Do Certain Anaesthetic Drugs Affect Postoperative Cancer Recurrence Rates? Implications for Drug Discovery
Abstract
1. Introduction
2. Primary Tumour Resection and the Risk of Metastasis
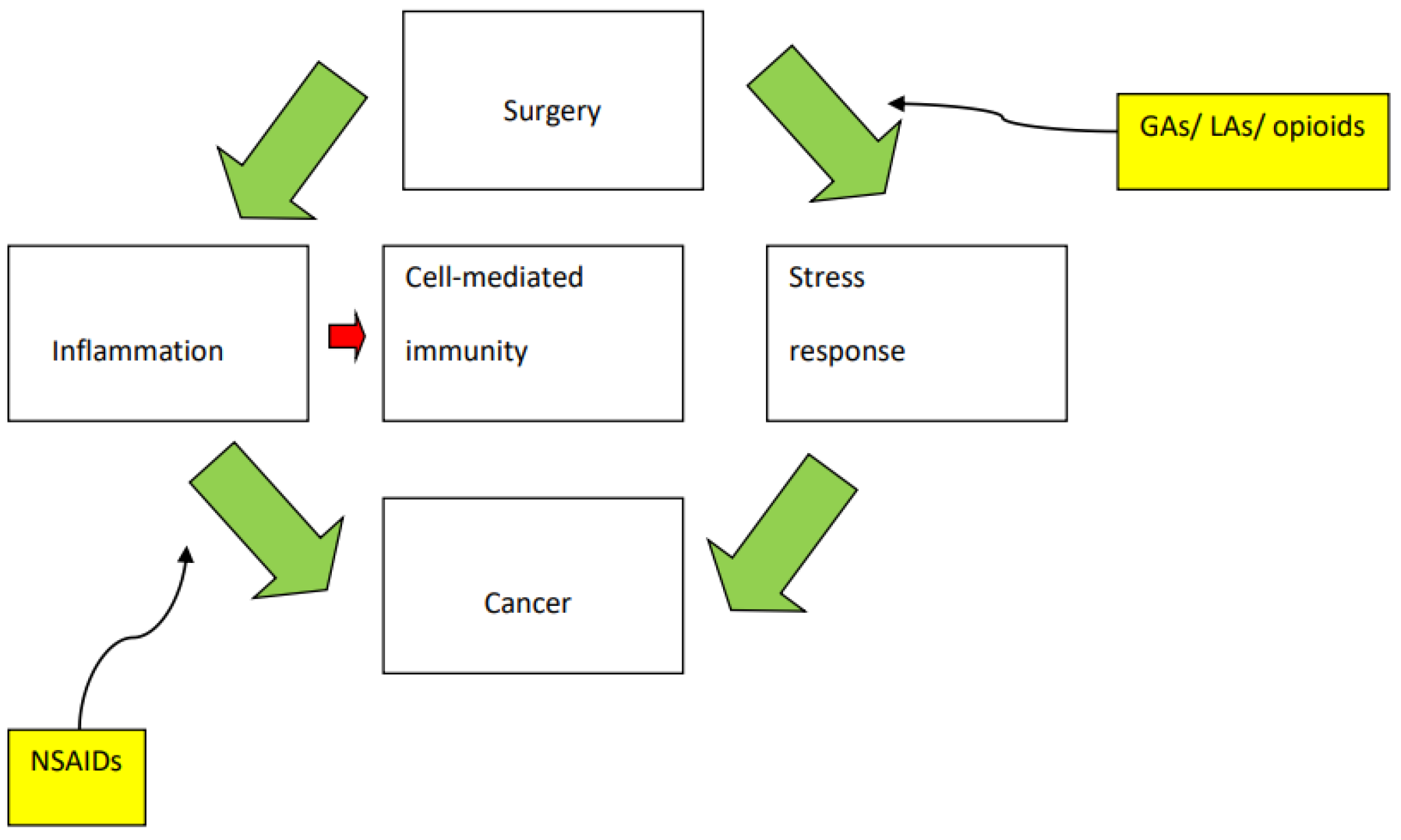
3. The Perioperative Period and the Immune System
4. General Anaesthesia: A Janus Effect?
4.1. Evidence of Anaesthesia Promoting Cancer Recurrence
4.2. Evidence of Anaesthesia Inhibiting Cancer Recurrence
4.3. The Janus Effect: Analgesics
5. Evidence from Clinical Research
5.1. Propofol
5.2. Local Anaesthetics
6. Conclusions: Drug Development
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.R. Effects of regional analgesia on stress responses to pediatric surgery. Pediatr. Anesth. 2012, 22, 19–24. [Google Scholar] [CrossRef]
- Angele, M.K.; Faist, E. Clinical review: Immunodepression in the surgical patient and increased susceptibility to infection. Crit. Care 2002, 6, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Heaney, Á.; Buggy, D.J. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br. J. Anaesth. 2012, 109 (Suppl. 1), i17–i28. [Google Scholar] [CrossRef] [PubMed]
- Page, G.G. Surgery-induced immunosuppression and postoperative pain management. AACN Clin. Issues 2005, 16, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.F.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Eschwège, P.; Dumas, F.; Blanchet, P.; Le Maire, V.; Benoit, G.; Jardin, A.; Lacour, B.; Loric, S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995, 346, 1528–1530. [Google Scholar] [CrossRef]
- O’Riain, S.C.; Buggy, D.J.; Kerin, M.J.; Watson, R.W.G.; Moriarty, D.C. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth. Analg. 2005, 100, 244–249. [Google Scholar] [CrossRef]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef]
- Lloyd, J.M.; McIver, C.M.; Stephenson, S.A.; Hewett, P.J.; Rieger, N.; Hardingham, J.E. Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin. Cancer Res. 2006, 12, 417–423. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takagi, Y.; Aoki, S.; Futamura, M.; Saji, S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000, 232, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tavare, A.N.; Perry, N.J.S.; Benzonana, L.L.; Takata, M.; Ma, D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 2012, 130, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Slingo, M.E.; Pandit, J.J. Oxygen sensing, anaesthesia and critical care: A narrative review. Anaesthesia 2022, 77, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xia, W.H.; Zheng, M.Q.; Lu, C.Q.; Han, X.; Sun, Y.J. Surgical excision promotes tumor growth and metastasis by promoting expression of MMP-9 and VEGF in a breast cancer mode. Exp. Oncol. 2008, 30, 60–64. [Google Scholar]
- Li, H.; Zhao, B.; Liu, Y.; Deng, W.; Zhang, Y. Angiogenesis in residual cancer and roles o HIF-1α, VEGF and MMP-9 in the development of residual cancer after radiofrequency ablation and surgical resection in rabbits with liver cancer. Folia Morphol. 2020, 79, 71–78. [Google Scholar]
- Demicheli, R.; Miceli, R.; Moliterni, A.; Zambetti, M.; Hrushesky, W.J.; Retsky, M.W.; Valagussa, P.; Bonadonna, G. Breast cancer recurrence dynamics following adjuvant CMF is consistent with tumor dormancy and mastectomy-driven acceleration of the metastatic process. Ann. Oncol. 2005, 16, 1449–1457. [Google Scholar] [CrossRef]
- Wang, H.L.; Ning, T.; Li, M.; Lu, Z.J.; Yan, X.; Peng, Q.; Lei, N.; Zhang, H.; Luo, F. Effect of endostatin on preventing postoperative progression of distant metastasis in a murine lung cancer model. Tumari 2011, 97, 787–793. [Google Scholar] [CrossRef]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.M.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann. Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef]
- Wu, F.P.K.; Westphal, J.R.; Hoekman, K.; Mels, A.K.; Statius Muller, M.G.; de Waal, R.W.; Beelen, R.H.; van Leeuwen, P.A.; Meijer, S.; Cuesta, M.A. The effects of surgery, with or without rhGM-CSF, on the angiogenic profile of patients treated for colorectal carcinoma. Cytokine 2004, 25, 68–72. [Google Scholar] [CrossRef]
- Peeters, C.F.J.M.; de Geus, L.F.; Westphal, J.R.; de Waal, R.M.; Ruiter, D.J.; Wobbes, T.; Oyen, W.J.; Ruers, T.J. Decrease in circulating anti-angiogenic factors (angiostatin and endostatin) after surgical removal of primary colorectal carcinoma coincides with increased metabolic activity of liver metastases. Surgery 2005, 137, 246–249. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Shakhar, G.; Ben-Eliyahu, S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann. Surg. Oncol. 2003, 10, 972–992. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliyahu, S.; Page, G.G.; Yirmiya, R.; Shakhar, G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer 1999, 80, 880–888. [Google Scholar] [CrossRef]
- Snyder, G.L.; Greenberg, S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br. J. Anaesth. 2010, 105, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Conrick-Martin, I.; Kell, M.R.; Buggy, D.J. Meta-analysis of the effect of central neuraxial regional anesthesia compared with general anesthesia on postoperative natural killer T lymphocyte function. J. Clin. Anesth. 2012, 24, 3–7. [Google Scholar] [CrossRef]
- Brittenden, J.; Heys, S.D.; Ross, J.; Eremein, O. Natural killer cells and cancer. Cancer 1996, 77, 1226–1243. [Google Scholar] [CrossRef]
- Melamed, R.; Rosenne, E.; Shakhar, K.; Schwartz, Y.; Abudarham, N.; Ben-Eliyahu, S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: Suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav. Immun. 2005, 19, 114–126. [Google Scholar] [CrossRef]
- Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Sijan, G.; Dimitrijevic, S.; Peric-Hajzler, Z.; Matovic, D.; Wollina, U.; Tirant, M.; Thuong, N.V.; et al. Beta blockers and melenoma. J. Med. Sci. 2019, 7, 3110–3112. [Google Scholar]
- Xu, P.; Zhang, P.; Sun, Z.; Wang, Y.; Chen, J.; Miao, C. Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol. Immunother. 2015, 64, 1383–1392. [Google Scholar] [CrossRef]
- Lin, E.; Calvano, S.E.; Lowry, S.F. Inflammatory cytokines and cell response in surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.N.; Knight, P.R.; Murasko, D.M. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology 1993, 78, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhao, H.; Hennah, L.; Ning, J.; Liu, J.; Tu, H.; Ma, D. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br. J. Anaesth. 2015, 114, 831–839. [Google Scholar] [CrossRef]
- Manicom, A.; Pandit, J.J. A narrative review of the role of anaesthesia and peri-operative medicine in improving outcomes after surgery for advanced ovarian cancer. Gynecol. Pelvic Med. 2022, 5, 21–28. [Google Scholar] [CrossRef]
- Ferrell, J.K.; Cattano, D.; Brown, R.E.; Patel, C.B.; Karni, R.J. The effects of anesthesia on the morphoproteomic expression of head and neck squamous cell carcinoma: A pilot study. Transl. Res. 2015, 166, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, G.C.; Gordon, J.; Forrest, J.B. Comparative effects of volatile anaesthetic agents and nitrous oxide on human leucocyte chemotaxis in vitro. Can. Anaesth. Soc. J. 1984, 31, 631–637. [Google Scholar] [CrossRef]
- Shapiro, J.; Jersky, J.; Katzav, S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J. Clin. Investig. 1981, 68, 678–685. [Google Scholar] [CrossRef]
- Loop, T.; Liu, Z.; Humar, M.; Benzing, A.; Pahl, H.L.; Geiger, K.K.; Pannen, B.H.J. Thiopental inhibits the activation of nuclear factor kappa B. Anesthesiology 2002, 96, 1202–1213. [Google Scholar] [CrossRef]
- Braun, S.; Gaza, N.; Werdehausen, R.; Hermanns, H.; Bauer, I.; Durieux, M.E.; Hollmann, M.W.; Stevens, M.F. Ketamine induces apoptosis via the mitochondrial pathway in human lymphocytes and neuronal cells. Br. J. Anaesth. 2010, 105, 347–354. [Google Scholar] [CrossRef]
- Roesslein, M.; Schibilsky, D.; Muller, L.; Goebel, U.; Schwer, C.; Humar, M.; Schmidt, R.; Geiger, K.K.; Pahl, H.L.; Pannen, B.H.; et al. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J. Pharmacol. Exp. Ther. 2008, 325, 217–225. [Google Scholar] [CrossRef]
- Cassinello, F.; Prieto, I.; del Olmo, M.; Rivas, S.; Strichartz, G.R. Cancer surgery: How may anesthesia influence outcome? J. Clin. Anesth. 2015, 27, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Inada, T.; Shingu, K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol. Immunotoxicol. 2007, 29, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.J.; Zhan, J.; Feng, X.B.; Wu, Y.; Rao, Y.; Wang, Y.L. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth. Intensive Care 2008, 36, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: Mediating mechanisms and prophylactic measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Zhang, J.L.; Li, Z.W.; Hu, Z.H.; Liu, Z.; Li, Y. Propofol inhibits proliferation, migration, invasion and promotes apoptosis through down-regulating miR-374a in hepatocarcinoma cell Lines. Cell. Physiol. Biochem. 2018, 49, 2099–2110. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Zhu, Z.; Zheng, Y.; Song, B. Propofol inhibits proliferation, migration and invasion of gastric cancer cells by up-regulating microRNA-195. Int. J. Biol. Macromol. 2018, 120, 975–984. [Google Scholar] [CrossRef]
- Liu, W.Z.; Liu, N. Propofol inhibits lung cancer a549 cell growth and epithelial-mesenchymal transition process by upregulation of microrna-1284. Oncol. Res. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Hsu, S.-S.; Jan, C.-R.; Liang, W.-Z. Evaluation of cytotoxicity of propofol and its related mechanism in glioblastoma cells and astrocytes. Environ. Toxicol. 2017, 32, 2440–2454. [Google Scholar] [CrossRef]
- Xu, Y.-B.; Du, Q.H.; Zhang, M.Y.; Yun, P.; He, C.Y. Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2486–2494. [Google Scholar]
- Looney, M.; Doran, P.; Buggy, D.J. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor β in women undergoing anesthesia and surgery for breast cancer. Anesthesiology 2010, 113, 1118–1125. [Google Scholar] [CrossRef]
- Martinez-Vazquez, P.; Lindner, C.; Melia, U.; Pandit, J.J.; Martinez-Vazquez, P. Be Aware, Unaware and Confusion Everywhere: TIVA and Awareness. In Taking on TIVA.; Irwin, M.G., Wong, G.T.C., Lam, S.K., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 63–72. [Google Scholar]
- Beilin, B.; Martin, F.C.; Shavit, Y.; Gale, R.P.; Liebeskind, J.C. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav. Immun. 1989, 3, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Kumar, S.; Khanna, S.; Mehta, Y. Are we causing the recurrence-impact of perioperative period on long-term cancer prognosis: Review of current evidence and practice. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 153. [Google Scholar] [CrossRef]
- Gupta, K.; Kshirsagar, S.; Chang, L.; Schwartz, R.; Law, P.Y.; Yee, D.; Hebbel, R.P. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002, 62, 4491–4498. [Google Scholar] [PubMed]
- Shavit, Y.; Ben-Eliyahu, S.; Zeidel, A.; Beilin, B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Neuroimmunomodulation 2004, 11, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, R.; Moser, D.; Salfinger, H.; Frass, M.; Kapiotis, S. Sufentanil inhibits migration of human leukocytes through human endothelial cell monolayers. Anesth. Analg. 1998, 87, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Gaspani, L.; Rossoni, G.; Panerai, A.E.; Bianchi, M. Effect of the opioid remifentanil on cellular immune response in the rat. Int. Immunopharmacol. 2001, 1, 713–719. [Google Scholar] [CrossRef]
- Singleton, P.A.; Mirzapoiazova, T.; Hasina, R.; Salgia, R.; Moss, J. Increased μ-opioid receptor expression in metastatic lung cancer. Br. J. Anaesth. 2014, 113 (Suppl. S1), i103–i108. [Google Scholar] [CrossRef]
- Mathew, B.; Lennon, F.E.; Siegler, J.; Mirzapoiazova, T.; Mambetsariev, N.; Sammani, S.; Gerhold, L.M.; LaRiviere, P.J.; Chen, C.T.; Garcia, J.G.; et al. The novel role of the mu opioid receptor in lung cancer progression: A laboratory investigation. Anesth. Analg. 2011, 112, 558–567. [Google Scholar] [CrossRef]
- Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Poroyko, V.A.; Salgia, R.; Moss, J.; Singleton, P.A. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and Epithelial Mesenchymal Transition (EMT) in human lung cancer. PLoS ONE 2014, 9, e91577. [Google Scholar] [CrossRef]
- Page, G.G.; Ben-Eliyahu, S.; Yirmiya, R.; Liebeskind, J.C. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain 1993, 54, 21–28. [Google Scholar] [CrossRef]
- Page, G.G.; Mcdonald, J.S.; Ben-Eliyahu, S. Pre-operative versus postoperative administration of morphine: Impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br. J. Anaesth. 1998, 81, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.P.; Cloote, A.; Weir, P.M.; Jenkins, I.; Murphy, P.J.; Pawade, A.K.; Rogers, C.A.; Wolf, A.R. Reducing stress responses in the pre-bypass phase of open heart surgery in infants and young children: A comparison of different fentanyl doses. Br. J. Anaesth. 2000, 84, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz-Praga, S. Reversal of tumor-induced immunosuppression by TGF-β inhibitors. Investig. New Drugs 2003, 21, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J.; DuBois, R.N. COX-2: A target for colon cancer prevention. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.; Soreghan, B.; Szabo, I.L.; Pavelka, M.; Baatar, D.; Tarnawski, A.S. Prostaglandin E2, transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat. Med. 2002, 8, 289–293. [Google Scholar] [CrossRef]
- Chang, S.H.; Liu, C.H.; Conway, R.; Han, D.K.; Nithipatikom, K.; Trifan, O.C.; Lane, T.F.; Hla, T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc. Natl. Acad. Sci. USA 2004, 101, 591–596. [Google Scholar] [CrossRef]
- Iñiguez, M.A.; Rodríguez, A.; Volpert, O.; Fresno, M.; Redondo, J.M. Cyclooxygenase-2: A therapeutic target in angiogenesis. Trends Mol. Med. 2003, 9, 73–78. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef]
- Farooqui, M.; Li, Y.; Rogers, T.; Griffin, R.J.; Song, C.W.; Gupta, K. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br. J. Cancer 2007, 97, 1523–1531. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Li, H. NSAIDs use and reduced metastasis in cancer patients: Results from a meta-analysis. Sci. Rep. 2017, 7, 1875. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Zhu, S.; Li, X.W.; Wang, F.; Hu, F.L.; Li, D.D.; Zhang, W.C.; Li, X. Association between NSAIDs use and breast cancer risk: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2009, 117, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Hankin, J.; Zhao, H.; Yao, S.; Ma, D. The potential benefits of the use of regional anesthesia in cancer patients. Int. J. Cancer 2015, 137, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Diss, J.K.; Chioni, A.M.; Mycielska, M.E.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Pandit, J.J.; McGuire, N. Unlicensed intravenous lidocaine for postoperative pain: Always a safer ‘licence to stop’ than to start. Anaesthesia 2021, 76, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Mazeki, F.; Fukai, K.; Sakamoto, A.; Arai, M.; Mikata, R.; Tokuhisa, T.; Yokosuka, O. Procaine inhibits the proliferation and DNA methylation in human hepatoma cells. Hepatol. Int. 2007, 1, 355–364. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Z.; Hung, M.S.; Lin, Y.C.; Wang, T.; Gong, M.; Zhi, X.; Jablons, D.M.; You, L. Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol. Rep. 2009, 22, 1479–1484. [Google Scholar] [PubMed]
- Zhou, H.; Xu, M.; Luo, G.; Zhang, Y. Effects of procaine on human nasopharyngeal carcinoma cell strain CNE-2Z. J. Clin. Otorhinolaryngol. Head Neck Surg. 2007, 21, 1118–1121. [Google Scholar]
- Lucchinetti, E.; Awad, A.E.; Rahman, M.; Feng, J.; Lou, P.H.; Zhang, L.; Ionescu, L.; Lemieux, H.; Thébaud, B.; Zaugg, M. Antiproliferative effects of local anesthetics on mesenchymal stem cells: Potential implications for tumor spreading and wound healing. Anesthesiology 2012, 116, 841–856. [Google Scholar] [CrossRef]
- Mammoto, T.; Higashiyama, S.; Mukai, M.; Mammoto, A.; Ayaki, M.; Mashimo, T.; Hayashi, Y.; Kishi, Y.; Nakamura, H.; Akedo, H. Infiltration anesthetic lidocaine inhibits cancer cell invasion by modulating ectodomain shedding of heparin-binding epidermal growth factor-like growth factor (HB-EGF). J. Cell. Physiol. 2002, 192, 351–358. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Kuroda, Y.; Hirose, M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth. Analg. 2006, 102, 1103–1107. [Google Scholar] [CrossRef]
- Yoon, J.R.; Whipple, R.A.; Balzer, E.M.; Cho, E.H.; Matrone, M.A.; Peckham, M.; Martin, S.S. Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells. Breast Cancer Res. Treat. 2011, 129, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.Y. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int. J. Biochem. Cell Biol. 2007, 39, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shen, Y.; Cai, J.; Lei, M.; Wang, Z. Expression of voltage-gated sodium channel · subunit in human ovarian cancer. Oncol. Rep. 2010, 23, 1293–1299. [Google Scholar] [PubMed]
- Andrikopoulos, P.; Fraser, S.P.; Patterson, L.; Ahmad, Z.; Burcu, H.; Ottaviani, D.; Diss, J.K.; Box, C.; Eccles, S.A.; Djamgoz, M.B. Angiogenic functions of voltage-gated Na+ channels in human endothelial cells: Modulation of vascular endothelial growth factor (VEGF) signaling. J. Biol. Chem. 2011, 286, 16846–16860. [Google Scholar] [CrossRef]
- Johnson, M.Z.; Crowley, P.D.; Foley, A.G.; Xue, C.; Connolly, C.; Gallagher, H.C.; Buggy, D.J. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br. J. Anaesth. 2018, 121, 76–85. [Google Scholar] [CrossRef]
- Perez-Castro, R.; Patel, S.; Garavito-Aguilar, Z.V.; Rosenberg, A.; Recio-Pinto, E.; Zhang, J.; Blanck, T.J.; Xu, F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth. Analg. 2009, 108, 997–1007. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Liu, C.L.; Chen, M.J.; Hsu, Y.W.; Chen, S.N.; Lin, C.H.; Chen, C.M.; Yang, F.M.; Hu, M.C. Local anesthetics induce apoptosis in human breast tumor cells. Anesth. Analg. 2014, 118, 116–124. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, Y.C.; Liu, C.L.; Huang, S.Y.; Hu, M.C.; Cheng, S.P. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS ONE 2014, 9, e89563. [Google Scholar] [CrossRef]
- Enlund, M.; Berglund, A.; Andreasson, K.; Cicek, C.; Enlund, A.; Bergkvist, L. The choice of anaesthetic-sevoflurane or propofol-and outcome from cancer surgery: A retrospective analysis. Upsala J. Med. Sci. 2014, 119, 251–261. [Google Scholar] [CrossRef]
- Wigmore, T.J.; Mohammed, K.; Jhanji, S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: A retrospective analysis. Anesthesiology 2016, 124, 69–79. [Google Scholar] [CrossRef]
- Sofra, M.; Fei, P.C.; Fabrizi, L.; Marcelli, M.E.; Claroni, C.; Gallucci, M.; Ensoli, F.; Forastiere, E. Immunomodulatory effects of total intravenous and balanced inhalation anesthesia in patients with bladder cancer undergoing elective radical cystectomy: Preliminary results. J. Exp. Clin. Cancer Res. 2013, 32, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, S.H.; Kim, Y.; Kim, H.A.; Kim, B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: A retrospective study. Korean J. Anesthesiol. 2016, 69, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, D.W.; Kim, J.H.; Lee, K.Y.; Park, S.; Yoo, Y.C. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? Oncotarget 2017, 8, 90477–90487. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, H.B.; Han, W.; Noh, D.Y.; Park, S.K.; Kim, W.H.; Kim, J.T. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: A retrospective cohort study. Anesthesiology 2019, 130, 31–40. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Lee, M.S.; Lou, Y.S.; Lai, H.C.; Yu, J.C.; Lu, C.H.; Wong, C.S.; Wu, Z.F. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in breast cancer surgery. PLoS ONE 2019, 14, e0224728. [Google Scholar] [CrossRef]
- Wu, Z.F.; Lee, M.S.; Wong, C.S.; Yeh, T.T.; Lai, H.C.; Wu, K.L.; Wu, Z.F.; Tseng, W.C. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology 2018, 129, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Kim, K.; Jheon, S.; Lee, J.; Do, S.H.; Hwang, J.W.; Song, I.A. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: A retrospective propensity matching analysis. Cancer Control 2018, 25, 1073274818775360. [Google Scholar] [CrossRef]
- Jun, I.J.; Jo, J.Y.; Kim, J.I.; Chin, J.H.; Kim, W.J.; Kim, H.R.; Lee, E.H.; Choi, I.C. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: A retrospective observational study. Sci. Rep. 2017, 7, 14020. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y.; Dong, L.; Zhao, S.; Wang, L.; Chen, H.; Xu, Y.; Wang, G. Effects of propofol-based total intravenous anesthesia on gastric cancer: A retrospective study. OncoTargets Ther. 2018, 11, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zeng, M.; Ji, N.; Hao, S.; Zhou, Y.; Gao, Z.; Gu, H.; Zhang, L.; Ma, D.; Peng, Y.; et al. Impact of anesthesia on long-term outcomes in patients with supratentorial high-grade glioma undergoing tumor resection. J. Neurosurg. Anesthesiol. 2019, 32, 227–233. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, X.P.; Sun, Z.; Wang, H.Q.; Yu, W.F. Distant survival for patients undergoing surgery using volatile versus IV anesthesia for hepatocellular carcinoma with portal vein tumor thrombus: A retrospective study. BMC Anesthesiol. 2020, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Jang, W.S.; Choi, Y.D.; Hong, J.H.; Noh, S.; Yoo, Y.C. Comparison of biochemical recurrence after robot-assisted laparoscopic radical prostatectomy with volatile and total intravenous anesthesia. Int. J. Med. Sci. 2020, 17, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, R.; Liu, J.; Lin, J. Long-term prognosis after cancer surgery with inhalational anesthesia and total intravenous anesthesia: A systematic review and meta-analysis. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 83–94. [Google Scholar] [PubMed]
- Sury, M.R.; Palmer, J.H.; Cook, T.M.; Pandit, J.J. The state of UK anaesthesia: A survey of National Health Service activity in 2013. Br. J. Anaesth. 2014, 113, 575–584. [Google Scholar] [CrossRef]
- Pandit, J.J.; Andrade, J.; Bogod, D.G.; Hitchman, J.M.; Jonker, W.R.; Lucas, N.; Mackay, J.H.; Nimmo, A.F.; O’Connor, K.; O’Sullivan, E.P.; et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: Summary of main findings and risk factors. Br. J. Anaesth. 2014, 113, 549–559. [Google Scholar] [CrossRef]
- Biki, B.; Mascha, E.; Moriarty, D.C.; Fitzpatrick, J.M.; Sessler, D.I.; Buggy, D.J. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology 2008, 109, 180–187. [Google Scholar] [CrossRef]
- Tsui, B.C.H.; Rashiq, S.; Schopflocher, D.; Murtha, A.; Broemling, S.; Pillay, J.; Finucane, B.T. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can. J. Anesth. 2010, 57, 107–112. [Google Scholar] [CrossRef]
- Wuethrich, P.Y.; Hsu Schmitz, S.F.; Kessler, T.M.; Thalmann, G.N.; Studer, U.E.; Stueber, F.; Burkhard, F.C. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: A retrospective study. Anesthesiology 2010, 113, 570–576. [Google Scholar] [CrossRef]
- Forget, P.; Tombal, B.; Scholtès, J.L.; Nzimbala, J.; Meulders, C.; Legrand, C.; Van Cangh, P.; Cosyns, J.P.; De Kock, M. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur. J. Anaesthesiol. 2011, 28, 830–835. [Google Scholar] [CrossRef]
- Wuethrich, P.Y.; Thalmann, G.N.; Studer, U.E.; Burkhard, F.C. Epidural analgesia during open radical prostatectomy does not improve long-term cancer-related outcome: A retrospective study in patients with advanced prostate cancer. PLoS ONE 2013, 8, e72873. [Google Scholar] [CrossRef]
- Roiss, M.; Schiffmann, J.; Tennstedt, P.; Kessler, T.; Blanc, I.; Goetz, A.; Schlomm, T.; Graefen, M.; Reuter, D.A. Oncological long-term outcome of 4772 patients with prostate cancer undergoing radical prostatectomy: Does the anaesthetic technique matter? Eur. J. Surg. Oncol. 2014, 40, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Sprung, J.; Scavonetto, F.; Yeoh, T.Y.; Kramer, J.M.; Karnes, R.J.; Eisenach, J.H.; Schroeder, D.R.; Weingarten, T.N. Outcomes after radical prostatectomy for cancer: A comparison between general anesthesia and epidural anesthesia with fentanyl analgesia: A matched cohort study. Anesth. Analg. 2014, 119, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Scavonetto, F.; Yeoh, T.Y.; Umbreit, E.C.; Weingarten, T.N.; Gettman, M.T.; Frank, I.; Boorjian, S.A.; Karnes, R.J.; Schroeder, D.R.; Rangel, L.J.; et al. Association between neuraxial analgesia, cancer progression, and mortality after radical prostatectomy: A large, retrospective matched cohort study. Br. J. Anaesth. 2014, 113 (Suppl. S1), i95–i102. [Google Scholar] [CrossRef]
- Tseng, K.S.; Kulkarni, S.; Humphreys, E.B.; Carter, H.B.; Mostwin, J.L.; Partin, A.W.; Han, M.; Wu, C.L. Spinal anesthesia does not impact prostate cancer recurrence in a cohort of men undergoing radical prostatectomy: An observational study. Reg. Anesth. Pain Med. 2014, 39, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, R.; James, K.E.; Tableman, M.; Marshall, P.; Johnson, F.E. Long-term survival after colon cancer surgery: A variation associated with choice of anesthesia. Anesth. Analg. 2008, 107, 325–332. [Google Scholar] [CrossRef]
- Gottschalk, A.; Ford, J.G.; Regelin, C.C.; You, J.; Mascha, E.J.; Sessler, D.I.; Durieux, M.E.; Nemergut, E.C. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology 2010, 113, 27–34. [Google Scholar] [CrossRef]
- Gupta, A.; Björnsson, A.; Fredriksson, M.; Hallböök, O.; Eintrei, C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: A retrospective analysis of data from 655 patients in Central Sweden. Br. J. Anaesth. 2011, 107, 164–170. [Google Scholar] [CrossRef]
- Cummings, K.C.; Xu, F.; Cummings, L.C.; Cooper, G.S. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: A population-based study. Anesthesiology 2012, 116, 797–806. [Google Scholar] [CrossRef]
- Day, A.; Smith, R.; Jourdan, I.; Fawcett, W.; Scott, M.; Rockall, T. Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. Br. J. Anaesth. 2012, 109, 185–190. [Google Scholar] [CrossRef]
- Holler, J.P.N.; Ahlbrandt, J.; Burkhardt, E.; Gruss, M.; Röhrig, R.; Knapheide, J.; Hecker, A.; Padberg, W.; Weigand, M.A. Peridural analgesia may affect long-term survival in patients with colorectal cancer after surgery (PACO-RAS-Study): An analysis of a cancer registry. Ann. Surg. 2013, 258, 989–993. [Google Scholar] [CrossRef]
- Vogelaar, F.J.; Abegg, R.; van der Linden, J.C.; Cornelisse, H.G.; van Dorsten, F.R.; Lemmens, V.E.; Bosscha, K. Epidural analgesia associated with better survival in colon cancer. Int. J. Color. Dis. 2015, 30, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- MacFater, W.S.; Xia, W.; Barazanchi, A.W.H.; MacFater, H.S.; Lightfoot, N.; Svirskis, D.; Kahokehr, A.A.; Hill, A.G. Association between perioperative intraperitoneal local anaesthetic infusion and long-term survival and cancer recurrence after colectomy: Follow-up analysis of a previous randomized controlled trial. Aust. N. Z. J. Surg. 2020, 90, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Hacking, M.B.; Link, E.K.; Wessels, K.L.; Riedel, B.J. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol. Scand. 2014, 58, 281–290. [Google Scholar] [CrossRef]
- Cummings, K.C.; Patel, M.; Htoo, P.T.; Bakaki, P.M.; Cummings, L.C.; Koroukian, S.A. A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: A population-based study. Reg. Anesth. Pain Med. 2014, 39, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, H.I.; Kim, N.Y.; Lee, K.Y.; Kim, D.W.; Yoo, Y.C. Effect of postoperative analgesia technique on the prognosis of gastric cancer: A retrospective analysis. Oncotarget 2017, 8, 104594–104604. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, H.; Xu, Y.; Zheng, X.; Wang, G. The effects of intra- and post-operative anaesthesia and analgesia choice on outcome after gastric cancer resection: A retrospective study. Oncotarget 2017, 8, 62658–62665. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Huang, Q.; Ye, S.; Ye, S.; Rong, T. Short and long-term outcomes of epidural or intravenous analgesia after esophagectomy: A propensity-matched cohort study. PLoS ONE 2016, 11, e0154380. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Tan, H.; Ouyang, H.; Zhang, Y.; Zeng, W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: A retrospective analysis. Br. J. Anaesth. 2011, 106, 814–822. [Google Scholar] [CrossRef]
- De Oliveira, G.S.; Ahmad, S.; Schink, J.C.; Singh, D.K.; Fitzgerald, P.C.; McCarthy, R.J. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg. Anesth. Pain Med. 2011, 36, 271–277. [Google Scholar] [CrossRef]
- Capmas, P.; Billard, V.; Gouy, S.; Lhommé, C.; Pautier, P.; Morice, P.; Uzan, C. Impact of epidural analgesia on survival in patients undergoing complete cytoreductive surgery for ovarian cancer. Anticancer Res. 2012, 32, 1537–1542. [Google Scholar]
- Lacassie, H.J.; Cartagena, J.; Brañes, J.; Assel, M.; Echevarría, G.C. The relationship between neuraxial anesthesia and advanced ovarian cancer-related outcomes in the Chilean population. Anesth. Analg. 2013, 117, 653–660. [Google Scholar] [CrossRef]
- Tseng, J.H.; Cowan, R.A.; Afonso, A.M.; Zhou, Q.; Iasonos, A.; Ali, N.; Thompson, E.; Sonoda, Y.; O’Cearbhaill, R.E.; Chi, D.S.; et al. Perioperative epidural use and survival outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer. Gynecol. Oncol. 2018, 151, 287–293. [Google Scholar] [CrossRef]
- Doiron, R.C.; Jaeger, M.; Booth, C.M.; Booth, C.M.; Wei, X.; Robert Siemens, D. Is there a measurable association of epidural use at cystectomy and postoperative outcomes? A population-based study. Can. Urol. Assoc. J. 2016, 10, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, T.N.; Taccolini, A.M.; Ahle, S.T.; Dietz, K.R.; Dowd, S.S.; Frank, I.; Boorjian, S.A.; Thapa, P.; Hanson, A.C.; Schroeder, D.R.; et al. Perioperative management and oncological outcomes following radical cystectomy for bladder cancer: A matched retrospective cohort study. Can. J. Anesth. 2016, 63, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Baek, S.; Joo, E.Y.; Yoon, S.H.; Kim, E.; Hong, B.; Hwang, J.H.; Kim, Y.K. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget 2017, 8, 87667–87674. [Google Scholar] [CrossRef] [PubMed]
- Koumpan, Y.; Jaeger, M.; Mizubuti, G.B.; Tanzola, R.; Jain, K.; Hosier, G.; Hopman, W.; Siemens, D.R. Spinal anesthesia is associated with lower recurrence rates after resection of nonmuscle invasive bladder cancer. J. Urol. 2018, 199, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Chipollini, J.; Alford, B.; Boulware, D.C.; Forget, P.; Gilbert, S.M.; Lockhart, J.L.; Pow-Sang, J.M.; Sexton, W.J.; Spiess, P.E.; Poch, M.A.; et al. Epidural anesthesia and cancer outcomes in bladder cancer patients: Is it the technique or the medication? A matched-cohort analysis from a tertiary referral center. BMC Anesthesiol. 2018, 18, 157. [Google Scholar] [CrossRef]
- Zimmitti, G.; Soliz, J.; Aloia, T.A.; Gottumukkala, V.; Cata, J.P.; Tzeng, C.W.; Vauthey, J.N. Positive impact of epidural analgesia on oncologic outcomes in patients undergoing resection of colorectal liver metastases. Ann. Surg. Oncol. 2016, 23, 1003–1011. [Google Scholar] [CrossRef]
- Gottschalk, A.; Brodner, G.; Van Aken, H.K.; Ellger, B.; Althaus, S.; Schulze, H.J. Can regional anaesthesia for lymph-node dissection improve the prognosis in malignant melanoma. Br. J. Anaesth. 2012, 109, 253–259. [Google Scholar] [CrossRef]
- Merquiol, F.; Montelimard, A.S.; Nourissat, A.; Molliex, S.; Zufferey, P.J. Cervical epidural anesthesia is associated with increased cancer-free survival in laryngeal and hypopharyngeal cancer surgery: A retrospective propensity-matched analysis. Reg. Anesth. Pain Med. 2013, 38, 398–402. [Google Scholar] [CrossRef]
- Myles, P.S.; Peyton, P.; Silbert, B.; Hunt, J.; Rigg, J.R.; Sessler, D.I.; ANZCA Trials Group Investigators. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: Randomised trial. Br. Med. J. 2011, 342, d1491. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Tai, Y.H.; Chan, M.Y.; Tsou, M.Y.; Chen, H.H.; Chang, K.Y. Effects of epidural analgesia on cancer recurrence and long-term mortality in patients after non-small-cell lung cancer resection: A propensity score-matched study. BMJ Open 2019, 9, e027618. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Singh Ghotra, V.; Karam, J.A.; Karam, J.A.; Hernandez, M.; Pratt, G.; Cata, J.P. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: A meta-analysis. Pain Manag. 2015, 5, 387–395. [Google Scholar] [CrossRef]
- Weng, M.; Chen, W.; Hou, W.; Li, L.; Ding, M.; Miao, C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: An updated meta-analysis. Oncotarget 2016, 7, 15262–15273. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, R.K.; Lee, S.; Abd-Elsayed, A. The relationship between regional anesthesia and cancer: A meta-analysis. Ochsner 2017, 17, 345–361. [Google Scholar]
- Cata, J.P.; Lasala, J.; Pratt, G.; Feng, L.; Shah, J.B. Association between perioperative blood transfusions and clinical outcomes in patients undergoing bladder cancer surgery: A systematic review and meta-analysis study. J. Blood Transfus. 2016, 2016, 9876394. [Google Scholar] [CrossRef]
- Agnes, A.; Lirosi, M.C.; Panunzi, S.; Santocchi, P.; Persiani, R.; D’Ugo, D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur. J. Surg. Oncol. 2018, 44, 404–419. [Google Scholar] [CrossRef]
- Li, S.-L.; Ye, Y.; Yuan, X.-H. Association between allogeneic or autologous blood transfusion and survival in patients after radical prostatectomy: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0171081. [Google Scholar] [CrossRef]
- Ben-Eliyahu, S.; Shakhar, G.; Rosenne, E.; Levinson, Y.; Beilin, B. Hypothermia in barbiturate-anesthetized rats suppresses natural killer cell activity and compromises resistance to tumor metastasis: A role for adrenergic mechanisms. Anesthesiology 1999, 91, 732–740. [Google Scholar] [CrossRef]
- Pandit, J.J. Monitoring (un)consciousness: The implications of a new definition of ‘anaesthesia’. Anaesthesia 2014, 69, 801–807. [Google Scholar] [CrossRef]
| Study | Study Type | Propofol/VA | Volatile Agent | Cancer Type | End Point | Hazard Ratio | 95% CI | Result |
|---|---|---|---|---|---|---|---|---|
| Enlund et al. (2014) [90] | RC | 1935/903 | Sevoflurane | Various | OS | 0.86 | 0.60–1.24 | − |
| Wigmore et al. (2016) [91] | RC | 3316/3714 | Sevoflurane or isoflurane | Various | OS | 0.68 | 0.60–0.78 | + |
| Sofra et al. (2013) [92] | RCT | 14/14 | Sevoflurane | Bladder | OS | nr | nr, p = 0.14 | − |
| Lee et al. (2016) [93] | RC | 152/173 | Sevoflurane | Breast | OS | nr | nr, p = 0.38 | − |
| RFS | 0.48 | 0.27–0.86 | + | |||||
| Kim et al. (2017) [94] | RC | 56/2589 | Sevoflurane, isoflurane, enflurane or desflurane | Breast | OS | 1.14 | 0.49–2.60 | − |
| Yoo et al. (2019) [95] | RC | 3085/2246 | Sevoflurane, isoflurane, enflurane or desflurane | Breast | OS | 0.96 | 0.69–1.33 | − |
| RFS | 0.96 | 0.69–1.32 | − | |||||
| Huang et al. (2019) [96] | RC | 344/632 | Desflurane | Breast | OS | 1.13 | 0.67–1.92 | − |
| Wu et al. (2018) [97] | RC | 657/706 | Desflurane | Colorectal | OS | 0.27 | 0.22–0.35 | + |
| Oh Et al. (2016) [98] | RC | 194/749 | Sevoflurane | Non-small cell lung | OS | 0.90 | 0.64–1.26 | − |
| RFS | 1.31 | 0.84–2.04 | − | |||||
| Jun et al. (2017) [99] | RC | 731/191 | Sevoflurane, isoflurane or desflurane | Oesophageal | OS | 0.63 | 0.50–0.81 | + |
| RFS | 0.70 | 0.56–0.89 | + | |||||
| Zheng et al. (2018) [100] | RC | 1506/1350 | Sevoflurane | Gastric | OS | 0.65 | 0.56–0.75 | + |
| Dong et al. (2019) [101] | RC | 154/140 | Sevoflurane | Glioma | OS | nr | nr, p = 0.76 | − |
| OS (Low Karnofsky) | 0.60 | 0.39–0.93 | + |
| Study | Study Type | LA/Control | LA Technique | Control Technique | Cancer Type | End Point | Hazard Ratio | 95% CI | Result |
|---|---|---|---|---|---|---|---|---|---|
| Sessler et al. (2019) [1] | RCT | 1043/1065 | LA PVB + propofol | Opioid + sevoflurane | Breast | RFS | 0.97 | 0.74–1.28 | − |
| Biki et al. (2008) [107] | RC | 102/123 | Epidural LA + GA | Opioid + GA | Prostate | BCR | 0.43 | 0.22–0.83 | + |
| Tsui et al. (2010) [108] | RCT | 49/50 | Epidural LA + GA | GA | Prostate | BCR | 1.33 | 0.64–2.77 | − |
| Wuethrich et al. (2010) [109] | RC | 103/158 | Epidural LA + GA | Opioid + NSAID + GA | Prostate | OS | 0.61 | 0.29–1.28 | − |
| PFS | 0.45 | 0.27–0.75 | + | ||||||
| Forget et al. (2011) [110] | RC | 578/533 | Epidural LA + GA | GA | Prostate | BCR | 0.84 | 0.52–1.17 | − |
| Wuethrich et al. (2013) [111] | RC | 67/81 | Epidural LA + GA | Opioid + NSAID + GA | Prostate | OS | 1.17 | 0.63–2.17 | − |
| Local RFS | 1.16 | 0.41–3.29 | − | ||||||
| Distant RFS | 0.56 | 0.26–1.25 | − | ||||||
| Roiss et al. (2014) [112] | RC | 3047/1725 | Spinal LA + GA | GA | Prostate | OS | 0.90 | 0.51–1.60 | − |
| RFS | 1.11 | 0.54–2.27 | − | ||||||
| BCR | 1.09 | 0.85–1.41 | − | ||||||
| Sprung et al. (2014) [113] | RC | 486/486 | Epidural LA + GA | Opioid + GA | Prostate | OS | 0.81 | 0.61–1.08 | − |
| RFS | 1.27 | 0.96–1.67 | − | ||||||
| Scavonetto et al. (2014) [114] | RC | 1642/1642 | Neuraxial LA + GA | GA | Prostate | OS | 0.76 | 0.57–1.00 | + |
| SP | 0.36 | 0.17–0.76 | + | ||||||
| Tseng et al. (2014) [115] | RC | 1166/798 | Spinal LA + Sedative | GA | Prostate | BCR | 0.91 | 0.70–1.18 | − |
| Christopherson et al. (2008) [116] | RCT | 85/92 | Epidural LA + GA | GA | Colorectal | OS | 1.43 | 0.75–2.70 | − |
| Gottschalk et al. (2010) [117] | RC | 256/253 | Epidural LA + GA | GA | Colorectal | RFS | 0.82 | 0.49–1.35 | − |
| Gupta et al. (2011) [118] | RC | 562/93 | Epidural LA + GA | PCA + GA | Colorectal | OS (colon) | 0.82 | 0.30–2.19 | − |
| OS (rectal) | 0.45 | 0.22–0.90 | + | ||||||
| Cummings et al. (2012) [119] | RC | 9278/40377 | Epidural LA + GA | GA | Colorectal | OS | 0.91 | 0.87–0.94 | + |
| RFS | 1.05 | 0.95–1.15 | − | ||||||
| Day et al. (2012) [120] | RC | 251/173 | Epidural or Spinal LA + GA | PCA + GA | Colorectal | OS | Nr | nr, p = 0.622 | − |
| Holler et al. (2013) [121] | RC | 442/307 | Epidural LA + GA | GA | Colorectal | OS | 0.73 | nr, p < 0.002 | + |
| Vogelaar et al. (2015) [122] | RC | 399/189 | Epidural LA + GA | GA | Colorectal | OS | 0.77 | 0.63–0.95 | + |
| MacFater et al. (2020) [123] | RCT | 37/19 | IP LA + GA | IP Saline +GA | Colorectal | OS | 0.65 | nr, p = 0.620 | − |
| Hiller etc. (2014) [124] | RC | 97/43 | Epidural LA + GA | GA | Gastric | OS | 0.42 | 0.0.21–0.83 | + |
| TTR | 0.33 | 0.17–0.63 | + | ||||||
| Cummings et al. (2014) [125] | RC | 766/1979 | Epidural LA + GA | GA | Gastric | OS | 0.93 | 0.84–1.03 | − |
| Shin et al. (2017) [126] | RC | 4325/374 | Epidural PCA | i.v. PCA | Gastric | OS | 0.67 | 0.43–1.13 | − |
| RFS | 1.10 | 0.86–1.40 | − | ||||||
| Wang et al. (2017) [127] | RC | 1390/2856 | Epidural LA + GA | GA | Gastric | OS | 0.65 | 0.58–0.73 | + |
| Li et al. (2016) [128] | RC | 178/178 | Epidural LA + GA | GA | Oesophageal | OS | Nr | nr, p = 0.470 | − |
| RFS | Nr | nr, p = 0.460 | − | ||||||
| Lin et al. (2011) [129] | RC | 106/37 | Epidural LA + GA | Opioid + GA | Ovarian | OS | 0.82 | 0.70–0.96 | + |
| de Oliviera et al. (2011) [130] | RC | 55/127 | Epidural LA + GA | GA | Ovarian | P/O TTR | 0.86 | 0.52–1.41 | − |
| I/O TTR | 0.37 | 0.19–0.73 | + | ||||||
| Capmas et al. (2012) [131] | RC | 47/47 | Epidural PCA + GA | GA | Ovarian | OS | 1.25 | 0.39–4.04 | − |
| RFS | 1.18 | 0.61–2.31 | − | ||||||
| Lacassie et al. (2013) [132] | RC | 37/43 | Epidural LA + GA | GA | Ovarian | TTR | 0.72 | 0.40–1.33 | − |
| Tseng et al. (2018) [133] | RC | 435/213 | Epidural LA + GA | GA | Ovarian | OS | 0.64 | 0.49–0.82 | + |
| RFS | 0.75 | 0.60–0.94 | + | ||||||
| Doiron et al. (2016) [134] | RC | 887/741 | Epidural LA + GA | GA | Bladder | OS | 0.91 | 0.80–1.03 | − |
| Weingarten et al. (2016) [135] | RC | 195/195 | Spinal LA + GA | GA | Bladder | OS | 1.09 | 0.77–1.53 | − |
| Choi et al. (2017) [136] | RC | 718/158 | Spinal LA | GA | Bladder | RFS | 0.62 | 0.48–0.79 | + |
| Koumpan et al. (2018) [137] | RC | 135/96 | Spinal LA | GA | Bladder | RFS | 0.49 | 0.27–0.88 | + |
| TTR | 0.64 | 0.46–0.88 | + | ||||||
| Chipollini et al. (2018) [138] | RC | 215/215 | Epidural LA. + GA | GA | Bladder | RFS | 1.67 | 1.14–2.45 | − |
| CSS | 1.53 | 1.04–2.25 | − | ||||||
| Zimmitti et al. (2016) [139] | RC | 390/120 | Epidural LA +GA | GA | Liver | OS | 0.72 | 0.49–1.07 | − |
| RFS | 0.74 | 0.56–0.95 | + | ||||||
| Gottschalk et al. (2012) [140] | RC | 52/221 | Spinal LA | GA | Melanoma | OS | Nr | nr, P = 0.087 | + |
| Merquiol et al. (2013) [141] | RC | 111/160 | Epidural LA + GA | Opioid + GA | Head and neck | OS | 0.82 | 0.70–0.96 | + |
| Myles et al. (2011) [142] | RCT | 230/216 | Epidural LA + GA | GA | Abdominal surgery (e.g., colorectal) | RFS | 0.95 | 0.76–1.17 | − |
| Wu et al. (2018) [143] | RC | 1799/392 | Epidural LA + GA | Opioid + GA | NSCLC | OS | 0.81 | 0.58–1.31 | − |
| RFS | 0.93 | 0.76–1.14 | − |
| Study | Study Design | Participation | Agents | Cancer Type | End Point | Expected Completion Date |
|---|---|---|---|---|---|---|
| NCT03034096 | Multicentre prospective | 2000 | Propofol TIVA vs. VA | Various | OS +RFS | December 2020 |
| NCT01975064 | Multicentre prospective | 8000 | Propofol TIVA vs. sevoflurane | Breast + Colorectal | OS | December 2023 |
| NCT02786329 | Single-centre prospective, 2 × 2 factorial | 450 | Propofol TIVA vs. VA and lidocaine vs. placebo | Colorectal | OS + RFS | December 2021 |
| NCT02840227 | Multicentre prospective | 2000 | Epidural LA + GA vs. opioid + GA | Non-small cell lung carcinoma | RFS | December 2021 |
| NCT01318161 | Single-centre prospective | 300 | Ropivacaine vs. morphine PCA | Colorectal | OS + RFS | December 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, B.A.; Pandit, J.J. Do Certain Anaesthetic Drugs Affect Postoperative Cancer Recurrence Rates? Implications for Drug Discovery. Drugs Drug Candidates 2023, 2, 121-136. https://doi.org/10.3390/ddc2010008
Wilson BA, Pandit JJ. Do Certain Anaesthetic Drugs Affect Postoperative Cancer Recurrence Rates? Implications for Drug Discovery. Drugs and Drug Candidates. 2023; 2(1):121-136. https://doi.org/10.3390/ddc2010008
Chicago/Turabian StyleWilson, Ben A., and Jaideep J. Pandit. 2023. "Do Certain Anaesthetic Drugs Affect Postoperative Cancer Recurrence Rates? Implications for Drug Discovery" Drugs and Drug Candidates 2, no. 1: 121-136. https://doi.org/10.3390/ddc2010008
APA StyleWilson, B. A., & Pandit, J. J. (2023). Do Certain Anaesthetic Drugs Affect Postoperative Cancer Recurrence Rates? Implications for Drug Discovery. Drugs and Drug Candidates, 2(1), 121-136. https://doi.org/10.3390/ddc2010008






