The Use of Virtual Reality to Improve Gait and Balance in Patients with Parkinson’s Disease: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
3. Results
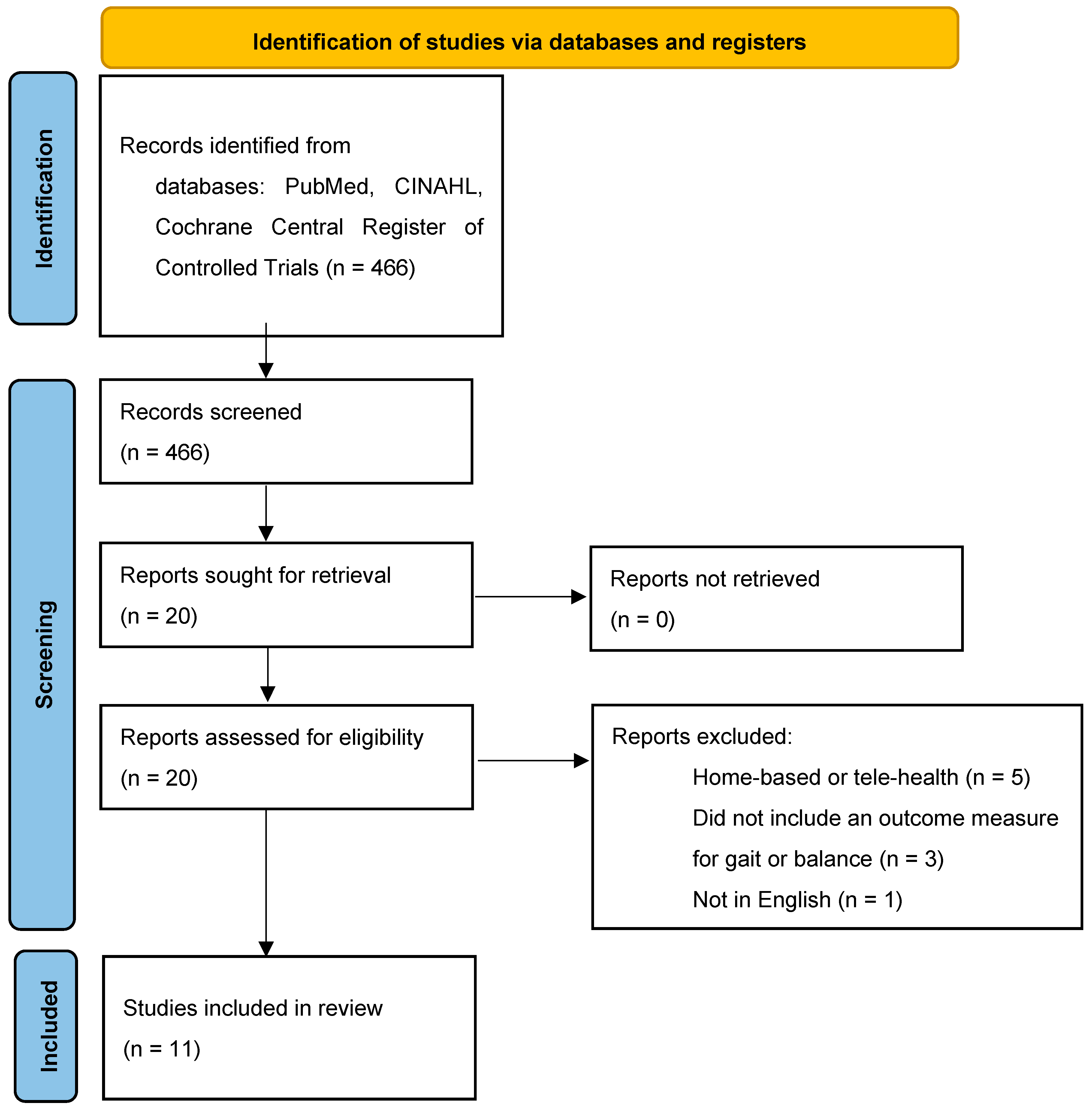
3.1. Search Results
3.2. Major Findings
3.3. Characteristics of Outcome Measures
3.4. Balance Outcomes
3.5. Gait Outcomes
3.6. Quality of Life
3.7. Motor Function
4. Discussion
4.1. Future Research
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| VR | Virtual Reality |
| PT | Physical Therapy |
| BBS | Berg Balance Scale |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| ABC | Activities-specific Balance Confidence Scale |
| 10-MWT | 10 min Walk Test |
| DGI | Dynamic Gait Index |
| PDQ | Parkinson’s Disease Questionnaire |
| TUGT | Timed Up-And-Go Test |
| FGA | Functional Gait Assessment |
| DASH | Disabilities of the Arm, Shoulder and Hand |
| SF-36/12 | Short Form 36/12 |
| POMA | Performance-Oriented Mobility Assessment |
| FES | Falls Efficacy Scale |
| BI | Barthel Index |
| MI | Motor Imagery |
References
- Kashif, M.; Ahmad, A.; Bandpei, M.; Gilani, S.; Hanif, A.; Iram, H. Combined effects of virtual reality techniques and motor imagery on balance, motor function and activities of daily living in patients with Parkinson’s disease: A randomized control trial. BMC Geriatr. 2022, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- DynaMed. Parkinson Disease. EBSCO Information Services. Available online: https://www-dynamed-com.georgefox.idm.oclc.org/condition/parkinson-disease (accessed on 11 August 2024).
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Dorsey, E.R.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Park. Dis. 2020, 6, 15. [Google Scholar]
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar]
- Pickering, R.M.; Grimbergen, Y.A.M.; Rigney, U.; Ashburn, A.; Mazibrada, G.; Wood, B.; Gray, P.; Kerr, G.; Bloem, B.R. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov. Disord. 2007, 22, 1892–1900. [Google Scholar]
- Olanow, C.; Schapira, A.V. Parkinson’s Disease. In Harrison’s Principles of Internal Medicine, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Osborne, J.; Botkin, R.; Colon-Semenza, C.; DeAngelis, T.R.; Gallardo, O.G.; Kosakowski, H.; Martello, J.; Pradhan, S.; Rafferty, M.; Readinger, J.L.; et al. Physical therapist management of Parkinson’s disease: A clinical practice guideline from the American Physical Therapy Association. Phys. Ther. 2022, 102, pzab302. [Google Scholar]
- Goodwin, V.A.; Richards, S.H.; Taylor, R.S.; Taylor, A.H.; Campbell, J.L. The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2008, 23, 631–640. [Google Scholar]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: A multicenter, single-blind, randomized, controlled trial. Biomed. Res. Int. 2017, 2017, 7962826. [Google Scholar] [CrossRef] [PubMed]
- Barry, G.; Galna, B.; Rochester, L. The role of exergaming in Parkinson’s disease rehabilitation: A systematic review of the evidence. J. Neuroeng. Rehabil. 2014, 11, 33. [Google Scholar] [CrossRef]
- American Physical Therapy Association. What Is Virtual Reality? Available online: https://www.apta.org/patient-care/interventions/virtual-reality#:~:text=Virtual%20reality%20refers%20to%20a,auditory%20feedback%20to%20enhance%20immersion (accessed on 20 March 2025).
- Mehrabi, S.; Munoz, J.E.; Basharat, A.; Boger, J.; Cao, S.; Barnett-Cowan, M.; Middleton, L.E. Immersive virtual reality exergames to promote the well-being of community dwelling older adults: Protocol for a mixed methods pilot study. JMIR Res. Protoc. 2022, 11, e32955. [Google Scholar]
- Grospretre, S.; Marcel-Millet, P.; Eon, P.; Wollesen, B. How exergaming with virtual reality enhances specific cognitive and visuo-motor abilities: An explorative study. Cogn. Sci. 2023, 47, e13278. [Google Scholar]
- Omlor, A.J.; Schwarzel, L.S.; Bewarder, M.; Casper, M.; Damm, E.; Danziger, G.; Mahfoud, F.; Rentz, K.; Sester, U.; Bals, R.; et al. Comparison of immersive and non-immersive virtual reality videos as substitute for in-hospital teaching during coronavirus lockdown: A survey with graduate medical students in Germany. Med. Educ. Online 2022, 27, 2101417. [Google Scholar] [CrossRef]
- Truijen, S.; Abdullahi, A.; Bijsterbosch, D.; van Zoest, E.; Conijn, M.; Wang, Y.; Struyf, N.; Saeys, W. Effect of home-based virtual reality training and telerehabilitation on balance in individuals with Parkinson disease, multiple sclerosis, and stroke: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 2995–3006. [Google Scholar] [CrossRef]
- Fandim, J.V.; Saragiotto, B.T.; Porfirio, G.J.M.; Santana, R.F. Effectiveness of virtual reality in children and young adults with cerebral palsy: A systematic review of randomized controlled trials. Braz. J. Phys. Ther. 2021, 25, 369–386. [Google Scholar] [CrossRef] [PubMed]
- De Natale, G.; Qorri, E.; Todri, J.; Lena, O. Impacto of virtual reality alone and in combination with conventional therapy on balance in Parkinson’s disease: A systematic review with a meta-analysis of randomzed controlled trials. Medicina 2025, 61, 524. [Google Scholar] [CrossRef]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.P.; Staniszewksi, K.; Hays, K.; Gerber, D.; Natale, A.; O’Dell, D. Virtual reality-based therapy for the treatment of balance deficits in patients receiving inpatient rehabilitation for traumatic brain injury. Brain Inj. 2014, 28, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.K.; Kumar, N.; Singhi, P. Effectiveness of virtual reality rehabilitation for children and adolescents with cerebral palsy: An updated evidence-based systematic review. Physiotherapy 2017, 103, 245–258. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, J.K.; Koh, Y.H. A systematic review and meta-analysis on the effect of virtual reality-based rehabilitaiton for people with Parkinson’s disease. J. Neuroeng. Rehabil. 2023, 20, 94. [Google Scholar] [CrossRef]
- Rodriguez-Mansilla, J.; Bedmar-Vargas, C.; Garrido-Ardiala, E.M.; Torres-Piles, S.T.; Gonzalez-Sanchez, B.; Rodriguez-Dominguez, M.T.; Ramirez-Duran, M.V.; Jimenez-Palomares, M. Effects of virtual reality in the rehabilitation of Parkinson’s disease: A systematic review. J. Clin. Med. 2023, 12, 4896. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Amirthalingam, J.; Paidi, G.; Alshowaikh, K.; Jayarathna, A.I.; Salibindla, D.B.A.M.R.; Karpinska-Leydier, K.; Ergin, H.E. Virtual reality intervention to help improve motor function in patients undergoing rehabilitation for cerebral palsy, Parkinson’s disease, or stroke: A systematic review of randomized controlled trials. Cureus 2021, 13, e16763. [Google Scholar] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Kastner, M.; Levac, D.; Ng, C.; Sharpe, J.P.; Wilson, K.; et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med. Res. Methodol. 2016, 16, 15. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson’s disease patients: A randomized controlled trial. Med. Sci. Monit. 2019, 25, 4186–4192. [Google Scholar]
- Santos, P.; Machado, T.; Santos, L.; Ribeiro, N.; Melo, A. Efficacy of the nintendo wii combination with conventional exercises in the rehabilitation of individuals with Parkinson’s disease: A randomized clinical trial. NeuroRehabilitation 2019, 45, 255–263. [Google Scholar]
- Bekkers, E.M.J.; Mirelman, A.; Alcock, L.; Rochester, L.; Nieuwhof, F.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; Cereatti, A.; Della Croce, U.; et al. Do patients with Parkinson’s disease with freezing of gait respond differently than those without to treadmill training augmented by virtual reality? Neurorehabil. Neural Repair 2020, 34, 440–449. [Google Scholar] [PubMed]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Lo Monaco, R.; Padua, L. Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: A randomized controlled trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [PubMed]
- Maranesi, E.; Casoni, E.; Baldoni, R.; Barboni, I.; Rinaldi, N.; Tramontana, B.; Amabili, G.; Benadduci, M.; Barbarossa, F.; Luzi, R.; et al. The effect of non-immersive virtual reality exergames versus traditional physiotherapy in Parkinson’s disease older patients: Preliminary results from a randomized-controlled trial. Int. J. Environ. Res. Public Health 2022, 19, 14818. [Google Scholar] [CrossRef]
- Gulcan, K.; Guclu-Gunduz, A.; Yasar, E.; Ar, U.; Karadag, Y.S.; Saygili, F. The effects of augmented and virtual reality gait training on balance and gait in patients with Parkinson’s disease. Acta Neurol. Belg. 2022, 123, 1917–1925. [Google Scholar]
- Liao, Y.Y.; Yang, Y.R.; Cheng, S.J.; Wu, Y.R.; Fuh, J.L.; Wang, R.Y. Virtual reality-based training to improve obstacle-crossing performance and dynamic balance in patients with Parkinson’s disease. Neurorehabilit. Neural Repair 2015, 29, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (v-time): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [PubMed]
- Kashif, M.; Albalwi, A.A.; Zulfiqar, A.; Bahir, K.; Alharbi, A.A.; Zaidi, S. Effects of virtual reality versus motor imagery versus routine physical therapy in patients with Parkinson’s disease: A randomized controlled trial. BMC Geriatr. 2024, 24, 229. [Google Scholar] [CrossRef]
- Shih, M.C.; Wang, R.Y.; Cheng, S.J.; Yang, Y.R. Effects of a balance-based exergaming intervention using the Kinect sensor on posture stability in individuals with Parkinson’s disease: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 78. [Google Scholar] [PubMed]
- International Parkinson and Movement Disorder Socitey. MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). Available online: https://www.movementdisorders.org/MDS/MDS-Rating-Scales/MDS-Unified-Parkinsons-Disease-Rating-Scale-MDS-UPDRS.htm (accessed on 20 August 2024).
- Skorvanek, M.; Martinez-Martin, P.; Kovacs, N.; Zezula, I.; Rodriguez-Violante, M.; Corvol, J.C.; Taba, P.; Seppi, K.; Levin, O.; Schrag, A.; et al. Relationship between the MDS-UPDRS and quality of life: A large multicenter study of 3206 patients. Park. Relat. Disord. 2018, 52, 83–89. [Google Scholar]
- Martinez-Martin, P.; Gil-Nagel, A.; Gracia, L.M.; Gomez, J.B.; Martinez-Sarries, J.; Bermejo, F. Unified Parkinson’s disease rating scale characteristics and structure. The Cooperative Multicenter Group. Mov. Disord. 1994, 9, 76–83. [Google Scholar]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. 2), S7–S11. [Google Scholar]
- Donoghue, D.; Physiotherapy Research and Older People (PROP) group; Stokes, E.K. How much change is true change? The minimum detectable change of the berg balance scale in elderly people. J. Rehabil. Med. 2009, 41, 343–346. [Google Scholar]
- Schlenstedt, C.; Brombacher, S.; Hartwigsen, G.; Weisser, B.; Moller, B.; Deuschel, G. Comparison of the Fullerton Advanced Balance Scale, Mini-BESTest, and Berg Balance Scale to Predict Falls in Parkinson Disease. Phys. Ther. 2016, 96, 494–501. [Google Scholar] [CrossRef]
- Botner, E.M.; Miller, W.C.; Eng, J.J. Measurement properties of the Activities-specific Balance Confidence scale among individuals with stroke. Disabil. Rehabil. 2009, 27, 156–163. [Google Scholar] [CrossRef]
- Mak, M.K.; Pang, M.Y. Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: A 1-year prospective study. J. Neurol. 2009, 256, 1689–1695. [Google Scholar] [PubMed]
- Chiaramonte, R.; Bonfiglio, M. Acoustic analysis of voice in Parkinson’s disease: A systematic review of voice disability and meta-analysis of studies. Rev. Neurol. 2020, 70, 393–405. [Google Scholar] [PubMed]
- Thangavelu, K.; Hayward, J.A.; Pachana, N.A.; Byrne, G.J.; Mitchell, L.K.; Wallis, G.M.; Au, T.R.; Dissanayaka, N.N. Designing virtual reality assisted psychotherapy for anxiety in older adults living with Parkinson’s disease: Integrating literature for scoping. Clin. Gerontol. 2022, 45, 235–251. [Google Scholar] [PubMed]
- Bektic, M.; Smith, B.E.; Ridgel, A.; Kim, K. Virtual reality game with haptic feedback for upper limb rehabilitation in Parkinson’s disease. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024; Volume 2024, pp. 1–4. [Google Scholar]
- Winser, S.J.; Kannan, P.; Bello, U.M.; Whitney, S.L. Measures of balance and falls risk prediction in people with Parkinson’s disease: A systematic review of psychometric properties. Clin. Rehabil. 2019, 33, 1949–1962. [Google Scholar]
| Search Term | Identified Articles | Articles Included in This Review |
|---|---|---|
| Parkinson’s disease OR Parkinson’s AND virtual reality OR VR OR augmented reality OR exergaming AND randomized controlled trials | 1756 | Not assessed |
| Parkinson’s disease OR Parkinson’s AND virtual reality OR VR OR augmented reality OR exergaming AND gait AND balance | 181 | 6 [28,29,30,31,32,33] |
| Virtual reality AND rehabilitation AND gait AND balance | 84 | 0 |
| Parkinson’s disease OR Parkinson’s AND virtual reality AND randomized controlled trials | 84 | 4 [1,34,35,36] |
| Parkinson’s disease AND rehabilitation AND virtual reality | 39 | 1 [37] |
| Parkinson’s disease AND physical therapy AND virtual reality | 27 | 0 |
| Parkinson’s disease AND exercise AND virtual reality | 26 | 0 |
| Parkinson’s disease AND exergaming | 19 | 0 |
| Parkinson’s disease AND augmented reality | 8 | 0 |
| Reference | Year | Country | Subject Population | Sample Size |
|---|---|---|---|---|
| Liao et al. [34] | 2015 | Taiwan | (17M, 19F), mean age range 64.6 ± 8.6 y to 67.3 ± 7.1 y (group mean not provided), disease duration range 6.4 ± 3.0 y to 7.9 ± 2.7 y (group mean not provided), no baseline UPDRS | 36 |
| Mirelman et al. [35] | 2016 | Israel, Belgium, Italy, Netherlands, UK | (182M, 100F), mean age range 73.3 ± 6.4 y to 74.2 ± 6.9 y (group mean not provided), disease duration range not reported, no baseline UPDRS | 282 |
| Shih et al. [37] | 2016 | Taiwan | (16M, 4F), mean age 68.15 ± 9.82 y, disease duration 4.62 ± 4.30 y, no baseline UPDRS | 20 |
| Feng et al. [28] | 2019 | China | (17M, 13F), mean age 67.2± 4.72 y, disease duration 6.84± 1.45 y, baseline UPDRS 24.93 ± 6.81 | 28 |
| Santos et al. [29] | 2019 | Brazil | (31M, 14F), mean age 64.3 ± 8.5 y, disease duration 7.1 ± 0.5 y, no baseline UPDRS | 45 |
| Bekkers et al. [30] | 2020 | Israel, Belgium, Italy, Netherlands, UK | (74M, 47F), mean age range 70.57 ± 6.04 y (FOG+) to 71.66 ± 6.3 y (FOG-) (group mean not provided), disease duration range 7.25 ± 5.1 y (FOG-) to 10.43 ± 6.7 y (FOG+) (group mean not provided), baseline UPDRS-III range 26.11 ± 12.2 (FOG-) to 31.83 ± 13.2 (FOG+) | 121 |
| Pazzaglia et al. [31] | 2020 | Italy | (35M, 16F), mean age 71 ± 8.5 y, disease duration 6.1 ± 6.29 y, baseline UPDRS 24 ± 1 | 51 |
| Kashif et al. [1] | 2022 | Pakistan | (25M, 19F), mean age 63.09 ± 4.59 y, disease duration 6.39± 1.77 y, no baseline UPDRS | 44 |
| Maranesi et al. [32] | 2022 | Italy | (15M, 15F), mean age 74.1 ± 5.85 y, no baseline UPDRS | 30 |
| Gulcan et al. [33] | 2023 | Turkey | (26M, 4F), mean age 60.5± 6.0 y, disease duration 6.0 ± 4.5 y, no baseline UPDRS | 30 |
| Kashif et al. [36] | 2024 | Pakistan | (33M, 27F), mean age 63.33 ± 4.86 y, disease duration 6.43 ± 1.95 y, baseline UPDRS 33.23 ± 3.23 | 60 |
| Reference | Traditional Physical Therapy Intervention | VR Intervention | Outcome Measurements | Results | Summary of Conclusions |
|---|---|---|---|---|---|
| Liao et al., 2015 [34] | 60 min program consisting of 10 min of stretching exercises, 15 min of strengthening exercises, 20 min of balance exercises, and 15 min of treadmill walking 2 days a week for 6 weeks. Treadmill training was performed using a safety harness with subjects walking at 80% of their walking speed for the first 5 min, then increasing every 5 min by 0.2 km/h. | 60 min program consisting of 45 min of exercise using the Wii Fit and 15 min of treadmill walking. The Wii Fit program consisted of 10 min of yoga exercises, 15 min of strengthening exercises, and 20 min of balance exercises. The treadmill training program was the same as the one performed by the traditional physical therapy intervention group. | Obstacle crossing performance, dynamic balance performance, SOT, PDQ-39, FES-I, TUGT. | At 6 weeks and 1 month after completing training: significant improvement in each group; however, no between group differences for obstacle crossing performance, TUGT, SOT, PDQ-39, FES-I. Significant between group differences, with VR group demonstrating greater improvements in dynamic balance at 6 weeks and 1 month after completing training: maximum velocity (forward): p < 0.001; p < 0.001; maximum velocity (sideward): p < 0.001; p < 0.001. | VR training program was as effective as the traditional PT program for many variables. VR training was superior than traditional PT for improving forward and sideward maximal velocity during dynamic balance testing. |
| Mirelman et al., 2016 [35] | 45 min treadmill program performed 3 times a week for 6 weeks. | 45 min treadmill training program with VR system. VR system utilized Microsoft Kinect camera that provided real-time feedback to the patient. Program performed three times a week for six weeks. | Incident rate of falls, short physical performance battery (SPPB), 2 min walk test, leading foot clearance, gait speed, SF-36 | At 6 months: significantly lower rate of falls in the VR group: p = 0.01. Significantly greater improvements in SPPB gait, gait speed variability during obstacle negotiation, and 2 min walk test (at 6 weeks); SPPB total, SPPB balance, and leading foot clearance (at 6 months). | The VR-based treadmill program was superior to the traditional PT treadmill program at reducing the rate of falls. VR intervention superior at improving some measures of balance and gait. |
| Shih et al., 2016 [37] | 50 min conventional balance training (warm up, reaching activities, weight-shifting activities, marching activities, cool down) 2 days per week for 8 weeks | 10 min warm up, 30 min balance-based exergaming with the Kinect sensor (stationary object reaching, moving object reaching, obstacle avoidance, and marching), and 10 min cool down 2 days per week for 8 weeks. | Limits of stability and one-leg stance for postural control, BBS for balance, and TUGT for gait and balance. | At 8 weeks, BBS and TUGT were improved significantly in both groups. Only the VR group showed significant improvement in postural control (limits of stability and one-leg stance). | Both training programs were effective at improving BBS and TUGT scores. The VR group was superior at improving limits of stability and one-leg stance measures. |
| Feng et al., 2019 [28] | 45 min traditional rehabilitation training (warm up, balance training, physical conditioning to include rhythm training, coordination training, and cool down), 5 times per week for 12 weeks | 45 min VR training (warm up, hands and feet touch the ball, hard boating, take the maze, and cool down) 5 times per week for 12 weeks. | BBS for balance, TUGT for gait and balance, UPDRS-III for motor function, and FGA for gait. | At 12 weeks, statistically significant improvements in BBS, TUGT, and FGA scores occurred in both the VR and traditional PT groups. UPDRS-III scores only improved in the VR group. VR group scores after training were significantly greater than traditional PT group scores: BBS: p-value < 0.05. TUGT: p-value < 0.05. UPDRS-III: p-value < 0.05. FGA: p-value < 0.05. | Both groups experienced significant improvements in BBS, TUGT, and FGA scores; however, the improvements were greater in the VR group. VR group also had significant improvements in UPDRS-III scores. |
| Santos et al., 2019 [29] | 40 min sessions performed twice a week for 8 weeks consisting of proprioceptive neuromuscular facilitation patterns and gait training consisting of manual resistance applied to the hip by the physical therapist when stepping. | Group 1: 40 min VR program (2x a week for 8 weeks) consisting of playing four games (boxing, soccer heading, golf, running) with the Nintendo Wii and Wii Balance Board. Group 2: 40 min combination (VR and traditional exercise); 20 min of Wii gaming and 20 min of traditional exercise. Performed twice a week for eight weeks. | BBS, DGI, TUGT, PDQ-39. | Each group experienced significant improvements in all outcome measures; however, there was no difference between groups. The effect size for each outcome measure was highest in the VR plus exercise group. | The VR and VR plus traditional exercise was as effective as the traditional PT group at improving measures of gait, balance, and PDQ-39 scores. |
| Bekkers et al., 2020 [30] | 45 min treadmill program performed 3 times a week for 6 weeks. | 45 min treadmill training program with VR system. VR system utilized Microsoft Kinect camera that provided real-time feedback to the patient. Program performed 3 times a week for six weeks. | Mini-BEST, NFOG-Q, SPPB, FSST, TMT-B, FES-I, PASE. | Both groups experienced significant improvements in Mini-BEST and TMT-B scores at 6 weeks (p = 0.001). Both groups demonstrated improved overall mobility performance in SPPB scores, with scores generally higher in the VR group (p = 0.001). Patients who were FOG+ and FOG− who were in the VR group had a greater reduction of falls (p = 0.008). | VR training was as good as traditional PT at improving Mini-BEST and TMT-B scores in the short-term. VR training reduced falls in those who were FOG+ or FOG- more than the traditional PT group. |
| Pazzaglia et al., 2020 [31] | 40 min conventional rehabilitation program (warm-up phase, active phase both standing and seated to include motor coordination/balance/walking, cool-down phase seated) 3 times per week for 6 weeks. | 40 min VR rehabilitation with the NIRVANA system (7 exercises involving coordination of the lower and upper limbs, and trunk control, such as leading a dog, tapping falling leaves, and maintaining balance between two projected bars) 3 times per week for 6 weeks. | BBS for balance, DGI for gait response, DASH for upper limb performance, and SF-36 for quality of life. | At 6 weeks, there was statistically significant improvement in BBS, DGI, DASH, and the mental composite score (MCS) of the SF-36 in the VR group, and only statistically significant improvement in the DASH score in the traditional PT group: BBS: p-value = 0.003. DGI: p-value = 0.003. DASH: traditional PT p-value = 0.007, VR p-value = 0.009. MCS (SF-36): p-value = 0.037. | The VR program was more effective than the traditional PT program for improving a variety of outcome scores. |
| Kashif et al., 2022 [1] | 40 min routine PT (warm-up, stretching, strengthening, limb coordination, core, neck, and gait training, relaxation), 20 min walking and cycling 3 days per week for 12 weeks. | 40 min routine PT, 10–15 min of VR training with Wii Fit (tennis, boxing, bowling, soccer, kicking, table tilt, penguin slide, tilt city, single-leg extension, torso twist), and 5–10 min of MI techniques (watching and analyzing videos of a normal movement and a video of the patient performing the movement, visualizing and meditating on the movement, then performing the movement) 3 days per week for 12 weeks. | UPDRS-III for motor function, BBS for balance, ABC for balance confidence, UPDRS-II for ADLs. | At 12 weeks, statistically significant improvements in the VR group and traditional PT group were noted, with significant group main effect: UPDRS-III: p-value < 0.001. BBS: p-value < 0.001. ABC: p-value < 0.001. UPDRS-II: p-value < 0.001. | The VR group was significantly better at improving balance and function scores. |
| Maranesi et al., 2022 [32] | 50 min traditional therapy (breathing and relaxation, task-oriented exercise, walking, stretching, static and dynamic balance, flexibility, and unilateral and contralateral coordination exercises) 2 times per week for 5 weeks. | 30 min traditional therapy and 20 min of treatment with the Tymo VR system (one-dimensional or two-dimensional exergames where the patient’s body is the joystick, including apple picking, the hot air balloon, and the labyrinth) 2 times per week for 5 weeks. | POMA scale (POMA balance, POMA gait, and POMA total), FES-I for fear of falling, BI for ADLs, gait speed, and SF-12 for quality of life. | At 5 weeks, there was a statistically significant improvement in POMA balance for each group, though VR group improved to a greater degree. The VR group also improved with statistical significance in the POMA total, and the mental component of the SF-12: POMA total: p-value = 0.010. POMA balance: traditional PT p-value = 0.017, VR p-value = 0.004. SF-12 mental (MCS-12): p-value = 0.022. | Subjects in the VR group experienced significantly greater gains in POMA balance, POMA total, and SF-12 (MCS-12) scores. |
| Gulcan et al., 2023 [33] | 60 min conventional training (supine exercise, sitting exercise, standing exercise, stretching, relaxation) 3 days per week for 6 weeks. | 90 min augmented and virtual reality gait training using the C-Mill VR+, starting with a conventional warm up, AR/VR gait training (stepping stones, random stepping stones, obstacle avoidance, speed adaptation, slalom, monster game, balls track, auditory cueing, nature island, and the Italian Alps) and ending with stretching and relaxation, 3 days per week for 6 weeks. | BBS for balance, UPDRS-III for motor function, ABC for balance confidence, TUGT for gait and balance, double-leg stability test, single-leg stability test, step length, step width, stride length, stance duration, swing duration, and total double support duration. | At 6 weeks, both groups showed statistically significant improvement in UPDRS-III, BBS, ABC, step length, and stride length (p-value <0.001), with group main effect significant in the VR group for step length and stride length: Step length (m): right p-value = 0.001, left p-value = 0.005. Stride length (m): p-value = 0.001. In all other outcomes, only the VR group showed significant improvement: TUGT: p-value = 0.002. Double-leg stability: p-value = 0.036. Single-leg stability: p-value = 0.031. Step width (m): p-value = 0.010. Stance duration (s): p-value = 0.001. Swing duration (s): p-value = 0.003. Total double support duration (s): p-value = 0.011. | Subjects in the VR group experienced significantly greater gains in measures for gait and stability. |
| Kashif et al., 2024 [37] | 40 min routine physical therapy (warm-up, stretching, strength training, cool-down) and 20 min walking and cycling 3 days per week for 12 weeks | VR + RPT (Group A) 40 min routine physical therapy, 15–20 min of VR training with Wii Fit (tennis, bowling, boxing, kicking, table tilt, penguin slide, tilt city, soccer, torso twists, single-leg stance) 3 days per week for 12 weeks. MI+ RPT (Group B) 40 min routine physical therapy, 15–20 min of MI training (watching and analyzing videos of a normal movement and a video of the patient performing the movement, visualizing and meditating on the movement, then performing the movement) 3 days per week for 12 weeks. | UPDRS-III for motor function, ABCs for balance confidence, BBS for balance, and UPDRS-II for ADLs. | At 12 weeks, the VR + RPT group showed statistically significant results in all outcomes, versus the traditional PT group and the test MI + RPT group: UPDRS-III: p-value = 0.011. UPDRS-II: p-value = 0.000. ABCs: p-value = 0.010. BBS: p-value = 0.019. | Subjects in the VR group experienced greater gains in balance and functional scores compared with the traditional PT and MI + RPT groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surridge, R.; Stilp, C.; Johnson, C.; Brumitt, J. The Use of Virtual Reality to Improve Gait and Balance in Patients with Parkinson’s Disease: A Scoping Review. Virtual Worlds 2025, 4, 13. https://doi.org/10.3390/virtualworlds4020013
Surridge R, Stilp C, Johnson C, Brumitt J. The Use of Virtual Reality to Improve Gait and Balance in Patients with Parkinson’s Disease: A Scoping Review. Virtual Worlds. 2025; 4(2):13. https://doi.org/10.3390/virtualworlds4020013
Chicago/Turabian StyleSurridge, Rachel, Curt Stilp, Christen Johnson, and Jason Brumitt. 2025. "The Use of Virtual Reality to Improve Gait and Balance in Patients with Parkinson’s Disease: A Scoping Review" Virtual Worlds 4, no. 2: 13. https://doi.org/10.3390/virtualworlds4020013
APA StyleSurridge, R., Stilp, C., Johnson, C., & Brumitt, J. (2025). The Use of Virtual Reality to Improve Gait and Balance in Patients with Parkinson’s Disease: A Scoping Review. Virtual Worlds, 4(2), 13. https://doi.org/10.3390/virtualworlds4020013







