Towards a New Dynamic Interaction Model of Adolescent CUD Manifestation, Prevention, and Treatment: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. CUD and DSM
3.2. Adverse Effects of Cannabis Use
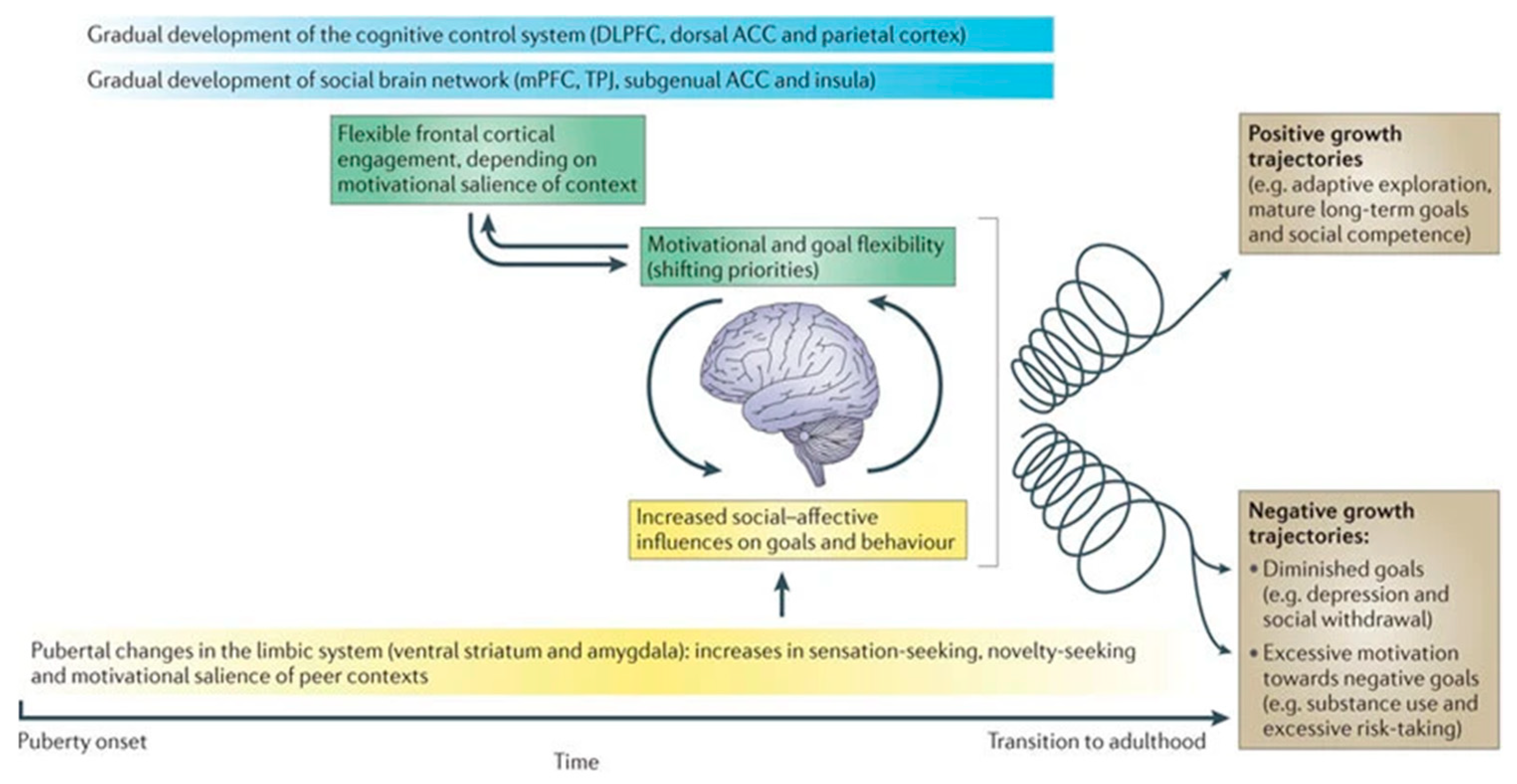
3.3. Adolescent Brain Development
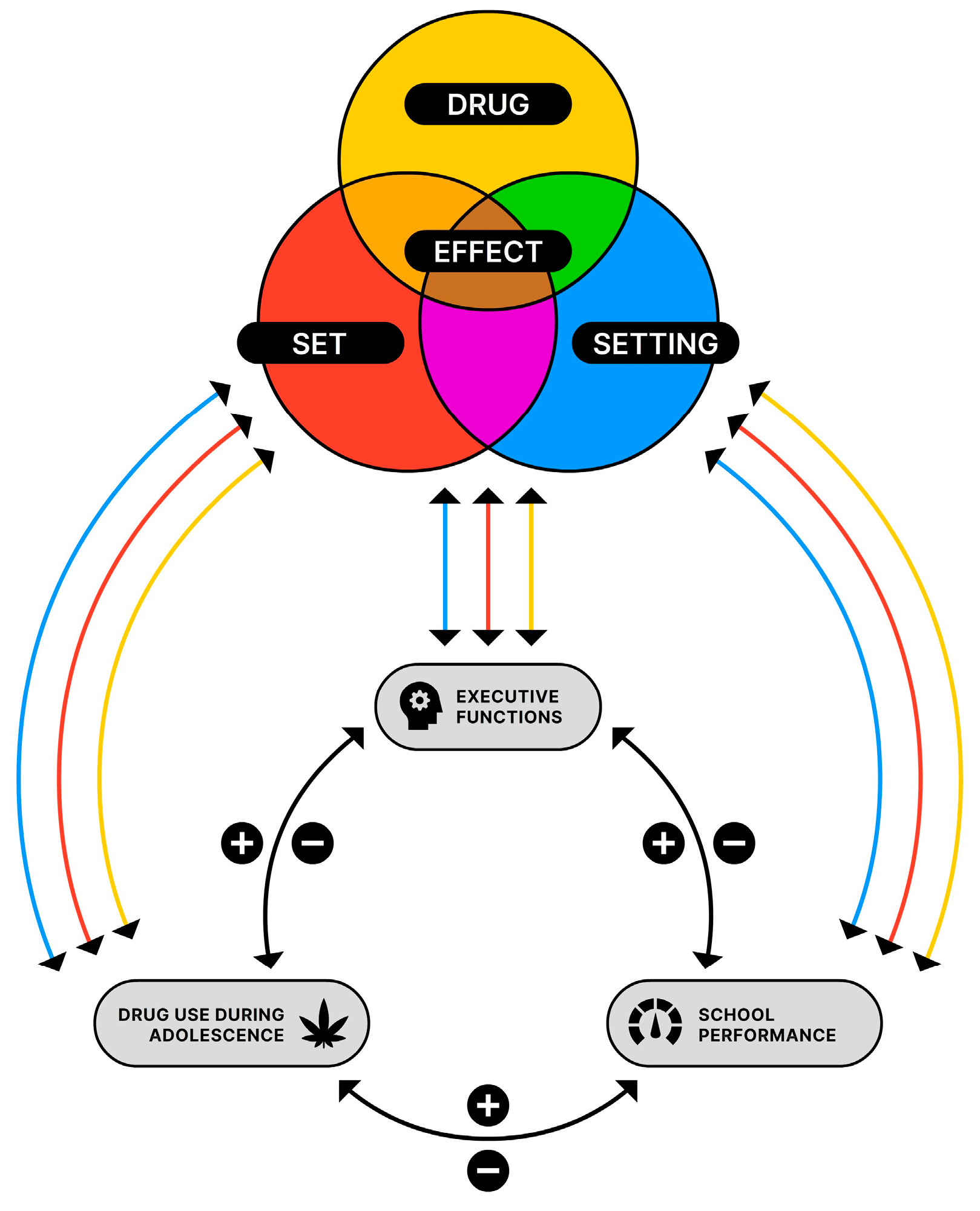
3.4. Drug, Set, and Setting of Cannabis Use
3.4.1. Cannabis (Drug)
Mechanism of Action
Effects of Cannabis Use
Neuropsychological Effects of Cannabis Use
3.4.2. Individual Motives and Risk Factors (Set)
3.4.3. Environmental Risk Factors (Setting)
3.5. Landscape of Therapeutic Interventions
3.6. Integrated Dynamic Interaction Model
3.6.1. Interaction between Cannabis, EFs and School Performance
3.6.2. Model of Interaction
4. Discussion
4.1. Prevention-Based Interventions
4.2. Therapeutic Interventions
4.3. Limitations and Future Research Recommendations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Oomen, P.P.; van Hell, H.H.; Bossong, M.G. The acute effects of cannabis on human executive function. Behav. Pharmacol. 2018, 29, 605–616. [Google Scholar] [CrossRef]
- Trimbos Instituut. Cijfers Drugs: Gebruik en Trends. 2022. Available online: https://www.trimbos.nl/kennis/cijfers/drugs/ (accessed on 15 July 2023).
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef]
- Blest-Hopley, G.; Colizzi, M.; Giampietro, V.; Bhattacharyya, S. Is the adolescent brain at greater vulnerability to the effects of cannabis? A narrative review of the evidence. Front. Psychiatry 2020, 11, 859. [Google Scholar] [CrossRef]
- Chye, Y.; Christensen, E.; Yücel, M. Cannabis use in adolescence: A review of neuroimaging findings. J. Dual Diagn. 2020, 16, 83–105. [Google Scholar] [CrossRef]
- Lorenzetti, V.; Hoch, E.; Hall, W. Adolescent cannabis use, cognition, brain health and educational outcomes: A review of the evidence. Eur. Neuropsychopharmacol. 2020, 36, 169–180. [Google Scholar] [CrossRef]
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Kakuma, R. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef]
- Castellanos-Ryan, N.; Pingault, J.B.; Parent, S.; Vitaro, F.; Tremblay, R.E.; Seguin, J.R. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: A longitudinal study from early adolescence to young adulthood. Dev. Psychopathol. 2017, 29, 1253. [Google Scholar] [CrossRef]
- Over, E.; Van Gils, P.; Suijkerbuijk, A.; Lokkerbol, J.; De Wit, G. Maatschappelijke Kosten-Baten Analyse van Cognitieve Gedragstherapie Voor Alcohol-en Cannabisverslaving; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 2016; p. 63. [Google Scholar]
- Townsend, L.; Flisher, A.J.; King, G. A systematic review of the relationship between high school dropout and substance use. Clin. Child Fam. Psychol. Rev. 2007, 10, 295–317. [Google Scholar] [CrossRef]
- Sherman, B.J.; McRae-Clark, A.L. Treatment of Cannabis Use Disorder: Current Science and Future Outlook. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 511–535. [Google Scholar] [CrossRef]
- Gray, K.M.; Carpenter, M.J.; Baker, N.L.; DeSantis, S.M.; Kryway, E.; Hartwell, K.J.; McRae-Clark, A.L.; Brady, K.T. A Double-Blind Randomized Controlled Trial of N-Acetylcysteine in Cannabis-Dependent Adolescents. Am. J. Psychiatry 2012, 169, 805–812. [Google Scholar] [CrossRef]
- Tomko, R.L.; Gilmore, A.K.; Gray, K.M. The role of depressive symptoms in treatment of adolescent cannabis use disorder with N-Acetylcysteine. Addict. Behav. 2018, 85, 26–30. [Google Scholar] [CrossRef]
- Van Benthem, P.; Spijkerman, R.; Blanken, P.; Kleinjan, M.; Vermeiren, R.R.; Hendriks, V.M. A dual perspective on first-session therapeutic alliance: Strong predictor of youth mental health and addiction treatment outcome. Eur. Child Adolesc. Psychiatry 2020, 29, 1593–1601. [Google Scholar] [CrossRef]
- Kadden, R.M.; Litt, M.D.; Kabela-Cormier, E.; Petry, N.M. Abstinence rates following behavioral treatments for marijuana dependence. Addict. Behav. 2007, 32, 1220–1236. [Google Scholar] [CrossRef]
- Larsen, B.; Luna, B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 2018, 94, 179–195. [Google Scholar] [CrossRef]
- Hartogsohn, I. Set and setting, psychedelics and the placebo response: An extra pharmacological perspective on psychopharmacology. J. Psychopharmacol. 2016, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Snelders, S.; Kaplan, C. LSD therapy in Dutch psychiatry: Changing socio-political settings and medical sets. Med. Hist. 2002, 46, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Zinberg, N.E. Drug, Set, and Setting: The Basis for Controlled Intoxicant Use; Yale University Press: New Haven, CT, USA, 1984. [Google Scholar]
- Ataiants, J.; Roth, A.M.; Mazzella, S.; Lankenau, S.E. Circumstances of overdose among street-involved, opioid-injecting women: Drug, set, and setting. Int. J. Drug Policy 2020, 78, 102691. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Blair, C.B.; Willoughby, M.T. Executive Function: Implications for Education. NCER 2017-2000. National Center for Education Research. 2016. Available online: https://files.eric.ed.gov/fulltext/ED570880.pdf (accessed on 10 July 2023).
- Crone, E.A.; Dahl, R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012, 13, 636–650. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Hsu, M.; Shah, A.; Hill, K.P. Is your patient’s cannabis use problematic? J. Fam. Pract. 2020, 69, 379–385. [Google Scholar] [CrossRef]
- Hooper, S.R.; Woolley, D.; De Bellis, M.D. Intellectual, neurocognitive, and academic achievement in abstinent adolescents with cannabis use disorder. Psychopharmacology 2014, 231, 1467–1477. [Google Scholar] [CrossRef]
- Pijlman, F.T.A.; Rigter, S.M.; Hoek, J.; Goldschmidt, H.M.J.; Niesink, R.J.M. Strong increase in total delta-THC in cannabis preparations sold in Dutch coffee shops. Addict. Biol. 2005, 10, 171–180. [Google Scholar] [CrossRef]
- Wilson, J.; Freeman, T.P.; Mackie, C.J. Effects of increasing cannabis potency on adolescent health. Lancet Child Adolesc. Health 2019, 3, 121–128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adolescent Health. 2019. Available online: https://www.who.int/health-topics/adolescent-health#tab=tab_1 (accessed on 26 November 2019).
- Colver, A.; Longwell, S. New understanding of adolescent brain development: Relevance to transitional healthcare for young people with long term conditions. Arch. Dis. Child. 2013, 98, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Pattij, T.; Wiskerke, J.; Schoffelmeer, A.N. Cannabinoid modulation of executive functions. Eur. J. Pharmacol. 2008, 585, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Executive Functions: What They Are, How They Work, and Why They Evolved; Guilford Publications: New York, NY, USA, 2012. [Google Scholar]
- Best, J.R.; Miller, P.H.; Naglieri, J.A. Relations between Executive Function and Academic Achievement from Ages 5 to 17 in a Large, Representative National Sample. Learn Individ. Differ. 2011, 21, 327–336. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Romer, D.; Reyna, V.F.; Satterthwaite, T.D. Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev. Cogn. Neurosci. 2017, 27, 19–34. [Google Scholar] [CrossRef]
- Khurana, A.; Loan, C.M.; Romer, D. Predicting cigarette use initiation and dependence in adolescence using an affect-driven exploration model. Front. Psychol. 2022, 13, 887021. [Google Scholar] [CrossRef]
- Kwon, S.; Turpyn, C.C.; Duell, N.; Telzer, E.H. Neural Underpinnings of Social Contextual Influences on Adolescent Risk-Taking. Curr. Addict. Rep. 2020, 7, 413–420. [Google Scholar] [CrossRef]
- Casey, B.C. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annu. Rev. Psychol. 2015, 66, 295–319. [Google Scholar] [CrossRef]
- Steinberg, L.; Icenogle, G.; Shulman, E.P.; Breiner, K.; Chein, J.; Bacchini, D.; Chang, L.; Chaudhary, N.; Di Giunta, L.; Dodge, K.A.; et al. Around the world, adolescence is a time of heightened sensation seeking and immature self-regulation. Dev. Sci. 2017, 21, e12532. [Google Scholar] [CrossRef]
- Kim-Spoon, J.; Deater-Deckard, K.; Holmes, C.; Lee, J.; Chiu, P.H.; King-Casas, B. Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia 2016, 91, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Goddings, A.; Dumontheil, I.; Viner, R.; Blakemore, S. Puberty and risky decision making in male adolescents. Dev. Cogn. Neurosci. 2023, 60, 101230. [Google Scholar] [CrossRef]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and long term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Ruehle, S.; Rey, A.A.; Remmers, F.; Lutz, B. The endocannabinoid system in anxiety, fear memory and habituation. J. Psychopharmacol. 2011, 26, 23–39. [Google Scholar] [CrossRef]
- Caballero, A.; Granberg, R.; Tseng, K.Y. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev. 2016, 70, 4–12. [Google Scholar] [CrossRef]
- Reggio, P.H. Endocannabinoid binding to the cannabinoid receptors: What is known and what remains unknown. Curr. Med. Chem. 2010, 17, 1468–1486. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Solowij, N.; Broyd, S.J.; Greenwood, L.; Van Hell, H.; Martelozzo, D.; Rueb, K.; Todd, J.; Liu, Z.; Galettis, P.; Martin, J.H.; et al. A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: Acute intoxication effects. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 17–35. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Manzoni, O.J.; Pletnikov, M.V.; Lee, F.S.; Bhattacharyya, S.; Melis, M. Cannabis and the developing brain: Insights into its long-lasting effects. J. Neurosci. 2019, 39, 8250–8258. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use. JAMA 2015, 313, 2456. [Google Scholar] [CrossRef]
- Chohan, H.; Greenfield, A.L.; Yadav, V.; Graves, J. Use of Cannabinoids for Spasticity and Pain Management in MS. Curr. Treat. Options Neurol. 2015, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Haroutounian, S.; Ratz, Y.; Ginosar, Y.; Furmanov, K.; Saifi, F.; Meidan, R.; Davidson, E. The Effect of Medicinal Cannabis on Pain and Quality-of-Life Outcomes in Chronic Pain. Clin. J. Pain 2016, 32, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Velzeboer, R.; Malas, A.; Boerkoel, P.; Cullen, K.; Hawkins, M.; Roesler, J.; Lai, W.W.K. Cannabis dosing and administration for sleep: A systematic review. Sleep 2022, 45, zsac218. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.; Livny, A.; Weizman, A. Brain Imaging Studies on the Cognitive, Pharmacological and Neurobiological Effects of Cannabis in Humans: Evidence from Studies of Adult Users. Curr. Pharm. Des. 2017, 22, 6366–6379. [Google Scholar] [CrossRef]
- Hartman, R.L.; Huestis, M.A. Cannabis Effects on Driving Skills. Clin. Chem. 2013, 59, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.D.; Zois, V.; McKeown, D.A.; Lee, T.D.; Holt, D.W.; Powell, J.F.; Kapur, S.; Murray, R.M. The acute effects of synthetic intravenous Δ9 tetrahydrocannabinol on psychosis, mood, and cognitive functioning. Psychol. Med. 2009, 39, 1607. [Google Scholar] [CrossRef]
- Sewell, R.A.; Poling, J.; Sofuoglu, M. The Effect of Cannabis Compared with Alcohol on Driving. Am. J. Addict. 2009, 18, 185–193. [Google Scholar] [CrossRef]
- Murray, R.M.; Quigley, H.; Quattrone, D.; Englund, A.; Di Forti, M. Traditional marijuana, high-potency cannabis and synthetic cannabinoids: Increasing risk for psychosis. World Psychiatry 2016, 15, 195–204. [Google Scholar] [CrossRef]
- Leung, J.; Chan, G.C.; Hides, L.; Hall, W.D. What is the prevalence and risk of cannabis use disorders among people who use cannabis? a systematic review and meta-analysis. Addict. Behav. 2020, 109, 106479. [Google Scholar] [CrossRef]
- Blanco, C.; Hasin, D.S.; Wall, M.M.; Flórez-Salamanca, L.; Hoertel, N.; Wang, S.; Kerridge, B.T.; Olfson, M. Cannabis Use and Risk of Psychiatric Disorders. JAMA Psychiatry 2016, 73, 388. [Google Scholar] [CrossRef]
- Chen, C.; Storr, C.L.; Anthony, J.C. Early-onset drug use and risk for drug dependence problems. Addict. Behav. 2009, 34, 319–322. [Google Scholar] [CrossRef]
- Rubino, T.; Prini, P.; Piscitelli, F.; Zamberletti, E.; Trusel, M.; Melis, M.; Parolaro, D. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol. Dis. 2015, 73, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Burns, H.D.; Van Laere, K.; Sanabria-Bohórquez, S.; Hamill, T.G.; Bormans, G.; Eng, W.S.; Krause, S. [18F] MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 9800–9805. [Google Scholar] [CrossRef]
- Weiland, B.J.; Thayer, R.E.; Depue, B.E.; Sabbineni, A.; Bryan, A.D.; Hutchison, K.E. Daily Marijuana Use Is Not Associated with Brain Morphometric Measures in Adolescents or Adults. J. Neurosci. 2015, 35, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Fried, P.A.; Watkinson, B.; Gray, R. Neurocognitive consequences of marihuana—A comparison with pre-drug performance. Neurotoxicol. Teratol. 2005, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Rubino, T.; Parolaro, D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biol. Psychiatry 2016, 79, 578–585. [Google Scholar] [CrossRef]
- Lubman, D.I.; Cheetham, A.; Yücel, M. Cannabis and adolescent brain development. Pharmacol. Ther. 2015, 148, 1–16. [Google Scholar] [CrossRef]
- Hanson, K.L.; Winward, J.L.; Schweinsburg, A.D.; Medina, K.L.; Brown, S.A.; Tapert, S.F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict. Behav. 2010, 35, 970–976. [Google Scholar] [CrossRef]
- Medina, K.L.; Hanson, K.L.; Schweinsburg, A.D.; Cohen-Zion, M.; Nagel, B.J.; Tapert, S.F. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. JINS 2007, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.A.; Sellman, J.D.; Porter, R.J.; Frampton, C.M. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007, 26, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lisdahl, K.M.; Price, J.S. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J. Int. Neuropsychol. Soc. JINS 2012, 18, 678. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, M.; Radhakrishnan, R.; Addy, P.H.; Schnakenberg-Martin, A.M.; Williams, H.; Carbuto, M.; Elander, J.; Pittman, B.; Andrew Sewell, R.; Skosnik, P.D.; et al. Tetrahydrocannabinol (THC) impairs encoding but notretrieval of verbal information. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 176–183. [Google Scholar] [CrossRef]
- Gustavson, D.E.; Stallings, M.C.; Corley, R.P.; Miyake, A.; Hewitt, J.K.; Friedman, N.P. Executive functions and substance use: Relations in late adolescence and early adulthood. J. Abnorm. Psychol. 2017, 126, 257. [Google Scholar] [CrossRef]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yuecel, M.; Solowij, N. Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Jager, G.; Block, R.I.; Luijten, M.; Ramsey, N.F. Cannabis use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 561–572. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Schreiner, A.M.; Dunn, M.E. Residual effects of cannabis use on neurocognitiveperformance after prolonged abstinence: A meta-analysis. Exp. Clin. Psychopharmacol. 2012, 20, 420. [Google Scholar] [CrossRef]
- Schulte, M.H.; Cousijn, J.; den Uyl, T.E.; Goudriaan, A.E.; van den Brink, W.; Veltman, D.J.; Wiers, R.W. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin. Psychol. Rev. 2014, 34, 531–550. [Google Scholar] [CrossRef]
- Sagar, K.A.; Gruber, S.A. Interactions between recreational cannabis use and cognitive function: Lessons from functional magnetic resonance imaging. Ann. N. Y. Acad. Sci. 2019, 1451, 42. [Google Scholar] [CrossRef] [PubMed]
- Miech, R.A.; Johnston, L.D.; Patrick, M.E.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E. Monitoring the Future National Survey Results on Drug Use, 1975–2022, Secondary School Students. Institute for Social Research. 2023. Available online: http://monitoringthefuture.org/results/publications/monographs/ (accessed on 9 August 2023).
- Mariani, A.C.; Williams, A.E. Perceived risk of harm from monthly cannabis use among US adolescents: National Survey on drug Use and Health, 2017. Prev. Med. Rep. 2021, 23, 101436. [Google Scholar] [CrossRef] [PubMed]
- Norberg, M.M.; Olivier, J.; Schmidt, N.B.; Zvolensky, M.J. Cannabis Use among Treatment-Seeking Smokers: Motives and the Moderating Effects of Anxiety Sensitivity. Am. J. Addict. 2014, 23, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bonn-Miller, M.O.; Boden, M.T.; Bucossi, M.M.; Babson, K.A. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am. J. Drug Alcohol Abus. 2014, 40, 23–30. [Google Scholar] [CrossRef]
- Hecimovic, K.; Barrett, S.; Darredeau, C.; Stewart, S.H. Cannabis use motives and personality risk factors. Addict. Behav. 2014, 39, 729–732. [Google Scholar] [CrossRef]
- Defoe, I.N.; Rap, S.E.; Romer, D. Adolescents’ own views on their risk behaviors, and the potential effects of being labeled as risk-takers: A commentary and review. Front. Psychol. 2022, 13, 945775. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Vujanovic, A.A.; Zvolensky, M.J. Emotional Dysregulation: Association with Coping-Oriented Marijuana Use Motives Among Current Marijuana Users. Subst. Use Misuse 2008, 43, 1653–1665. [Google Scholar] [CrossRef]
- Taylor, O.D. Life stressors and substance abuse in African American adolescents residing in a public housing community. J. Hum. Behav. Soc. Environ. 2015, 25, 288–303. [Google Scholar] [CrossRef]
- Ketcherside, A.; Filbey, F.M. Mediating processes between stress and problematic marijuana use. Addict. Behav. 2015, 45, 113–118. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Nguyen-Louie, T.T.; Tapert, S.F. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology 2014, 28, 782. [Google Scholar] [CrossRef]
- White, J.; Batty, G.D. Intelligence across childhood in relation to illegal drug use in adulthood: 1970 British Cohort Study. J. Epidemiol. Community Heal. 2012, 66, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, R.M.D.S.; Hammonds, R.; Filbey, F.M.; Choudhary, P.K.; Biswas, S. A preliminary risk prediction model for cannabis use disorder. Prev. Med. Rep. 2020, 20, 101228. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Bravo, A.J.; Morizot, J.; Pilatti, A.; Pearson, M.R.; Ibáñez, M.I.; Ortet, G.; Team, C.A.S. Cross-cultural examination of the Big Five Personality Trait Short Questionnaire: Measurement invariance testing and associations with mental health. PLoS ONE 2019, 14, e0226223. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Kevorkian, S.; Sheerin, C.M.; Zvolensky, M.J.; Berenz, E.C. Examination of the Association Among Personality Traits, Anxiety Sensitivity, and Cannabis Use Motives in a Community Sample. J. Psychopathol. Behav. Assess. 2016, 38, 373–380. [Google Scholar] [CrossRef]
- Dash, G.F.; Slutske, W.S.; Martin, N.G.; Statham, D.J.; Agrawal, A.; Lynskey, M.T. Big Five personality traits and alcohol, nicotine, cannabis, and gambling disorder comorbidity. Psychol. Addict. Behav. 2019, 33, 420–429. [Google Scholar] [CrossRef]
- Dass-Brailsford, P.; Myrick, A.C. Psychological trauma and substance abuse: The need for an integrated approach. Trauma Violence Abus. 2010, 11, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.D.; Morris, P.E.; Abarno, C.N.; Glover, N.I.; Lewis, E.M. Biopsychosocial Model Social Anxiety and Substance Use Revised. Curr. Psychiatry Rep. 2021, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Didden, R.; VanDerNagel, J.; Delforterie, M.; Van Duijvenbode, N. Substance use disorders in people with intellectual disability. Curr. Opin. Psychiatry 2020, 33, 124–129. [Google Scholar] [CrossRef]
- Artigas, M.S.; Sánchez-Mora, C.; Rovira, P.; Richarte, V.; Garcia-Martínez, I.; Pagerols, M.; Neale, B.M. Attention-deficit/hyperactivity disorder and lifetime cannabis use: Genetic overlap and causality. Mol. Psychiatry 2020, 25, 2493–2503. [Google Scholar] [CrossRef]
- van der Gronde, T.; Los, L.; Herremans, A.; Oosting, R.; Zorzanelli, R.; Pieters, T. Toward a New Model of Understanding, Preventing, and Treating Adolescent Depression Focusing on Exhaustion and Stress. Front. Psychiatry 2020, 11, 412. [Google Scholar] [CrossRef]
- Prom-Wormley, E.; Ebejer, J.L.; Dick, D.M.; Bowers, M.W. The genetic epidemiology of substance use disorder: A review. Drug Alcohol Depend. 2017, 180, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Deak, J.D.; Johnson, E.C. Genetics of substance use disorders: A review. Psychol. Med. 2021, 51, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Urbanoski, K.A.; Kelly, J.F. Understanding genetic risk for substance use and addiction: A guide for non-geneticists. Clin. Psychol. Rev. 2012, 32, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Demontis, D.; Thorgeirsson, T.E.; Walters, R.K.; Polimanti, R.; Hatoum, A.S.; Sanchez-Roige, S.; Paul, S.K.; Wendt, F.R.; Clarke, T.; et al. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 2020, 7, 1032–1045. [Google Scholar] [CrossRef]
- Vink, J.M.; Willemsen, G.; Boomsma, D.I. Heritability of Smoking Initiation and Nicotine Dependence. Behav. Genet. 2005, 35, 397–406. [Google Scholar] [CrossRef]
- Sizoo, B.; Van Den Brink, W.; Koeter, M.W.J.; Van Eenige, M.G.; Van Wijngaarden Cremers, P.J.M.; Van Der Gaag, R.J. Treatment seeking adults with autism or ADHD and co-morbid Substance Use Disorder: Prevalence, risk factors and functional disability. Drug Alcohol Depend. 2010, 107, 44–50. [Google Scholar] [CrossRef]
- Allen, J.P.; Chango, J.M.; Szwedo, D.E.; Schad, M.M.; Marston, E.G. Predictors of Susceptibility to Peer Influence Regarding Substance Use in Adolescence. Child Dev. 2012, 83, 337–350. [Google Scholar] [CrossRef]
- McDonough, M.H.; Jose, P.E.; Stuart, J. Bi-directional Effects of Peer Relationships and Adolescent Substance Use: A Longitudinal Study. J. Youth Adolesc. 2016, 45, 1652–1663. [Google Scholar] [CrossRef]
- Schaub, M.P.; Henderson, C.E.; Pelc, I.; Tossmann, P.; Phan, O.; Hendriks, V.; Rigter, H. Multidimensional family therapy decreases the rate of externalizing behavioural disorder symptoms in cannabis abusing adolescents: Outcomes of the INCANT trial. BMC Psychiatry 2014, 14, 26. [Google Scholar] [CrossRef]
- Ryabov, I. Relation of peer effects and school climate to substance use among Asian American adolescents. J. Adolesc. 2015, 42, 115–127. [Google Scholar] [CrossRef]
- Poulin, F.; Kiesner, J.; Pedersen, S.; Dishion, T.J. A short-term longitudinal analysis of friendship selection on early adolescent substance use. J. Adolesc. 2011, 34, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lynskey, M.; Hall, W. The effects of adolescent cannabis use on educational attainment: A review. Addiction 2000, 95, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.H.; Hill, M.L.; Small, P.J.; Luthar, S.S. Associations of adolescent cannabis use with academic performance and mental health: A longitudinal study of upper middle class youth. Drug Alcohol Depend. 2015, 156, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.M.; Gilman, J.; Schoenfeld, D.; Evenden, J.; Hareli, M.; Ulysse, C.; Nip, E.; Hanly, A.; Zhang, H.; Evins, A.E. One Month of Cannabis Abstinence in Adolescents and Young Adults Is Associated with Improved Memory. J. Clin. Psychiatry 2018, 79, 2484. [Google Scholar] [CrossRef] [PubMed]
- Ministerie van Justitie en Veiligheid. Gedoogbeleid Softdrugs en Coffeeshops. Drugs|Rijksoverheid.nl. Available online: https://www.rijksoverheid.nl/onderwerpen/drugs/gedoogbeleidsoftdrugs-en-coffeeshops (accessed on 9 October 2019).
- Taylor, M.; Cousijn, J.; Filbey, F. Determining Risks for Cannabis Use Disorder in the Face of Changing Legal Policies. Curr. Addict. Rep. 2019, 6, 466–477. [Google Scholar] [CrossRef] [PubMed]
- ESPAD Group. Results from the European School Survey Project on Alcohol and Other Drugs; ESPAD Report 2019; EMCDDA Joint Publications: Luxembourg, 2019. [Google Scholar]
- Meulenbroeks, R.; Reijerkerk, M.; Angerer, E.; Pieters, T.; Bakker, A. Academic discourse on education during the early part of the pandemic. Heliyon 2022, 8, e11170. [Google Scholar] [CrossRef]
- Pelikan, E.R.; Lüftenegger, M.; Holzer, J.; Korlat, S.; Spiel, C.; Schober, B. Learning during COVID-19, the role of self-regulated learning, motivation, and procrastination for perceived competence. Z Erzieh. 2021, 24, 393–418. [Google Scholar] [CrossRef]
- Taylor, S.; Paluszek, M.M.; Rachor, G.S.; McKay, D.; Asmundson, G.J. Substance use and abuse, COVID-19-related distress, and disregard for social distancing: A network analysis. Addict. Behav. 2021, 114, 106754. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, D.J.; Kuijpers, W.G.T.; Kerssies, J.P.; Van Der Slink, J.B.; Kersten, A.; Jansen, B.A.L.; Van Der Hoop-Terwindt, V.A.M. Tussenrapportage Kerncijfers Verslavingszorg 2016–2021. In Ladis.eu. Stichting Informatievoorziening Zorg (IVZ). 2023. Available online: https://cdn.bluenotion.nl/209c705755afb5baca6501d22c0ddc6e05c586540b886fe813b7a1b3cb76695.pdf (accessed on 2 April 2023).
- Dennis, M.L.; Clark, H.W.; Huang, L.N. The need and opportunity to expand substance use disorder treatment in school-based settings. Adv. Sch. Ment. Health Promot. 2014, 7, 75–87. [Google Scholar] [CrossRef]
- Walker, A.A.; Stephens, R.S.; Blevins, C.E.; Banes, K.E.; Mathews, L.; Roffman, R.A. Augmenting brief interventions for adolescent marijuana users: The impact of motivational check-ins. J. Consult. Clin. Psychol. 2016, 84, 983–992. [Google Scholar] [CrossRef]
- Hendriks, V.M.; Van Der Schee, E.; Blanken, P.D. Treatment of adolescents with a cannabis use disorder: Main findings of a randomized controlled trial comparing multidimensional family therapy and cognitive behavioral therapy in The Netherlands. Drug Alcohol Depend. 2011, 119, 64. [Google Scholar] [CrossRef] [PubMed]
- Hogue, A.; Henderson, C.E.; Becker, S.J.; Knight, D.K. Evidence Base on Outpatient Behavioral Treatments for Adolescent Substance Use, 2014–2017, Outcomes, Treatment Delivery, and Promising Horizons. J. Clin. Child Adolesc. Psychol. 2018, 47, 499–526. [Google Scholar] [CrossRef] [PubMed]
- Calomarde-Gómez, C.; Jiménez-Fernández, B.; Balcells-Oliveró, M.; Gual, A.; López Pelayo, H. Motivational Interviewing for Cannabis Use Disorders: A Systematic Review and Meta-Analysis. Eur. Addict. Res. 2021, 27, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Chazal, C.; Roux, C.; Kinouani, S.; Schuers, M.; Fortin, F.; Pereira, B.; Blanc, O.; Piñol Domenech, N.; Brousse, G.; Vorilhon, P.; et al. Effectiveness of brief interventions in primary care for cannabis users aged from 12 to 25 years old: A systematic review. Fam. Pract. 2022, 39, 1156–1168. [Google Scholar] [CrossRef]
- Blevins, C.E.; Banes, K.E.; Stephens, R.S.; Walker, D.D.; Roffman, R.A. Change in motives among frequent cannabis-using adolescents: Predicting treatment outcomes. Drug Alcohol Depend. 2016, 167, 175–181. [Google Scholar] [CrossRef]
- Westermann, G.; Thomas, M.S.C.; Karmiloff-Smith, A. Neuroconstructivism. In The Blackwell Handbook of Childhood Cognitive Development; Goswami, U., Ed.; Wiley-Blackwell: New York, NY, USA, 2011; pp. 723–747. [Google Scholar]
- Silins, E.; Horwood, L.J.; Patton, G.C.; Fergusson, D.M.; Olsson, C.A.; Hutchinson, D.M.; Coffey, C. Young adult sequelae of adolescent cannabis use: An integrative analysis. Lancet Psychiatry 2014, 1, 286–293. [Google Scholar] [CrossRef]
- Brinch, C.N.; Galloway, T.A. Schooling in adolescence raises IQ scores. Proc. Natl. Acad. Sci. USA 2012, 109, 425–430. [Google Scholar] [CrossRef]
- Kogan, S.M.; Luo, Z.; Brody, G.H.; Murry, V.M. The influence of high school dropout on substance use among African American youth. J. Ethn. Subst. Abus. 2005, 4, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Green, K.M.; Ensminger, M.E. Adult social behavioral effects of heavy adolescent marijuana use among African Americans. Dev. Psychol. 2006, 42, 1168. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Boden, J.M. Cannabis use and later life outcomes. Addiction 2008, 103, 969–976. [Google Scholar] [CrossRef]
- Verweij, K.J.H.; Huizink, A.C.; Agrawal, A.; Martin, N.G.; Lynskey, M.T. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug Alcohol Depend. 2013, 133, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.J.; Isen, J.D.; Khoddam, R.; Irons, D.; Tuvblad, C.; Iacono, W.G.; McGue, M.; Raine, A.; Baker, L.A. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proc. Natl. Acad. Sci. USA 2016, 113, E500–E508. [Google Scholar] [CrossRef]
- Brook, J.S.; Lee, J.P.; Finch, S.J.; Seltzer, N.; Brook, D.W. Adult Work Commitment, Financial Stability, and Social Environment as Related to Trajectories of Marijuana Use Beginning in Adolescence. Subst. Abus. 2013, 34, 298–305. [Google Scholar] [CrossRef]
- Conrod, P.J.; Castellanos-Ryan, N.; Strang, J. Brief, personality-targeted coping skills interventions and survival as a non–drug user over a 2-year period during adolescence. Arch. Gen. Psychiatry 2010, 67, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, V.M.; Dom, G. Proactieve psychiatrie bij verslaving. Tijdschr. Voor Psychiatr. 2021, 63, 125–128. [Google Scholar] [CrossRef]
- Das, J.K.; Arshad, A.; Finkelstein, Y.; Bhutta, Z.A. Interventions for Adolescent Substance Abuse: An Overview of Systematic Reviews. J. Adolesc. Health 2016, 59, S61–S75. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, E.J.; Houck, J.M.; Hunter, S.B.; Miles, J.N.V.; Osilla, K.C.; Ewing, B. Group motivational interviewing for adolescents: Change talk and alcohol and marijuana outcomes. J. Consult. Clin. Psychol. 2015, 83, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Hall, W.; Degenhardt, L. Adolescent Cannabis Use Disorders; Elsevier EBooks: Amsterdam, The Netherlands, 2020; pp. 111–135. [Google Scholar] [CrossRef]
- Hawes, S.W.; Trucco, E.M.; Duperrouzel, J.C.; Coxe, S.; Gonzalez, R.S. Developmental pathways of adolescent cannabis use: Risk factors, outcomes and seksspecific differences. Subst. Use Misuse 2019, 54, 271281. [Google Scholar] [CrossRef]
- Creemers, H.E.; Dijkstra, J.K.; Vollebergh, W.A.M.; Ormel, J.; Verhulst, F.C.; Huizink, C. Predicting life-time and regular cannabis use during adolescence; the roles of temperament and peer substance use: The TRAILS study. Addiction 2010, 105, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Luo, S.; Zhang, L.; Wu, S.; Chi, I. Acceptance and Commitment Therapy (ACT) to reduce depression: A systematic review and meta-analysis. J. Affect. Disord. 2020, 260, 728–737. [Google Scholar] [CrossRef]
- Osaji, J.; Ojimba, C.; Ahmed, S. The Use of Acceptance and Commitment Therapy in Substance Use Disorders: A Review of Literature. J. Clin. Med. Res. 2020, 12, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Suárez-Relinque, C.; Jiménez, T.I. Effects of family therapy for substance abuse: A systematic review of recent research. Family Process. 2022, 62, 49–73. [Google Scholar] [CrossRef] [PubMed]
| 1. | Taking more cannabis than was intended |
| 2. | Difficulty controlling or cutting down cannabis use |
| 3. | Spending a lot of time obtaining, using, or recovering from cannabis |
| 4. | Craving cannabis |
| 5. | Problems at work, school, and home as a result of cannabis use |
| 6. | Continuing cannabis use despite related social or relationship problems |
| 7. | Giving up or reducing other activities in favour of cannabis use |
| 8. | Taking cannabis in high-risk situations |
| 9. | Continuing to use cannabis despite physical or psychological problems |
| 10. | Tolerance to cannabis |
| 11. | Withdrawal symptoms when discontinuing cannabis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oosten, W.; Vos, E.; Los, L.; Nelwan, M.; Pieters, T. Towards a New Dynamic Interaction Model of Adolescent CUD Manifestation, Prevention, and Treatment: A Narrative Review. Psychoactives 2023, 2, 294-316. https://doi.org/10.3390/psychoactives2040019
Oosten W, Vos E, Los L, Nelwan M, Pieters T. Towards a New Dynamic Interaction Model of Adolescent CUD Manifestation, Prevention, and Treatment: A Narrative Review. Psychoactives. 2023; 2(4):294-316. https://doi.org/10.3390/psychoactives2040019
Chicago/Turabian StyleOosten, Wesley, Elena Vos, Leontien Los, Michel Nelwan, and Toine Pieters. 2023. "Towards a New Dynamic Interaction Model of Adolescent CUD Manifestation, Prevention, and Treatment: A Narrative Review" Psychoactives 2, no. 4: 294-316. https://doi.org/10.3390/psychoactives2040019
APA StyleOosten, W., Vos, E., Los, L., Nelwan, M., & Pieters, T. (2023). Towards a New Dynamic Interaction Model of Adolescent CUD Manifestation, Prevention, and Treatment: A Narrative Review. Psychoactives, 2(4), 294-316. https://doi.org/10.3390/psychoactives2040019







