Abstract
The ansa cervicalis (AC) is a neural loop within the carotid triangle of the anterior neck. The loop is traditionally formed by nerve roots C1–C3 of the cervical plexus and extends multiple motor branches. The current case was discovered during a routine dissection as an anatomical variation of the right AC in an 86-year-old Caucasian male cadaver. In this variation, the C1 nerve root did not form the typical loop with the C2 and C3 nerve roots, but instead, remained independent, traveling deep to the superior belly of the omohyoid muscle to supply the sternothyroid muscle. Because no loop was formed, the anatomy of the current case was not an ansa according to the Latin origin of the word, meaning handle or loop. The AC is an important anatomical landmark within the neck and is implicated in laryngeal reinnervation and respiratory nerve stimulation for patients with sleep apnea. The current anatomical variant contributes to a relatively limited catalog of identified anomalies. Knowledge of new AC variations can guide future surgical interventions and further develop the current base of knowledge surrounding the neuromuscular structures of the head and neck.
1. Introduction
The ansa cervicalis (AC) is a neural structure within the anterior neck region, providing significant motor innervation to muscles involved in swallowing, vocalization, and neck stabilization. The neural loop of the AC is composed of two major roots, superiorly by C1 fibers and inferiorly by the C2 and C3 fibers of the ventral rami of the cervical plexus (C1–C3). These superior and inferior roots join distally near the carotid bifurcation, forming a communicating loop from which various motor branches arise []. Motor components of the AC exist to innervate three of the four infrahyoid muscles of the neck (sternothyroid, sternohyoid, and omohyoid), while the thyrohyoid is innervated directly by C1 fibers branching from the hypoglossal nerve. These muscles play an important role in swallowing and depression of the hyoid bone [].
As the AC serves a multitude of functions, the disruption of the structure can result in various disabilities, ranging from difficulty speaking to neck pain []. The dysfunction of the infrahyoid muscles due to AC injury can lead to significant changes in vocal tone, the ability to produce speech, and swallowing deficits []. In addition, the AC plays a crucial role in surgical procedures of the neck, as iatrogenic injury can lead to critical postoperative complications []. Therefore, healthcare professionals must be aware of the AC’s anatomy and function to ensure optimal patient outcomes during both clinical and surgical interventions.
Beyond the native motor and sensory functions, the AC is useful as a viable donor nerve for reconstructions. Historically, the AC has been used as a donor nerve in laryngeal reinnervation following damage to the recurrent laryngeal nerve (RLN) []. The RLN is a distal branch of the vagus nerve that is in close proximity to the thyroid gland and innervates multiple endothyroid muscles important for speech production []. One review reported damage to the RLN in up to 1% of thyroid surgeries, rendering it a major source of malpractice litigation following thyroidectomy, leading to use of the AC for reinnervation a technique under frequent consideration []. Recent research has also implicated the AC as a potential target for respiratory nerve stimulation in patients with obstructive sleep apnea that has not responded to the more common hypoglossal nerve stimulation [,].
The complex branching of the AC renders it prone to anatomical variation, and the implications of damage to this structure render it important to understand its function and form. The current case describes a unilateral, right-sided variation of the AC in which the C1 root from the cervical plexus does not join roots C2 and C3, traveling independently to innervate the sternothyroid muscle.
2. Materials and Methods
2.1. Disclaimer Regarding Use of Human Donor Patients
The cadaver referenced in this study was obtained through a university anatomical donation program through which the patient provided written informed consent for the utilization of their remains for the purposes of medical education and research such as this. The dissection of the donor body, specimen preparation, and variation discovery were performed following established university protocols of good practice. The authors of this case report offer their sincerest thanks to all those who have participated in the anatomical donation program. Their donation aids in the education of future physicians and the discovery of anatomical variations and anomalies such as this one, which can improve patient care in the future and further our knowledge in the fields of anatomy and medicine as a whole [].
2.2. Anatomical Dissection
The current case report was discovered during routine dissection in an 86-year-old Caucasian male human body donor. After removing the skin and superficial fascia bilaterally from the anterior cervical region, the right sternocleidomastoid muscle was reflected superiorly to expose the anterior carotid and muscular triangles of the right neck. The extraneous tissue was removed, the courses of the hypoglossal, vagus, and C1, C2, and C3 nerve roots were noted, and the AC was identified. Following the identification of the anatomical variation, the left cervical region was dissected and investigated to determine if the variation was bilateral.
3. Results
3.1. Anatomical Variant of the Right Ansa Cervicalis
In the right anterior triangle of the neck, we identified the hypoglossal nerve (Figure 1A, black arrow), along with nerve roots C1–C3 of the cervical plexus (Figure 1A, yellow, red, and blue arrows, respectively), which typically join distally to form the AC (Figure 1A). Two branches of C1 emerged medially from the hypoglossal nerve, one diving under the mylohyoid (Figure 1B, pink double-headed arrow) and another innervating the thyrohyoid (Figure 1B, brown double-headed arrow). Upon further dissection, it was discovered that the third and most lateral branch of C1, which typically forms the superior root of the AC, remained isolated throughout the carotid triangle (Figure 1B, yellow arrows). Rather than joining the inferior root of the AC (IRAC, Figure 1, purple arrow) near the carotid bifurcation, this branch traveled independently, anterior to the common carotid artery, under the superior belly of the omohyoid (Figure 1C, yellow arrows), before branching to supply the sternothyroid and sternohyoid muscles (Figure 1C, green arrowheads). The fibers from C2 and C3 (Figure 1A,B, red and blue arrows, respectively) joined to form the inferior root of the AC (Figure 1A–C, purple arrows), which continued independently until it divided into multiple smaller branches, deep to the tendon separating the superior and inferior belly of the omohyoid (Figure 1B, green arrowheads).
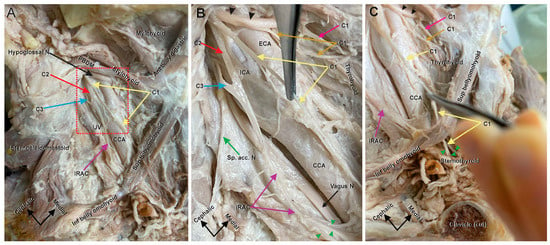
Figure 1.
Right ansa cervicalis and carotid triangle structures. The anomalous AC. (A) An anterior view of deep dissection of the right carotid triangle. The muscular outlines of the carotid triangle are shown and labeled. The triangle is bound superiorly by the posterior belly of the digastric muscle (PBDM), anteriorly by the superior belly of the omohyoid and posterior by the sternocleidomastoid. Other major structures in the carotid triangle are also shown and labeled; common carotid artery (CCA), internal jugular vein (IJV), hypoglossal nerve (black arrow), and inferior root of the AC (IRAC) (purple arrow). The three roots forming the AC are shown: the anomalous C1 (yellow arrows) from the hypoglossal nerve, C2 (red arrow), and C3 (blue arrow); (B) Zoom in of the red boxed area in (A) to show the details and abnormal pattern of the AC. In this view, the spinal accessory nerve (Sp. Acc. N, green arrow), vagus nerve (black double-headed arrow), internal carotid artery (ICA), and external carotid artery (ECA) are seen. The normal C1 components from the hypoglossal nerve (black arrowheads) extend two branches, a medial branch (pink arrow) to supply the geniohyoid muscle and a more lateral branch (brown arrow) to supply the thyrohyoid muscle. The third C1 branch is the anomalous branch (yellow arrows). This branch typically forms the superior root of the AC, which joins an inferior root comprised of C2 and C3 (red and blue arrows, respectively). In this case, the C1 branch ran downward independently without joining the inferior trunk formed by C2 and C3. C2 and C3 (red and blue arrows, respectively) emerged in the carotid triangle behind the ICA and joined to form the IRAC (purple arrows) at the level of bifurcation of the CCA. The IRAC did not receive any contributions from C1 and broke into several smaller branches (green arrowheads) at the tendon between the superior and inferior belly of the omohyoid. (C) Anterior view of the inferolateral angle of the carotid triangle showing the lower end of the C1 branch (yellow arrows). The terminal end of the C1 nerve crossed under the superior belly of the omohyoid muscle without supplying it and broke into smaller branches (green arrowheads) which supplied the sternohyoid and sternothyroid muscles. Cadaver orientation is indicated in by arrows in the bottom left corner of each panel.
3.2. Bilateral Comparison of the Left Ansa Cervicalis
The hypoglossal nerve was identified (Figure 2A,B, red arrow), along with the associated branching C1 fibers. As with the right dissection, the most medial of the C1 branches emerged from the hypoglossal to dive under the mylohyoid (Figure 2A, yellow arrowhead), and the next branch emerged to innervate the thyrohyoid (Figure 2A, yellow double arrowhead). Unlike the anatomy on the right, however, the most lateral C1 branch (Figure 2A, yellow arrow) combined with the C2 and C3 roots (Figure 2A,B; blue and green arrow, respectively) to form a single trunk (Figure 2A–C, purple arrows). This single trunk continued a vertical course superficial to the longus colli, between the common carotid artery and internal jugular vein, until branching at its terminal end. Three terminal branches were visualized and believed to supply the sternothyroid, sternohyoid, and omohyoid (Figure 2C, green arrowheads). During the dissection of the left carotid triangle, the omohyoid muscle was reflected; therefore, the branch innervating it was believed to have been cut (Figure 2B). The anatomy of this left carotid triangle is similar to short AC variants but not completely consistent with previous cases. Rather than a long superior root forming an ansa distally with multiple terminal branches emerging from the loop, this anatomy shows the three cervical roots joining almost simultaneously into a single trunk that descends to innervate distal infrahyoid muscles.
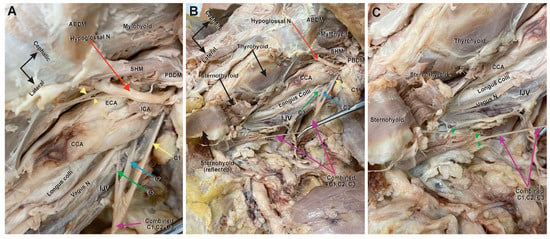
Figure 2.
Left ansa cervicalis and carotid triangle structures. Anterior view of the left carotid and digastric triangles. Cadaver orientation is indicated by a black arrow in (A) and maintained in (B) and (C). (A) High magnification view of the nerve roots. The upper area (the base) of the carotid and lower area of the digastric triangle with the posterior belly of the digastric muscle (PBDM) and stylohyoid muscle (SHM) separating the two triangles. The hypoglossal nerve (red arrow) runs below the intermediate tendon of the digastric and crosses over the ECA and ICA. The hypoglossal nerve here provided the three traditional C1 fibers contingents; one C1 nerve to the geniohyoid muscle (yellow arrowhead), a second C1 contingent as a nerve to the thyrohyoid (yellow double arrowhead), and a third C1 contingent (yellow arrow) joining the C2 (blue arrow) and C3 (green arrow) roots just below the bifurcation of the CCA and anterior to the IJV to form a combined nerve trunk (purple arrow). (B) An anterior view at a lower magnification of the entire carotid triangle which shows the entire combined (C1–C3) nerve trunk (purple arrows) running a downward vertical course between the CCA and IJV, and on top of the vagus nerve and longus colli muscle. (C) A magnified distal view of the terminal end of the combined nerve trunk (purple arrows) near the sternoclavicular joint in the root of the neck. At the lower end of the trunk, the nerve splayed into three visible branches (green arrowheads), which ended by diving into the lower ends of the sternohyoid and sternothyroid muscles. In this dissection, the omohyoid muscle was reflected, and the innervating branch was cut in the process.
A simplified diagram of the typical anatomy of the AC (Figure 3A) and the anatomy of the anatomical variant discovered in the right AC can be found below. The diagram highlights the anomalous nerve branch in orange, with traditional anatomic features illustrated in yellow Figure 3B).
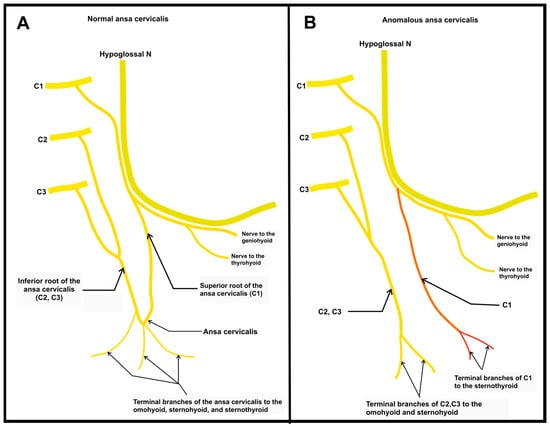
Figure 3.
Diagram of normal and anomalous ansa cervicalis. A simplified drawing of the presented anatomy. (A) This panel illustrates the most common standard anatomy of the AC. Of note, the terminal branches innervating the omohyoid, sternohyoid, and sternothyroid all emerge from the ansa loop formed by nerve roots C1, C2, and C3; (B) The anomalous branch of the right AC is indicated in orange. The most lateral nerve root of C1 can be seen descending between the two moremedial branches and the more lateral trunk formed by nerve roots C2 and C3. These two nerves never anastomose as in the traditional AC, instead traveling independently to innervate their respective target muscles.
4. Discussion
The AC is an important neural structure within the neck that stems from the ventral rami of the cervical plexus, commonly the first three or four cervical spinal nerves. The major components of a true AC are a superior and inferior root that converge to form a loop within the carotid triangle []. Due to the complex branching of the AC and its descent from the cervical plexus, there are a variety of reported anatomical variations within human subjects. Of note, all but one anatomical variation of the AC examined during a literature review for the current study was found to be unilateral [].
Variations in the length of and branching pattern of the distal ansa loop have been described previously by Chhetri and Caloit [,]. The most common form is the double classic form, in which two cervical branches contribute to the inferior root of the AC, and accounted for 40% of cases [,]. This structure can be seen in Figure 3A. The anatomy of the left AC in our cadaver is similar to the double short form described by Chhetri, with one single terminal branch descending the carotid triangle []. With the vagus nerve traveling near the AC, some cases have shown nerve variants in which the cervical roots join the vagus nerve before emerging again to innervate the infrahyoid muscles [,,,,]. The term “vagus ansa” can be used when the cervical nerve roots, typically C1 or C2, coalesce with the vagus nerve before reemerging as a single root that forms an ansa loop with the inferior cervical roots, C2 and/or C3 [,]. Other vagal variants have been described in which the cervical roots join the vagus nerve but never form a structural loop, instead extending various branches along the course of the vagus nerve to innervate various infrahyoid muscles [,,]. During the literature review, our team described these as absent ansa vagal variants.
Common variations in the structure of the AC are well summarized by Jelev into five morphological classifications based on the previous literature []. Within this classification system, an absent ansa exists when the superior and inferior roots do not converge to form a loop, represented by type I and type V. Type I has distinctly separate hypoglossal and cervical components, with only the cervical components from C2/C3 descending to innervate the infrahyoid muscles. Type V has distinctly separate hypoglossal and vagal components, in which the cervical components from C1,2, and 3 converge onto the vagal nerve before emerging as a single root to continue their descent to the infrahyoid muscles []. Type V is an absent ansa vagal variant.
One report identified a small C1 nerve branching from the hypoglossal nerve to innervate the distal one-third of the sternocleidomastoid muscle []. To our knowledge, there has not been a case reporting a major C1 nerve root descending independently to innervate the omohyoid muscle without anastomosing with the inferior root of the AC. Previously reported absent ansa variants involve some form of union between the anomalous branch and the vagus or hypoglossal nerves. What renders our report unique is that the two medial C1 branches from the hypoglossal nerve and the inferior root of the AC, follow traditional anatomic form and courses, but the branch of C1 that would normally form the upper root of the AC travels through the carotid triangle without any connections to nearby nerves (Figure 3). This case is most like Jelev type I, with the addition of this independent C1 branch [].
The importance of reporting anatomical variations of the AC is paramount due to its use in the clinical setting for laryngeal reinnervation, respiratory nerve stimulation in patients with sleep apnea, and other head and neck procedures of this region [,]. Iatrogenic damage to the AC may occur during operations involving the head and neck region, such as in the setting of a total thyroidectomy, especially when there is an unknown variation from normal anatomy. Unfortunately, the rate of vocal paralysis following these procedures is alarmingly high, with some reports showing up to 23% of vocal paralysis occurred due to iatrogenic causes []. Another rare but potentially relevant pathology involving the AC is the development of a schwannoma. Schwannomas of the AC are very uncommon, with as few as six cases reported. However, schwannomas of other nearby nerves can put the AC at risk of iatrogenic or collateral damage [,]. In the current case report, three nerves travelled independently through the cervical region: the vagus nerve, combined inferior root of the AC, and the anomalous independent superior root. This arrangement of nerves further complicates the anatomy of the already complex carotid triangle, increasing the risk of inadvertent damage. It is for this reason that it is vital to understand the variations in anatomical structure that can occur in the AC.
The use of the AC for laryngeal reinnervation is due to its similar size and proximity to the recipient site. Unknown variants of the AC may preclude its use in graft harvest or place interconnected structures at risk of further damage []. In one case, a patient underwent a parotidectomy for adenoid cystic carcinoma with planned upper-division facial nerve resection to be repaired using the AC. Following the identification of a vagal ansa variant, surgery was aborted to preserve vagal nerve function []. Without careful exploration of the associated structures, unexpected anatomical variation can predispose to complications. The lack of a true ansa loop can also complicate reinnervation procedures. The sternohyoid branch of the AC loop is the most commonly used nerve for laryngeal reinnervation []. In the current case, no loop is formed, and the terminal branches are unusually short as they emerge near their target muscle groups. This has the potential to complicate reinnervations by shortening the length of donor nerves and potentially paralyzing other muscles that may not be implicated or affected when traditional anatomy is present. Awareness of new anatomical variations is necessary for successful nerve dissection with current surgical approaches and can guide future education and development of novel surgical treatments for conditions involving the neuromuscular structures of the anterior neck.
When considering the current case report, there are some limitations that we should be aware of. Regarding the dissection, this case report was discovered during a student anatomy course for first year medical students, therefore the quality of the dissection could have been improved. This is primarily the reason some of the dissection images are not as clear as they could be. Secondly, the cadaver used was preserved with formalin and not fresh frozen, therefore some detail and biomechanical properties of the tissue have been altered. This is not particularly relevant to our study of the nerves in the neck region but is worth noting. Finally, image quality could be improved, however, the cadaver was replaced shortly after the initial discovery and pictures were taken of the anomalous AC.
5. Conclusions
The current case report describes a novel anatomical variation in the structure of the right AC in an 86-year-old male cadaver. The structure differs from the typical anatomy and previously published variants in three specific ways:
- There is no ansa according to the true Latin derivative of the word (meaning “loop”);
- The root composed of C2 and C3, which would typically form the inferior root of the AC, descends independently to innervate the omohyoid and sternohyoid muscles;
- A C1 root exists that does not connect with the vagus nerve or C2/C3 branch, traveling independently to innervate the sternothyroid muscle.
This current case report adds to a relatively limited set of reported anatomical variants of the AC. This anatomical variant is clinically important in any surgical procedure of the anterior neck to avoid iatrogenic nerve injury and must be considered when planning to use the AC as a donor nerve for reconstruction.
Author Contributions
Conceptualization, A.M. and E.L.; methodology, K.T.; validation, J.M.K., S.H. and S.Y.; investigation, S.Y., K.T., B.B. and K.S.; resources, B.B. and J.M.K.; writing—original draft preparation, K.T., B.B., S.Y., S.H.; writing—review and editing, E.L., K.S., J.M.K. and A.M.; visualization, A.M.; supervision, A.M.; project administration, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All donors contributing to the cadaver lab at The University of Toledo College of Medicine and Life Sciences have been required to complete documentation before donating their remains for educational purposes.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are thankful to all those involved in medical education at the University of Toledo, and specifically those who work in the gross anatomy lab. The work performed by these individuals is paramount to a robust medical education curriculum, and the lessons learned through gross dissection are difficult to obtain from any other medium.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
Ansa cervicalis, AC; Recurrent laryngeal nerve, RLN; inferior root of ansa cervicalis, IRAC; Anterior belly of digastric muscle, ABDM; posterior belly of digastric muscle, PBDM; Common Carotid artery, CCA; spinal accessory nerve, Sp. Acc. N.; external carotid artery, ECA; internal carotid artery, ICA; internal jugular vein, IJV; sternocleidomastoid, SCM; stylohyoid, SHM; posterior belly of digastric muscle, PBDM.
References
- Kikuta, S.; Jenkins, S.; Kusukawa, J.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. Ansa cervicalis: A comprehensive review of its anatomy, variations, pathology, and surgical applications. Anat. Cell Biol. 2019, 52, 221–225. [Google Scholar] [CrossRef]
- Mnatsakanian, A.; Al Khalili, Y. Anatomy, Head and Neck, Thyroid Muscles. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Quadros, L.S.; Prasanna, L.C.; D’Souza, A.S.; Singh, A.; Kalthur, S.G. Unilateral anatomical variation of the ansa cervicalis. Australas. Med. J. 2015, 8, 170–173. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Blumin, J.H. Laryngeal reinnervation for unilateral vocal fold paralysis using ansa cervicalis nerve to recurrent laryngeal nerve anastomosis. Oper. Tech. Otolaryngol. Head Neck Surg. 2012, 23, 173–177. [Google Scholar] [CrossRef]
- Masuoka, H.; Miyauchi, A.; Yabuta, T.; Fukushima, M.; Miya, A. Innervation of the cricothyroid muscle by the recurrent laryngeal nerve. Head Neck 2016, 38 (Suppl. S1), E441–E445. [Google Scholar] [CrossRef]
- Lynch, J.; Parameswaran, R. Management of unilateral recurrent laryngeal nerve injury after thyroid surgery: A review. Head Neck 2017, 39, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.T.; Scott, W.C.; Zealear, D.; Schwartz, A.R. Ansa cervicalis stimulation increases pharyngeal patency in patients with obstructive sleep apnea. J. Appl. Physiol. 2021, 131, 487–495. [Google Scholar] [CrossRef]
- Kent, D.T.; Zealear, D.; Schwartz, A.R. Ansa Cervicalis and Hypoglossal Nerve Stimulation in a Patient With Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2021, 165, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Singh, V.; Ohtsuka, A.; Hwang, Y.; Kim, H.J.; Moryś, J.; Ravi, K.S.; Ribatti, D.; Trainor, P.A.; Sañudo, J.R.; et al. Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clin. Anat. 2021, 34, 2–4. [Google Scholar] [CrossRef]
- Abu-Hijleh, M.F. Bilateral absence of ansa cervicalis replaced by vagocervical plexus: Case report and literature review. Ann. Anat. Anat. Anz. 2005, 187, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.K.; Berke, G.S. Ansa Cervicalis Nerve: Review of the Topographic Anatomy and Morpholog. Laryngoscope 1997, 107, 1366–1372. [Google Scholar] [CrossRef]
- Caliot, P.; Dumont, D.; Bousquet, V.; Midy, D. A note on the anastomoses between the hypoglossal nerve and the cervical plexus. Surg. Radiol. Anat. 1986, 8, 75–79. [Google Scholar] [CrossRef]
- Ayyoubian, M.; Koruji, M. A rare anatomical variant of ansa cervicalis: Case report. Med. J. Islam. Repub. Iran 2011, 24, 238–240. [Google Scholar]
- D’Souza, A.S.; Ray, B. Study of the formation and distribution of the ansa cervicalis and its clinical significance. Eur. J. Anat. 2010, 14, 143–148. [Google Scholar]
- Rath, G.; Anand, C. Vagocervical complex replacing an absent ansa cervicalis. Surg. Radiol. Anat. 1994, 16, 441–443. [Google Scholar] [CrossRef]
- Manjunath, K.Y. Vagal origin of the ANSA cervicalis nerve—Report of two cases. Indian J. Otolaryngol. Head Neck Surg. 2000, 52, 257–258. [Google Scholar] [CrossRef]
- Kikuchi, T. A contribution to the morphology of the ansa cervicalis and the phrenic nerve. Kaibogaku Zasshi 1970, 45, 242–281. [Google Scholar]
- Jelev, L. Some unusual types of formation of the ansa cervicalis in humans and proposal of a new morphological classification. Clin. Anat. 2013, 26, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Blythe, J.N.; Matharu, J.; Reuther, W.J.; Brennan, P.A. Innervation of the lower third of the sternocleidomastoid muscle by the ansa cervicalis through the C1 descendens hypoglossal branch: A previously unreported anatomical variant. Br. J. Oral Maxillofac. Surg. 2015, 53, 470–471. [Google Scholar] [CrossRef]
- Anghel, A.; Anghel, I.; Dumitru, M.; Soreanu, C. Respiratory and phonatory impairment due to iatrogenic vocal fold paralysis and paresis. A retrospective study of 188 patients. Rom. J. Leg. Med. 2012, 20, 287–290. [Google Scholar] [CrossRef]
- Vrinceanu, D.; Dumitru, M.; Popa-Cherecheanu, M.; Marinescu, A.N.; Patrascu, O.-M.; Bobirca, F. Extracranial Facial Nerve Schwannoma—Histological Surprise or Therapeutic Planning? Medicina 2023, 59, 1167. [Google Scholar] [PubMed]
- Rath, S.; Sasmal, P.K.; Saha, K.; Deep, N.; Mishra, P.; Mishra, T.S.; Sharma, R. Ancient Schwannoma of Ansa Cervicalis: A Rare Clinical Entity and Review of the Literature. Case Rep. Surg. 2015, 2015, 578467. [Google Scholar] [CrossRef] [PubMed]
- Paniello, R.C. Laryngeal reinnervation. Otolaryngol. Clin. N. Am. 2004, 37, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Mohan, S.; Richmon, J.D.; Jowett, N. An Anatomic Variant of the Ansa Cervicalis Precluding Its Use as a Donor Nerve. Ann. Otol. Rhinol. Laryngol. 2020, 129, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.M.; Gavid, M.; Dubois, M.D.; Dumollard, J.M.; Timoshenko, A.T.; Peoc’h, M. Surgical anatomy of the ansa cervicalis nerve: Which branch to use for laryngeal reinnervation in humans? Surg. Radiol. Anat. 2015, 37, 139–145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).