The Integration and Application of Extended Reality (XR) Technologies within the General Practice Primary Medical Care Setting: A Systematic Review
Abstract
:1. Introduction
1.1. Extended Reality (XR)
1.2. The Metaverse
2. Materials and Methods
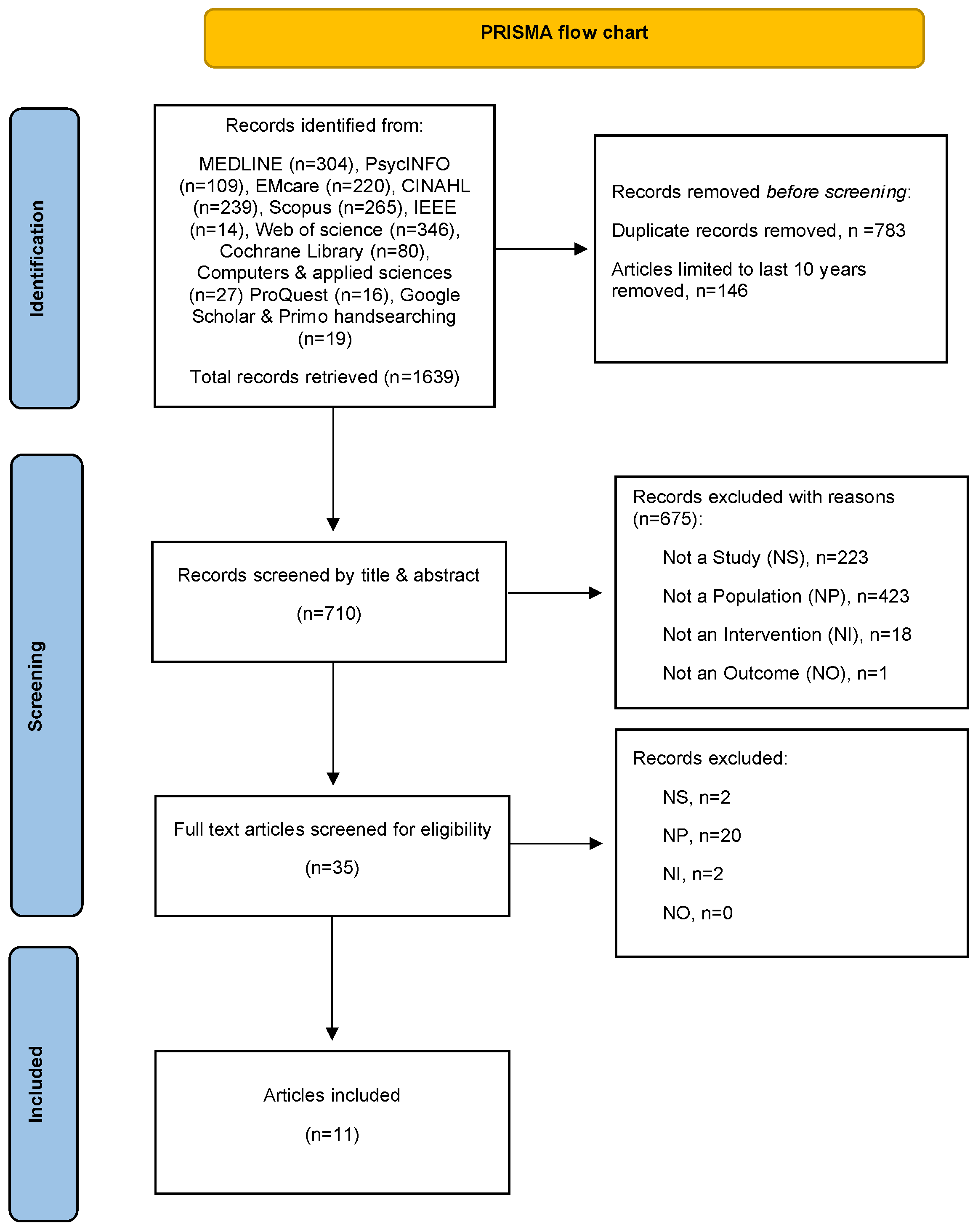
2.1. Data Sources and Searches
2.2. Eligibility Criterion
2.3. Study Selection
2.4. Quality Appraisal
2.5. Data Extraction
3. Results
3.1. Study Characteristics
3.2. IVR for Childhood Vaccinations
3.3. IVR for Mental Health Therapy or Assessment
3.4. IVR for Health Promotion/Disease Prevention
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matenge, S.; Sturgiss, E.; Desborough, J.; Dykgraaf, S.H.; Dut, G.; Kidd, M. Ensuring the continuation of routine primary care during the COVID-19 pandemic: A review of the international literature. Fam. Pract. 2021, 39, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S. Use of Extended Reality in Medicine During the COVID-19 Pandemic. In Extended Reality Usage During COVID 19 Pandemic; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–14. [Google Scholar]
- Chengoden, R.; Victor, N.; Huynh-The, T.; Yenduri, G.; Jhaveri, R.H.; Alazab, M.; Bhattacharya, S.; Hegde, P.; Maddikunta, P.K.R.; Gadekallu, T.R. Metaverse for Healthcare: A Survey on Potential Applications, Challenges and Future Directions. arXiv 2022, arXiv:2209.04160. [Google Scholar] [CrossRef]
- Musamih, A.; Yaqoob, I.; Salah, K.; Jayaraman, R.; Al-Hammadi, Y.; Omar, M.; Ellahham, S. Metaverse in Healthcare Applications Challenges and Future Directions. IEEE Consum. Electron. Mag. 2022, 12, 33–46. [Google Scholar] [CrossRef]
- Sun, M.; Xie, L.; Liu, Y.; Li, K.; Jiang, B.; Lu, Y.; Yang, Y.; Yu, H.; Song, Y.; Bai, C.; et al. The Metaverse in Current Digital Medicine. Clin. eHealth 2022, 5, 52–57. [Google Scholar] [CrossRef]
- Milgram, P.; Kishino, F. A taxonomy of mixed reality visual displays. IEICE Trans. Inf. Syst. 1994, 77, 1321–1329. [Google Scholar]
- Bansal, G.; Rajgopal, K.; Chamola, V.; Xiong, Z.; Niyato, D. Healthcare in Metaverse: A Survey on Current Metaverse Applications in Healthcare. IEEE Access 2022, 10, 119914–119946. [Google Scholar] [CrossRef]
- Fealy, S.; Jones, D.; Hutton, A.; Graham, K.; McNeill, L.; Sweet, L.; Hazelton, M. The integration of immersive virtual reality in tertiary nursing and midwifery education: A scoping review. Nurse Educ. Today 2019, 79, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.K.; Paul, S. Augmented Reality and Virtual Reality Creating Ripple in Medical and Pharmaceutical World; Springer Singapore Pte. Limited: Singapore, 2022; Volume 998. [Google Scholar]
- Stephenson, N. Snow Crash; Bantum Books: New York, NY, USA, 1992. [Google Scholar]
- Tacgin, Z.; Hagin, A. Enhanced Learning Environments Technology and Innovation, 1st ed.; Zeynep Tacgin, A.H., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2022. [Google Scholar]
- Skalidis, I.; Fournier, S.; Skalidis, E.; Maurizi, N. Virtual hospitals and digital doctors: How far are we from the CardioVerse? Eur. Heart J. 2022, 44, 7–9. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, J.; Chen, R.; Song, Y.; Song, Z.; Zhang, X.; Wang, Q.; Wang, K.; Zhou, C.; Sun, J.; et al. Expert consensus on the metaverse in medicine. Clin. eHealth 2022, 5, 1–9. [Google Scholar] [CrossRef]
- Tang, Y.M.; Chau, K.Y.; Kwok, A.P.K.; Zhu, T.; Ma, X. A systematic review of immersive technology applications for medical practice and education—Trends, application areas, recipients, teaching contents, evaluation methods, and performance. Educ. Res. Rev. 2021, 35, 100429. [Google Scholar] [CrossRef]
- López-Ojeda, W.; Hurley, R.A. Extended-reality technologies: An overview of emerging applications in medical education and clinical care. J. Neuropsychiatry Clin. Neurosci. 2021, 33, A4-177. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.; Lionis, C. Compassionate Care within the Primary Health Care Setting: Before and During a Public Health Crisis. In The Art and Science of Compassionate Care: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2023; pp. 43–59. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Rosella, L.; Bowman, C.; Pach, B.; Morgan, S.; Fitzpatrick, T.; Goel, V. The development and validation of a meta-tool for quality appraisal of public health evidence: Meta Quality Appraisal Tool (MetaQAT). Public Health 2016, 136, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Kang, G.C.-Y.; Koh, E.Y.L.; Fong, R.J.K.; Tang, J.; Goh, C.K.; Tan, N.C. Immersive Virtual Reality in Alleviating Pain and Anxiety in Children During Immunization in Primary Care: A Pilot Randomized Controlled Trial. Front. Pediatr. 2022, 10, 847257. [Google Scholar] [CrossRef]
- Lim, J.E.; Wong, W.T.; Teh, T.A.; Lim, S.H.; Allen, J.C.; Quah, J.H.M.; Malhotra, R.; Tan, N.C. A Fully-Immersive and Automated Virtual Reality System to Assess the Six Domains of Cognition: Protocol for a Feasibility Study. Front. Aging Neurosci. 2021, 12, 604670. [Google Scholar] [CrossRef]
- Tan, N.C.; Lim, J.E.; Allen, J.C.; Wong, W.T.; Quah, J.H.M.; Muthulakshmi, P.; Teh, T.A.; Lim, S.H.; Malhotra, R. Age-Related Performance in Using a Fully Immersive and Automated Virtual Reality System to Assess Cognitive Function. Front. Psychol. 2022, 13, 847590. [Google Scholar] [CrossRef]
- Wong, W.T.; Tan, N.C.; Lim, J.E.; Allen, J.C.; Lee, W.S.; Quah, J.H.M.; Paulpandi, M.; Teh, T.A.; Lim, S.H.; Malhotra, R. Comparison of Time Taken to Assess Cognitive Function Using a Fully Immersive and Automated Virtual Reality System vs. the Montreal Cognitive Assessment. Front. Aging Neurosci. 2021, 13, 756891. [Google Scholar] [CrossRef]
- Herrera, M.d.l.C.; Fuster-Casanovas, A.; Catalina, Q.M.; Mensa, M.C.; Pinillos, P.A.; Guitart, I.V.; Carrión, S.G.; Vidal-Alaball, J. Use of Virtual Reality in the Reduction of Pain After the Administration of Vaccines Among Children in Primary Care Centers: Protocol for a Randomized Clinical Trial. JMIR Res. Protoc. 2022, 11, e35910. [Google Scholar] [CrossRef]
- Navarro-Haro, M.V.; Modrego-Alarcón, M.; Hoffman, H.G.; López-Montoyo, A.; Navarro-Gil, M.; Montero-Marin, J.; García-Palacios, A.; Borao, L.; García-Campayo, J. Evaluation of a Mindfulness-Based Intervention With and Without Virtual Reality Dialectical Behavior Therapy® Mindfulness Skills Training for the Treatment of Generalized Anxiety Disorder in Primary Care: A Pilot Study. Front. Psychol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Palomo, P.; Rodrigues de Oliveira, D. Study Protocol for a randomized controlled trial of mindfulness training with immersive technology (virtual reality) to improve the quality of life of patients with multimorbidity in Primary Care- the Mindful-VR study. Annu. Rev. Cyber Ther. Telemed. 2018, 16, 140–147. [Google Scholar]
- Lundin, J.; Lundström, A.; Gulliksen, J.; Blendulf, J.; Ejeby, K.; Nyman, H.; Björkander, D.; Hedman-Lagerlöf, E. Using 360-degree videos for virtual reality exposure in CBT for panic disorder with agoraphobia: A feasibility study. Behav. Cogn. Psychother. 2022, 50, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.; Simonsen, S.; Jensen, J.D.; Yingling, L.; Schaefer, J.; Sundaresh, V.; Zhang, Y.; Altizer, R. Mobile Virtual Reality Versus Mobile 360° Video to Promote Enrollment in the Diabetes Prevention Program Among Hispanic Adults: Pilot Study. JMIR Diabetes 2022, 7, e26013. [Google Scholar] [CrossRef] [PubMed]
- Ellerton, K.; Tharmarajah, H.; Medres, R.; Brown, L.; Ringelblum, D.; Vogel, K.; Dolphin, A.; McKellar, S.; Bridson, F.; John-White, M.; et al. The VRIMM study: Virtual Reality for IMMunisation pain in young children—Protocol for a randomised controlled trial. BMJ Open 2020, 10, e038354. [Google Scholar] [CrossRef]
- Althumairi, A.; Sahwan, M.; Alsaleh, S.; Alabduljobar, Z.; Aljabri, D. Virtual Reality: Is It Helping Children Cope with Fear and Pain During Vaccination? J. Multidiscip. Healthc. 2021, 14, 2625–2632. [Google Scholar] [CrossRef]
- Eijlers, R.; Utens, E.M.W.J.; Staals, L.M.; Nijs, P.F.A.d.; Berghmans, J.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Dierckx, B.; Legerstee, J.S. Systematic Review and Meta-analysis of Virtual Reality in Pediatrics: Effects on Pain and Anxiety. Anesth. Analg. 2019, 129, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lin, J.; Han, R.; Peng, C.; Huang, A. Using Virtual Reality Exposure Therapy in Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Value Health 2022, 25, 288–301. [Google Scholar] [CrossRef]
- Riva, G.; Serino, S. Virtual Reality in the Assessment, Understanding and Treatment of Mental Health Disorders. J. Clin. Med. 2020, 9, 3434. [Google Scholar] [CrossRef]
- Thunström, A.O.; Sarajlic Vukovic, I.; Ali, L.; Larson, T.; Steingrimsson, S. Prevalence of virtual reality (VR) games found through mental health categories on STEAM: A first look at VR on commercial platforms as tools for therapy. Nord. J. Psychiatry 2022, 76, 474–485. [Google Scholar] [CrossRef]
- Asiain, J.; Braun, M.; Roussos, A.J. Virtual reality as a psychotherapeutic tool: Current uses and limitations. Br. J. Guid. Couns. 2022, 50, 1–28. [Google Scholar] [CrossRef]
- Baghaei, N.; Chitale, V.; Hlasnik, A.; Stemmet, L.; Liang, H.-N.; Porter, R. Virtual Reality for Supporting the Treatment of Depression and Anxiety: Scoping Review. JMIR Ment. Health 2021, 8, e29681. [Google Scholar] [CrossRef] [PubMed]
- Best, P.; Meireles, M.; Schroeder, F.; Montgomery, L.; Maddock, A.; Davidson, G.; Galway, K.; Trainor, D.; Campbell, A.; Van Daele, T. Freely Available Virtual Reality Experiences as Tools to Support Mental Health Therapy: A Systematic Scoping Review and Consensus Based Interdisciplinary Analysis. J. Technol. Behav. Sci. 2022, 7, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, A.; Alabdulkreem, E.; Alduailij, M.; Alduailij, M.; Alhalabi, W.; Alharbi, S.; Lytras, M.D. Virtual Reality in the Treatment of Patients with Overweight and Obesity: A Systematic Review. Sustainability 2022, 14, 3324. [Google Scholar] [CrossRef]
- Keijsers, M.; Vega-Corredor, M.C.; Hoermann, S.; Tomintz, M. Virtual Reality Technology Use in Cigarette Craving and Smoking Interventions (I “Virtually” Quit): Systematic Review. J. Med. Internet Res. 2021, 23, e24307. [Google Scholar] [CrossRef] [PubMed]
- Saab, M.M.; Landers, M.; Cooke, E.; Murphy, D.; Hegarty, J. Feasibility and usability of a virtual reality intervention to enhance men’s awareness of testicular disorders (E-MAT). Virtual Real. J. Virtual Real. Soc. 2019, 23, 169–178. [Google Scholar] [CrossRef]
- Bryant, L.; Sedlarevic, N.; Stubbs, P.; Bailey, B.; Nguyen, V.; Bluff, A.; Barnett, D.; Estela, M.; Hayes, C.; Jacobs, C.; et al. Collaborative co-design and evaluation of an immersive virtual reality application prototype for communication rehabilitation (DISCOVR prototype). Disabil. Rehabil. Assist. Technol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Desselle, M.R.; Holland, L.R.; McKittrick, A.; Kennedy, G.; Yates, P.; Brown, J. “A Wanderer’s Tale”: The development of a virtual reality application for pain and quality of life in Australian burns and oncology patients. Palliat. Support. Care 2023, 21, 454–460. [Google Scholar] [CrossRef]
- Liddicoat, S. Mental health facility codesign: A new research method for integrating the service user voice in design processes using virtual reality. Gen. Psychiatry 2019, 32, e100061. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Pati, D.; Lorusso, L.N. How to Write a Systematic Review of the Literature. HERD Health Environ. Res. Des. J. 2018, 11, 15–30. [Google Scholar] [CrossRef]
| Citation | Application of XR | Application in Primary Care | XR Design and Contents | Study Design and Measures | Study Outcomes |
|---|---|---|---|---|---|
| Palomo et al., (2018) [26], Brazil. | Immersive virtual reality (VR). Mindfulness-based intervention (MBI). Mobile phone gear VR HMD. | MBI intervention with VR for adults > 18 years with multimorbidity. | 8-week mindfulness-based health promotion program based on teachings by Jon Kabat-Zinn. Meditation room environment. Use of avatars (instructor and user). Instructor avatar conducts the formal practice of mindfulness using 10 imaginative visualisation scenarios. | Randomised controlled trial (RCT) with pre/post and qualitative components. Mindfulness Scale (MAAS). Quality of Life Scale (SF-36). Self-Compassion Scale (SCS). Hospital Anxiety and Depression Scale (HADS). Viability of Technology Scale (VTS). Sociodemographic Questionnaire (DEMO). Qualitative Questionnaire about User Experience (QQUE). | Protocol. |
| Navarro-Haro et al., (2019) [25], Spain. | Immersive virtual reality. Mindfulness-based intervention. Oculus Rift, DK2 HMD with head tracking. | Adults > 18 years diagnosed with generalised anxiety disorder. | Mindful river world. Users float slowly down a virtual river with trees, boulders and mountains and practice mindfulness skills. Participants listened to mindfulness skill education audios during the VR. | RCT (pre/post) MBI vs. MBI + VR. Screening: Mini international neuropsychiatric interview (MINI). Primary outcome: General Anxiety Disorder 7 (GAD-7). Secondary outcomes: Hospital Anxiety and Depression Scale (HADS). Five Facets of Mindfulness Questionnaire (FFMQ). Difficulties of Emotion Regulation Scale (DERS). Multidimensional Assessment of Interoceptive Awareness (MAIA). Independent Television Company SOP Inventory (TC-SOPI). Visual analogue scale (VAS). The Sense of Presence questionnaire. | N = 39, MBI + VR (n = 19). Primary outcome: Pre/post differences, lower GAD-7 scores, p < 0.001. Secondary outcomes: Pre–post improvements on HADS, FFMQ, DERS, and MAIA subscales were evident. |
| Ellerton et al., (2020) [29], Australia. | Immersive virtual reality. Distraction technique to reduce pain and distress. Google Pixel XL and Google Daydream HMD. | Well children receiving 4-year-old vaccinations (diphtheria, tetanus, pertussis, and poliomyelitis). | Marine adventure with relaxation sequence. Underwater scenes, including gaze-based tracking of virtual fish. Time: 1 min with the vaccination provided approximately 30 sec after commencement of the VR experience. | RCT (VR vs. standard care). Primary outcome: The Faces Pain Scale–Revised (FPS-R). Secondary outcome: Poker Chip Tool. Observer (parents and healthcare providers) ratings of pain and distress: VAS. Qualitative satisfaction survey. | Protocol. |
| Althumairi et al., (2021) [30], Saudi Arabia. | Immersive virtual reality. Distraction technique to reduce pain and distress. Future Sight All-in-One, VR HMD, Grey, Melkco Inc. | Well children 4–6 years old receiving routine vaccinations. | Animated adventure story (with audio). Time: 2 min synchronised with vaccination procedure. | Non-experimental (VR vs. routine care). Post-intervention measures: Wong–Baker FACES scale. Pain Rating Scale. McMurtry Children’s Fear Scale (CFS). | N = 103 (n = 53, VR group). Lower mean pain and fear scores observed for VR group vs. usual care group scores. |
| Lim et al., (2021) [21], Singapore. | Immersive virtual reality. Cognitive assessment. VR HMD. | Adults 35 to 84 years without cognitive impairment or dementia. Feasibility of using VR to assess cognitive function in primary care. | CAIVRE Cognitive Assessment—a tutorial session followed by 13 VR tasks measuring 6 cognitive assessment domains (using hand gestures and head movements, speech recognition). Embedded automated scoring system. Time: tutorial and assessment < 15 min. | Pilot feasibility study. Feasibility = proportion of participants who completed the entire VR assessment in each age group. Screening: Montreal Cognitive Assessment (MoCA). Pre-VR measures: demographic characteristics, Abbreviated Mental Test (AMT), Mini-Mental State Examination (MMSE), Barthel Index—Basic Activities of Daily Living (BADLs), and Instrumental Activities of Daily Living Scale (IADLs). Post-VR measures: Adapted Spatial Presence Experience Scale (SPES). | Protocol. |
| Wong et al., (2021) [23], Singapore. | Immersive virtual reality. Cognitive assessment. HTC VIVE Pro with head and hand movements recorded using a Leap Motion controller and voice recognition software. | Cognitively healthy Asian adults aged between 35 and 74 years, (10-year age groups 35–44, 45–54, 55–64, 65–74). | CAIVRE Cognitive Assessment—a tutorial session followed by 13 VR tasks measuring 6 cognitive assessment domains. Embedded automated scoring system. Time: tutorial and assessment < 15 min. | Pilot feasibility study to compare VR assessment completion times with standard cognitive assessment. Demographic characteristics. Proportion completed. Time taken to complete. | N = 99/100. Proportion completed > 90%. Mean time taken to complete VR assessment compared with standard assessment was shorter across all age groups (p < 0.001). |
| Chang et al., (2022) [20], Singapore. | Immersive virtual reality. Distraction technique to reduce pain and distress. Oculus Quest HMD. | Multi-ethnic Asian children aged 4–10 years receiving vaccinations. | SILVER—Soothing Immunization Leveraging on Virtual Reality Experience. VR story centered on the “Burp’s Magic Tower” to portray a cosy room filled with shelves of books and other magical items. Time: 2 min with injection administered at 1 min. | RCT pilot feasibility study. Feasibility = recruitment response rate. Children: FPS-R. CFS. Parents and nurse: VAS. | N = 30/34. Recruitment response rate = 88%. Intention to treat analysis. Lower median CFS scores in intervention group (p 0.04). No difference in median FPS-R scores between groups (p 0.13). Lower median VAS scores for parents of children in the intervention group (p 0.04). No difference in nurse VAS scores between groups. |
| Gibson et al., (2022) [28], Utah, United States. | Immersive virtual reality. Health risk presentation and risk perceptions for prevention of type 2 diabetes (T2DM). Cardboard VR headset utilising smartphones and headphones. | Hispanic adults identified as being pre-diabetic. | Two codesigned 360° videos for use on a smartphone that can also be used with cardboard VR. Video 1: demonstrates how vision worsens with diabetic eye disease. Video 2: a first-person narrative of an individual who progressively develops T2DM, oral health issues, and heart disease. Post-video message: enrolling in the T2DM prevention program (DPP) may prevent negative outcomes. | RCT pilot feasibility study of cardboard VR versus smartphone. Primary outcome was self-reported enrolment in the DPP. Pre-intervention: baseline demographics. 18-item validated risk perception measure. Post-intervention: Feedback survey. 18-item validated risk perception measure. Health literacy measure. Purposive barrier/facilitator survey. Qualitative feedback. | N = 116/209, DPP enrolment 44/116 (37.9%), 25/56 from VR group, and 19/60 from smartphone group. No difference in pre/post-risk perception scores between groups. |
| Herrera et al., (2022) [24], Spain. Protocol. | Immersive virtual reality. Distraction technique to reduce pain and anxiety. Pico G2 VR goggles with Android tablet connected as controller. | Well children aged 3–6 years of age due to receive: (1) the triple viral + varicella vaccine at 3 years of age; (2) the hepatitis A+ diphtheria–tetanus–pertussis vaccine at age 6. | Leia’s World—experience for vaccinations. | RCT VR experience vs. standard care. Baseline demographics. Outcomes (pain and anxiety) Wong–Baker FACES scale. CFS. Purposive post-satisfaction survey. | Protocol. |
| Lundin et al., (2022) [27], Sweden. | Immersive virtual reality. Cognitive behavioural therapy (CBT) HTC Vive HMD. | Adults >18 years with agoraphobia or previous panic disorder (PDA). Using videoed environments converted to 360° video as a treatment for PDA. | VR-CBT using 360° video virtual environments (VE). VEs were situations feared and avoided by individuals with PDA: inside a subway carriage, a walking tunnel, a busy train station, an elevator, an auditorium, and a tall bridge. 10–12 weekly 60 min CBT program. VR used in weeks 4–9. | Feasibility/acceptability trial. Pre/post and 6 months follow-up. Primary outcomes: Mobility Inventory for Agoraphobia (MIA). Panic Disorder Severity Scale–Self Rated (PDSS-SR). Secondary outcomes: Patient Health Questionnaire-9 (PHQ9). World Health Organization Disability Assessment (WHODAS). World Health Organization Quality of Life questionnaire (WHOQOL). Post-acceptability measure: Client Satisfaction Questionnaire-8 (CSQ-8). | N = 12 (pre/post measures), N = 10 (6 months follow-up). Intention to treat. Primary outcomes: lower mean MIA (p < 0.001), PDSS-SR (p < 0.001) and PHQ9 scores (p < 0.001) pre/post and at 6 months follow-up (p < 0.05). |
| Tan et al., (2022) [22], Singapore. | Immersive virtual reality. Cognitive assessment. HTC VIVE Pro with head and hand movements recorded by a Leap Motion controller and voice recognition software. | Cognitively healthy Asian adults aged between 35 and 74 years (10-year age groups 35–44, 45–54, 55–64, 65–74). | CAIVRE Cognitive Assessment—a tutorial session followed by 13 VR tasks measuring 6 cognitive assessment domains. Embedded automated scoring system. Time: tutorial and assessment < 15 min. | Pilot feasibility study. Age-related aggregated cognitive performance scores (across 6 assessment domains using the within VR automatic scoring matrix) and completion times. | N = 99. Younger participants achieved higher performance scores across all 6 cognitive domains (p < 0.05). Younger participants took less time to complete the assessments compared with the older participants (p < 0.01). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, D.; Galvez, R.; Evans, D.; Hazelton, M.; Rossiter, R.; Irwin, P.; Micalos, P.S.; Logan, P.; Rose, L.; Fealy, S. The Integration and Application of Extended Reality (XR) Technologies within the General Practice Primary Medical Care Setting: A Systematic Review. Virtual Worlds 2023, 2, 359-373. https://doi.org/10.3390/virtualworlds2040021
Jones D, Galvez R, Evans D, Hazelton M, Rossiter R, Irwin P, Micalos PS, Logan P, Rose L, Fealy S. The Integration and Application of Extended Reality (XR) Technologies within the General Practice Primary Medical Care Setting: A Systematic Review. Virtual Worlds. 2023; 2(4):359-373. https://doi.org/10.3390/virtualworlds2040021
Chicago/Turabian StyleJones, Donovan, Roberto Galvez, Darrell Evans, Michael Hazelton, Rachel Rossiter, Pauletta Irwin, Peter S. Micalos, Patricia Logan, Lorraine Rose, and Shanna Fealy. 2023. "The Integration and Application of Extended Reality (XR) Technologies within the General Practice Primary Medical Care Setting: A Systematic Review" Virtual Worlds 2, no. 4: 359-373. https://doi.org/10.3390/virtualworlds2040021
APA StyleJones, D., Galvez, R., Evans, D., Hazelton, M., Rossiter, R., Irwin, P., Micalos, P. S., Logan, P., Rose, L., & Fealy, S. (2023). The Integration and Application of Extended Reality (XR) Technologies within the General Practice Primary Medical Care Setting: A Systematic Review. Virtual Worlds, 2(4), 359-373. https://doi.org/10.3390/virtualworlds2040021







