1. Introduction
Medical devices created from thermoplastics are widely used in patient treatment. Applications include their use in lieu of metal plates, screws, and nails in orthopedic surgeries, along with surgical sutures, wound dressing, scaffolds for tissue engineering, and the long-term delivery of drugs [
1]. Thermoplastics have low melting temperatures that allow for the easy fabrication of movement-restricting devices. This includes immobilization forms (masks) used to position patients during high-dose radiation therapy for head and neck tumors and custom-fabricated orthoses worn to immobilize the affected area in patients with burns, surgical wounds, or open traumatic injuries (
Figure 1).
Sampling of patient thermoplastic devices revealed contamination by several different healthcare-associated infection (HAI) microbial pathogens, including Gram-positive cocci, Gram-negative rods, yeast, and Gram-positive rods [
2,
3,
4]. HAIs are a major concern in inpatient and outpatient treatment settings. They contribute to extended hospital stays, higher treatment costs, and increased antibiotic resistance [
5].
In 1989, Wright et al. examined 20 patient orthoses in a burn unit for contaminants [
2]. Ten orthoses failed to grow any microbes upon initial sampling or subsequent resampling. The ten remaining orthoses demonstrated several microbes, including Gram-positive cocci (n = 4), Gram-negative rods (n = 3), yeasts (n = 2), and a Gram-positive rod (n = 1).
Bacillus species (spp.) is the Gram-positive rod. Wounds were dressed using either silver sulfadiazine, silver nitrate, mafenide acetate, nystatin, or 1% peroxide. A quaternary ammonium compound (QAC) disinfectant was likewise used to clean patient orthoses. This group recommended routine orthosis cleaning each time it was removed for either a dressing change, after debridement, or post-grafting procedures.
In 1994, Faoagali et al. reported that contaminated orthoses could be a source of infection in burn patients [
3]. One hundred samples were taken from ten patients wearing a total of twenty-eight orthoses. The recovered microbes included Gram-positive cocci like coagulase-negative staphylococci or CoNS (n = 11),
Staphylococcus aureus (n = 4),
Bacillus spp. (n = 4), and Gram-negative rods (n = 2). Sampling was conducted before and after orthosis cleaning by cold disinfection using 4% chlorhexidine followed by air drying. Of note,
Bacillus was still recovered from 3 out of 4 orthoses after thorough cleaning.
In 2020, Ravine et al. sampled 12 in-use patient masks at three different radiation therapy treatment facilities [
4]. The recovered bacteria included
Bacillus spp. (n = 21), coagulase-negative staphylococci (n = 19),
Staphylococcus aureus (n = 2),
Enterococcus spp. (n = 1), a Gram-negative rod (n = 1), and a viridans (alpha-hemolytic)
Streptococcus (n = 1). In some instances, more than one
Bacillus species was recovered from a single mask. Furthermore, a different
Bacillus spp. was recovered from one facility’s water bath used to heat patient masks. The recovery of seven different
Bacillus spp. from patient masks was completely unexpected. CoNS, like
Staphylococcus epidermidis, are part of the expected normal skin microbiota (flora), whereas
Bacillus is not. Accordingly, it was expected that CoNS would be seen more often than any non-microbiota bacteria like
Bacillus.
The recovered Bacillus spp. were identified by MALDI-TOFI technology as B. cereus (n = 12), B. subtilis (n = 2), B. megaterium (n = 2), B. pumilus (n =2), B. luciferensis (n = 1), B. amyloliquefaciens (n = 1), and B. circulans (n = 1). The water bath isolate was identified as B. halosaccharovorans. The B. subtilis isolates and B. amyloliquefaciens were very mucoid and proved difficult to identify. The importance of mucoid B. subtilis will be discussed later.
The current study assessed the capability of either B. cereus, B. megaterium, or B. subtilis recovered from patient masks to similarly attach two different thermoplastic materials after 1 h of contact time. Similarly measured was the ability of attached bacteria to be recovered from these materials at different time intervals up to eight weeks. To the authors’ knowledge, no comparative study of this type has ever been performed using orthotic thermoplastic material. We now share our findings with the journal’s readership.
2. Materials and Methods
2.1. Experimental Design
This was a quantitative research study. Investigators hypothesized that Bacillus spp. recovered from thermoplastic patient radiation therapy masks would show similar attachment capability and subsequent recovery from a thermoplastic material used to create patient orthotic splints. The specific aims were (1) to assess Bacillus adherence during 1 h of initial contact and (2) to determine if applied Bacillus bacteria could be recovered after 2 months.
2.2. Bacteria Tested
B. cereus (MAB03F) and
B. megaterium (DAB01F) were recovered from the forehead region of two different patient masks.
B. subtilis (MAB03N) was recovered from the nose region of the same mask as
B. cereus (MAB03F) [
4]. They are all hereafter referred to as
B. cereus,
B. megaterium, and
B. subtilis unless otherwise indicated. Prior thermoplastic material testing showed that the
B. cereus MAB03F strain produced abundant spores during 96 h of prolonged incubation. Moreover, the increased spore numbers corresponded to an increased attachment capability to thermoplastic material [
6]. Given the predictability of previous results,
B. cereus MAB03F served as an internal positive control in the current study. Stock cultures were maintained by periodic transfer to fresh nutrient trypticase soy agar (TSA) and incubated under ambient conditions at 37 °C (
Figure 2). A fresh TSA plate was separately inoculated with each bacterium and incubated at 37 °C for 96 h before testing to promote spore formation by the nutrient depletion method. All the statistical data reported in this manuscript were derived using GraphPad Prism software version 9.5.1.
2.3. Identification of Mask Isolates
A Bruker MALDI Biotyper model MBT Smart Biotyper system (Bruker, Billerica, MA, USA) was used to identify mask-recovered
Bacillus isolates. MALDI-TOF stands for matrix-assisted laser desorption/ionization/time-of-flight. Microbe identification methodology involves mass spectrometry analysis. MALDI-TOF converts microbial protein molecules into ions in the gas phase that are measured for their mass-to-charge (
m/
z) ratio. Bound to a solid surface, a laser strikes the sample converting the protein molecules into gas without altering them. The time of the ion flight paths are monitored, and the resulting pattern of the mass peaks is compared to a library of known patterns [
7]. Each
Bacillus isolate was tested twice for biomolecule spectral agreement. Sample processing and testing were accomplished by laboratory professionals working in a College of American Pathologist (CAP)-accredited clinical microbiology laboratory employing strict quality control procedures.
2.4. Material Processing
The tested thermoplastic sheets were unperforated and 2.5 cm thick. They included uncoated Model R-3200A Klarity ThermoSheets (lot number 909296(1); (Klarity Medical Heath, OH, USA) and coated Model NC12979 North Coast (NC) Spectrum Beige (lot number 201099); (North Coast Medical, Morgan Hill, CA, USA). The Klarity material is typically used in radiation therapy applications and not for making upper extremity orthoses. It was included for comparison since it does not have an additional anti-stick coating, as does NC Spectrum Beige. A 220 mm × 300 mm rectangle was cut from each thermoplastic sheet and treated as follows.
A drawing template was prepared from an X-ray film containing thirty-five 20 mm × 20 mm squares spaced 20 mm apart (
Figure 3). Both materials were separately heated in a water bath at 130 °F (71 °C) for 3 min and cooled. The template was cleaned with methanol, air-dried, and clamped to either material’s face. A sterile gentian violet marker (Medline Industries, Northfield, IL, USA) was used to outline the target sample squares. The water bath used to heat both materials was sampled on the day of use, and subsequent cultures revealed that it was not contaminated.
2.5. Inoculum Preparation
B. cereus, B. megaterium, and B. subtilis inoculums were separately prepared by removing sufficient colony material from a 96 h TSA plate and transferring it to a sterilized glass tube containing 3 mL of sterile physiologic (0.9%) saline. Suspensions were adjusted to match a 1.0% McFarland turbidity standard using a DEN-1 densitometer (Grant Inc., Beaver Falls, PA, USA).
2.6. Material Inoculation
A 100 μL sample of B. cereus was distributed to the end of a sterile cotton-tipped swab. The dampened swab was then applied to a designated sample square and rolled vertically over the entire square, taking care not to go outside the marked borders. The same swab was then rolled in horizontal and oblique (45°) directions without adding more samples. B. cereus inoculum was applied to triplicate sample squares on both materials. B. megaterium and B. subtilis inoculums were separately applied in the same manner. A sterile saline control without bacteria was applied to a central square on each material to determine if exogenous contamination was present. Inoculated materials were transferred to an open rack shelving unit until periodic sampling. The mean room temperature during the investigation was 22.1 °C (21 °Cmin–23 °Cmax), while the mean relative humidity was 45.6% (43%min–49%max).
2.7. Material Sampling
Target squares were separately sampled at designated time intervals (e.g., 1 h). Here, a sterile cotton-tipped swab was immersed into 1 mL of sterile saline contained in a sterile 15 mL conical tube. The wetted swab was withdrawn to just above the saline line, and excess saline was removed by wringing it gently against the inner tube wall. The dampened swab was rolled over the entire inner portion of the target sample square in vertical, horizontal, and oblique directions, staying within the drawn lines. The swab was inserted back into the same tube and vortexed vigorously for 10–15 s to dislodge bacteria. Triplicate squares were separately sampled in the same manner as the middle saline control square was.
2.8. Bacterial Counts
The direct plate count method was used to determine the number of bacteria in initial suspensions applied to either material, along with the number recovered at each sampling interval. Ten-fold serial dilutions were prepared from each sample tube. A 100 µL aliquot was removed from each dilution tube and separately transferred to a fresh TSA plate. The fluid was evenly distributed using an L-shaped plate spreader (Genesee Scientific, San Diego, CA, USA). Count plates were incubated for 24 h at 37 °C under ambient conditions. Plates exhibiting between 10 and 150 colonies were counted to determine the number of bacteria in each corresponding dilution. Colony-forming units/milliliter (CFU/mL
−1) were determined as follows:
2.9. Spore Stains and Count
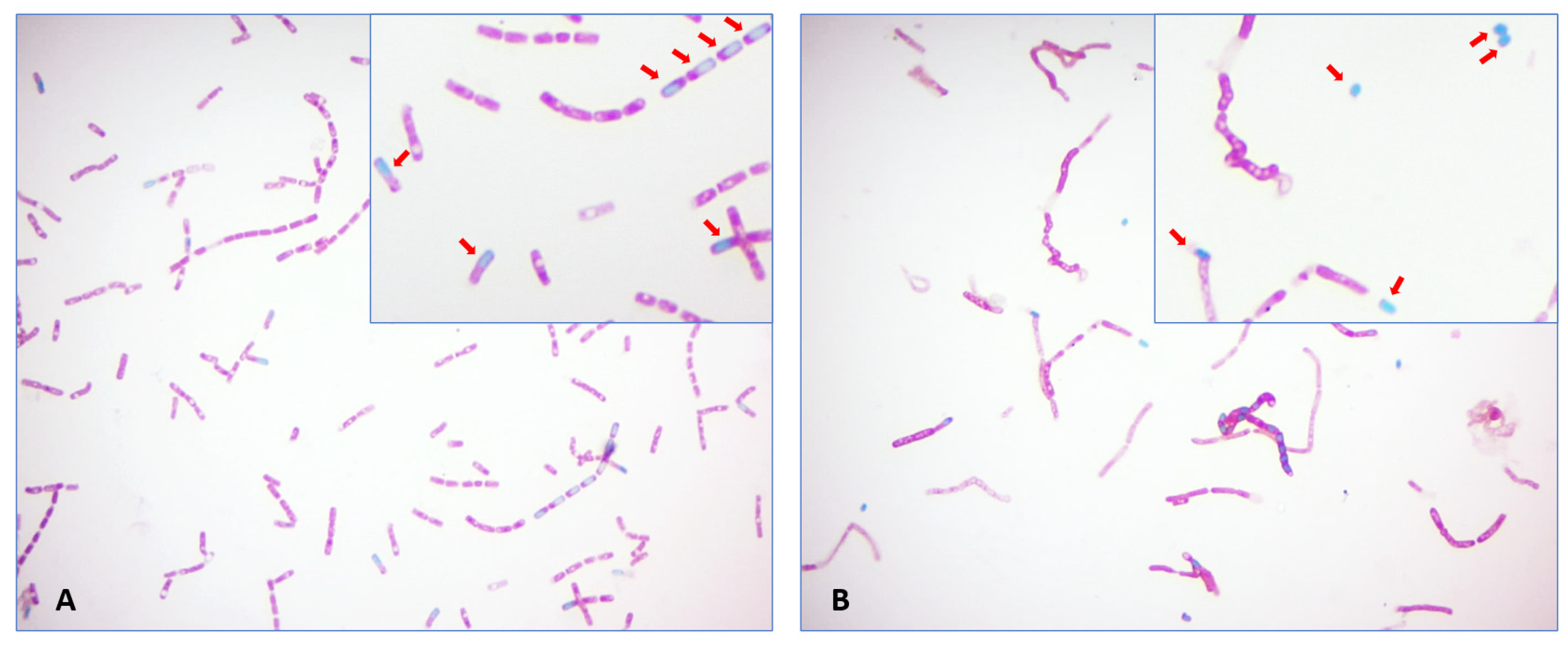
Endospore staining involved transferring a 20 μL drop of the initial turbidity-adjusted suspension to a clean glass slide, followed by air-drying and heat-fixing. Steam was applied for 5 min to drive the malachite green stain into the spore wall. Safranin counterstain was applied to each slide for 2 min. Spores were counted in 20 consecutive oil immersion fields using an Eclipse E200 brightfield microscope (Nikon Group) equipped with a 100× objective and 10× ocular eyepiece for a total magnification of 1000× (
Figure 4).
2.10. Water Contact Angles (WCAs)
The WCAs of both thermoplastic sheets were measured by optical tensiometry using the sessile drop technique using a Theta Lite tl100 optical tensiometer (Biolin Scientific, Paramus, NJ, USA). Here, a water drop is applied to the test material surface, and its degree of spreading is measured. If the measured angle (theta) between the waterdrop and the test material is >90°, the material is considered hydrophobic. In contrast, the material is considered hydrophilic if the angle is <90°.
4. Discussion
B. cereus is considered an emerging HAI pathogen that produces tissue-destroying exotoxins like hemolysins, phospholipases, and proteases [
8,
9,
10]. Both
B. cereus and
B. megaterium are associated with severe orthopedic wounds and burn infections [
11,
12,
13,
14]. What makes the control of
Bacillus so problematic is its ability to form dormant spores that can survive on contaminated surfaces for extended periods of time.
Bacillus is a widely distributed environmental bacterium that gains easy access to medical treatment facilities by various routes, including medical staff [
15]. For example, sampling of 93 of 100 mobile phones present in an orthopedic operating room recovered coagulase-negative
Staphylococcus (62%),
Micrococcus (41%), and
Bacillus (26%) [
16].
Bacillus has also been recovered from healthcare workers’ hands. The use of an alcohol-based disinfectant proved insufficient to eliminate its spores [
17].
4.1. Spores
Spore-forming bacteria belong to the genera
Clostridium and
Bacillus. Enterotoxin-producing
Clostridioides difficile, formerly
Clostridium difficile (or
C. diff.), is a frequently encountered HAI pathogen and a significant health threat [
18]. Like
C. diff.,
B. cereus also generates enterotoxins responsible for severe diarrhea [
19]. In general, spores are resistant to antibiotics and specifically resistant to disinfectants routinely used to clean patient treatment surfaces like exam tables and countertops [
20].
Spores are also very durable at high temperatures [
15,
19]. In this regard, heat-resistant
Bacillus spores are used to determine if a steam autoclave reached the required sterilization temperature of 121 °C (250 °F). Previous studies recovered
Bacillus from two heating appliances (e.g., water baths) used to heat immobilization masks. Heating devices were set to operating temperatures between 68 and 98 °C (~154–208 °F), with a mean temperature of 77.1 °C (~171 °F). Resampling of the contaminated devices after thorough cleaning did not eliminate
Bacillus spores [
21].
4.2. Medical Biofilms
Medical biofilms can contain a single microbe or be composed of several different microbes embedded in extracellular polymeric substances (EPSs). The EPS is typically composed of “polysaccharides, proteins, lipids, enzymes, extracellular-DNA (eDNA), and water” [
22] (p. 1). Only actively growing or vegetative
Bacillus cells produce EPSs.
B. subtilis is a strong EPS producer. In the current study, the mucoid
B. subtilis colonies seen on 96 h cultures indicated a considerable level of EPS expression [
23]. A large amount of
B. subtilis EPS material was seen as a red-staining stranded material in both Gram and spore stains. Large numbers of spores are grouped together in these strands. This resulted in an unequal distribution in each examined field. Thus, it prevented an accurate determination of both
B. subtilis spores and recovered bacterial numbers from the 1 h sampling interval.
Medical biofilms are associated with both increased antibiotic resistance and reduced susceptibility to disinfectant susceptibility urinary catheter infections, pneumonia, and medical devices [
24]. Medical biofilms involving either
B. cereus or
B. licheniformis have been reported in intravenous catheters, central venous access devices, and prosthetic aortic valve endocarditis [
25,
26,
27].
B. subtills has not been likewise implicated in medical biofilms.
B. cereus biofilms are widely documented in foodborne illnesses from the ingestion of spores. One source involves commercial food preparation facilities.
B. cereus spores readily attach to polymers (e.g., film wraps) used during food processing and stainless-steel food preparation surfaces [
28]. A search of the relevant literature using the PubMed search engine did not reveal the incidence of
B. cereus biofilm formation on thermoplastics used to construct externally applied medical devices like orthoses.
Environmental strains of
B. cereus (MK517567) have been shown to biodegrade polyethylene and polyester, but they require 90 days before there is any noticeable degradation [
29]. Polycaprolactone (PCL) thermoplastic is a polyester used to construct the Klarity material (personal communication). The base material of the NC Spectrum Beige is not known but is suspected to also be PCL due to its wide use. The biodegradation of PCL would not be an issue for a study of 60 days’ duration. In the case of radiation therapy masks, they are typically worn by patients for treatments given over 4–6 weeks. Moreover, it is highly improbable that
B. cereus (MAB03F) is related to the
B. cereus (MK517567) strain.
4.3. Attraction to Thermoplastics
Dormant
Bacillus spores are more hydrophobic than their vegetative counterparts. The increased hydrophobicity of bacterial spores has been attributed to protein in the outer coats and its exosporium when compared with peptidoglycan on actively growing vegetative cell surfaces (
Figure 8). “The hydrophobic nature of
Bacillus spores suggests that hydrophobic interactions may play a role in the adhesion of these spores to surfaces” [
30] (p. 2603). Spores suspended in aqueous solutions are strongly attracted to hydrophobic thermoplastic surfaces like PCL [
31].
Adherence is the property governing this initial attraction between bacterial and thermoplastic surfaces. In contrast, adhesion is the tendency of contacting surfaces to hold on to one another as if they are kept in place by an adhesive. It determines how strongly attached bacteria and/or spores remain associated with thermoplastic surfaces over time. The results indicated that differences in the WCA of either material did not affect the number of B. cereus or B. megaterium adhering to either material after 1 h of contact.
However, a trend in decreasing spore recovery from the more hydrophobic Klarity material was noted over time. This indicated that spores of both
Bacillus spp. exhibited greater adhesiveness to this material than the more hydrophilic NC Spectrum Beige (
Figure 6), showing an opposite trend. Here, increased spore recovery over time from the NC Spectrum Beige material indicated the decreased adhesiveness of either
Bacillus spores. It is speculated that the additional anti-stick coating contributed to the decreased NC Spectrum Beige surface hydrophobicity, as indicated by a WCA of 83.1°. A similar coating was absent from the more hydrophobic Klarity thermoplastic sheet with a higher WCA of 98.3°. The decreased adhesiveness of
Bacillus spores to the NC Spectrum Beige hydrophilic surface implies a greater probability of spore transfer to a patient wearing a contaminated device. However, this speculation requires further study using additional thermoplastic materials.
Microscopic surface topography may have also played a role in these trends. Scanning electron microscopy (SEM) is needed to assess the comparative surface features of each different orthotic material. Examination by SEM may reveal substantial differences in surface topography between Klarity-uncoated and NC Spectrum-coated thermoplastic materials. The presence of numerous microscopic spaces may offer
Bacillus spores abundant “hiding” places [
31]. If present, spores would be protected from mechanical removal by wiping the splint.
4.4. Cleaning Orthoses
A survey of three occupational therapy outpatient treatment facilities yielded differences in patient cleaning/disinfection instructions (
Table 1). Preferred cleaning and/or disinfection methods are specified by manufacturers in instructions for use (IFU) information sheets packaged with their product. This ensures that the disinfectant does not degrade or alter the intrinsic properties of thermoplastic. The Klarity ThermoSheets IFU indicated that cleaning and disinfecting should be conducted using soapy water or an isopropanol-based disinfectant applied with a soft cloth. It further stated, “do not use aerosol sprays, corrosive cleaning agents, solvents, or abrasive detergents” [
32]. North Coast Spectrum Beige IFU specifies to “Clean with mild soap and lukewarm water. However, do not immerse in water greater than 120 °F (49 °C)” [
33]. Neither method would be sufficient for eliminating heat- and chemical-resistant
Bacillus spores [
19].
4.5. Commercial Products
Disposable wipes containing from 1% to 4% hydrogen peroxide (H
2O
2) are available from several manufacturers. Clorox Healthcare Hydrogen Peroxide Disinfectant Wipes contain 1.4% H
2O
2 (Clorox Healthcare Oakland, CA), while Pharma-C-Wipes contain 3% H
2O
2 (Kleen Test Products, Port Washington, WI). The PDI Healthcare Sani-HyPerCide Germicidal Disposable Wipe (PDI Healthcare Brooklyn, NY) has a higher concentration of 4.04% H
2O
2 [
34]. The PDI Technical Bulletin claims that their product is effective against
C. diff spores with an exposure time of 5 min at 73.4°–75.2 °F. Product assessment was performed using a modified ASTM E 2197-02 test [
35]. The PDI literature indicated that these conditions were sufficient to achieve a minimum reduction of 6 Log
10 in viable
C. diff. spore numbers. ASTM E2197 testing is performed using hard-surface stainless-steel discs. The suitability and/or effectiveness of PDI Sani-HyPerCide 4.04% H
2O
2 wipes to achieve a similar 6 Log
10 reduction of either
C. diff or
Bacillus spores on thermoplastics cannot be predicted.
4.6. Disinfection Recommendation
Reports of
Bacillus being recovered from surgical sites after orthopedic surgery and from burn patients suggest that caution should be exercised in preventing infections. Using a disinfectant wipe containing only 70% isopropyl alcohol to clean patient orthoses will not eliminate
Bacillus spores [
17]. The demonstrated presence of additional HAI pathogens like
S. aureus,
Enterococcus species, and
Pseudomonas aeruginosa only serves to increase this concern.
Providing outpatients with a container of wipes like PDI Sani-HyPerCide to clean their orthoses may appear to be an appropriate measure. However, a wipe containing only H
20
2 may not eliminate
S. aureus and
Enterococcus spp. bacteria producing high levels of catalase [
36]. Additionally, the PDI wipe has only been shown to reduce
C. diff spores and not
Bacillus spores. It is explicitly stated that the authors are not in any way endorsing the use of PDI Sani-HyPerCide wipes. They are only mentioned as an example of a H
2O
2 wipe. From a practical consideration, wipes containing 3% H
2O
2 are readily available from several retail sources. This makes it a more practical alternative for outpatients to use in routinely disinfecting their orthotic splint. However, the disinfection of orthoses worn by burn patients should continue to follow local Infection Control guidelines.
Until studies are performed revealing the effectiveness of different solutions, we recommend a two-step cleaning process for outpatients to use in cleaning/disinfecting their splints. This method involves using disposable wipes readily available from most retailers. The first step involves wiping the skin contact area with a 3–4% H
20
2 saturated wipe to reduce and/or eliminate attached bacterial spores and letting it air dry to extend the contact time. The second step is to use a 70% alcohol wipe to kill catalase-producing bacteria and remove any residual H
2O
2. Individual thermoplastic manufacturers should be consulted to ensure that treatment with 3–4% hydrogen peroxide will not alter the material integrity or negatively impact an anti-stick coating. We also support the suggestion by Wright et al. that orthoses be cleaned each time they are removed [
2]. Finally, we also recommend that water baths used to heat thermoplastic sheets be regularly drained and disinfected to eliminate stubborn
Bacillus spores.
4.7. Study Limitations
The current investigation represents an initial look at associations created and maintained between Bacillus bacteria and its spores with thermoplastic material used to fashion patient orthoses. It is limited in its scope for two reasons. First, only 3 of 7 mask-recovered Bacillus spp. were selected for testing. Second, only two thermoplastic sheets were used when several others were also available. Accordingly, our results should only be interpreted under the stated conditions. However, the choice of B. cereus and B. megaterium for this initial evaluation was considered prudent since both bacteria have been associated with post-surgical infections. Although B. subtilis testing could not be similarly completed, it has not been associated with wound infections. Lastly, further studies are needed to establish the resistance of Bacillus spores derived from each mask isolate to commonly used commercial disinfectants like 70% isopropyl alcohol.
4.8. Future Directions
The recovery of additional HAI pathogens like
S. aureus and
Enterococcus spp. from patient radiation therapy masks represents a similar concern for their contamination of orthotic thermoplastics. In this respect,
S. aureus has already been recovered from patient orthotic forms by Faoagali et al. [
4]. Accordingly, a similar study to this one is planned to examine the associations of three different antibiotic-resistant HAI pathogens with additional coated and uncoated thermoplastic sheets. Test bacteria include methicillin-resistant
Staphylococcus aureus (MRSA), vancomycin-resistant
Enterococcus faecalis (VRE), and extended-spectrum beta-lactamase (ESBL)
Escherichia coli. Moreover, the authors intend to work together with a thermoplastic manufacturer to develop a modified ASTM E2197 testing method that uses thermoplastic material in lieu of stainless-steel discs. This would allow for the direct testing of different antimicrobial solutions in determining which one provides optimal pathogen elimination, including dormant spores, but does not alter the inherent properties of thermoplastics that make it useful in the construction of patient medical devices.