.0 | 35.20 ± 0.59 | 0.947 |
| 2 | 60.0 | 4.0 | 10.80 ± 0.09 | 0.648 |
| 3 | 80.0 | 2.0 | 5.75 ± 0.24 | 0.448 |
| 4 | 80.0 | 4.0 | 2.87 ± 0.12 | 0.151 |
| 5 | 55.9 | 3.0 | 21.27 ± 0.28 | 0.876 |
| 6 | 84.1 | 3.0 | 2.92 ± 0.12 | 0.188 |
| 7 | 70.0 | 1.6 | 26.00 ± 0.71 | 0.919 |
| 8 | 70.0 | 4.4 | 4.85 ± 0.05 | 0.195 |
| 9 | 70.0 | 3.0 | 6.31 ± 0.06 | 0.505 |
| 10 | 70.0 | 3.0 | 5.35 ± 0.30 | 0.428 |
| Bioactive Compound | Statistical Analysis | ||||
|---|---|---|---|---|---|
| R2 | SSE | RMSE | Significative Parameters | p-Value | |
| TPC | 0.8295 | 48.3322 | 2.1985 | 0.00587 | |
| 0.01975 | |||||
| 0.02160 | |||||
| TFC | 0.7695 | 0.0450 | 0.0671 | 0.00406 | |
| 0.04739 | |||||
| CA | 0.8284 | 400.2782 | 6.3268 | 0.09753 | |
| 0.08877 | |||||
| 0.01146 | |||||
| 0.09114 | |||||
| AA | 0.9314 | 0.3461 | 0.1860 | 0.06231 | |
| 0.00623 | |||||
| 0.00278 | |||||
| 0.01852 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.C.; Santos, A.O.; Duarte, C.R.; Barrozo, M.A.S. Refractance Window Drying as an Alternative Method for Brewer’s Spent Grain Preservation. Appl. Biosci. 2024, 3, 71-86. https://doi.org/10.3390/applbiosci3010005
Silva NC, Santos AO, Duarte CR, Barrozo MAS. Refractance Window Drying as an Alternative Method for Brewer’s Spent Grain Preservation. Applied Biosciences. 2024; 3(1):71-86. https://doi.org/10.3390/applbiosci3010005
Chicago/Turabian StyleSilva, Neiton C., Andressa O. Santos, Claudio R. Duarte, and Marcos A. S. Barrozo. 2024. "Refractance Window Drying as an Alternative Method for Brewer’s Spent Grain Preservation" Applied Biosciences 3, no. 1: 71-86. https://doi.org/10.3390/applbiosci3010005
APA StyleSilva, N. C., Santos, A. O., Duarte, C. R., & Barrozo, M. A. S. (2024). Refractance Window Drying as an Alternative Method for Brewer’s Spent Grain Preservation. Applied Biosciences, 3(1), 71-86. https://doi.org/10.3390/applbiosci3010005






