Failed Repurposing of Lysosomotropic Drugs for COVID-19 Treatment or Prevention
Abstract
:1. Introduction
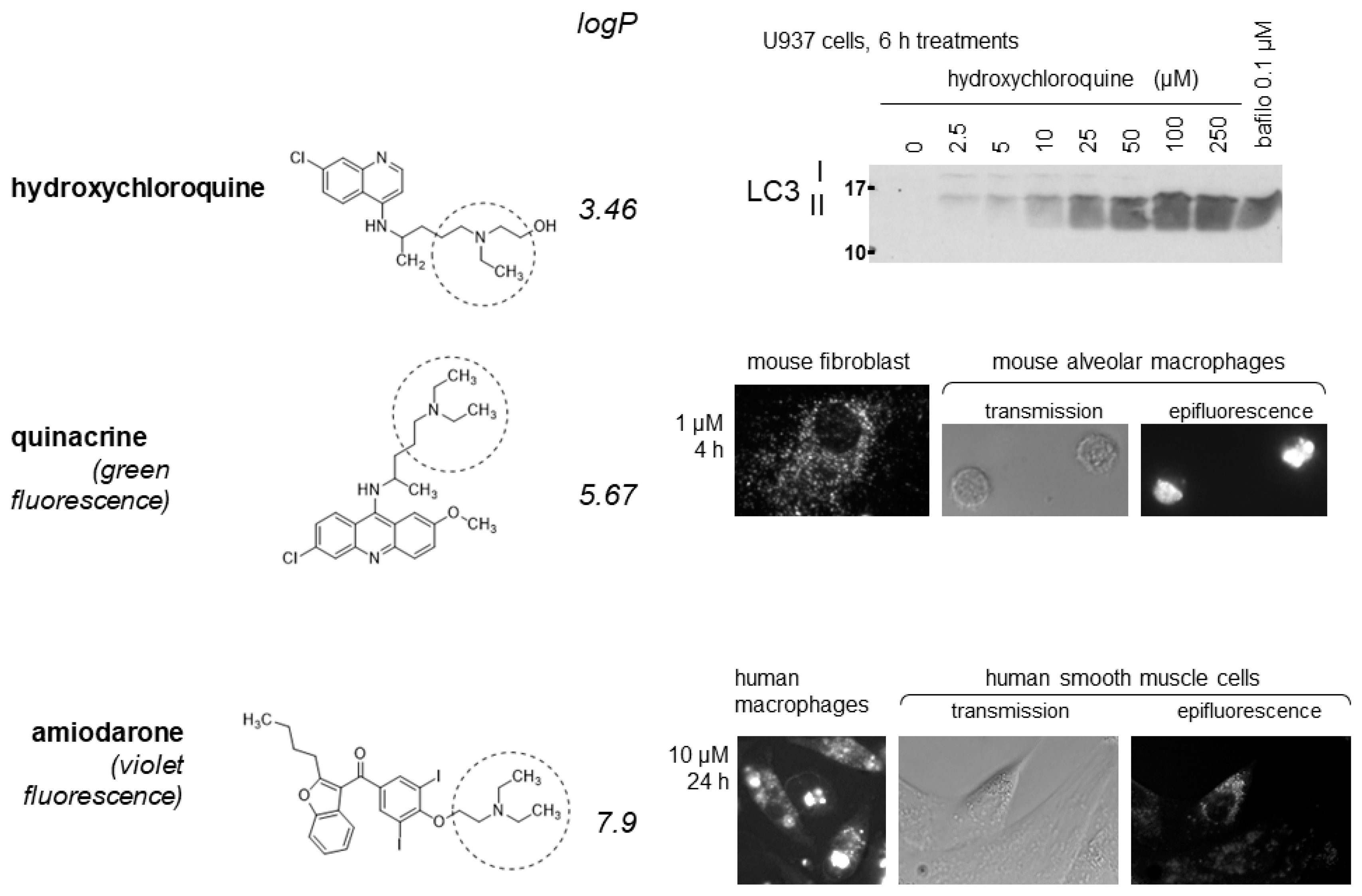
2. Discussion
2.1. Lysosomotropic Drugs
2.2. The Transport and Cellular Retention of Lysosomotropic Drugs Is Driven by V-ATPase
2.3. Lysosomotropic Drug Accumulation Precedes the Cytopathology
2.4. The Cytopathology Induced by Lysosomotropic Drugs Is Complex and Evolving over Time
2.5. Why Was the Life Cycle of SARS-CoV-2 Expected to Be Susceptible to Lysosomotropic Drugs?
2.6. Drug Accumulation Has Some Cell Type Specificity In Vitro and In Vivo
3. Conclusions: Are the Effects of Lysosomotropic Drugs Clinically Exploitable?
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kupferschmidt, K.; Cohen, J. Race to find COVID-19 treatments accelerates. Science 2020, 367, 1412–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanden Eynde, J.J. COVID-19: Failure of the DisCoVeRy Clinical Trial, and Now-New Hopes? Pharmaceuticals 2021, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Tummino, T.A.; Rezelj, V.V.; Fischer, B.; Fischer, A.; O’Meara, M.J.; Monel, B.; Vallet, T.; White, K.M.; Zhang, Z.; Alon, A.; et al. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science 2021, 373, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Salata, C.; Calistri, A.; Parolin, C.; Baritussio, A.; Palù, G. Antiviral activity of cationic amphiphilic drugs. Expert Rev. Anti Infect. Ther. 2017, 15, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Sauvat, A.; Ciccosanti, F.; Colavita, F.; Di Rienzo, M.; Castilletti, C.; Capobianchi, M.R.; Keep, O.; Zitvogel, L.; Fimia, G.M.; Piacentini, M.; et al. On-target versus off-target effects of drugs inhibiting the replication of SARS-CoV-2. Cell Death Dis. 2020, 11, 656. [Google Scholar] [CrossRef]
- Yang, L.; Pei, R.J.; Li, H.; Ma, X.N.; Zhou, Y.; Zhu, F.H.; He, P.L.; Tang, W.; Zhang, Y.C.; Xiong, J.; et al. Identification of SARS-CoV-2 entry inhibitors among already approved drugs. Acta Pharmacol. Sin. 2021, 42, 1347–1353. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.H.; Hardin, J.W.; Sutton, S.S.; Ambati, J. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Medcine 2020, 18, 114–127. [Google Scholar] [CrossRef]
- Mitjà, O.; Corbacho-Monné, M.; Ubals, M.; Alemany, A.; Suñer, C.; Tebé, C.; Tobias, A.; Peñafiel, J.; Ballana, E.; Pérez, C.A.; et al. BCN-PEP-CoV2 Research Group. A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of COVID-19. N. Engl. J. Med. 2021, 384, 417–427. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Agoramoorthy, G.; Lee, S.S. The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies. Front. Pharmacol. 2021, 12, 704205. [Google Scholar] [CrossRef]
- Navarese, E.P.; Podhajski, P.; Andreotti, F.; La Torre, G.; Gajda, R.; Radziwanowski, A.; Nowicka, M.; Bukowski, P.; Gajda, J.; Omyła, M.; et al. Ion channel inhibition with amiodarone or verapamil in symptomatic hospitalized nonintensive-care COVID-19 patients: The ReCOVery-SIRIO randomized trial. Cardiol. J. 2022, 29, 739–750. [Google Scholar] [CrossRef]
- Tirupakuzhi Vijayaraghavan, B.K.; Jha, V.; Rajbhandari, D.; Myatra, S.N.; Ghosh, A.; Bhattacharya, A.; Arfin, S.; Bassi, A.; Donaldson, L.H.; Hammond, N.E.; et al. Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: A multicentre, parallel-group randomised controlled trial from India. BMJ Open 2022, 12, 059540. [Google Scholar] [CrossRef] [PubMed]
- Marceau, F.; Bawolak, M.T.; Lodge, R.; Bouthillier, J.; Gagné-Henley, A.; Gaudreault, R.C.; Morissette, G. Cation trapping by cellular acidic compartments: Beyond the concept of lysosomotropic drugs. Toxicol. Appl. Pharmacol. 2012, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Ha, H.R.; Ciminale, V.; Spirli, C.; Saletti, G.; Schiavon, M.; Bruttomesso, D.; Bigler, L.; Follath, F.; Pettenazzo, A.; et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am. J. Respir. Cell. Mol. Biol. 2008, 39, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Morissette, G.; Ammoury, A.; Rusu, D.; Marguery, M.C.; Lodge, R.; Poubelle, P.E.; Marceau, F. Intracellular sequestration of amiodarone: Role of vacuolar ATPase and macroautophagic transition of the resulting vacuolar cytopathology. Br. J. Pharmacol. 2009, 157, 1531–1540. [Google Scholar] [CrossRef] [Green Version]
- Parks, A.; Marceau, F. Lysosomotropic cationic drugs induce cytostatic and cytotoxic effects: Role of liposolubility and autophagic flux and antagonism by cholesterol ablation. Toxicol. Appl. Pharmacol. 2016, 305, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, A.; Charest-Morin, X.; Boivin-Welch, M.; Bouthillier, J.; Marceau, F. Autophagic flux inhibition and lysosomogenesis ensuing cellular capture and retention of the cationic drug quinacrine in murine models. PeerJ 2015, 3, e1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Duve, C.; De Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y. Membrane energization by proton pumps is important for compartmentalization of drugs and toxins: A new type of active transport. J. Exp. Biol. 1996, 199, 1447–1454. [Google Scholar] [CrossRef]
- Bawolak, M.T.; Morissette, G.; Marceau, F. Vacuolar ATPase-mediated sequestration of local anesthetics in swollen macroautophagosomes. Can. J. Anaesth. 2010, 57, 230–239. [Google Scholar] [CrossRef]
- Atilla, H.; Tekeli, O.; Can, B.; Karel, F.; Saran, Y. Effects of intracameral lidocaine on ocular tissues. Clin. Exp. Ophthalmol. 2003, 31, 73–77. [Google Scholar] [CrossRef]
- Myers, J.L.; Kennedy, J.I.; Plumb, V.J. Amiodarone lung: Pathologic findings in clinically toxic patients. Hum. Pathol. 1987, 18, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ammoury, A.; Michaud, S.; Paul, C.; Prost-Squarcioni, C.; Alvarez, F.; Lamant, L.; Launay, F.; Bazex, J.; Chouini-Lalanne, N.; Marguery, M.C. Photodistribution of blue-gray hyperpigmentation after amiodarone treatment: Molecular characterization of amiodarone in the skin. Arch. Dermatol. 2008, 144, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruangchira-Urai, R.; Colby, T.V.; Klein, J.; Nielsen, G.P.; Kradin, R.L.; Mark, E.J. Nodular amiodarone lung disease. Am. J. Surg. Pathol. 2008, 32, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Bedrossian, C.W.; Warren, C.J.; Ohar, J.; Bhan, R. Amiodarone pulmonary toxicity: Cytopathology, ultrastructure, and immunocytochemistry. Ann. Diagn. Pathol. 1997, 1, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, C.; Rodrigues, L.R.; Côrte-Real, M. Emerging insights on the role of V-ATPase in human diseases: Therapeutic challenges and opportunities. Med. Res. Rev. 2021, 41, 1927–1964. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Davies, S.A.; Guo, Y.; Graham, S.; Finbow, M.E.; Kaiser, K. Molecular genetic analysis of V-ATPase functions in Drosophila melanogaster. J. Exp. Biol. 1997, 200, 237–245. [Google Scholar] [CrossRef]
- Inoue, H.; Noumi, T.; Nagata, M.; Murakami, H.; Kanazawa, H. Targeted disruption of the gene encoding the proteolipid subunit of mouse vacuolar H+-ATPase leads to early embryonic lethality. Biochim. Biophys. Acta 1999, 1413, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Morissette, G.; Lodge, R.; Marceau, F. Intense pseudotransport of a cationic drug mediated by vacuolar ATPase: Procainamide-induced autophagic cell vacuolization. Toxicol. Appl. Pharmacol. 2008, 228, 364–377. [Google Scholar] [CrossRef]
- Marceau, F.; Bawolak, M.T.; Bouthillier, J.; Morissette, G. Vacuolar ATPase-mediated cellular concentration and retention of quinacrine: A model for the distribution of lipophilic cationic drugs to autophagic vacuoles. Drug Metab. Dispos. 2009, 37, 2271–2274. [Google Scholar] [CrossRef] [Green Version]
- Roy, C.; Gagné, V.; Fernandes, M.J.G.; Marceau, F. High affinity capture and concentration of quinacrine in polymormonuclear neutrophils via vacuolar ATPase-mediated ion trapping: Comparison with other peripheral blood leukocytes and implications for the distribution of cationic drugs. Tox. Appl. Pharmacol. 2013, 270, 77–86. [Google Scholar] [CrossRef]
- Reasor, M.J.; Hastings, K.L.; Ulrich, R.G. Drug-induced phospholipidosis: Issues and future directions. Expert Opin. Drug Saf. 2006, 5, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, E.; Nadai, M.; Caretta, C.M.; Bergonzini, V.; Del Vecchio, C.; Ha, H.R.; Bigler, L.; Dal Zoppo, D.; Faggin, E.; Pettenazzo, A.; et al. Amiodarone impairs trafficking through late endosomes inducing a Niemann-Pick C-like phenotype. Biochem. Pharmacol. 2011, 82, 1234–1249. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Toprak, M.; Noory, M.A.; Robertson, G.P. Effect of lysosomotropic molecules on cellular homeostasis. Pharmacol. Res. 2017, 117, 177–184. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, D.C.; Steele, J.C.; Adagu, I.S.; Craig, J.C.; Cullander, C. Hydroxychloroquine is much less active than chloroquine against chloroquine-resistant Plasmodium falciparum, in agreement with its physicochemical properties. J. Antimicrob. Chemother. 2003, 52, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Durcan, L.; Petri, M. Immunomodulators in SLE: Clinical evidence and immunologic actions. J. Autoimmun. 2016, 74, 73–84. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marceau, F. Failed Repurposing of Lysosomotropic Drugs for COVID-19 Treatment or Prevention. Drugs Drug Candidates 2022, 1, 22-28. https://doi.org/10.3390/ddc1010003
Marceau F. Failed Repurposing of Lysosomotropic Drugs for COVID-19 Treatment or Prevention. Drugs and Drug Candidates. 2022; 1(1):22-28. https://doi.org/10.3390/ddc1010003
Chicago/Turabian StyleMarceau, François. 2022. "Failed Repurposing of Lysosomotropic Drugs for COVID-19 Treatment or Prevention" Drugs and Drug Candidates 1, no. 1: 22-28. https://doi.org/10.3390/ddc1010003
APA StyleMarceau, F. (2022). Failed Repurposing of Lysosomotropic Drugs for COVID-19 Treatment or Prevention. Drugs and Drug Candidates, 1(1), 22-28. https://doi.org/10.3390/ddc1010003






