1. Introduction
Delirium is a complex neuropsychiatric condition characterized by sudden impairments in attention and awareness that fluctuate throughout the day. As per the DSM-V [
1,
2,
3], the diagnostic criteria for delirium encompass an impairment in attention and is characterized as a transient, comprehensive disruption of cognitive functions like memory and language [
4]. Delirium can be categorized into three types: hyperactive, hypoactive, and mixed. These classifications are based on psychomotor changes, such as verbal communication, speech patterns, sleep behaviors, physical activity, and agitation [
5]. Within the hypoactive category of delirium, the person displays a diminished level of psychomotor activity, characterized by a state of sluggishness and lethargy that can approach a stupor. Delirium can further be seen as an extreme manifestation of a maladaptive sickness behavior response, where the goal of preserving energy expenditure is exceeded. The hypoactive phase of delirium can thus be considered a type of “sickness behavior” [
6,
7].
The hypoactive category may be more common compared to the other subtypes, with studies indicating that it accounts for approximately 50% of delirium cases [
8,
9]. This subcategory may have a worse prognosis and is associated with prolonged hospitalization, a heightened mortality rate, and a significant decline in cognitive awareness [
10,
11]. It is reported to be related to less reversible causes, such as conditions like hypoxia, metabolic imbalances, and organ failure. In contrast, hyperactive delirium is more commonly associated with factors like substance intoxication, infections, or medication withdrawal [
10,
11].
One of the foremost challenges in managing hypoactive delirium is early detection. The subtle and often overshadowed symptoms can easily be attributed to the patient’s primary illness, leading to delayed or missed diagnosis. Furthermore, distinguishing hypoactive delirium from other conditions such as depression or dementia poses a diagnostic challenge, necessitating comprehensive clinical assessments and specialized tools for accurate identification. Even after the diagnosis and extensive tests, treating delirium of all types can be difficult and treatment is debatable. Antipsychotics are the most common medications used in treating delirium, with haloperidol the drug of choice [
12]. Antipsychotics are not proven to be helpful in randomized clinical trials (RCTs) and do not increase the number of days alive without delirium or coma in terminally ill patients [
13,
14]. Non-pharmacologic interventions are effective but are not cost-effective and take time to bear results [
12]. While no standard pharmacotherapy is available for hypoactive delirium, the literature suggests addressing the root causes, hydration, hearing, and visual assistance, and promoting patient mobility when feasible may help [
15,
16].
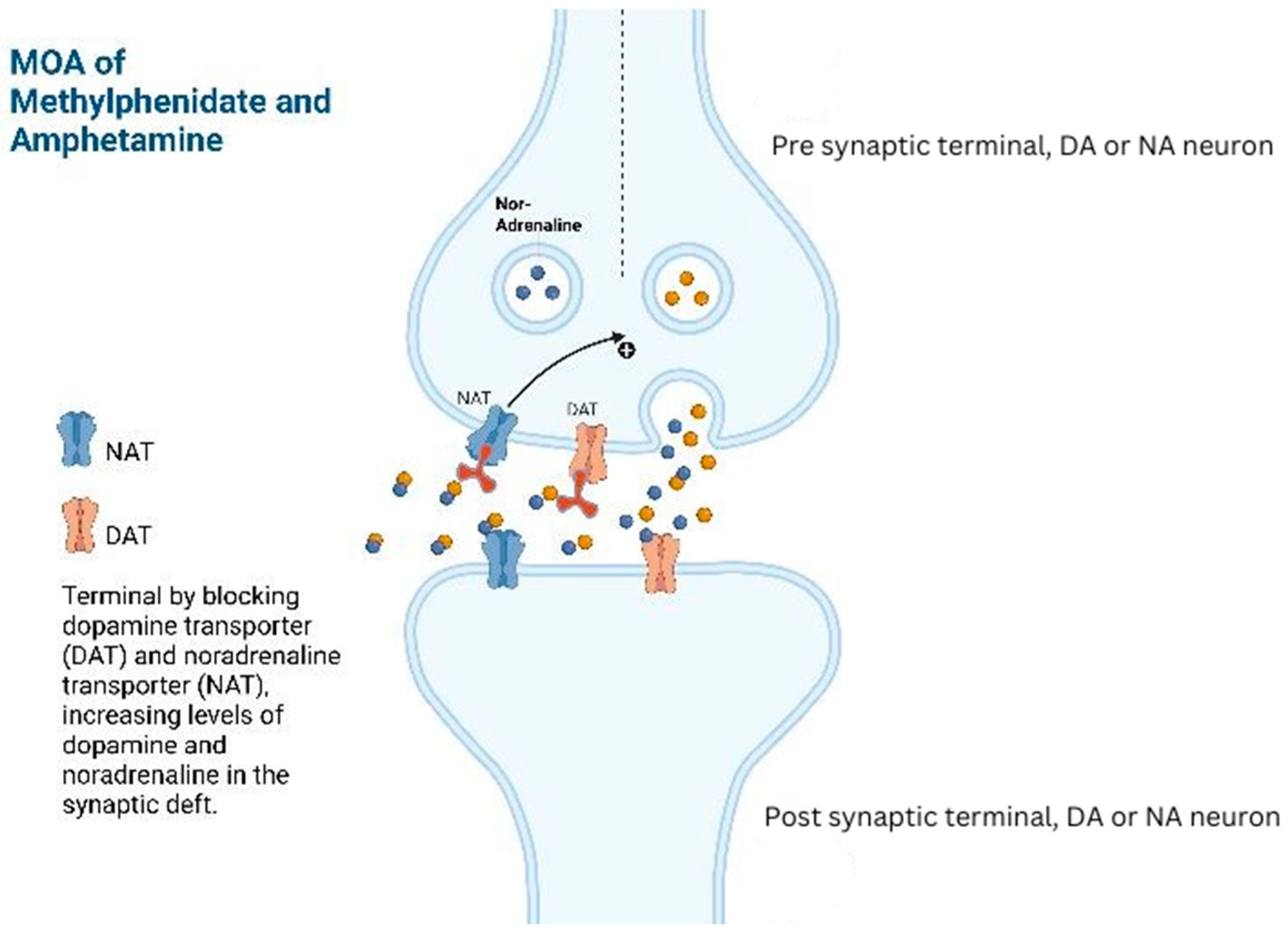
Methylphenidate or amphetamine analogues (e.g., lisdexamfetamine and dextroamphetamine) have proven benefits to counteract drowsiness, improving cognition, alleviating neurobehavioral symptoms, and managing conditions like post-traumatic narcolepsy, brain injury-related anger, attention-deficit hyperactivity disorder (ADHD), and reducing coma duration caused by brain trauma [
17]. In patients with hypoactive delirium, specific nuclei in the brain are impacted by disrupted phasic–tonic firing in the mesolimbic–nucleus accumbens–anterior cingulate–prefrontal circuitry. Amphetamine analogues may affect this circuitry by promoting the recovery of the phasic–tonic balance. By inhibiting the reuptake, it increases the dopaminergic and noradrenergic activity in the prefrontal cortex, leading to increased alertness which can help resolve psychomotor retardation associated with hypoactive delirium (
Figure 1) [
17,
18].
Our aim with this narrative review is to explore the potential role amphetamine analogues may play as a treatment option for hypoactive delirium.
2. Methods
We conducted a systematic literature search of PubMed, MEDLINE, Cochrane, and clinical trial registries from 1990 to 15 March 2023, in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [
19] statement. This manuscript does not require registration for PROSPERO. The following keyword combinations were used: “hypoactive delirium AND dextroamphetamine”, “hypoactive delirium AND amphetamine”, “hypoactive delirium AND lisdexamfetamine”, “hypoactive delirium AND stimulants,” “hypoactive delirium AND methylphenidate,” and “hypoactive delirium AND Ritalin.” We included “Ritalin” and “stimulants” in our search words to include all the relevant studies in our literature review. Our inclusion criteria were all original studies conducted in humans, including case reports, case series, cross-sectional studies, prospective clinical studies, clinical trials, retrospective chart reviews, case–control, and cohort studies. We excluded animal and non-original studies like letters to editors, review articles, systematic reviews, meta-analyses, commentaries, short reviews, and editorials. The data collection, screening, and extraction were performed using MS Excel, and at least two reviewers completed the study procedures. Demographics, comorbidities, and outcomes of the patients treated with methylphenidate for hypoactive delirium were reported in the results section.
We retrieved a total of 68 articles from all the databases after removing duplicates, and two independent authors worked on it separately, and finally, this conclusion was reached. Any disagreement between the two authors regarding the inclusion or exclusion criteria of studies in this systematic review was resolved by in-depth discussion among authors and independently verified by another author. After applying our exclusion/inclusion criteria, we removed 55 articles based on title and abstract screening and 6 articles upon full-text screening. We could not find the full text for four articles, so they were removed during data extraction. In total, we retrieved three articles to include in our systematic review (
Figure 2).
3. Results
Three studies representing 17 participants in total were pooled, with 9 males and 8 females, depicted in
Table 1. The ages ranged from 21 to 80 years. All patients were diagnosed with advanced cancer and admitted to hospice care before the diagnosis of hypoactive delirium and initiation of methylphenidate. The starting dose for Methylphenidate was 5 mg/day and maintained at 10–20 mg/day. All patients showed sudden and drastic improvement, including increased alertness, ability to perform activities of daily living, improved communication, and resolution of delirium.
In a prospective clinical trial carried out by Gagnon et al. [
17], a study group consisting of 14 patients between the ages of 41 and 80, all suffering from advanced metastatic cancer and hypoactive delirium, were administered methylphenidate. The treatment demonstrated favorable results, manifesting as heightened awareness, substantial improvement or complete resolution of psychomotor retardation, normalization of speech difficulties, and a significant surge in energy levels. Notably, the patients’ scores on the Mini-Mental Status Exam (MMSE) also exhibited progressive enhancement, with the median score jumping from 21 to 27 after the initial dose of methylphenidate.
For the majority of patients, an appropriate daily dosage ranging from 20 to 30 mg was found to be effective. Increasing the dosage beyond this range did not yield any further improvement and instead resulted in heightened adverse effects. The observable progression of improvement was seen initially in the patient’s ability to draw and write, followed by increased attention. However, partial impairment of recall was still evident in most cases. Remarkably, patients experienced a sustained response until a few days before their inevitable passing. The survival duration ranged from 4 days to 205 days, with a median survival period of 39.5 days. Notably, all three patients who survived for less than two weeks were afflicted by severe cancer cachexia.
Keen and Brown [
20] examined two cases in their report. The first case involved a 65-year-old woman with inoperable gastric adenocarcinoma and non-insulin-dependent diabetes mellitus who was admitted to hospice with various symptoms of nausea, fatigue, and epigastric pain. Medications in hospice included transdermal fentanyl, cyclizine, and insulin. On day 13, the patient was diagnosed with delirium with features of a mixed-type delirium. Her symptoms included increased confusion and withdrawal, scarce eye contact, and increased hallucinations. The patient was more attuned to a state of hypoactive delirium, and she was started on 2.5 mg methylphenidate BID. The patient became active and interactive within the first 3.5 h after the first dose. The dosage was increased to 5 mg BID but had to be stopped after 5 days due to increased nausea. Within 2 days of stopping methylphenidate, the patient became delirious again. Methylphenidate was restarted, and she showed improvement and was eventually discharged from hospice with the medication. She later died at home.
The second case involved a 51-year-old woman diagnosed with metastatic renal carcinoma who was admitted to hospice care due to limited mobility caused by lower limb lymphoedema, a low mood state, and controlled mixed nociceptive/neuropathic pain. She had a previous history of depression and was not compliant with antidepressants, had chronic mild hypercalcemia, hypertension, and deep venous thrombosis of the lymphoedematous leg. The patient was started on paroxetine on day 7; however, she became increasingly withdrawn and was diagnosed with hypoactive delirium using the Confusion Assessment Method (CAM) criteria. On day 19, the patient was completely withdrawn, without verbal communication, and her behavior suggested intermittent hallucinations. The initial dose of 2.5 mg methylphenidate was started and continued to 5 mg BID. The patient was more active, made eye contact, and initiated conversation 48 h after starting methylphenidate. Methylphenidate was increased to 15 mg in doses of 10 mg with breakfast and 5 mg with lunch. Delirium was resolved, and the patient’s mood and communication improved from day 22 to 30. The patient was unable to take methylphenidate due to a urinary tract infection on day 80, following which she developed paranoia and hallucinations, which resolved after restarting the treatment. The methylphenidate dose was gradually increased and maintained at 15 mg BID until the patient died of septicemia on day 111.
Morita et al. [
21] discussed a 51-year-old woman with a history of gastric cancer who underwent a total gastrectomy. Five years later, the patient was admitted to a palliative care unit for continuous pain in her neck, right arm, and hip due to multiple bone metastases to cervical and lumbar vertebrae. She required significant assistance in daily activities due to uncontrolled pain. Her mental status was clear, and she communicated appropriately with the carers. The patient’s symptoms were managed using subcutaneous fentanyl, betamethasone, and metoclopramide, which provided acceptable relief within five days without any signs of cognitive impairment. However, on day 16, the patient exhibited severe somnolence, short-term memory disturbance, disorientation to time, and inability to maintain communication and concentration, despite no change in medication. She was diagnosed with hypoactive delirium based on the Memorial Delirium Assessment Scale (MDAS) and Delirium Rating Scale (DRS); she scored 21 points and 20 points, respectively. After clinical evaluation and lab findings, she was diagnosed with delirium due to disseminated intravascular coagulation (DIC) leading to multi-organ failure. Methylphenidate was administered orally at 10–20 mg/day for symptom resolution, significantly improving the patient’s somnolence and communication ability. Her scores on the MDAS and DRS were 4 and 10, respectively. She continued to receive oral methylphenidate for 14 days with adequate symptom control and a score of less than five on the MDAS until her underlying disease worsened, and she could no longer take the medication orally.
4. Discussion
This review aimed to investigate the effectiveness of amphetamine analogues for hypoactive delirium. Our findings suggest that methylphenidate may be a useful treatment option for this condition. As managing hypoactive delirium is often challenging, underdiagnosed, and difficult to treat, the potential effectiveness of methylphenidate represents a significant advancement in palliative care. In the studies that were reviewed, all of the patients demonstrated positive effects on symptoms related to hypoactive delirium. Thus, methylphenidate appears to be a potentially beneficial treatment option for managing hypoactive delirium in terminally ill patients. Improvement in cognitive function on Mini-Mental State Examination (MMSE) and in delirious symptoms on methylphenidate shows that it could be a potentially useful treatment option and can reduce morbidity around stressful conditions [
17,
20,
21]. Additional therapies like music therapy and transcranial direct current stimulation (tDCS) can potentiate these effects by activating dopaminergic and noradrenergic pathways, respectively, warranting controlled studies [
22,
23,
24].
Albeit, it is pivotal for treating physicians to mitigate clinical efficacy with potential side effects of methylphenidate. In a meta-analysis by Chings et al., participants in randomized controlled trials (RCTs) who received methylphenidate treatment for ADHD had a higher risk of adverse events compared to those who were given a placebo. These events included headaches, anorexia, insomnia, and abdominal pain. Additionally, in cohort studies, a significant percentage of participants experienced adverse events, with anorexia, insomnia, and headaches being the most common [
25].
The potential efficacy of methylphenidate in palliative care is a notable development. However, the off-label use of medications raises ethical, legal, and safety concerns in the broader clinical context. Moreover, the use of methylphenidate may be limited to patients with advanced tumor disease due to specific considerations. First, methylphenidate is primarily indicated for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. Its use in the context of advanced tumor disease may be off-label, meaning it is not approved specifically for that use, but it may be considered based on specific considerations such as managing fatigue and improving cognitive function in palliative care situations. Additionally, patients with advanced tumor disease may be taking multiple medications, and the potential side effects and interactions of methylphenidate with other medications need to be carefully monitored. Finally, the decision to use specific medications, including methylphenidate, in patients with advanced tumor disease is often made on an individual basis. It is pivotal to take into account the patient’s overall condition, other available treatment options, and potential benefits and risks associated with the use of methylphenidate.
The off-label use of methylphenidate may impact clinicians’ decision to not use it in patient groups that could potentially benefit. Off-label use poses concerns regarding legality, ethics, and liability for healthcare professionals. However, in certain cases, clinicians may still consider off-label use of methylphenidate if they believe the potential benefits outweigh the risks. This could be the case in situations such as treatment-resistant depression or cognitive impairment where methylphenidate has shown potential efficacy. Overall, the decision to use methylphenidate off-label should be based on careful evaluation of available evidence and patient-specific factors.
Providers, healthcare staff, and caregivers of chronically ill patients or acute trauma patients should be educated on the early signs and symptoms of delirium to treat the reversible causes of delirium, especially hypoactive delirium which is the most difficult to diagnose [
8]. The early detection and reversal of cause and risk factors can prevent morbidity and mortality in these patients. This review included three studies that were focused on hypoactive delirium. Studies were underpowered and had a short treatment duration. Although methylphenidate has shown benefits for treating hypoactive delirium, it has only been studied widely in terminally ill patients.
Future studies investigating the use of methylphenidate for hypoactive delirium should consider a longitudinal design with diverse patient populations to assess long-term safety and efficacy, incorporating controlled trials to determine optimal dosing and application intervals. Robust adverse event monitoring, pharmacokinetic, and pharmacodynamic analyses are crucial, as is a thorough investigation of the dose–response relationship and patient stratification based on clinical characteristics. Integrating real-time monitoring technologies, considering ethnic and genetic variability, establishing standardized safety assessment protocols, and implementing long-term follow-up assessments are essential for a comprehensive understanding of the drug’s safety and efficacy in this context.
5. Study Limitations and Potential Biases
This review has several limitations that affect the interpretation and generalizability of the findings. Firstly, the narrow scope of the review, which encompassed only three studies, limits the comprehensive understanding of the efficacy and safety of methylphenidate for hypoactive delirium. This limitation highlights the need for more extensive literature reviews and meta-analyses to provide a more holistic perspective on the topic.
Moreover, the inclusion of studies with susceptible biases, such as small sample sizes, short treatment durations, and potential selection biases, calls for a cautious interpretation of the findings. The absence of a placebo-controlled group in some of the studies may introduce a significant risk of bias in evaluating the true effects of methylphenidate. Additionally, the restriction to English-language publications could result in language bias and the exclusion of relevant studies conducted in other languages.
Furthermore, the heterogeneity in patient populations and the lack of standardized diagnostic criteria for hypoactive delirium among the included studies might have introduced inconsistencies in the assessment and classification of the condition. These variations in study design and patient characteristics could impact the overall robustness and generalizability of the findings.
6. Conclusions
Our study reviews the potential use of methylphenidate as a treatment option for individuals with hypoactive delirium, particularly in the terminally ill population. However, further research should consider a longitudinal design with diverse patient populations to assess long-term safety and efficacy, incorporating controlled trials to determine optimal dosing and application intervals.
Author Contributions
A.B. conceived and designed the study, collected the data, contributed to the review of literature, wrote the manuscript, edited the manuscript, and oversaw and coordinated the workflow with other authors. P.K. collected and updated the data, contributed to the review of literature, wrote the manuscript, and revised/edited the manuscript. T.K. and G.Y. updated the data collection, and revised and edited the manuscript. S.A. and S.P. were involved in software, methodology, and formal analysis. B.N.P. wrote the manuscript, and revised and edited the manuscript. K.K. provided the final edits and corrections, expert-level guidance, and final approval of the study. N.A.Y. provided the revisions and corrections, oversaw and coordinated the workflow with other authors, and provided expert-level guidance and final approval of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
In this study, no new data were generated, and any additional information or clarifications regarding the data used in this study can be requested from the corresponding author or the responsible ethics committee, subject to the applicable privacy and ethical guidelines.
Conflicts of Interest
Youssef discloses that in the last 5 years, he received research support from the National Institute of Health (NIH), Department of Veteran Affairs, Department of Defense, and research support but not salary support from MECTA Corporation, Vistagen, and Merck. He receives royalties from Elsevier Publishing.
References
- Morrison, J. DSM-5-TR® Made Easy: The Clinician’s Guide to Diagnosis; Guilford Publications: New York, NY, USA, 2023; 690p, Available online: https://books.google.com/books/about/DSM_5_TR_Made_Easy.html?hl=&id=t0SKEAAAQBAJ (accessed on 7 May 2023).
- Black, D.W.; Grant, J.E. DSM-5 Guidebook: The Essential Companion to the Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2014; 570p, Available online: https://books.google.com/books/about/DSM_5_Guidebook.html?hl=&id=lKeTAwAAQBAJ (accessed on 7 May 2023).
- First, M.B. DSM-5 Handbook of Differential Diagnosis; American Psychiatric Publishing: Washington, DC, USA, 2013; 340p, Available online: https://books.google.com/books/about/DSM_5_Handbook_of_Differential_Diagnosis.html?hl=&id=SqeTAwAAQBAJ (accessed on 7 May 2023).
- Burry, L.; Mehta, S.; Perreault, M.M.; Luxenberg, J.S.; Siddiqi, N.; Hutton, B.; A Fergusson, D.; Bell, C.; Rose, L. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 2018, 6, CD005594. [Google Scholar] [CrossRef]
- Meagher, D. Motor subtypes of delirium: Past, present and future. Int. Rev. Psychiatry 2009, 21, 59–73. [Google Scholar] [CrossRef]
- Dantzer, R.; Kelley, K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef]
- Cunningham, C.; Maclullich, A.M. At the extreme end of the psychoneuroimmunological spectrum: Delirium as a maladaptive sickness behaviour response. Brain Behav. Immun. 2013, 28, 1–13. [Google Scholar] [CrossRef]
- Hosker, C.; Ward, D. Hypoactive delirium. BMJ 2017, 357, j2047. [Google Scholar] [CrossRef]
- Oh, E.S.; Fong, T.G.; Hshieh, T.T.; Inouye, S.K. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA 2017, 318, 1161–1174. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Stelfox, H.T.; Leigh, J.P.; Ely, E.W.; Fiest, K.M. Incidence and Prevalence of Delirium Subtypes in an Adult ICU: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 2029–2035. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, S.-W.; Kim, J.-M.; Shin, I.-S.; Bae, K.-Y.; Shim, H.-J.; Bae, W.-K.; Cho, S.-H.; Chung, I.-J.; Yoon, J.-S. Differential Associations Between Delirium and Mortality According to Delirium Subtype and Age: A Prospective Cohort Study. Psychosom. Med. 2015, 77, 903–910. [Google Scholar] [CrossRef]
- Yano, M.; Steiner, H. Methylphenidate and cocaine: The same effects on gene regulation? Trends Pharmacol. Sci. 2007, 28, 588–596. [Google Scholar] [CrossRef]
- van Velthuijsen, E.L.; Zwakhalen, S.M.G.; Mulder, W.J.; Verhey, F.R.J.; Kempen, G.I.J.M. Detection and management of hyperactive and hypoactive delirium in older patients during hospitalization: A retrospective cohort study evaluating daily practice. Int. J. Geriatr. Psychiatry 2018, 33, 1521–1529. [Google Scholar] [CrossRef]
- Kappenschneider, T.; Meyer, M.; Maderbacher, G.; Parik, L.; Leiss, F.; Quintana, L.P.; Grifka, J. Delir—Eine interdisziplinäre Herausforderung [Delirium—An interdisciplinary challenge]. Orthopade 2022, 51, 106–115. [Google Scholar] [CrossRef]
- Girard, T.D.; Exline, M.C.; Carson, S.S.; Hough, C.L.; Rock, P.; Gong, M.N.; Douglas, I.S.; Malhotra, A.; Owens, R.L.; Feinstein, D.J.; et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N. Engl. J. Med. 2018, 379, 2506–2516. [Google Scholar] [CrossRef]
- Challman, T.D.; Lipsky, J.J. Methylphenidate: Its pharmacology and uses. Mayo Clin. Proc. 2000, 75, 711–721. [Google Scholar] [CrossRef]
- Grover, S.; Avasthi, A. Clinical Practice Guidelines for Management of Delirium in Elderly. Indian J. Psychiatry 2018, 60 (Suppl. 3), S329–S340. [Google Scholar] [CrossRef]
- Gagnon, B.; Low, G.; Schreier, G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: A prospective clinical study. J. Psychiatry Neurosci. 2005, 30, 100–107. [Google Scholar]
- Verghese, C.; Abdijadid, S. Methylphenidate. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482451/ (accessed on 7 January 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Keen, J.C.; Brown, D. Psychostimulants and delirium in patients receiving palliative care. Palliat. Support. Care 2004, 2, 199–202. [Google Scholar] [CrossRef]
- Morita, T.; Otani, H.; Tsunoda, J.; Inoue, S.; Chihara, S. Successful palliation of hypoactive delirium due to multi-organ failure by oral methylphenidate. Support. Care Cancer 2000, 8, 134–137. [Google Scholar] [CrossRef]
- Plazier, M.; Tchen, S.; Ost, J.; Joos, K.; De Ridder, D.; Vanneste, S. Is Transcranial Direct Current Stimulation an Effective Predictor for Invasive Occipital Nerve Stimulation Treatment Success in Fibromyalgia Patients? Neuromodulation 2015, 18, 623–629. [Google Scholar] [CrossRef]
- Menon, V.; Levitin, D.J. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage 2005, 28, 175–184. [Google Scholar] [CrossRef]
- Ching, C.; Eslick, G.D.; Poulton, A.S. Evaluation of Methylphenidate Safety and Maximum-Dose Titration Rationale in Attention-Deficit/Hyperactivity Disorder: A Meta-analysis. JAMA Pediatr. 2019, 173, 630–639. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).