Effects of Bone Marrow Sparing and TGF-β3 Treatment in Total Body Irradiation of C57BL/6J Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. α-SMA Detecting Assay
2.3. Animals
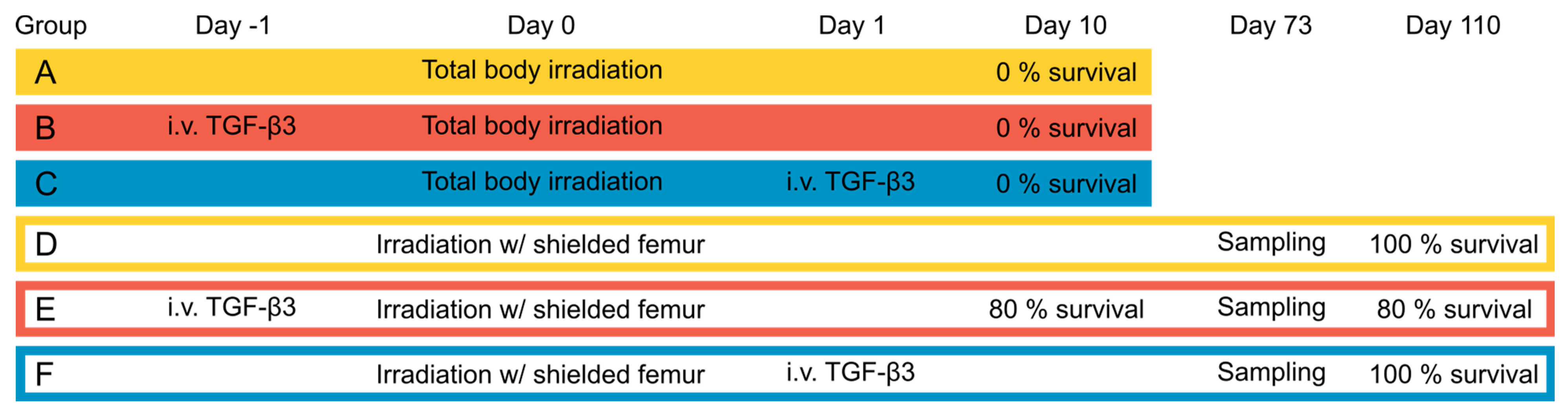
2.4. Treatment
2.5. Follow-Up
2.6. Histological Evaluations
2.7. Statistical Analyses
3. Results
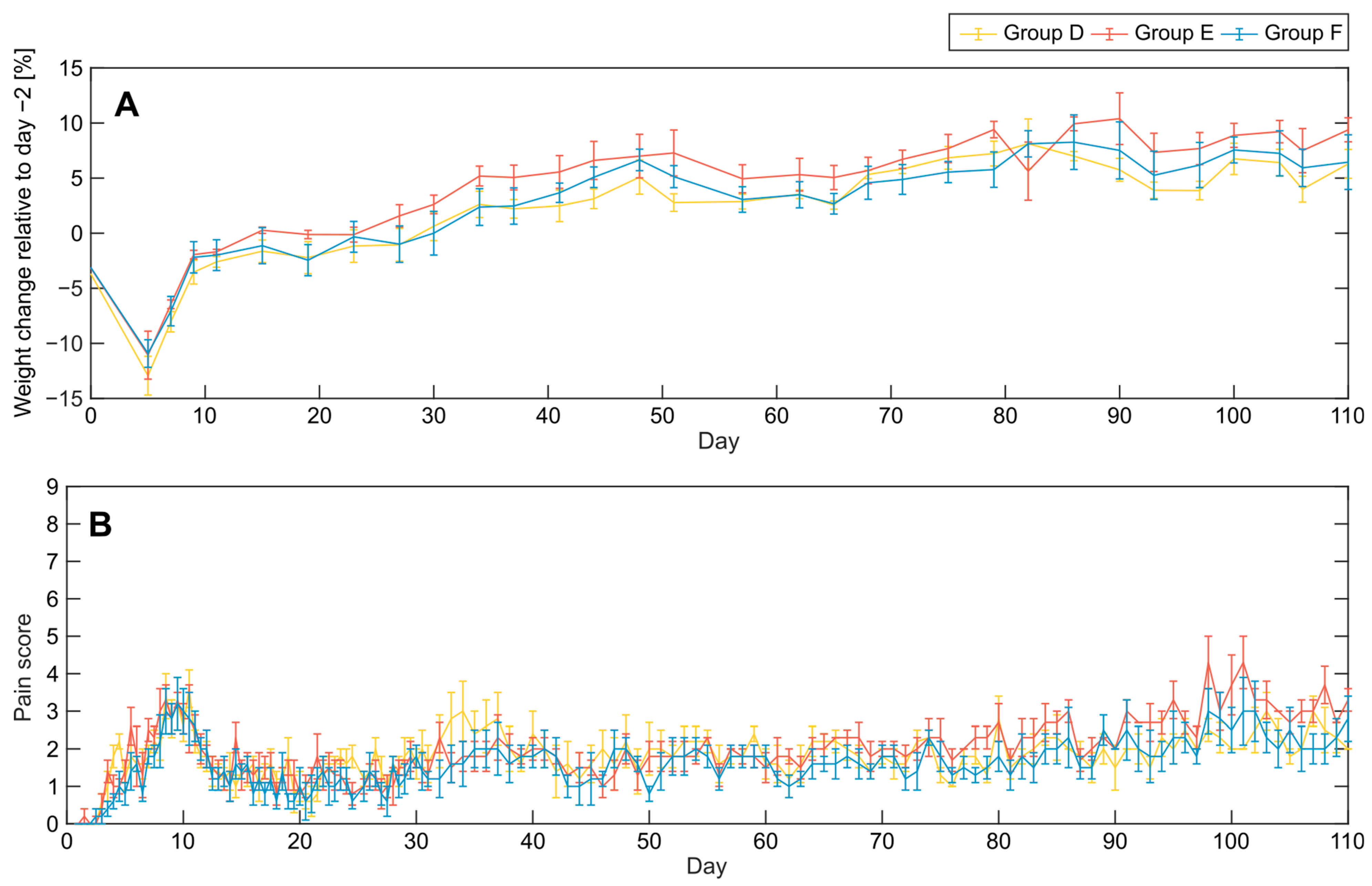
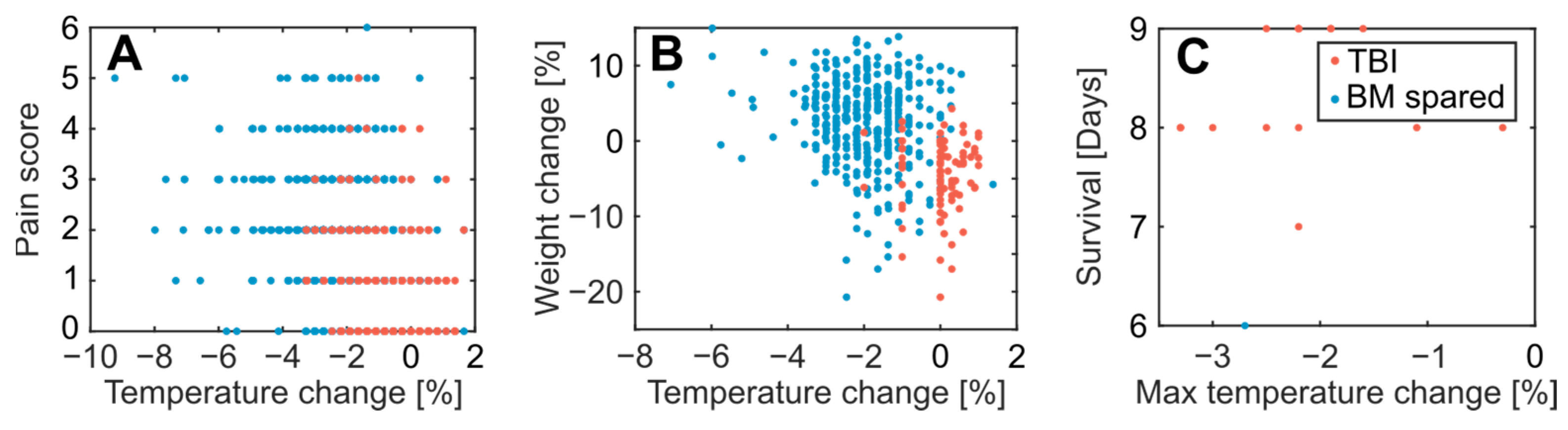
3.1. Acute Effects and Survival after 8.5 Gy Irradiation
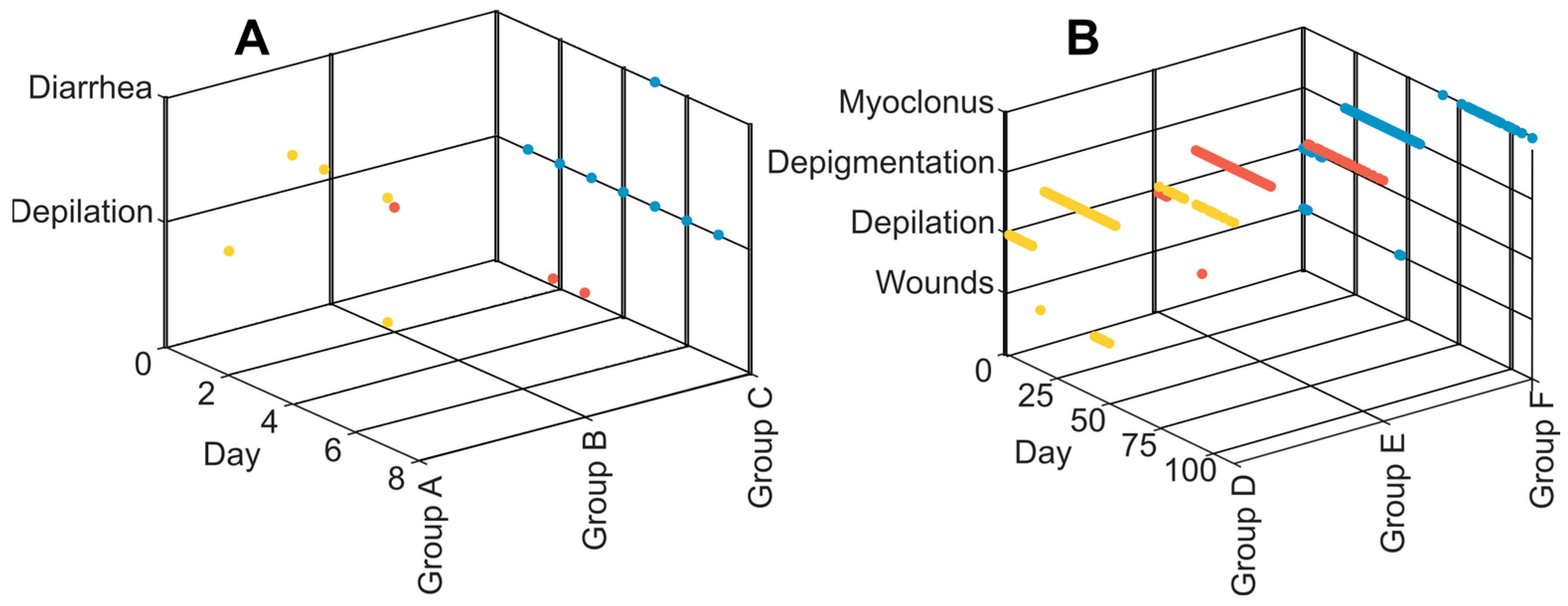
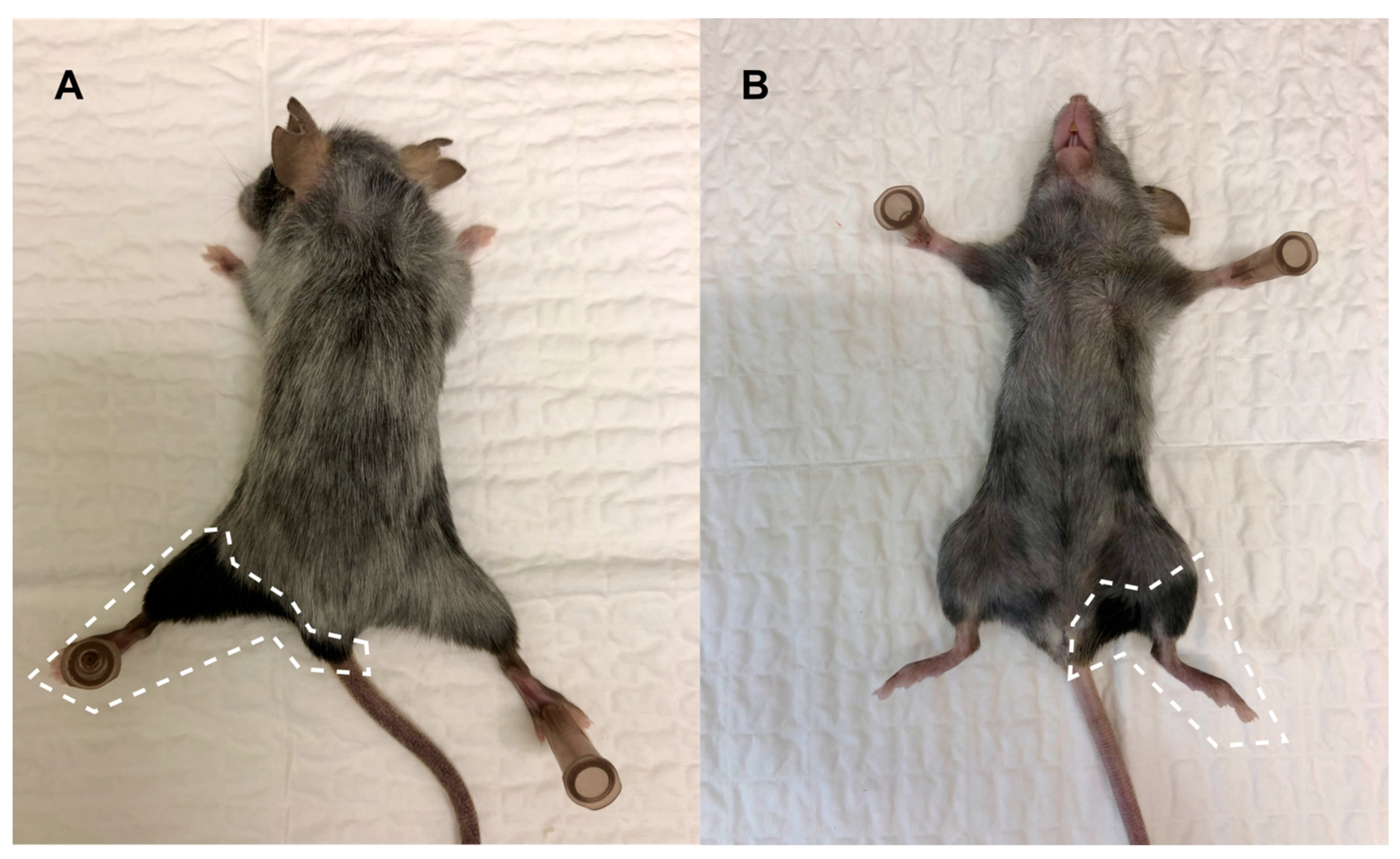
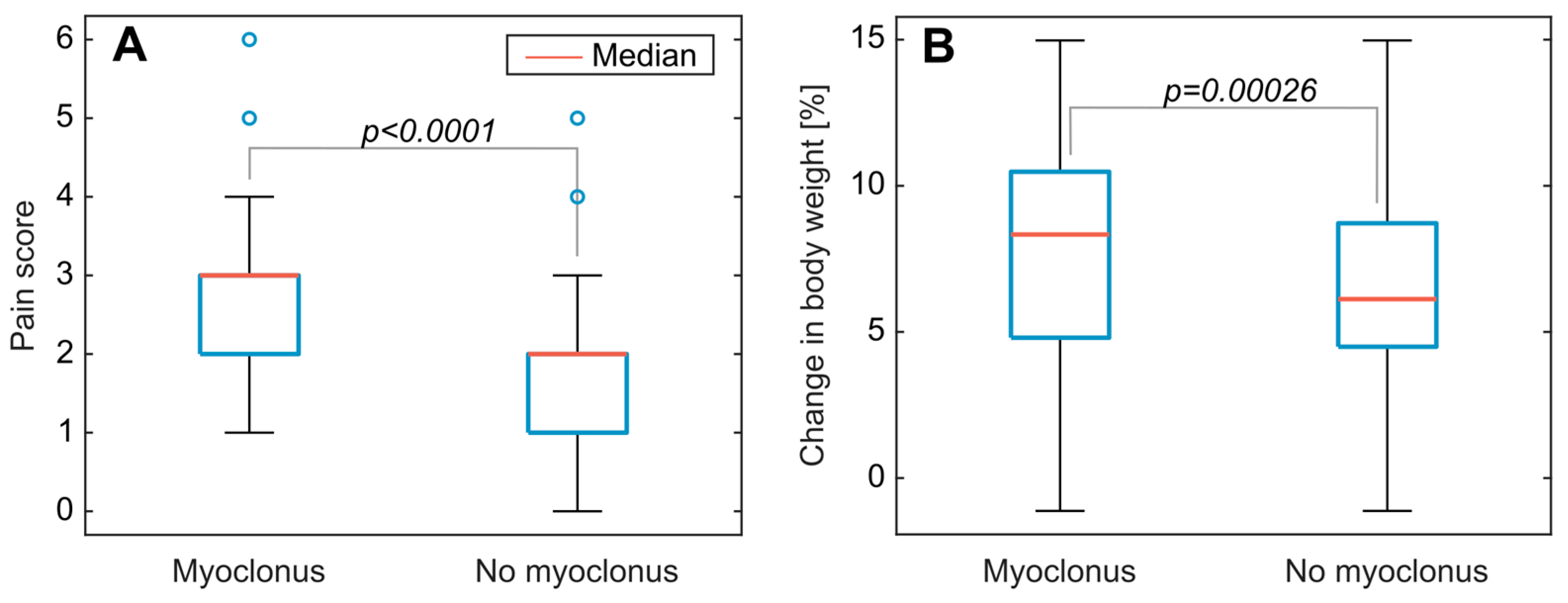
3.2. Clinical Symptoms and Necropsy Assessments
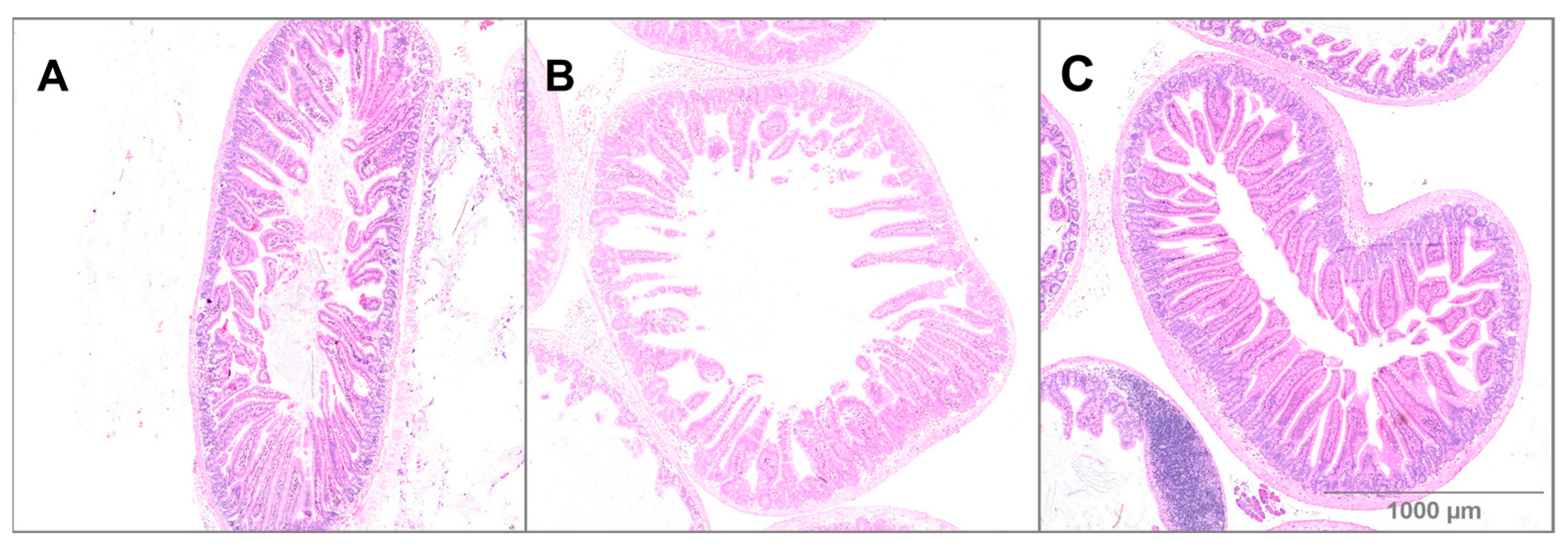
3.3. Small Intestine Histology
3.4. In Vitro α-SMA Expression after Treatment with TGF-β3 and Radiation
3.5. Collagen Content in Lung and Liver Sections from BM-Spared Mice
4. Discussion
4.1. Acute Effects of Total Body and Bone Marrow-Spared Irradiation
4.2. Fibrogenesis In Vitro and In Bone Marrow-Spared Animals
4.3. Evaluation of Animal Monitoring Methods
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- i Garau, M.M.; Lucas Calduch, A.; López, E.C. Radiobiology of the acute radiation syndrome. Rep. Pract. Oncol. Radiother. 2011, 16, 123–130. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Acute Radiation Syndrome. In Radiobiology for the Radiologst, 7th ed.; Wolters Kluwer Health/Lipincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 114–128. [Google Scholar]
- International Atomic Energy Agency. Diagnosis and Treatment of Radiation Injuries; Safety Reports Series No. 2; International Atomic Energy Agency: Vienna, Austria, 1998. [Google Scholar]
- Farese, A.M.; MacVittie, T.J. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today 2015, 51, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Legesse, B.; Kaur, A.; Kenchegowda, D.; Hritzo, B.; Culp, W.E.; Moroni, M. Neulasta Regimen for the Hematopoietic Acute Radiation Syndrome: Effects Beyond Neutrophil Recovery. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; McManus, J.; Gale, R.P. Sargramostim in acute radiation syndrome. Expert Opin. Biol. Ther. 2022, 22, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. An update on romiplostim for treatment of acute radiation syndrome. Drugs Today 2022, 58, 133–145. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Radiological and Nuclear Emergency Preparedness Information from FDA: Medical Countermeasures; US Food and Drug Administration: Silver Spring, MD, USA, 2023.
- Booth, C.; Tudor, G.; Tudor, J.; Katz, B.P.; MacVittie, T.J. The acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012, 103, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Jin, H.; Yoo, Y.; Kim, Y.; Kim, Y.; Cho, J.; Lee, Y.S. Radiation-induced lung fibrosis: Preclinical animal models and therapeutic strategies. Cancers 2020, 12, 1561. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Lindquist, P.B.; Lee, A.; Wen, D.; Tamm, J.; Graycar, J.L.; Rhee, L.; Mason, A.J.; Miller, D.A.; Coffey, R.J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988, 7, 3737–3743. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Hansen, P.; Iwata, K.K.; Pieler, C.; Foulkes, J.G. Identification of another member of the transforming growth factor type beta gene family. Proc. Natl. Acad Sci. USA 1988, 85, 4715–4719. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-B Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, K.H.; Lin, X.; Feng, X.-H. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009, 19, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Kaartinen, V. Tgfb1 expressed in the Tgfb3 locus partially rescues the cleft palate phenotype of Tgfb3 null mutants. Dev. Biol. 2007, 312, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Komai, T.; Okamura, T.; Inoue, M.; Yamamoto, K.; Fujio, K. Reevaluation of pluripotent cytokine TGF-β3 in immunity. Int. J. Mol. Sci. 2018, 19, 2261. [Google Scholar] [CrossRef] [PubMed]
- Hanson, I.; Pitman, K.E.; Edin, N.F.J. The Role of TGF-β3 in Radiation Response. Int. J. Mol. Sci. 2023, 24, 7614. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, V.; Voncken, J.W.; Shuler, C.; Warburton, D.; Bu, D.; Heisterkamp, N.; Groffen, J. Abnormal lung development and cleft palate in mice lacking TGF–β3 indicates defects of epithelial–mesenchymal interaction. Nat. Genet. 1995, 11, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Proetzel, G.; Pawlowski, S.A.; Wiles, M.V.; Yin, M.; Boivin, G.P.; Howles, P.N.; Ding, J.; Ferguson, M.W.J.; Doetschman, T. Transforming growth factor-β3 is required for secondary palate fusion. Nat. Genet. 1995, 11, 409–414. [Google Scholar] [CrossRef]
- Laverty, H.G.; Wakefield, L.M.; Occleston, N.L.; O’Kane, S.; Ferguson, M.W.J. TGF-β3 and cancer: A review. Cytokine Growth Factor. Rev. 2009, 20, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Robson, H.; Spence, K.; Anderson, E.; Potten, C.S.; Hendry, J.H. Differential influence of TGFβ1 and TGFβ3 isoforms on cell cycle kinetics and postirradiation recovery of normal and malignant colorectal epithelial cells. Int. J. Radiat. Oncol. 1997, 38, 183–190. [Google Scholar] [CrossRef]
- Khalil, N.; O’Connor, R.N.; Flanders, K.C.; Shing, W.; Whitman, C.I. Regulation of type II alveolar epithelial cell proliferation by TGF-β during bleomycin-induced lung injury in rats. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1994, 267, 498–507. [Google Scholar] [CrossRef]
- McCormack, E.S.; Borzillo, G.V.; Ambrosino, C.; Mak, G.; Hamablet, L.; Qu, G.Y.; Haley, J.D. Transforming growth factor-β3 protection of epithelial cells from cycle-selective chemotherapy in vitro. Biochem. Pharmacol. 1997, 53, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.I.; Kawai, T.; Kamakura, T.; Ookura, T. TGF-β3 is expressed in taste buds and inhibits proliferation of primary cultured taste epithelial cells. In Vitro Cell. Dev. Biol.-Anim. 2010, 46, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Edin, N.J.; Sandvik, J.A.; Cheng, C.; Bergersen, L.; Pettersen, E.O. The roles of TGF-β3 and peroxynitrite in removal of hyper-radiosensitivity by priming irradiation. Int. J. Radiat. Biol. 2014, 90, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen Edin, N.; Altaner, Č.; Altanerova, V.; Ebbesen, P. TGF-Β3 Dependent Modification of Radiosensitivity in Reporter Cells Exposed to Serum from Whole-Body Low Dose-Rate Irradiated Mice. Dose-Response 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Booth, D.; Haley, J.D. Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo. Br. J. Cancer 1997, 75, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.; Haley, J.D.; Bruskin, A.M.; Potten, C.S. Transforming growth factor-B3 protects murine small intestinal stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int. J. Cancer 2000, 86, 53–59. [Google Scholar] [CrossRef]
- Xu, L.; Xiong, S.; Guo, R.; Yang, Z.; Wang, Q.; Xiao, F.; Wang, H.; Pan, X.; Zhu, M. Transforming growth factor β3 attenuates the development of radiation-induced pulmonary fibrosis in mice by decreasing fibrocyte recruitment and regulating IFN-γ/IL-4 balance. Immunol. Lett. 2014, 162, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170. [Google Scholar] [CrossRef]
- Nunamaker, E.A.; Anderson, R.J.; Artwohl, J.E.; Lyubimov, A.V.; Fortman, J.D. Predictive Observation-Based Endpoint Criteria for Mice Receiving Total Body Irradiation. Comp. Med. 2013, 63, 313–322. [Google Scholar]
- Singh, V.K.; Newman, V.L.; Berg, A.N.; MacVittie, T.J. Animal models for acute radiation syndrome drug discovery. Expert Opin. Drug Discov. 2015, 10, 497–517. [Google Scholar] [CrossRef]
- Nunamaker, E.A.; Artwohl, J.E.; Anderson, R.J.; Fortman, J.D. Endpoint Refinement for Total Body Irradiation of C57BL/6 Mice. Comp. Med. 2013, 63, 22–28. [Google Scholar] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hanson, I.; Juvkam, I.S.; Zlygosteva, O.; Søland, T.M.; Galtung, H.K.; Malinen, E.; Edin, N.F.J. TGF-β3 increases the severity of radiation-induced oral mucositis and salivary gland fibrosis in a mouse model. Int. J. Radiat. Biol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Kendall tau Rank Correlation (v1.0.13). 2017. Available online: https://www.wessa.net/ (accessed on 4 March 2024).
- Juvkam, I.S.; Zlygosteva, O.; Malinen, E.; Edin, N.J.; Galtung, H.K.; Søland, T.M. Fractionated irradiation of murine salivary glands resulted in focal acinar cell atrophy, immune cell infiltration, fibrosis, and hyposalivation. BioRxiv 2023. [Google Scholar] [CrossRef]
- Juvkam, I.S.; Zlygosteva, O.; Arous, D.; Galtung, H.K.; Malinen, E.; Søland, T.M.; Jeppesen Edin, N. A preclinical model to investigate normal tissue damage following fractionated radiotherapy to the head and neck. J. Radiat. Res. 2022, 64, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Plett, P.A.; Sampson, C.H.; Lin Chua, H.; Joshi, M.; Booth, C.; Gough, A.; Johnson, C.S.; Katz, B.P.; Farese, A.M.; Parker, J.; et al. Establishing a murine model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Phys. 2012, 103, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.X. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lin, X.; Feng, F.; Wei, Y.; Wei, S.; Gong, Y.; Guo, C.; Wang, Q.; Shuai, P.; Wang, T.; et al. Gut microbiota and ionizing radiation-induced damage: Is there a link? Environ. Res. 2023, 229, 115947. [Google Scholar] [CrossRef]
- Wymenga, A.N.M.; van der Graaf, W.T.A.; Hofstra, L.S.; Spijkervet, F.K.L.; Timens, W.; Timmer-Bosscha, H.; Sluiter, W.J.; van Buuren, A.H.J.A.W.; Mulder, N.H.; de Vries, E.G.E. Phase I Study of Transforming Growth Factor-3 Mouthwashes for Prevention of Chemotherapy-induced Mucositis. Clin. Cancer Res. 1999, 5, 1363–1368. [Google Scholar]
- Foncuberta, M.C.; Cagnoni, P.J.; Brandts, C.H.; Mandanas, R.; Fields, K.; Derigs, H.G.; Reed, E.; Sonis, S.T.; Fay, J.; LeVeque, F.; et al. Topical Transforming Growth Factor-β3 in the Prevention or Alleviation of Chemotherapy-Induced Oral Mucositis in Patients with Lymphomas or Solid Tumors. J. Immunother. 2001, 24, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.A.; Withers, H.R.; McBride, W.H.; Davis, C.A.; Smathers, J.B. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat. Res. 1989, 117, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.H.A.; Travis, E.L. The influence of bone marrow depletion on intestinal radiation damage. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Bhanja, P.; Kabarriti, R.; Liu, L.; Alfieri, A.A.; Guha, C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS ONE 2011, 6, e24072. [Google Scholar] [CrossRef] [PubMed]
- Ch’Ang, H.J.; Lin, L.M.; Chang, P.Y.; Luo, C.W.; Chang, Y.H.; Chou, C.K.; Chen, H.H. Bone marrow transplantation enhances trafficking of host-derived myelomonocytic cells that rescue intestinal mucosa after whole body radiation. Radiother. Oncol. 2012, 104, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Wang, W.; Prabath, B.G.; Boerma, M.; Wang, J.; Zhou, D.; Hauer-Jensen, M. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat. Res. 2014, 181, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Pejchal, J.; Šinkorová, Z.; Tichý, A.; Kmochová, A.; Ďurišová, K.; Kubelková, K.; Pohanka, M.; Bureš, J.; Tachecí, I.; Kuča, K.; et al. Attenuation of radiation-induced gastrointestinal damage by epidermal growth factor and bone marrow transplantation in mice. Int. J. Radiat. Biol. 2015, 91, 703–714. [Google Scholar] [CrossRef]
- Brodin, N.P.; Velcich, A.; Guha, C.; Tomé, W.A.; Tomé, T. A Model for Precise and Uniform Pelvic-and Limb-Sparing Abdominal Irradiation to Study the Radiation-Induced Gastrointestinal Syndrome in Mice Using Small Animal Irradiation Systems. Dose-Response 2017, 15, 1559325816685798. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, B.J.; Wei, L.; Zhang, L.; Ping, X.; Epperly, M.; Greenberger, J.; Cheng, T.; Yu, J. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat. Commun. 2014, 5, 3494. [Google Scholar] [CrossRef]
- Shaposhnikov, V.L. Distribution of bone marrow cells in the mouse skeleton. Bull. Exp. Biol. Med. 1979, 87, 510–512. [Google Scholar] [CrossRef]
- Withers, H.R.; Elkind, M.M. Microcolony Survival Assay for Cells of Mouse Intestinal Mucosa Exposed to Radiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1970, 17, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S. Radiation, the Ideal Cytotoxic Agent for Studying the Cell Biology of Tissues such as the Small Intestine. Radiat. Res. 2004, 161, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Lefaix, J.-L.; Sylvie, D. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. 2000, 47, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Serini, G.; Gabbiani, G. Modulation of a-smooth muscle actin expression in fibroblasts by transforming growth factor-P isoforms: An in vivo and in vitro study. Wound Repair. Regen. 1996, 3, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Loewen, M.S.; Walner, D.L.; Caldarelli, D.D. Improved airway healing using transforming growth factor beta-3 in a rabbit model. Wound Repair. Regen. 2001, 9, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ask, K.; Bonniaud, P.; Maass, K.; Eickelberg, O.; Margetts, P.J.; Warburton, D.; Groffen, J.; Gauldie, J.; Kolb, M. Progressive pulmonary fibrosis is mediated by TGF-β isoform 1 but not TGF-β3. Int. J. Biochem. Cell Biol. 2008, 40, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Peng, Y.; Gao, D.; Feng, C.; Yuan, X.; Li, H.; Wang, Y.; Yang, L.; Huang, S.; Fu, X. Mesenchymal Stem Cells Suppress Fibroblast Proliferation and Reduce Skin Fibrosis through a TGF-β3-Dependent Activation. Int. J. Low. Extrem. Wounds 2015, 14, 50–62. [Google Scholar] [CrossRef]
- Xue, K.; Zhang, J.; Li, C.; Li, J.; Wang, C.; Zhang, Q.; Chen, X.; Yu, X.; Sun, L.; Yu, X. The role and mechanism of transforming growth factor beta 3 in human myocardial infarction-induced myocardial fibrosis. J. Cell. Mol. Med. 2019, 23, 4229–4243. [Google Scholar] [CrossRef] [PubMed]
- Escasany, E.; Lanzón, B.; García-Carrasco, A.; Izquierdo-Lahuerta, A.; Torres, L.; Corrales, P.; Rodríguez, A.E.R.; Luis-Lima, S.; Álvarez, C.M.; Ruperez, F.J.; et al. Transforming growth factor β 3 deficiency promotes defective lipid metabolism and fibrosis in murine kidney. Dis. Model. Mech. 2021, 14, dmm048249. [Google Scholar] [CrossRef]
- Wilson, S.E. TGF beta −1, −2 and −3 in the modulation of fibrosis in the cornea and other organs. Exp. Eye Res. 2021, 207, 108594. [Google Scholar] [CrossRef]
- Reggio, S.; Rouault, C.; Poitou, C.; Bichet, J.-C.; Prifti, E.; Bouillot, J.-L.; Rizkalla, S.; Lacasa, D.; Tordjman, J.; Clément, K. Increased Basement Membrane Components in Adipose Tissue During Obesity: Links With TGF and Metabolic Phenotypes. J. Clin. Endocrinol. Metab. 2016, 101, 2578–2587. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, W.; Zeng, Z.; Lin, J.; Zhang, X.; Chen, L. Tgfb3 and Mmp13 regulated the initiation of liver fibrosis progression as dynamic network biomarkers. J. Cell. Mol. Med. 2021, 25, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Huang, Z.; Liang, W.C.; Yin, J.; Lin, W.Y.; Wu, J.; Vernes, J.M.; Lutman, J.; Caplazi, P.; Jeet, S.; et al. TGFβ2 and TGFβ3 isoforms drive fibrotic disease pathogenesis. Sci. Transl. Med. 2021, 13, eabe0407. [Google Scholar] [CrossRef] [PubMed]
- Juvista EU Phase 3 Trial Results. Fierce Biotech. 2011. Available online: https://www.fiercebiotech.com/biotech/juvista-eu-phase-3-trial-results (accessed on 13 March 2023).
- Ferguson, M.W.; Duncan, J.; Bond, J.; Bush, J.; Durani, P.; So, K.; Taylor, L.; Chantrey, J.; Mason, T.; James, G.; et al. Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet 2009, 373, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.; Duncan, J.A.L.; Bond, J.S.; Durani, P.; So, K.; Mason, T.; O’Kane, S.; Ferguson, M.W.J. Scar-Improving Efficacy of Avotermin Administered into the Wound margins of Skin Incisions as Evaluated by a Randomized, Double-Blind, Placebo-Controlled, Phase II Clinical Trial. Plast. Reconstr. Surg. 2010, 126, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- So, K.; McGrouther, D.A.; Bush, J.A.; Durani, P.; Taylor, L.; Skotny, G.; Mason, T.; Metcalfe, A.; Okane, S.; Ferguson, M.W.J. Avotermin for scar improvement following scar revision surgery: A randomized, double-blind, within-patient, placebo-controlled, phase II clinical trial. Plast. Reconstr. Surg. 2011, 128, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Heer, R.; Clarke, N.; Rigas, A.C.; Cheek, T.R.; Pickard, R.; Leung, H.Y. Phenotypic modulation of human urinary tract stroma-derived fibroblasts by transforming growth factor β3. Urology 2010, 76, 509.e13–509.e20. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Diaz-Arrastia, C.R.; Kilic, G.S.; Kamel, M.W. 2-Methoxyestradiol causes functional repression of transforming growth factor β3 signaling by ameliorating Smad and non-Smad signaling pathways in immortalized uterine fibroid cells. Fertil. Steril. 2012, 98, 178–184.e1. [Google Scholar] [CrossRef]
- Yeung, V.; Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Zieske, J.D.; Karamichos, D.; Ciolino, J.B. Fak inhibition attenuates corneal fibroblast differentiation in vitro. Biomolecules 2021, 11, 1682. [Google Scholar] [CrossRef]
- Liu, M.; Honjo, M.; Yamagishi, R.; Igarashi, N.; Nakamura, N.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Aihara, M. Fibrotic Response of Human Trabecular Meshwork Cells to Transforming Growth Factor-Beta 3 and Autotaxin in Aqueous Humor. Biomolecules 2022, 12, 1231. [Google Scholar] [CrossRef]
- Carrington, L.M.; Albon, J.; Anderson, I.; Kamma, C.; Boulton, M. Differential regulation of key stages in early corneal wound healing by TGF-β isoforms and their inhibitors. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cho, H.G.; Lee, J.W.; Oh, E.J.; Kim, H.M.; Ko, U.H.; Kang, M.; Shin, J.H.; Chung, H.Y. Influence of Transforming Growth Factors beta 1 and beta 3 in the Scar Formation Process. J. Craniofac. Surg. 2023, 34, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, K.; Hyun, J.; Lee, Y.; Kim, Y.; Jung, Y. Hedgehog signaling influences gender-specific response of liver to radiation in mice. Hepatol. Int. 2013, 7, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hyun, J.; Youn, B.; Jung, Y. Hedgehog signaling regulates the repair response in mouse liver damaged by irradiation. Radiat. Res. 2013, 179, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, Y.; Kim, J.; Hyun, J.; Lee, K. Potential Role of Hedgehog Pathway in Liver Response to Radiation. PLoS ONE 2013, 8, e74141. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Foreman, D.; Ferguson, M.W.J. Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-β3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [CrossRef]
- Lanning, D.A.; Diegelmann, R.F.; Yager, D.R.; Wallace, M.L.; Bagwell, C.E.; Haynes, J.H. Myofibroblast induction with transforming growth factor-β1 and -β3 in cutaneous fetal excisional wounds. J. Pediatr. Surg. 2000, 35, 183–188. [Google Scholar] [CrossRef]
- Hosokawa, R.; Nonaka, K.; Morifuji, M.; Shum, L.; Ohishi, M. TGF-β3 Decreases Type I Collagen and Scarring after Labioplasty. J. Dent. Res. 2003, 82, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Kishimoto, Y.; Hasan, A.; Welham, N. V TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Model. Mech. 2014, 7, 83–91. [Google Scholar] [CrossRef]
- Finkelstein, J.N.; Johnston, C.J.; Baggs, R.; Rubin, P. Early alterations in extracellular matrix and transforming growth factor β gene expression in mouse lung indicative of late radiation fibrosis. Int. J. Radiat. Oncol. 1994, 28, 621–631. [Google Scholar] [CrossRef]
- Johnston, C.J.; Piedboeuf, B.; Baggs, R.; Rubin, P.; Finkelstein, J.N. Differences in Correlation of mRNA Gene Expression in Mice Sensitive and Resistant to Radiation-Induced Pulmonary Fibrosis. Radiat. Res. 1995, 142, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Rubin, P.; Johnston, C.J.; Williams, J.P.; McDonald, S.; Finkelstein, J.N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int. J. Radiat. Oncol. 1995, 33, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Kim, S.H.; Chung, E.J.; Lee, W.J.; Suh, C.O. Early alteration in TGF-β mRNA expression in irradiated rat liver. Int. J. Radiat. Oncol. 2000, 46, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Gulani, J.; King, G.; Hieber, K.; Chappell, M. Establishment of Early Endpoints in Mouse Total-Body Irradiation Model. PLoS ONE 2016, 11, e0161079. [Google Scholar] [CrossRef]
- The Jackson Laboratory. Body Weight Information for C57BL/6J. 2023. Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664 (accessed on 4 March 2024).
- Taliaferro, L.P.; Agarwal, R.K.; Coleman, C.N.; Andrea, L.; Hofmeyer, K.A.; Loelius, S.G.; Molinar-inglis, O.; Tedesco, D.C.; Satyamitra, M.M.; Taliaferro, L.P.; et al. Sex differences in radiation research. Int. J. Radiat. Biol. 2023, 100, 466–485. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanson, I.; Vatne, J.T.; Edin, N.F.J. Effects of Bone Marrow Sparing and TGF-β3 Treatment in Total Body Irradiation of C57BL/6J Mice. Appl. Biosci. 2024, 3, 165-185. https://doi.org/10.3390/applbiosci3020011
Hanson I, Vatne JT, Edin NFJ. Effects of Bone Marrow Sparing and TGF-β3 Treatment in Total Body Irradiation of C57BL/6J Mice. Applied Biosciences. 2024; 3(2):165-185. https://doi.org/10.3390/applbiosci3020011
Chicago/Turabian StyleHanson, Ingunn, Jenny T. Vatne, and Nina F. J. Edin. 2024. "Effects of Bone Marrow Sparing and TGF-β3 Treatment in Total Body Irradiation of C57BL/6J Mice" Applied Biosciences 3, no. 2: 165-185. https://doi.org/10.3390/applbiosci3020011




