Bacterial Sialidases: Biological Significance and Application
Abstract
1. Introduction
2. Structural Properties, Catalytic Mechanism and Substrate Specificity

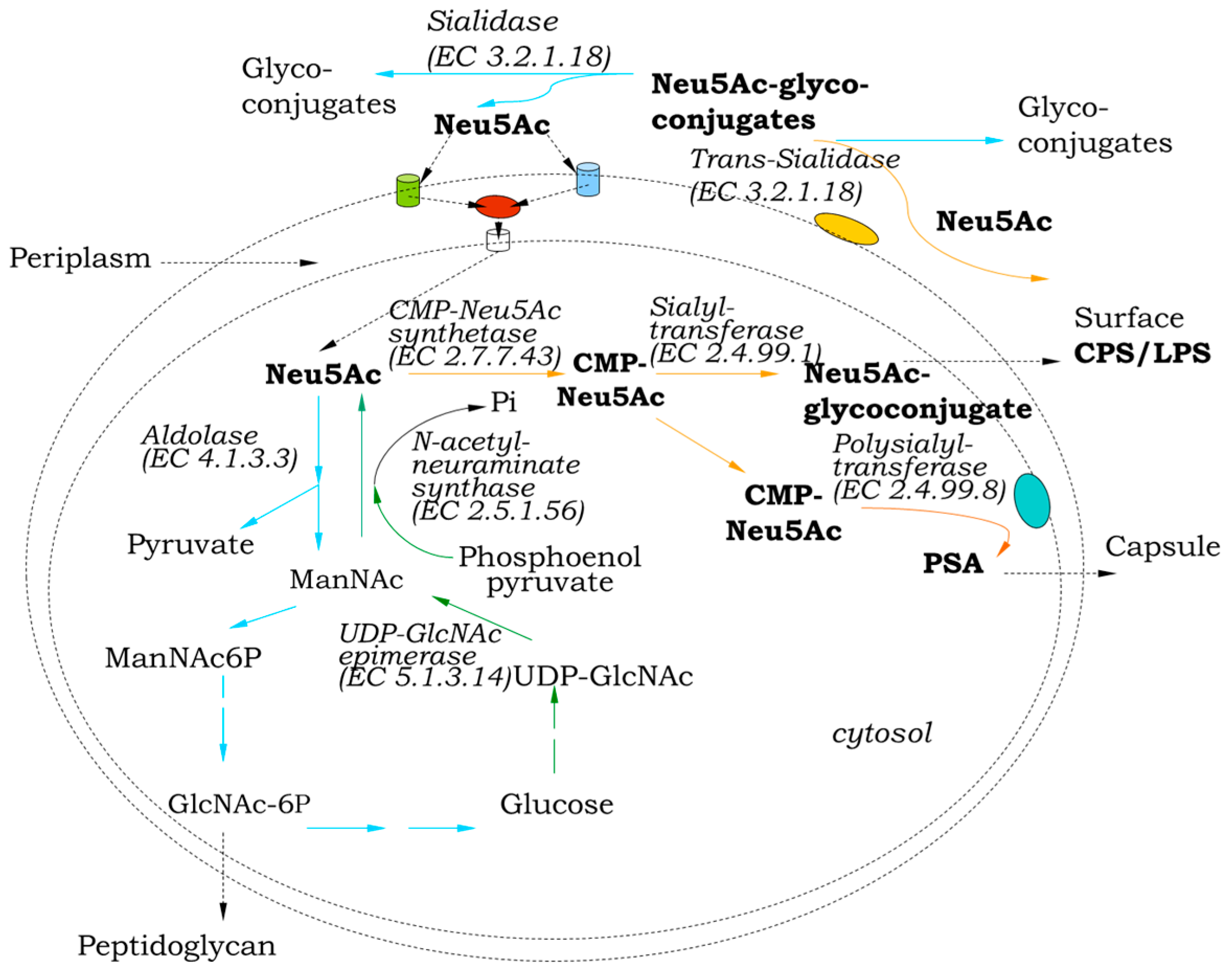
3. Role in Sialo Metabolism
4. Influence of External Factors on Sialidase Synthesis
5. Role in Pathogenicity
5.1. Pathogen Penetration and Adhesion
5.2. Disclosure of Receptors
5.3. Biofilm Formation
5.4. Modulation of Immune Mechanisms
5.5. Synergism Between Bacterial and Viral Sialidases
5.6. Sialidases as Targets for Inhibition
6. Sialidase Production in Saprophytes
7. Application of Bacterial Sialidases
7.1. In Medicine
7.1.1. In Tumor Immunotherapy
7.1.2. The Potential of Bacterial Sialidases as Antiviral Agents and Vaccines
7.1.3. As Diagnostic Preparations
7.1.4. Others
7.2. In Enzymatic Synthesis of Sialylated Glycans
7.3. As a Tool for Structural Analysis
7.4. As a Tool for Bioconversion of Polysialogangliosides to GM1
8. Future Perspectives
| Producer | References |
|---|---|
| |
| Clostridium perfringens | [3,6,11,94,111,112] |
| Clostridium chauvoei | [13,39] |
| Streptococcus pneumoniae | [3,26,51,53,57] |
| Vibrio cholerae | [2,3,6,14,34,47,48,61,77,78,89,115,123] |
| Pseudomonas aeruginosa | [3,49,50,79] |
| Corynebacterium diphtheriae | [17] |
| Pasteurella multocida | [14,103] |
| Erysipelothrix rhusiopathiae | [3,43] |
| Mycoplasma gallisepticum, M. synoviae | [38] |
| Haemophilus parasuis | [35] |
| Propionibacterium acnes | [126] |
| Prevotella timonensis | [68,76] |
| Gadnerella vaginalis | [26,75] |
| Porphyromonas gingivalis | [26,54,71] |
| Edwardsiella tarda | [107] |
| |
| Bacteroides fragilis | [3,6] |
| Bacteroidetes thetaiotaomicron | [6,70,72] |
| Bifidobacterium bifidum | [6,25] |
| Bifidobacterium infantis | [5] |
| Actinomyces viscosus | [3] |
| |
| Micromonospora viridifaciens | [8,16,83] |
| Arthrobacter ureafaciens | [3,5,63,85] |
| Arthrobacter sialophilus | [84] |
| Arthrobacter nicotianae | [77,78,86] |
| Oerskovia paurometabola | [22,75,77,78] |
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Traving, C.; Schauer, R. Structure, function and metabolism of sialic acids. Cell. Mol. Life Sci. 1998, 54, 1330–1349. [Google Scholar] [CrossRef]
- Vimr, E.; Kalivoda, K.; Deszo, E.; Steenbergen, S. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 2004, 68, 132–153. [Google Scholar] [CrossRef]
- Schwerdtfeger, S.; Melzig, M. Sialidases in biological systems. Pharmazie 2010, 65, 551–561. [Google Scholar] [CrossRef]
- Eneva, R.; Engibarov, S.; Abrashev, R.; Krumova, E.; Angelova, M. Sialic acids, sialoconjugates and enzymes of their metabolism in fungi. Biotechnol. Biotechnol. Equip. 2021, 35, 346–357. [Google Scholar] [CrossRef]
- Muñoz-Provencio, D.; Yebra, M. Gut microbial sialidases and their role in the metabolism of human milk sialylated glycans. Int. J. Mol. Sci. 2023, 24, 9994. [Google Scholar] [CrossRef]
- Juge, N.; Tailford, L.; Owen, C. Sialidases from gut bacteria: A mini-review. Biochem. Soc. Trans. 2016, 44, 166–175. [Google Scholar] [CrossRef]
- Kim, S.; Oh, D.; Kang, H.; Kwon, O. Features and applications of bacterial sialidases. Appl. Microbiol. Biotechnol. 2011, 91, 1–15. [Google Scholar] [CrossRef]
- Gaskell, A.; Crennell, S.; Taylor, G. The three domains of a bacterial sialidase: A beta propeller, an immunoglobulin module and galactose-binding jelly-roll. Structure 1995, 3, 1197–1205. [Google Scholar] [CrossRef]
- Quistgaard, E.; Thirup, S. Sequence and structural analysis of the Asp-box motif and Asp-box beta-propellers; a widespread propeller-type characteristic of the Vps10 domain family and several glycoside hydrolase families. BMC Struct. Biol. 2009, 9, 46. [Google Scholar] [CrossRef]
- Van Dijk, A.; Cyplenkova, N.; Dekker, P.; Efimova, Y. Novel Sialidases. Patent Application Number 20100167344, 1 July 2010.
- Newstead, S.; Potter, J.; Wilson, J.; Xu, G.; Chien, C.; Watts, A.; Withers, S.; Taylor, G. The structure of Clostridium perfringens NanI sialidase and its catalytic intermediates. J. Biol. Chem. 2008, 283, 9080–9088. [Google Scholar] [CrossRef]
- Keil, J.; Rafn, G.; Turan, I.; Aljohani, M.; Sahebjam-Atabaki, R.; Sun, X. Sialidase Inhibitors with Different Mechanisms. J. Med. Chem. 2022, 65, 13574–13593. [Google Scholar] [CrossRef]
- Vilei, E.; Johansson, A.; Schlatter, Y.; Redhead, K.; Frey, J. Genetic and functional characterization of the NanA sialidase from Clostridium chauvoei. Vet. Res. 2011, 42, 1–9. [Google Scholar] [CrossRef]
- Eneva, R.; Engibarov, S.; Petrova, P.; Abrashev, R.; Strateva, T.; Kolyovska, V.; Abrashev, I. High production of neuraminidase by a Vibrio cholerae non-O1 strain—The first possible alternative to toxigenic producers. Appl. Biochem. Biotechnol. 2015, 176, 412–427. [Google Scholar] [CrossRef]
- Mochalova, L.; Korchagina, E.; Kurova, V.; Shtyria, I.; Gambaryan, A.; Bovin, N. Fluorescent assay for studying the substrate specificity of neuraminidase. Anal. Biochem. 2005, 341, 190–193. [Google Scholar] [CrossRef]
- Sakurada, K.; Ohta, T.; Hasegawa, M. Cloning, expression, and characterization of the Micromonospora viridifaciens neuraminidase gene in Streptomyces lividans. J. Bacteriol. 1992, 174, 6896–6903. [Google Scholar] [CrossRef]
- Kim, S.; Oh, D.; Kwon, O.; Kang, H. Identification and functional characterization of the NanH extracellular sialidase from Corynebacterium diphtheriae. J. Biochem. 2010, 147, 523–533. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ugai, H.; Nakayama-Imaohji, H.; Tada, A.; Elahi, M.; Houchi, H.; Kuwahara, T. Characterization of a recombinant Bacteroides fragilis sialidase expressed in Escherichia coli. Anaerobe 2018, 50, 69–75. [Google Scholar] [CrossRef]
- Corfield, T. Bacterial sialidases: Roles in pathogenecty and nutrition. Glycobiology 1992, 2, 509–521. [Google Scholar] [CrossRef]
- Almagro-Moreno, S.; Boyd, E. Insights into the evolution of sialic acid catabolism among bacteria. BMC Evol. Biol. 2009, 26, 118. [Google Scholar] [CrossRef]
- Vimr, E. Unified Theory of Bacterial Sialometabolism: How and Why Bacteria Metabolize Host Sialic Acids. Int. Sch. Res. Not. 2013, 2013, 816713. [Google Scholar] [CrossRef]
- Chen, X.; Varki, A. Advances in the Biology and Chemistry of Sialic Acids. ACS Chem. Biol. 2010, 5, 163–176. [Google Scholar] [CrossRef]
- Todeschini, A.; Mendonça-Previato, L.; Previato, J.; Varki, A.; Halbeek, H. Trans-sialidase from Trypanosoma cruzi catalyzes sialoside hydrolysis with retention of configuration. Glycobiology 2000, 10, 213–221. [Google Scholar] [CrossRef]
- Severi, E.; Hood, D.; Thomas, G. Sialic acid utilization by bacterial pathogens. Microbiology 2007, 153, 2817–2822. [Google Scholar] [CrossRef]
- Yokoi, T.; Nishiyama, K.; Kushida, Y.; Uribayashi, K.; Kunihara, T.; Fujimoto, R.; Yamamoto, Y.; Ito, M.; Miki, T.; Haneda, T.; et al. O-acetylesterase activity of Bifidobacterium bifidum sialidase facilities the liberation of sialic acid and encourages the proliferation of sialic acid scavenging Bifidobacterium breve. Environ. Microbiol. Rep. 2022, 14, 637–645. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Xiao, B. The role of sialidases in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis. Front. Cell Infec. Microbiol. 2024, 14, 1367233. [Google Scholar] [CrossRef]
- Gualdi, L.; Hayre, J.; Gerlini, A.; Bidossi, A.; Colomba, L.; Trappetti, C.; Pozzi, G.; Docquier, J.; Andrew, P.; Ricci, S.; et al. Regulation of neuraminidase expression in Streptococcus pneumoniae. BMC Microbiol. 2012, 12, 200. [Google Scholar] [CrossRef]
- Eneva, R.; Engibarov, S.; Gocheva, Y.; Mitova, S.; Arsov, A.; Petrov, K.; Abrashev, R.; Lazarkevich, I.; Petrova, P. Safe sialidase production by the saprophyte Oerskovia paurometabola: Gene sequence and enzyme purification. Molecules 2022, 27, 8922. [Google Scholar] [CrossRef]
- Li, J.; Evans, D.; Freedman, J.; McClane, B.A. NanR regulates nanI sialidase expression by Clostridium perfringens F4969, a human enteropathogenic strain. Infect. Immunn. 2017, 85, e00241-17. [Google Scholar] [CrossRef]
- Hoyer, L.; Roggentin, P.; Schauer, R.; Vimr, E. Purification and properties cloned Salmonela typhimurium LT-2 sialidase with virus-typical kinetic preference for sialyl alpha 2–3 linkages. J. Biochem. 1991, 110, 462–467. [Google Scholar] [CrossRef]
- Bateman, R.; Sharpe, M.; Singer, M.; Ellis, C. The effect of sepsis on the erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef]
- Iijima, R.; Takahashi, H.; Namme, R.; Ikegami, S.; Yamazaki, M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004, 561, 163–166. [Google Scholar] [CrossRef]
- Cacalano, G.; Kays, M.; Saiman, L.; Prince, A. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J. Clin. Investig. 1992, 89, 1866–1874. [Google Scholar] [CrossRef]
- Almagro-Moreno, S.; Boyd, E. Sialic acid catabolism confers a competitive advantage to pathogenic Vibrio cholerae in the mouse intestine. Infect. Immun. 2009, 77, 3807–3816. [Google Scholar] [CrossRef]
- Lichtensteiger, C.; Vimr, E. Neuraminidase (sialidase) activity of Haemophilus parasuis. FEMS Microbiol. Lett. 1997, 152, 269–274. [Google Scholar] [CrossRef]
- Sudhakara, P.; Sellamuthu, I.; Aruni, A. Bacterial sialoglycosidases in virulence and pathogenesis. Pathogens 2019, 8, 39. [Google Scholar] [CrossRef]
- Abrashev, I.; Dulgerova, G. Neuraminidases (sialidases) from bacterial origin. Exp. Pathol. Parasitol. 2000, 4, 35–40. [Google Scholar]
- Berčič, R.; Cizelj, I.; Dušanić, D.; Narat, M.; Zorman-Rojs, O.; Dovč, P.; Benčina, D. Neuraminidase of Mycoplasma synoviae desialylates heavy chain of the chicken immunoglobulin G and glycoproteins of chicken tracheal mucus. Avian Pathol. 2011, 40, 299–308. [Google Scholar] [CrossRef]
- Useh, N.; Arimie, D.; Balogun, E.; Ibrahim, N.; Nok, A.; Esievo, K. Desialylation modulates alkaline phosphatase activity in zebu cattle experimentally infected with Clostridium chauvoei: A novel report. Jord. J. Bio Sci. 2012, 5, 265–268. [Google Scholar]
- Kastelic, S.; Bercic, R.L.; Cizelj, I.; Bencina, M.; Makrai, L.; Zorman-Rojs, O.; Narat, M.; Bisgaard, M.; Christiansen, H.; Bencina, D. Ornithobacterium rhinotracheale has neuraminidase activity causing desialylation of chicken and turkey serum and tracheal mucus glycoproteins. Vet. Microbiol. 2013, 162, 707–712. [Google Scholar] [CrossRef]
- Wong, A.; Grau, M.; Singh, A.; Woodiga, S.; King, S. Role of neuraminidase producing bacteria in exposing cryptic carbohydrate receptors for Streptococcus gordonii adherence. Infect. Immun. 2018, 86, e00068-18. [Google Scholar] [CrossRef]
- Hussain, M.; Hassan, M.; Shaik, N.; Iqbal, Z. The role of galactose in human health and disease. Cent. Eur. J. Med. 2012, 7, 409–419. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, B.; Riley, T. Erysipelothrix rhusiopathiae. Vet. Microbiol. 2010, 140, 405–417. [Google Scholar] [CrossRef]
- Jost, B.; Songer, J.; Billington, S. Identification of a second Arcanobacterium pyogenes neuraminidase and involvement of neuraminidase activity in host cell adhesion. Infect. Immun. 2002, 70, 1106–1112. [Google Scholar] [CrossRef]
- Schauer, R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009, 19, 507–514. [Google Scholar] [CrossRef]
- Grewal, P.; Aziz, P.; Uchiyama, S.; Rubio, G.; Lardone, R.; Le, D.; Varki, N.; Nizet, V.; Marth, J. Inducing host protection in pneumococcal sepsis by preactivation of the Ashwell-Morell receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 20218–20223. [Google Scholar] [CrossRef]
- Galen, J.; Ketley, J.; Fasano, A.; Richardson, S.; Wasserman, S.; Kaper, J. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect. Immun. 1992, 60, 406–415. [Google Scholar] [CrossRef]
- Moustafa, I.; Connaris, H.; Taylor, M.; Zaitsev, V.; Wilson, J.; Kiefel, M.; von Itzstein, M.; Tailor, G. Sialic acid recognition by Vibrio cholerae neuraminidase. J. Biol. Chem. 2004, 279, 40819–40826. [Google Scholar] [CrossRef]
- Soong, G.; Muir, A.; Gomez, M.I.; Waks, J.; Reddy, B.; Planet, P.; Singh, P.K.; Kanetko, Y.; Wolfgang, M.C.; Hsiao, Y.S.; et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Investig. 2006, 116, 2297–2305. [Google Scholar] [CrossRef]
- Xu, G.; Ryan, C.; Kiefel, M.; Wilson, J.; Taylor, G. Structural studies on the Pseudomonas aeruginosa sialidase-like enzyme PA2794 suggest substrate and mechanistic variations. J. Mol. Biol. 2009, 386, 828–840. [Google Scholar] [CrossRef]
- Parker, D.; Soong, G.; Planet, P.; Brower, J.; Ratner, A.J.; Prince, A. The NanA Neuraminidase of Streptococcus pneumoniae is involved in biofilm formation. Infect. Immun. 2009, 77, 3722. [Google Scholar] [CrossRef]
- Wren, J.; Blevins, L.; Pang, B.; Roy, A.; Oliver, M.; Reimche, J.; Wozniak, J.; Alexander-Miller, M.; Swords, W. Pneumococcal neuraminidase A (NanA) promotes biofilm formation and synergizes with influenza A virus in nasal colonization and middle ear infection (e01044-16). Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Blanchette, K.; Shenoy, A.; Milner, J., 2nd; Gilley, R.; McClure, E.; Hinojosa, C.; Kumar, N.; Daugherty, S.; Tallon, L.; Ott, S.; et al. Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect. Immun. 2016, 84, 2922–2932. [Google Scholar] [CrossRef]
- Li, C.; Kurniyati, K.; Hu, B.; Bian, J.; Sun, J.; Zhang, W.; Liu, J.; Pan, Y.; Li, C. Abrogation of neuraminidase reduces biofilm formation, capsule biosynthesis, and virulence of Porphyromonas gingivalis. Infect. Immun. 2012, 80, 3–13. [Google Scholar] [CrossRef]
- Reinholdt, J.; Tomana, M.; Mortensen, S.; Kilian, M. Molecular aspects of immunoglobulin-A1 degradation by oral streptococci. Infect. Immun. 1990, 58, 1186–1194. [Google Scholar] [CrossRef]
- Lewis, W.; Robinson, L.; Perry, J.; Bick, J.; Peipert, J.; Allsworth, J.; Lewis, A. Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J. Biol. Chem. 2012, 287, 2079–2089. [Google Scholar] [CrossRef]
- Dalia, A.; Standish, A.; Weiser, J. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH promote resistance to opsophagocytic killing by human neutrophils. Infect. Immun. 2010, 78, 2108–2116. [Google Scholar] [CrossRef]
- Mally, M.; Shin, H.; Paroz, C.; Landmann, R.; Cornelis, G. Capnocytophaga canimorsus: A human pathogen feeding at the surface of epithelial cells and phagocytes. PLoS Pathog. 2008, 4, e1000164. [Google Scholar] [CrossRef]
- Carlson, C.; Turpin, E.; Moser, L.; O’Brien, K.; Cline, T.; Jones, J.; Tumpey, T.; Katz, J.; Kelley, L.; Gauldie, J.; et al. Transforming growth factor-β: Activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 2010, 6, e1001136. [Google Scholar] [CrossRef]
- Karhadkar, T.; Meek, T.; Gomer, R. Inhibiting Sialidase-Induced TGF- β1 Activation Attenuates Pulmonary Fibrosis in Mice. J. Pharmacol. Exp. Ther. 2021, 376, 106–117. [Google Scholar] [CrossRef]
- Knop, J.; Ax, W.; Sedlacec, H.; Seiler, F. Effect of Vibrio cholerae neuraminidase on the phagocytosis of E. coli by macrophages in vivo and in vitro. Immunology 1978, 34, 555–563. [Google Scholar]
- Sun, Y.; Luxardi, G.; Xu, G.; Zhu, K.; Reid, B.; Guo, B.; Lebrilla, C.; Maverakis, E.; Zhao, M. Surface Glycans Regulate Salmonella Infection-Dependent Directional Switch in Macrophage Galvanotaxis Independent of NanH. Infect. Immun. 2022, 90, e00516-21. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Barbeau, B.; Sato, S.; Tremblay, M. Neuraminidase from bacterial source enhances both HIV-1-mediated syncytium formation and the virus binding/entry process. Virology 2001, 284, 26–36. [Google Scholar] [CrossRef]
- Schenkman, S.; Jiang, M.; Hart, G.; Nussenzweig, V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell 1991, 65, 1117–1125. [Google Scholar] [CrossRef]
- Nishikawa, T.; Shimizu, K.; Tanaka, T.; Kuroda, K.; Takayama, T.; Yamamoto, T.; Hanada, N.; Hamada, Y. Bacterial neuraminidase rescues influenza virus replication from inhibition by a neuraminidase inhibitor. PLoS ONE 2012, 7, e45371. [Google Scholar] [CrossRef]
- McCullers, J.; Rehg, J. Lethal synergism between influenza virus and Streptococcus pneumoniae: Characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef]
- Peltola, V.; McCullers, J. Respiratory viruses predisposing to bacterial infections: Role of neuraminidase. J. Pediatr. Infect. Dis. 2004, 23, S87–S97. [Google Scholar] [CrossRef]
- Segui-Perez, C.; de Jongh, R.; Jonkergouw, R.; Pelayo, P.; Balskus, E.; Zomer, A.; Strijbis, K. Prevotella timonensis degrades the vaginal epithelial glycocalyx through high fucosidase and sialidase activities. MBio 2024, 15, e00691-24. [Google Scholar] [CrossRef]
- Huang, Y.; Chassard, C.; Hausmann, M.; Von Itzstein, M.; Hennet, T. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat. Commun. 2015, 6, 8141. [Google Scholar] [CrossRef]
- Park, K.; Kim, M.; Ahn, H.; Lee, D.; Kim, J.; Kim, Y.; Woo, E. Structural and biochemical characterization of the broad substrate specificity of Bacteroides thetaiotaomicron commensal sialidase. Biochim. Biophys. Acta. 2013, 34, 1510–1519. [Google Scholar] [CrossRef]
- Dong, W.; Jiang, Y.; Zhu, Z.; Zhu, J.; Li, Y.; Xia, R.; Zhou, K. Structural and enzymatic characterization of the sialidase SiaPG from Porphyromonas gingivalis. Acta Crystallog. F Struct. Biol. Commun. 2023, 79, 87–94. [Google Scholar] [CrossRef]
- Assailly, C.; Bridot, C.; Saumonneau, A.; Lottin, P.; Roubinet, B.; Krammer, E.; François, F.; Vena, F.; Landemarre, L.; Alvarez Dorta, D.; et al. Polyvalent Transition-State Analogues of Sialyl Substrates Strongly Inhibit Bacterial Sialidases. Chem. Eur. J. 2021, 27, 3142–3150. [Google Scholar] [CrossRef]
- Glanz, V.; Myasoedova, V.; Grechko, A.; Orekhov, A. Inhibition of sialidase activity as a therapeutic approach. Drug Des. Devel. Ther. 2018, 12, 3431–3437. [Google Scholar] [CrossRef] [PubMed]
- Walther, E.; Xu, Z.; Richter, M.; Kirchmair, J.; Grienke, U.; Rollinger, J.M.; Krumbholz, A.; Saluz, H.P.; Pfister, W.; Saurbrei, A.; et al. Dual acting neuraminidase inhibitors open new opportunities to disrupt the lethal synergism between Streptococcus pneumoniae and influenza virus. Front. Microbiol. 2016, 7, 357. [Google Scholar] [CrossRef]
- Govinden, G.; Parker, J.; Naylor, K.; Frey, A.; Anumba, D.; Stafford, G. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch. Microbiol. 2018, 200, 1129–1133. [Google Scholar] [CrossRef]
- Pelayo, P.; Hussain, F.; Werlang, C.; Wu, C.; Woolston, B.; Xiang, C.; Rutt, L.; France, M.; Ravel, J.; Ribbeck, K.; et al. Prevotella are major contributors of sialidases in the human vaginal microbiome. Proc. Natl. Acad. Sci. USA 2024, 121, e2400341121. [Google Scholar] [CrossRef]
- Gocheva, Y.; Nikolova, M.; Engıbarov, S.; Lazarkevich, I.; Eneva, R. Effective inhibition of bacterial sialidases by phenolic acids and flavonoids. Int. J. Second. Metab. 2024, 11, 514–521. [Google Scholar] [CrossRef]
- Gocheva, Y.; Nikolova, M.; Engıbarov, S.; Lazarkevich, I.; Mitova, S.; Eneva, R. Study of Bulgarian Plant Extracts Effect on Three Bacterial Sialidases. Acta Microbiol. Bulg. 2024, 40, 236–241. [Google Scholar] [CrossRef]
- Shehab, Z.; Al-Rubaii, B. Effect of D-mannose on gene expression of neuraminidase produced from different clinical isolates of Pseudomonas aeruginosa. Baghdad Sci. J. 2019, 16, 291–298. [Google Scholar] [CrossRef]
- Muller, H. Neuraminidases of bacteria and protozoa and their pathogenic role. Behring. Inst. Mitt. 1974, 55, 34–56. [Google Scholar]
- Abrashev, I.; Orozova, P. Mucins in natural mud as inductor of some Aeromonas strains neuraminidase secretion. Probl. Inf. Parasit. Dis. 2004, 32, 27–30. [Google Scholar] [CrossRef]
- Abrashev, R.; Krumova, E.; Petrova, P.; Eneva, R.; Kostadinova, N.; Miteva-Staleva, J.; Engibarov, S.; Stoyancheva, G.; Gocheva, Y.; Kolyovska, V.; et al. Distribution of a Novel Enzyme of Sialidase Family among Native Filamentous Fungi. Fungal Biol. 2021, 125, 412–425. [Google Scholar] [CrossRef]
- Aisaka, K.; Igarashi, A.; Uwajima, T. Purification, crystallization, and characterization of neuraminidase from Micromonospora viridifaciens. Agric. Biol. Chem. 1991, 55, 997–1004. [Google Scholar] [CrossRef]
- Kessler, J.; Heck, J.; Tannenbaum, S.; Flashner, M. Substrate and product specificity of Arthrobacter sialophilus neuraminidase. J. Biol. Chem. 1982, 277, 5056–5060. [Google Scholar] [CrossRef]
- Iwamori, M.; Kaido, T.; Iwamori, Y.; Ohta, Y.; Tsukamoto, K.; Kozaki, S. Involvement of the C-terminal tail of Arthrobacter ureafaciens sialidase isoenzyme M in cleavage of the internal sialic acid of ganglioside GM1. J. Biochem. 2005, 138, 327–334. [Google Scholar] [CrossRef]
- Abrashev, I.; Dulguerova, G.; Dolashka-Angelova, P.; Voelter, W. Purification and Characterization of a Novel Sialidase from a Strain of Arthrobacter nicotianae. J. Biochem. 2005, 137, 365–371. [Google Scholar] [CrossRef]
- Eneva, R.; Engibarov, S.; Gocheva, Y.; Mitova, S.; Petrova, P. Novel sialidase from non-pathogenic bacterium Oerskovia paurometabola strain O129. Z. Naturforsch. C 2022, 78, 49–55. [Google Scholar] [CrossRef]
- Daly, J.; Carlsten, M.; O’Dwyer, M. Sugar free: Novel immunotherapeutic approaches targeting siglecs and sialic acids to enhance natural killer cell cytotoxicity against cancer. Front. Immunol. 2019, 10, 1047. [Google Scholar] [CrossRef]
- Bekesi, J.; St-Arneault, G.; Holland, J. Increase of leukemia L1210 immunogenicity by Vibrio cholerae neuraminidase treatment. Cancer Res. 1971, 31, 2130–2132. [Google Scholar]
- Pincus, J.; Jameson, A.; Brandt, A. Immunotherapy of L1210 leukemia using neuraminidase-modified plasma membranes combined with chemotherapy. Cancer. Res. 1981, 41, 3082–3086. [Google Scholar]
- Sedlacek, H.H.; Hagmayer, J.; Seler, F.R. Tumor therapy of neoplastic deseases with tumor cells and neuraminidase. Further experimental studies on chessboard vaccination in canine mammary tumors. Cancer. Immunol. Immunother. 1986, 23, 192–199. [Google Scholar] [CrossRef]
- Spence, R.; Simon, R.; Baker, A. Failure of immunotherapy with neuraminidase-treated tumor cell vaccine in mice bearing established 3-methylcholanthrene induced sarcomas. J. Natl. Canc. Inst. 1978, 60, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Laftah, B.; Al-Shammary, A.; Salih, H. Study the role of bacterial neuraminidase against adenocarcinoma cells in vivo. AIP Conf. Proc. 2021, 2372, 030009. [Google Scholar] [CrossRef]
- Durgin, J.; Thokala, R.; Johnson, L.; Song, E.; Leferovich, J.; Bhoj, V.; Ghassemi, S.; Milone, M.; Binder, Z.; O’Rourke, D.; et al. Enhancing CAR T function with the engineered secretion of C. perfringens neuraminidase. Mol. Ther. 2022, 30, 1201–1214. [Google Scholar] [CrossRef]
- Chen, Q.-V.; Zhang, Y.; Bao, P.; Zhang, X.-Z. Sialidase-Chimeric Bioengeneered Bacteria for Tumor-Sialoglycan-Triggered Solid Tumor Therapy. Nano Lett. 2024, 24, 10362–10371. [Google Scholar] [CrossRef]
- He, D.; Sun, L.; Li, C.; Hu, N.; Sheng, Y.; Chen, Z.; Li, X.; Chi, B.; Jin, N. Anti-tumor effects of an oncolytic adenovirus expressing hemagglutinin-neuraminidase of newcastle disease virus in vitro and in vivo. Viruses 2014, 6, 856–874. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Jiang, L.; Li, D.; Qian, R. Sialidase-conjugated “NanoNiche” for efficient immune checkpoint blockade therapy. ACS Appl. Bio. Mater. 2021, 4, 5735–5741. [Google Scholar] [CrossRef]
- Bull, C.; Stoel, M.; den Brock, M.; Adema, G. Sialic Acids Sweeten a Tumor’s Life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Matrosovich, M.; Herrler, G.; Klenk, H. Sialic acid receptors of viruses. Top. Curr. Chem. 2015, 367, 1–28. [Google Scholar] [CrossRef]
- Kurnia, R.; Tarigan, S.; Nugroho, C.; Silaen, O.; Natalia, L.; Ibrahim, F.; Sudarmono, P. Potency of bacterial sialidase Clostridium perfringens as antiviral of Newcastle disease infections using embryonated chicken egg in ovo model. Vet. World 2022, 15, 1896. [Google Scholar] [CrossRef]
- Nicholls, J.; Moss, R.; Haslam, S. The use of sialidase therapy for respiratory viral infections. Antivir. Res. 2013, 98, 401–409. [Google Scholar] [CrossRef]
- Chemaly, R.; Marty, F.; Wolfe, C.; Lawrence, S.; Dadwal, S.; Soave, R.; Farthing, J.; Hawley, S.; Montanez, P.; Hwang, J.; et al. DAS181 Treatment of Severe Lower Respiratory Tract Parainfluenza Virus Infection in Immunocompromised Patients: A Phase 2 Randomized, Placebo-Controlled Study. Clin. Infect. Dis. 2021, 73, e773–e778. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, C.; Kurnia, R.; Tarigan, S.; Silaen, O.; Triwidyaningtyas, S.; Wibawan, I.; Natalia, L.; Takdir, A.K.; Soebandrio, A. Screening and Purification of NanB Sialidase from Pasteurella multocida with Activity in Hydrolyzing Sialic Acid Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal. Sci. Rep. 2022, 12, 9425. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological function of sialic acid and sialylation in human health and disease. Cell Death Discov. 2024, 10, 415. [Google Scholar] [CrossRef]
- Thompson, C.; Barclay, W.; Zambon, M.; Pickles, R. Infection of human airway epithelium by human and avian strains of influenza a virus. J. Virol. 2006, 80, 8060–8068. [Google Scholar] [CrossRef]
- Madoff, M.; Eylar, E.; Weinstein, L. Serologic studies of the neuraminidases of Vibrio cholerae, Diplococcus pneumoniae and influenza virus. J Immunol. 1960, 85, 603–613. [Google Scholar] [CrossRef]
- Worrall, E.; Priadi, A. Sialivac: An intranasal homologous inactivated split virus vaccine containing bacterial sialidase for the control of avian influenza in poultry. Vaccine 2009, 27, 4161–4168. [Google Scholar] [CrossRef]
- Jin, R.; Hu, Y.; Sun, B.; Zhang, X.; Sun, L. Edwardsiella tarda sialidase: Pathogenicity involvement and vaccine potential. Fish Shellfish Immunol. 2012, 33, 514–521. [Google Scholar] [CrossRef]
- Chamberlain, B.; Buttery, J.; Pannall, P. A simple electrophoretic method for separating elevated liver and bone alkaline phosphatase isoenzymes in plasma after neuraminidase treatment. Clin. Chim. Acta 1992, 208, 219–225. [Google Scholar] [CrossRef]
- Miura, M.; Sakagishi, Y.; Hata, K.; Komoda, T. Differences between the sugar moieties of liver- and bone-type alkaline phosphatases: A re-evaluation. Ann. Clin. Biochem. 1994, 31, 25–30. [Google Scholar] [CrossRef]
- Ohno, J.; Ohshima, Y.; Arakaki, Z.; Yokoyama, S.; Utsumi, N. Immunohistochemical detection of sialyl Lex antigen on mucosal Langerhans cells of human oral mucosa following neuraminidase pretreatment. Biotech. Histochem. 1993, 68, 284–289. [Google Scholar] [CrossRef]
- Roggentin, P.; Gutschker-Gdaniec, G.; Hobrecht, R.; Schauer, R. Early diagnosis of clostridial gas gangrene using sialidase antibodies. Clin. Chim. Acta 1988, 173, 251–262. [Google Scholar] [CrossRef]
- Wu, S.; Lin, X.; Hui, K.; Yang, S.; Wu, X.; Tan, Y.; Li, M.; Qin, A.; Wang, Q.; Zhao, Q.; et al. A Biochemiluminescent Sialidase Assay for Diagnosis of Bacterial Vaginosis. Sci. Rep. 2019, 9, 20024. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yu, W.; Zhang, W.; Jiang, J.; Gu, Q.; Wang, X.; Wu, Y. Evaluating the activity of neuraminidase in bacterial vaginosis microflora and imaging sialic acid on the cell membrane by boron and nitrogen codoped fluorescent carbon dots. ACS Sens. 2023, 8, 2556–2562. [Google Scholar] [CrossRef]
- Mountney, A.; Zahner, M.; Lorenzini, I.; Oudega, M.; Schramm, L.; Schnaar, R. Sialidase enhances recovery from spinal cord contusion injury. Proc. Natl. Acad. Sci. USA 2010, 107, 11561–11566. [Google Scholar] [CrossRef]
- Schmidt, D.; Sauerbrei, B.; Thiem, J. Chemoenzymatic synthesis of sialyl oligosaccharides with sialidases employing transglycosylation methodology. J. Org. Chem. 2000, 65, 8518–8526. [Google Scholar] [CrossRef]
- Ajisaka, H.; Fujimoto, H.; Isomura, M. Regioselective transglycosylation in the synthesis of oligosaccharide: Comparison of β-galactosidases and sialidases of various origin. Carbohydr. Res. 1994, 259, 103–115. [Google Scholar] [CrossRef]
- Mariño, K.; Bones, J.; Kattla, J.; Rudd, P. A systematic approach to protein glycosylation analysis: A path through the maze. Nat. Chem. Biol. 2010, 6, 713–723. [Google Scholar] [CrossRef]
- Estrella, R.; Whitelock, J.; Roubin, R.; Packer, N.; Karlsson, N. Small-scale enzymatic digestion of glycoproteins and proteoglycans for analysis of oligosaccharides by LC-MS and FACE gel electrophoresis. Methods Mol. Biol. 2009, 534, 171–192. [Google Scholar] [CrossRef]
- Peng, Y.F.; Wang, X.D.; Wei, D.Z. Development of a large scale process for the conversion of polysialogangliosides to monosialotetrahexosylganglioside with a novel strain of Brevibacterium casei producing sialidase. Biotechnol. Lett. 2007, 29, 885–889. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, D.; Shen, D.; Wang, X.; Wei, D. Efficient conversion from polysialogangliosides to monosialotetrahexosylganglioside using Oerskovia xanthineolytica YZ-2. Bioprocess Biosyst. Eng. 2011, 34, 493–498. [Google Scholar] [CrossRef]
- Dong, H.J.; Jiang, J.Y.; Chen, Q.L. Development of a cell immobilization technique for the conversion of polysialogangliosides to monosialotetrahexosylganglioside. Pharm. Biol. 2011, 49, 805–809. [Google Scholar] [CrossRef]
- Eneva, R.; Engibarov, S.; Sirakov, I.; Kolyovska, V.; Pavlova, M.; Petrov, P.; Nenova, R.; Abrashev, I. Sialidase nanH of the non-toxigenic Vibrio cholerae strain V13 is a glycoprotein. C. R. Acad. Bulg. Sci. 2017, 70, 375–380. [Google Scholar]
- Thom, R.; D’Elia, R.V. Future applications of host direct therapies for infectious disease treatment. Front. Immunol. 2024, 15, 1436557. [Google Scholar] [CrossRef]
- Pennock, G.; Chow, L. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist 2015, 20, 812–822. [Google Scholar] [CrossRef]
- Brüggemann, H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin. Cutan. Med. Surg. 2005, 24, 67–72. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engibarov, S.; Gocheva, Y.; Lazarkevich, I.; Eneva, R. Bacterial Sialidases: Biological Significance and Application. Appl. Biosci. 2025, 4, 17. https://doi.org/10.3390/applbiosci4020017
Engibarov S, Gocheva Y, Lazarkevich I, Eneva R. Bacterial Sialidases: Biological Significance and Application. Applied Biosciences. 2025; 4(2):17. https://doi.org/10.3390/applbiosci4020017
Chicago/Turabian StyleEngibarov, Stephan, Yana Gocheva, Irina Lazarkevich, and Rumyana Eneva. 2025. "Bacterial Sialidases: Biological Significance and Application" Applied Biosciences 4, no. 2: 17. https://doi.org/10.3390/applbiosci4020017
APA StyleEngibarov, S., Gocheva, Y., Lazarkevich, I., & Eneva, R. (2025). Bacterial Sialidases: Biological Significance and Application. Applied Biosciences, 4(2), 17. https://doi.org/10.3390/applbiosci4020017






