Abstract
Opioids such as morphine are the first choice in acute and chronic pain treatment. However, they lead to addiction. Several studies have searched (i) to find a molecule that can replace morphine use or (ii) to reduce its adverse effects. This work aimed to evaluate whether (–)-Borneol [(–)-BOR], a bicyclic monoterpene, in doses of 25, 50, and 100 mg/kg (i.p.), has an antiaddictive effect on morphine (5 mg/kg, i.p.) and reduces its withdrawal symptoms precipitated by naloxone (8 mg/kg, i.p.) in Swiss mice. Furthermore, the (–)-BOR genotoxic potential was also investigated by the comet assay. The antiaddictive effect of (–)-BOR was evaluated by the conditioned preference place (CPP). The CPP was induced by morphine administration during the conditioning phase. The effects of (–)-BOR on the rewarding characteristics of morphine were tested in mice with the administration of (–)-BOR, naloxone, or vehicle (NaCl 0.9%), 30 min before morphine. This work also investigated the (–)-BOR effect on morphine withdrawal symptoms precipitated by naloxone. Morphine withdrawal symptoms were induced by administering morphine twice daily for 5 days, precipitated by naloxone administration on the sixth day. The effect of (–)-BOR on reducing morphine withdrawal symptoms was evaluated in mice that received (–)-BOR before daily morphine administration. Finally, the comet assay was performed to assess the DNA damage degree caused by the (–)-BOR (100 mg/kg, i.p.) administration. The comet assay was performed on peripheral blood taken from the tail of each animal. Cyclophosphamide (50 mg/kg, i.p.) was used to induce DNA damage. After starting the protocol, analyses were performed for 4 h (acute effect) and 24 h (repair effect). The (–)-BOR (100 mg/kg, i.p.) significantly attenuated (*** p < 0.001) the acquisition of morphine-induced CPP and reduced only the jumping behavior in the morphine withdrawal model. The best-studied dose was 100 mg/kg, being evaluated, then, in the comet assay. (–)-BOR at 100 mg/kg did not show the genotoxic effect when compared with the cyclophosphamide group (CYCLO, 50 mg/kg, i.p.) after 4 h or 24 h, a period that corresponded to the repair time of DNA fragmentation. The study showed that (–)-BOR attenuated the acquisition of CPP by morphine and made opioid withdrawal milder. In the comet assay, although (–)-BOR caused DNA damage, this damage was significantly less than the damage by CYCLO, at either 4 h or 24 h after the treatments.
1. Introduction
Opioids such as morphine are the choice drugs in acute, moderate, and severe pain treatments [1,2]. Controlled prescription and recreational morphine use has increased, and this has led to a dependence epidemic and death by overdose [3]. It is a difficult challenge for prescribers to distinguish between the controlled substances’ correct prescribing and prescribing for misuse [4]. Morphine is an addictive drug that causes relapses even years after withdrawal [5].
Due to dependence, researchers have been developing ways to replace opioids in pain treatment [6,7]. However, until now, there are no drugs capable of replacing opioids in pain treatment. In parallel, new drugs are being developed with the perspective of reducing dependence and opioid withdrawal symptoms [8,9,10]. Medicinal plants [11,12,13] and molecules obtained from medicinal plants [14,15] are options for developing or identifying these new drugs.
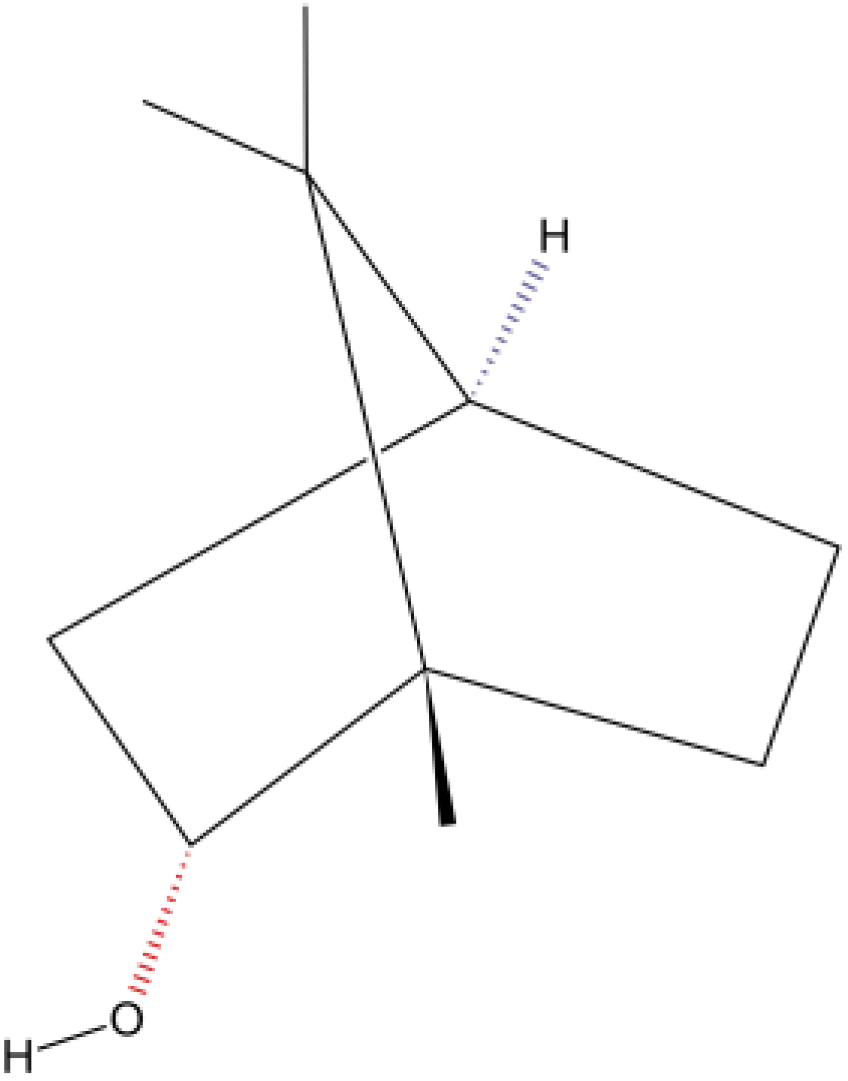
In the nutraceutical area [16,17], (–)-Borneol [(–)-BOR] (Figure 1) is a bicyclic monoterpene used in food, cosmetic, and pharmaceutical industries, which can be obtained either from essential oils of medicinal plants (e.g., Matricaria recutita L., Valeriana officinalis L., Micromeria macrosiphon Coss., Micromeria arganietorum (J. Emb.), and Cinnamomum camphora) [18,19,20] or as a synthetic product [21]. Pharmacological activities were attributed to (–)-BOR [22,23,24,25,26]. (–)-BOR acts as a partial agonist on the gamma-aminobutyric acid type A receptor (GABAAR) [27]. In addition, (–)-BOR has a vasorelaxant effect by blocking L-type voltage-operated calcium channels (CavL) [28]. Both the activation of GABA and the blocking Ca2+ channel are promising strategies for the development of new drugs in drug addiction therapy [29,30]. Recently, Xie et al. [31] investigated the neuroprotective effects of synthetic borneol and natural borneol based on the neurovascular unit against cerebral ischaemic injury: natural borneol showed a stronger effectiveness and had better regulation and neuroprotection on the neurovascular unit compared with synthetic borneol. In the emerging area of nanopharmaceutics and nanonutraceuticals [22,23,24], it is worth mentioning the work of Li et al. [32] and the recent progress on the synergistic antitumor effect of a borneol-modified nanocarrier drug delivery system. Wang et al. [25] developed and studied the physicochemical characteristics, storage stability, and enhanced antibacterial activities of nanoparticle-stabilized encapsulation of borneol and citral.
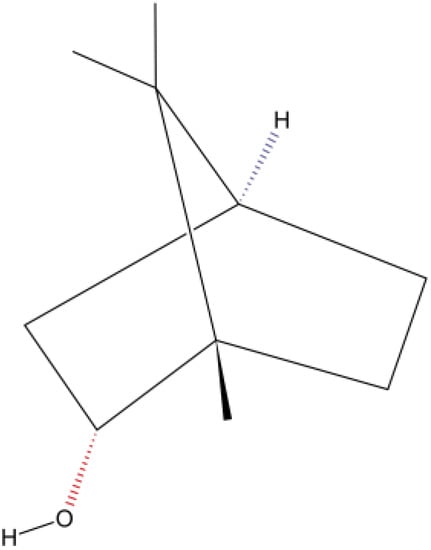
Figure 1.
Chemical structure of (–)-BOR (Adapted from: https://pubchem.ncbi.nlm.nih.gov/compound/1201518; accessed on 30 May 2023).
Furthermore, the pharmacokinetic study of this substance shows that its distribution in the central nervous system (CNS) is higher in the hippocampus [33], one of the mesolimbic areas that make up the rewarding system [34] and an important key in the development of opioid dependence [35,36,37,38]. Thus, this study aimed to evaluate the effect of synthetic (–)-BOR in addiction and withdrawal morphine preclinical models. In addition, as the work investigated the therapeutic potential of (–)-BOR on morphine addiction and withdrawal, its safety was also evaluated using the comet assay. The comet assay aims to determine whether a chemical substance administration is capable of causing DNA damage. It is the first time that this test with (–)-BOR has been reported in the literature.
2. Results
The results explored the following topics: (i) the effect of (–)-BOR on the CPP, (ii) the effect of (–)-BOR on morphine withdrawal symptoms precipitated by naloxone and (iii) the genotoxic evaluation of (–)-BOR by the comet assay.
2.1. Effect of Morphine and (–)-BOR on CPP
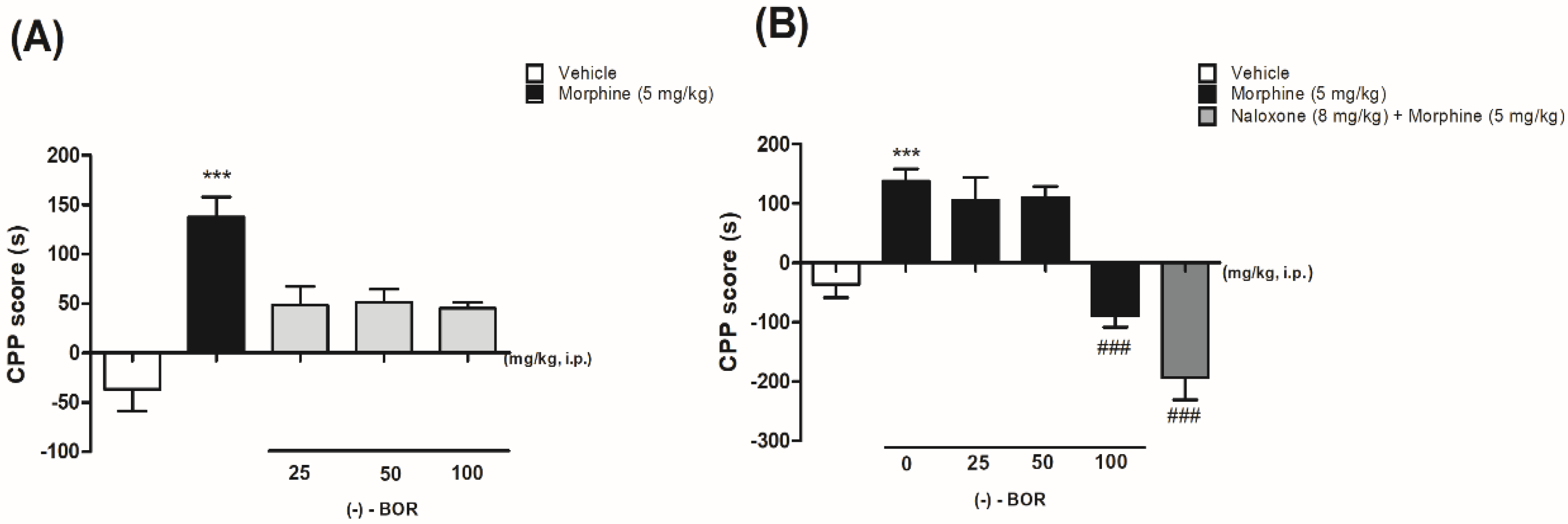
Figure 2 shows the effect of (–)-BOR on the CPP model by morphine. The administration of morphine (5 mg/kg, i.p.) increased the preference of the animals for the compartment in which they were paired (Figure 2A). The length of the stay in the compartment increased (137.6 ± 20.3 s) significantly (** p < 0.01) compared with the vehicle group (−30 ± 25.1 s). In Figure 2A, it is possible to observe that (–)-BOR administration (25, 50, and 100 mg/kg, i.p.) did not produce preference or aversion to the compartment.
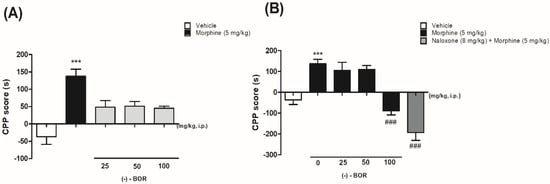
Figure 2.
(A) Effect of morphine and (–)-BOR on conditioned place preference (CPP) and (B) effect of the pre-administration of (–)-BOR (25, 50, and 100 mg/kg, i.p.) on CPP by morphine. Data are expressed as mean ± S.E.M. (n = 6 animals/group). (A,B) *** p ≤ 0.001 for morphine (5 mg/kg, i.p.) group compared with the vehicle group. (B) ### p ≤ 0.001 for (–)-BOR (100 mg/kg, i.p.) and naloxone (8 mg/kg, i.p.) compared with the morphine group (ANOVA and Tukey’s post hoc test).
2.2. Effect of (–)-BOR on the Acquisition of Morphine-Induced CPP
Only the pre-treatment with (–)-BOR (100 mg/kg, i.p.) reduced the preference (−89.0 ± 19.0 s) significantly (### p < 0.001) for the paired compartment compared with the morphine-treated group (Figure 2B).
2.3. Effect of (–)-BOR on Morphine Withdrawal Symptoms Precipitated by Naloxone
In the withdrawal model, it was possible to observe all withdrawal symptoms in the dependent group (Table 1). The naloxone (8 mg/kg, i.p.) administration triggered significantly jumping behavior (3.6 ± 0.4, *** p < 0.001), paw tremor (3.3 ± 0.7, * p < 0.05), wet dog shaking (3.1 ± 0.8, * p < 0.05), and reverse walking (1.0 ± 0.3, * p < 0.05) compared with the vehicle group. However, pretreatment with (–)-BOR (100 mg/kg, i.p.) significantly (### p < 0.001) reduced only the jumping behavior (0.8 ± 0.2), when compared with the morphine group.

Table 1.
Effect of (–)-BOR (25, 50, and 100 mg/kg, i.p.) on morphine withdrawal symptoms precipitated by naloxone (8 mg/kg, i.p.).
2.4. Genotoxic Evaluation of (–)-BOR by the Comet Assay
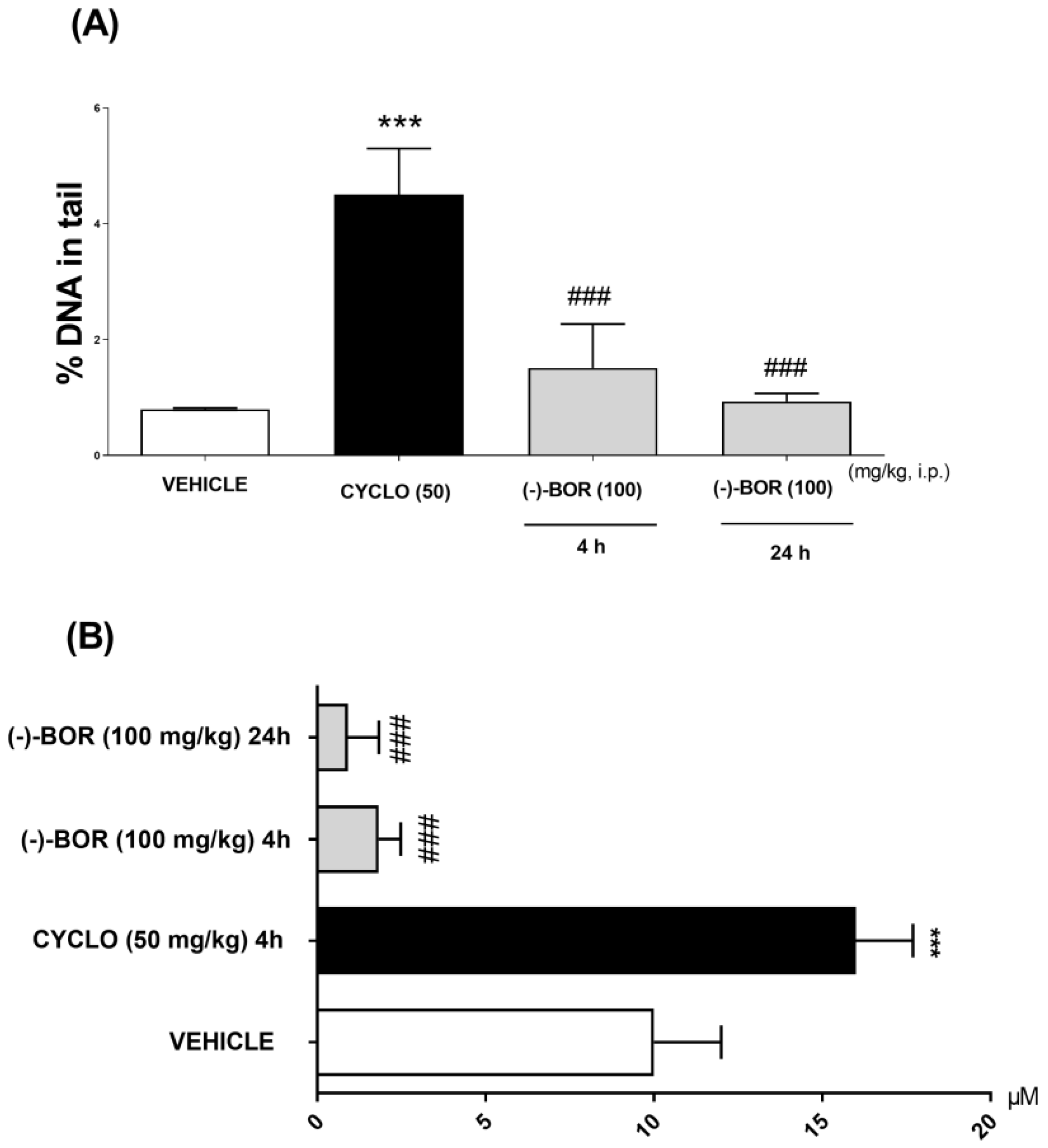
The (–)-BOR genotoxic potential evaluation was carried out through the comet test by means of two parameters: %DNA in the tail and the tail length (μM), at 4 and 24 h after the treatments. Thus, (–)-BOR significantly (### p < 0.05) reduced the % DNA in the tail and the tail length (μM) after the times of 4 (1.51 ± 0.76% and 0.93 ± 0.70 µM, respectively) and 24 h (0.93 ± 0.14% and 0.09 ± 0.08 µM, respectively) compared with CYCLO (4.5 ± 0.8% and 16 ± 2 μM) (Figure 3A,B).
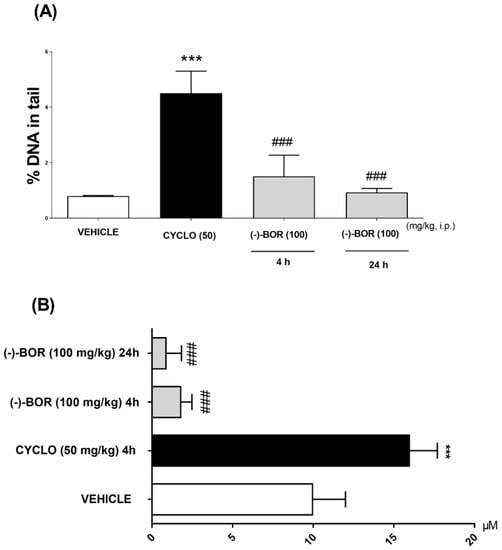
Figure 3.
Genotoxic evaluation of (–)-BOR by comet assay. (A) % DNA in the tail and (B) the tail length at 4 and 24 h after the treatments. Data are expressed as mean ± S.E.M. (n = 6 animals/group). *** p < 0.001 compared with the vehicle group, and ### p < 0.001 compared with the cyclophosphamide (CYCLO) group (ANOVA and Tukey’s post hoc test).
3. Discussion
Opioids have great relevance in acute, moderate, and chronic pain treatment. However, reducing adverse effects such as analgesia tolerance and drug addiction is still a major challenge for modern pharmacological research [1]. There are several animal models used in the drug addiction mechanisms study [39], and the CPP test is one of the most frequently used for this purpose [40]. The CPP paradigm is a model used to understand the mechanism of various addictive drugs such as psychostimulants. [41,42,43,44], cannabinoids [45,46], and opioids [47]. This model is based on the positive association hypothesis involving a specific context or stimulus during drug administration. In practice, it functions through a temporal overlapping of successive “pairings” of the animal, under the effect of the drug, to a certain compartment [39].
The present study examined the effects of (–)- BOR on the acquisition of morphine-induced CPP and morphine withdrawal symptoms precipitated by naloxone. According to the literature, the present research verified that morphine could induce CPP, confirming its reinforcing characteristics [48,49,50,51,52,53]. The increase in preference occurred through learning and memory, through the rewarding opioid effects associated with the morphine-paired compartment [40]. The mice that received morphine during the conditioning test exhibited a significant increase in morphine-paired compartment (–)- compared with the vehicle-paired compartment. The (–)-BOR administration could not induce CPP like morphine. In addition, prior administration of (–)-BOR (100 mg/kg, i.p.) decreased the acquisition of morphine-induced CPP.
One of the reported pharmacological activities of (–)-BOR is its anticonvulsive property [54,55], probably due to its ability to positively modulate GABAARs acting as a partial agonist [27]. Perhaps the effect on GABAAR may explain, in part, how (–)-BOR reduced the preference for the compartment in which the animals were paired. The inhibitory neurotransmitter GABA is crucial in the development and manifestation of opioid-addictive behavior [56,57]. The biochemical cascade that activates the rewarding system begins in the ventral tegmental area (VTA) and ends with the dopamine (DA) release in the D2 receptors located in the hippocampus and nucleus accumbens (NAc) cell membrane [58]. The cascade is triggered by serotonin (5-HT), which induces the release of an endogenous opioid peptide: met-enkephalin. This peptide, when binding to μ receptors, regulates the neurons responsible for the GABA release. The GABA’s role is to inhibit the DA release. When dopaminergic neurons in the VTA and some areas of the hippocampus and amygdala are disinhibited, DA is released in the NAc, producing pleasure and ending the cascade [59].
GABA modulation in mesolimbic system areas is important in morphine reinforcing and the acquisition of morphine-induced CPP [60]. For example, GABA-like drugs such as gabapentin and pregabalin, as well as diazepam, a classical benzodiazepine and receptor GABAA agonist, reduce both the development of CPP and DA release by morphine [61,62]. Therefore, it is possible that (–)-BOR is potentializing the GABAergic neurotransmission and strengthening the GABA inhibitory effect on the rewarding system cascade.
In addition to the GABAergic effect, (–)-BOR blocks voltage-activated Ca2+ channels (CaVL) [28]. This mechanism may contribute to the effect of (–)-BOR on morphine-induced CPP. Opioid dependence signaling involves Ca2+ through the Ca2+/calmodulin-dependent protein kinase (CaMK II) activation [14,63], triggering the phosphorylation of the cAMP response element-binding protein (CREB) [14,63]. CREB is an important transcription factor of memory formation, and its activation leads to morphological changes in neurons (e.g., formation of more spines or degeneration of spines) [64]. Therefore, it is also possible that pretreatment with (–)-BOR can interfere with the Ca2+ availability, decreasing its inflow and altering the memory and the CPP induced by morphine.
Moreover, there seems to be an interaction between GABA and Ca2+. The literature suggested that in the long-term memory formation, the excitatory synapse in both the cortex and the hippocampus is modified by GABA and Ca2+ [65]. Both the cortex and the hippocampus are areas rich in GABAergic and Glutamatergic interneurons. The NMDA receptor activation triggers Ca2+ influx, depolarizing the cell membrane and generating action potential. GABA interferes in long-term memory formation by reducing the influx of Ca2+ and thus the signaling triggered by the activation of NMDA receptors [66,67].
Opioid withdrawal is a complex phenomenon formed by many signs and symptoms [68]. The opioid withdrawal can be simulated in animal models with the administration of opioid antagonists such as naloxone [69]. Behaviors such as jumping and wet dog shaking are opioid withdrawal precipitation indicators in animals [70]. These symptoms are correlated with tremors, agitation, and nervousness [71]. Opioid addicts are more vulnerable to stress and irritability than the general population. Stress situations can trigger craving in humans [72]. In our study, pre-treatment with (–)-BOR was able to reduce only the jumping symptom, which could be correlated with the agitation and irritability characteristic of opioid withdrawal and craving.
To evaluate the safety of (–)-BOR, we performed the comet assay. The comet assay relies on the ability of negatively charged DNA fragments to be drawn through an agarose gel in response to a positive electric field. The extent of the DNA migration depends directly on the DNA damage present in each cell. The tail length reflects DNA damage [73]. The comet assay is being used to validate the safety of new products in several areas such as molecular biology, pharmacology, toxicology, genetics, and biotechnology [73]. It is advisable to use at least two parameters to ensure, to the maximum, the safety of the results [74]. The genotoxicity of (–)-BOR was evaluated through two parameters. Large quantities of (–)-BOR presented DNA damage lower than the damages caused by each CYCLO, either in the acute effect (4 h later) or in the repairing time (24 h after its administration).
A limitation of our study was that we focused exclusively on male mice and did not investigate the possible sex differences in the observed effects. Furthermore, the use of a specific animal model may limit our findings to other species. Future studies with more diverse samples, as well as the investigation of potential sex differences, are needed to explore the potential applications of our findings further.
4. Materials and Methods
4.1. Drugs
The (–)-BOR and naloxone were purchased from Sigma-Aldrich (St. Louis, MO, USA), whereas the morphine sulfate was obtained from Cristália (São Paulo, SP, Brazil). The (–)-BOR was previously solubilized in dimethylsulfoxide (1% DMSO) and then diluted in saline solution (0.9% NaCl). The other drugs were dissolved in saline.
4.2. Animals
For experiments, 264 male Swiss mice (Mus musculus), 25–30 g, two months old, were housed at the Agrarian Sciences Center of the Federal University of Piauí (CCA/UFPI), acclimated at a temperature of 24 ± 2 °C and relative humidity (45 ± 2%) under a light–dark cycle of 12–12 h (6 AM to 6 PM) with free access to food and water. All experiments followed protocols approved by the Ethics Committee for the Use of Animals (CEUA/UFPI) under the number 083/14 on 24 November 2014. The animals were acclimated for a period of 7 days before the beginning of the experimental protocols.
4.3. Conditioned Place Preference (CPP)
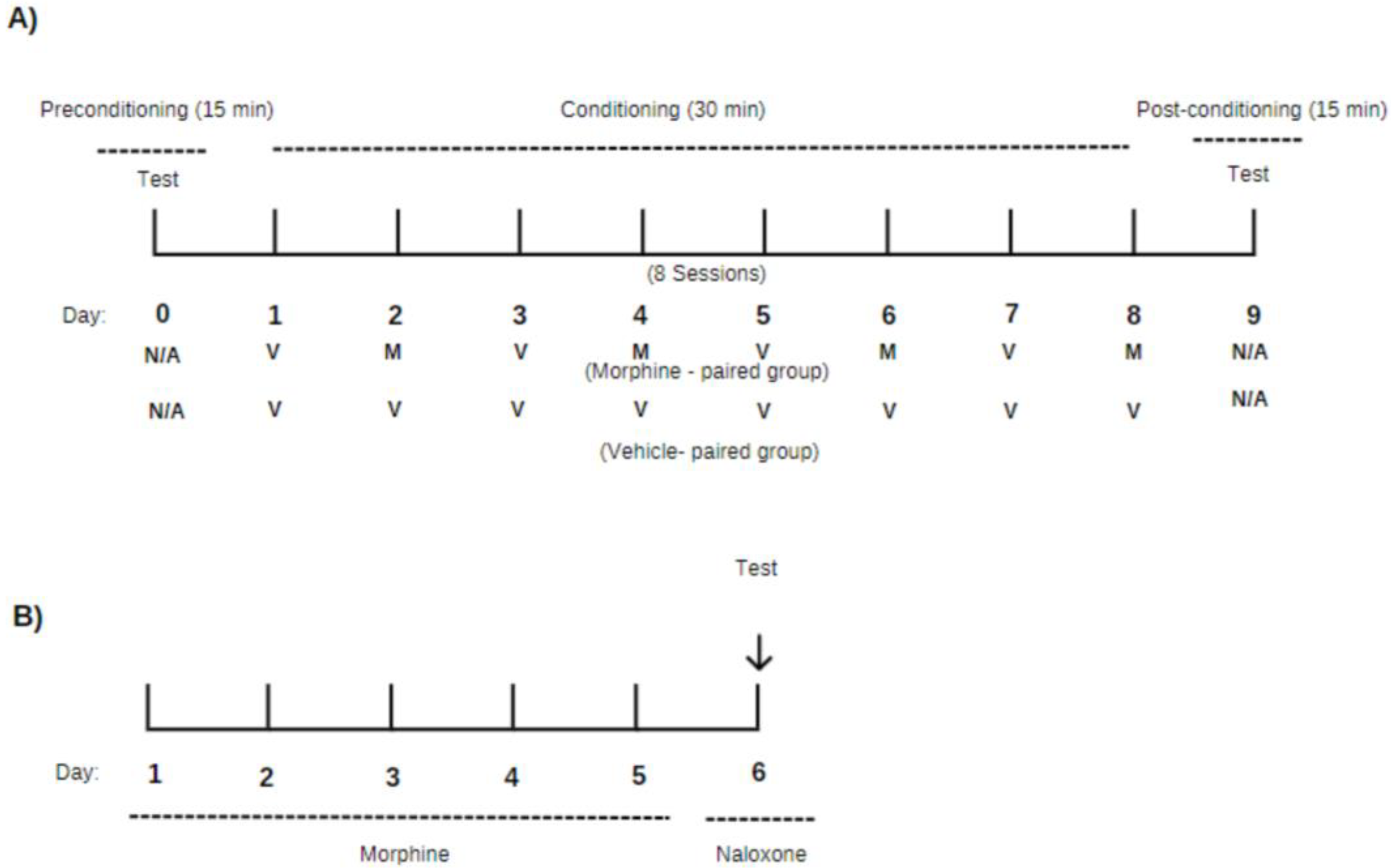
The CPP was performed according to the method described by Beach [75] with some modifications. The experiment was based on the Pavlovian conditioning model, in which an association between a given environment and the animal was established [40,50]. An apparatus made up of three distinct compartments (140 mm × 440 mm × 150 mm) was used, having floors and walls made of acrylic with different textures and colors. Compartment A (one of the ends) had black walls with stainless steel bars. Compartment B (the connecting tunnel) had light gray walls and floors. Compartment C (the other end) had white walls, black stripes, and a stainless-steel sheet cast floor. The compartments described above were connected by guillotine-shaped doors, which were closed and manually opened. The protocol was performed in 10 days from day zero (0) and divided into three phases: preconditioning, conditioning, and post-conditioning, according to the experimental design shown in Figure 4A.
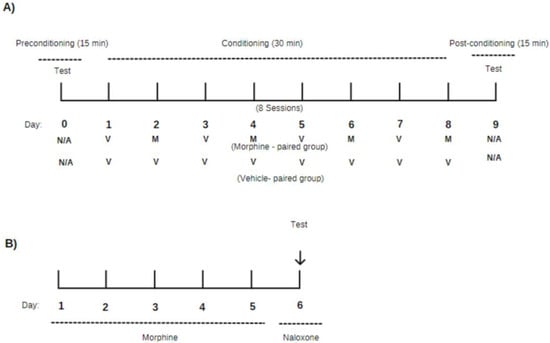
Figure 4.
Experimental design for (A) the conditioned place preference paradigm (CPP) and (B) morphine withdrawal symptoms precipitated by naloxone. The CPP (A) was divided into three phases: pre-conditioning, where the animals had access to all compartments for 15 min with no drug administration (N/A); conditioning, which was the morphine (M) administration phase and where the animals were paired with the compartment for 30 min; and post-conditioning, where the animals had access to all environments again for 15 min (test phase) with no drug administration (N/A). (B) Morphine was administered twice daily for five consecutive days. On the sixth day, naloxone was given to watch withdrawal signs.
4.3.1. Preconditioning Phase
During this phase (day 0), one animal at a time was placed in compartment B, and afterward, the doors were manually removed so that they had access to all environments of the apparatus during a 15 min period.
4.3.2. Conditioning Phase-Effect of Morphine and (–)-BOR on CPP
This phase was performed one day after the preconditioning phase and for eight consecutive days. First, it was investigated whether (–)-BOR was able to induce acquisition like morphine. During the conditioning phase, the animals received morphine (5 mg/kg, i.p.) or only (–)-BOR (25, 50, and 100 mg/kg) on days 2, 4, 6, and 8 and vehicle (NaCl 0.9%, i.p.) on days 1, 3, 5, and 7, as shown in Table 2 and Figure 4A. After each administration, the animals, one at a time, were immediately isolated in the conditioning chamber for 30 min. After that period, they were transferred back to their original cages. The morphine compartment was randomly chosen. On the day that morphine or (–)-BOR was not administered, the animals received a vehicle.

Table 2.
Representation of different phases of conditioned place preference (CPP).
4.3.3. Conditioning Phase—Effect of (–)-BOR on the Acquisition of Morphine-Induced CPP
To evaluate the effect of (–)-BOR on the acquisition of morphine-induced CPP, mice received naloxone (8 mg/kg, i.p.), (–)-BOR (25, 50, and 100 mg/kg, i.p.), or vehicle 30 min before each morphine administration, as described in Table 2.
4.3.4. Post-Conditioning Phase (Test Phase)
On day 9, the animals were placed, one at a time, in compartment B of the apparatus, and the guillotine-style doors were removed, leaving them with free access to the compartment of the apparatus for 15 min as shown Figure 4A. The time spent in each compartment was recorded and subtracted from the time recorded in the preconditioning phase. The protocol was performed by an experimenter, and the results were expressed as the CPP score.
4.4. Effect of (–)-BOR on Morphine Withdrawal Symptoms Precipitated by Naloxone
This model was performed according to the method described by Marshal and Grahame-Smith [76] with some modifications. Male Swiss mice (Mus musculus) (25–30 g), six per group, were treated with vehicle and (–)-BOR (25, 50, and 100 mg/kg, i.p.) prior to the administration of morphine (5 mg/kg, i.p.). Morphine dependence was induced by the administration of morphine (5 mg/kg, i.p.) twice daily (6 AM to 6 PM) for 5 days. On the 6th day, 2 h after the first administration of morphine, naloxone (8 mg/kg, i.p.) was administered for the precipitation of the withdrawal syndrome (Figure 4B). Immediately after the administration of the naloxone, the mice were placed in metabolic cages for a period of 30 min for the observation of withdrawal signs (jumping, reverse walking, paw tremor, and wet dog shaking) [76].
4.5. Genotoxic Evaluation of (–)-BOR by the Comet Assay
The comet assay was performed according to the method described by Singh et al. [77] with some modifications. Male Swiss mice (Mus musculus) (25–30 g), six per group, were treated with vehicle, (–)-BOR (100 mg/kg, i.p.), or cyclophosphamide (CYCLO, 50 mg/kg, i.p.). The comet assay was performed on the peripheral blood taken from the tail of each animal. The first sample was processed 4 h after the end of exposure (acute effect), and the second sample was processed after 24 h (repairing time). At the end of each period, 40 μL of blood was collected and transferred to microtubes containing 120 μL of low-melting-point agarose (1.5%) at 37 °C. The homogenized mixture was transferred to agarose-coated slides receiving coverslips and stored at 4 °C for 30 min [74]. The slides were then removed and immersed in vertical glass vials containing a lysis solution [NaCl (2.5 M), EDTA (100 mM), and 1.2 g TRIS (10 mM)]. After use, 1% Triton X-100 and 10% DMSO were added. The slides were placed in a vessel containing electrophoretic buffer, pH > 13.0 (300 mM NaOH and 1 mM EDTA, prepared from a stock solution of 10 N NaOH and 200 mM EDTA, pH 10.0), and allowed to stand for 20 min. The electrophoretic “run” was performed with 25 V and 300 mA at a temperature of 40 °C for 15 min in the dark. After electrophoresis, the slides were removed from the vessel and immersed in neutralizing solution (0.4 M Tris, pH 7.5, for 5 min). This process was performed in triplicate. Finally, they were rinsed with distilled water, dried, and washed with GelRed (dilution 2.0:10,000 μL for 10 min). All slides were analyzed by immunofluorescence microscopy (×40 magnification), equipped with an excitation filter (420–490 nm) and a barrier filter (520 nm). The images were captured using an Opton system (digital CCD camera, 5.0 mega pixel for immunofluorescence). The DNA damage was assessed by measuring the percentage of DNA in the tail (% of DNA measurement of the proportion of total DNA present in the tail) [74]. These parameters were calculated in 100 nucleoids/sample (two slides per individual) using the OpenComet version 1.3.1 software (https://cometbio.org/; accessed on 26 May 2023) [78].
4.6. Statistical Analysis
Values were expressed as the mean ± standard error of mean (S.E.M.). Differences between groups were determined using analysis of variance (ANOVA), followed by Tukey’s post hoc test. Values were considered significant when p < 0.05. All analyses were performed using the GraphPad Prism software, version 8.0 (GraphPad Software, Inc., San Diego, CA, USA).
5. Conclusions
Our study demonstrated that (–)-BOR was able to interfere in PCP by morphine. In addition, with regard to the withdrawal symptoms triggered by naloxone administration, the (–)-BOR was shown to be able to reduce only the jumping behavior, clinically related to agitation and irritability in patients addicted to opioids. Moreover, (–)-BOR was shown to be a safe molecule in that it had no significative genotoxic effect. These results open the possibility of applying this drug in chronic treatments with morphine and other opioids, reducing the possibility of patients developing dependence and irritability during the abstinence period.
Author Contributions
Conceptualization, M.P.d.M.d.A. and F.P.d.M.d.A.; methodology, M.P.d.M.d.A. and F.P.d.M.d.A.; validation, M.P.d.M.d.A., M.d.R.V. and A.T.O.; formal analysis, L.d.S.L. and R.d.C.M.O.; investigation, M.P.d.M.d.A., M.d.R.V. and A.T.O.; resources, L.d.S.L., F.P.d.M.d.A., M.L., A.D., D.D.R.A. and R.d.C.M.O.; writing—original draft preparation, M.P.d.M.d.A.; writing—review and editing, M.P.d.M.d.A., M.L., A.D., D.D.R.A. and F.P.d.M.d.A.; visualization, M.L., A.D., L.d.S.L. and D.D.R.A.; supervision, R.d.C.M.O.; project administration, M.P.d.M.d.A.; funding acquisition, M.P.d.M.d.A. and R.d.C.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Animal study protocols were approved by Ethics Committee for the Use of Animals of Universidade Federal do Piauí (CEUA/UFPI), No. 083/2014, approved on 24 November 2014.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CaMK II: Ca2+/calmodulin-dependent protein kinase; CaVL: voltage-operated calcium channels; CNS: central nervous system; CPP: conditioned place preference; CREB: cAMP-response element binding protein; CYCLO: cyclophosphamide; DA: dopamine; DNA: deoxyribonucleic acid; GABA: gamma-aminobutyric acid; GABAAR: type-A gamma-aminobutyric acid receptors; NAc: nucleus accumbens; NMDA: N-methyl-D-aspartate; VTA: ventral tegmental area; (–)-BOR: (–)-Borneol; 5-HT: serotonin.
References
- Abdolrazaghnejad, A.; Banaie, M.; Tavakoli, N.; Safdari, M.; Rajabpour-Sanati, A. Pain Management in the Emergency Department: A Review Article on Options and Methods. Adv. J. Emerg. Med. 2018, 2, e45. [Google Scholar]
- Murphy, P.B.; Bechmann, S.; Barrett, M.J. Morphine. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Holt, C.T.; McCall, K.L.; Cattabriga, G.; Tu, C.; Smalley, E.K.; Nichols, S.D. Using Controlled Substance Receipt Patterns to Predict Prescription Overdose Death. Pharmacology 2018, 101, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Preuss, C.V.; Kalava, A.; King, K.C. Prescription of Controlled Substances: Benefits and Risks. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Pierre, F.; Ugur, M.; Faivre, F.; Doridot, S.; Veinante, P.; Massotte, D. Morphine-dependent and abstinent mice are characterized by a broader distribution of the neurons co-expressing mu and delta opioid receptors. Neuropharmacology 2019, 152, 30–41. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Magnusson, P.; LeQuang, J.A.; Breve, F.; Taylor, R.; Wollmuth, C.; Varrassi, G. Can NSAIDs and Acetaminophen Effectively Replace Opioid Treatment Options for Acute Pain? Expert Opin. Pharmacother. 2021, 22, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Caldas da Silva Dantas Viegas, C.; Sérgio Silva, A.; Marinho Braga, R.; Nunes de Andrade, H.H.; Felício de Sousa Santos, A.K.; Leite Ferreira, M.D.; Ribeiro, M.D.; Agra Cavalcante Silva, L.H.; Alves de Lima, L.; Nobrega de Almeida, R.; et al. Antinociceptive, anti-inflammatory and antioxidant activities of the crude ethanolic extract and alkaloid fraction of Waltheria viscosissima A. St.-Hil. (Malvaceae). J. Ethnopharmacol. 2022, 292, 115173. [Google Scholar] [CrossRef] [PubMed]
- Taddesse, Y.; Kyunghwa, Y.; Soyong, J.; Seikwan, O. Morphine dependence is attenuated by red ginseng extract and ginsenosides Rh2, Rg3, and compound K. J. Ginseng Res. 2016, 40, 445–452. [Google Scholar]
- Glasser, M.; Chen, J.; Alzarah, M.; Wallace, M. Non-opioid Analgesics and Emerging Therapies. Cancer Treat. Res. 2021, 182, 125–142. [Google Scholar]
- Giordano, G.; Marsida, K.; Giulia, S.; Gregory, D.; George, G.; Roberto, C. Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology 2017, 234, 223–234. [Google Scholar]
- Dos Reis Izolan, L.; da Silva, D.M.; Oliveira, H.B.L.; de Oliveira Salomon, J.L.; Peruzzi, C.P.; Garcia, S.C.; Dallegrave, E.; Zanotto, C.; Elisabetsky, E.; Gonçalves, C.A.; et al. Sintocalmy, a Passiflora incarnata Based Herbal, Attenuates Morphine Withdrawal in Mice. Neurochem. Res. 2021, 46, 1092–1100. [Google Scholar] [CrossRef]
- Kiashemshaki, B.; Safakhah, H.A.; Ghanbari, A.; Khaleghian, A.; Miladi-Gorji, H. Saffron (Crocus sativus L.) stigma reduces symptoms of morphine-induced dependence and spontaneous withdrawal in rats. Am. J. Drug Alcohol Abus. 2021, 47, 170–181. [Google Scholar] [CrossRef]
- Ehtemami, Z.; Shafaroodi, H.; Asgarpanah, J. Effect of Essential Oil of Zhumeria majdae on Morphine Tolerance and Dependence in Mice. Chin. J. Integr. Med. 2020, 26, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, F.; Szymusiak, M.; Liu, Y.; Wang, Z.J. Curcumin attenuates opioid tolerance and dependence by inhibiting Ca2+/calmodulin-dependent protein kinase II alpha activity. J. Pharmacol. Exp. Ther. 2015, 352, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B. Ellagic acid enhances morphine analgesia and attenuates the development of morphine tolerance and dependence in mice. Eur. J. Pharmacol. 2014, 741, 272–280. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals—Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential application of peppermint (Mentha piperita L.), german chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.) as active fillers in natural rubber biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the Potential Use of Medicinal Plants from Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef]
- El Yaagoubi, M.; Mechqoq, H.; Ortiz, S.; Cavaleiro, C.; Lecsö-Bornet, M.; Pereira, C.G.; Rodrigues, M.J.; Custódio, L.; El Mousadik, A.; Picot, L.; et al. Chemical Composition and Biological Screening of the Essential Oils of Micromeriamacrosiphon and M. arganietorum (Lamiaceae). Chem. Biodivers. 2021, 18, e2100653. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, H.; Xie, Y.; Guo, Y.; Fan, J.; Yao, W. Evaluation of the analgesic potential and safety of Cinnamomum camphora chvar. Borneol essential oil. Bioengineered 2021, 12, 9860–9871. [Google Scholar]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II-Production Scales and Clinically Compliant Production Methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I-Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, Q.; Ma, R.; Li, Y.; Yuan, J.; Ren, M.; Li, H.; Wang, J.; Lu, D.; Xu, Z.; et al. Recent Progress on the Synergistic Antitumor Effect of a Borneol-Modified Nanocarrier Drug Delivery System. Front. Med. 2021, 8, 750170. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, Z.; Wang, L.; Cai, Y.; Ma, H.; Fang, L.; Su, J. Nanoparticle-stabilized encapsulation of borneol and citral: Physicochemical characteristics, storage stability, and enhanced antibacterial activities. J. Food Sci. 2021, 86, 4554–4565. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Xiao, Z.; Hui, Z.; Jian, X.; Xiao, C.; Yong, L.; Qi, W.; Zi, S.; Hong, W. Natural borneol recycling from Cinnamomum camphor chvar. Borneol oil residue by fractional distillation and recrystallization. Trop. J. Pharm. Res. 2014, 13, 1463–1470. [Google Scholar]
- Granger, E.; Campbell, L.; Johnston, R. (+)- and (–)-borneol: Efficacious positive modulators of GABA action at human recombinant GABAA receptors. Biochem. Pharmacol. 2005, 69, 1101–1111. [Google Scholar] [CrossRef]
- José, F.; Nelma, O.; Daniel, A.; Lucindo, J.; Sócrates, C.; Márcio, S.; Rita, O.; Aldeídia, O. Investigation of Mechanisms Involved in (–)-Borneol-Induced Vasorelaxant Response on Rat Thoracic Aorta. Basic Clin. Pharmacol. Toxicol. 2011, 10, 171–177. [Google Scholar]
- Engin, E.; Benham, R.S.; Rudolph, U. An Emerging Circuit Pharmacology of GABAA Receptors. Trends Pharmacol. Sci. 2018, 39, 710–732. [Google Scholar] [CrossRef]
- Morikawa, H.; Young, C.C.; Smits, J.A. Usage of L-type calcium channel blockers to suppress drug reward and memory driving addiction: Past, present, and future. Neuropharmacology 2022, 221, 109290. [Google Scholar] [CrossRef]
- Xie, Q.; Li, J.; Dong, T.; Yuan, J.; Lu, D.; Ma, R.; Li, H.; Li, Y.; Ren, M.; Chen, H.; et al. Neuroprotective effects of synthetic borneol and natural borneol based on the neurovascular unit against cerebral ischaemic injury. J. Pharm. Pharmacol. 2021, 74, 236–249. [Google Scholar] [CrossRef]
- Li, Q.; Xia, L.; Sun, C.; Zhang, H.; Zheng, M.; Zhang, H.; Lu, H.; Wang, Z. Role of Borneol Induced Autophagy in Enhancing Radiosensitivity of Malignant Glioma. Front. Oncol. 2021, 11, 749987. [Google Scholar] [CrossRef]
- Li, W.R.; Chen, R.Y.; Yang, L.; Huang, T.L.; Xu, Q.W.; Mi, S.Q.; Wang, N.S. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, Z.; Zeinaddini-Meymand, A.; Karimi-Haghighi, S.; Haghparast, A.; Khodagholi, F.; Haghparast, A. BDNF and p-GSK3β in the hippocampus mediate the impairment of delay-based decision making in morphine-dependent rats. NeuroReport 2020, 31, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Ujcikova, H.; Hejnova, L.; Eckhardt, A.; Roubalova, L.; Novotny, J.; Svoboda, P. Impact of three-month morphine withdrawal on rat brain cortex, hippocampus, striatum and cerebellum: Proteomic and phosphoproteomic studies. Neurochem. Int. 2021, 144, 104975. [Google Scholar] [CrossRef]
- Osmanlıoğlu, H.Ö.; Yıldırım, M.K.; Akyuva, Y.; Yıldızhan, K.; Nazıroğlu, M. Morphine Induces Apoptosis, Inflammation, and Mitochondrial Oxidative Stress via Activation of TRPM2 Channel and Nitric Oxide Signaling Pathways in the Hippocampus. Mol. Neurobiol. 2020, 57, 3376–3389. [Google Scholar] [CrossRef] [PubMed]
- Massaly, N.; Morón, A.; Al-hasani, A. Trigger for Opioid Misuse: Chronic Pain and Stress Dysregulate the Mesolimbic Pathway and Kappa Opioid System. Front. Neurosci. 2016, 10, 480. [Google Scholar] [CrossRef]
- Spanagel, R. Animal models of addiction. Dialogues Clin. Neurosci. 2017, 19, 247–258. [Google Scholar] [CrossRef]
- McKendrick, G.; Graziane, N.M. Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Front. Behav. Neurosci. 2020, 14, 582147. [Google Scholar] [CrossRef]
- Atehortua Martinez, L.A.; Curis, E.; Mekdad, N.; Larrieu, C.; Courtin, C.; Jourdren, L.; Blugeon, C.; Laplanche, J.L.; Megarbane, B.; Marie-Claire, C.; et al. Individual differences in cocaine-induced conditioned place preference in male rats: Behavioral and transcriptomic evidence. J. Psychopharmacol. 2022, 36, 1161–1175. [Google Scholar] [CrossRef]
- Schmill, M.P.; Cadney, M.D.; Thompson, Z.; Hiramatsu, L.; Albuquerque, R.L.; McNamara, M.P.; Castro, A.A.; Kay, J.C.; Buenaventura, D.G.; Ramirez, J.L.; et al. Conditioned place preference for cocaine and methylphenidate in female mice from lines selectively bred for high voluntary wheel-running behavior. Genes Brain Behav. 2021, 20, e12700. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Guo, L.K.; Han, X.; Song, R.; Dong, G.M.; Ma, C.M.; Wu, N.; Li, J. Naltrexone attenuates methamphetamine-induced behavioral sensitization and conditioned place preference in mice. Behav. Brain Res. 2021, 399, 112971. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, M.; Shang, Q.; Qian, H.; An, R.; Liu, H.; Shao, G.; Li, T.; Liu, X. Psilocin suppresses methamphetamine-induced hyperlocomotion and acquisition of conditioned place preference via D2R-mediated ERK signaling. CNS Neurosci. Ther. 2023, 29, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.F.; Davis, C.M.; Sempio, C.; Klawitter, J.; Christians, U.; Weerts, E.M. Δ9-Tetrahydrocannabinol Vapor Exposure Produces Conditioned Place Preference in Male and Female Rats. Cannabis Cannabinoid Res. 2022. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.; Havlickova, T.; Lapka, M.; Puskina, N.; Šlamberová, R.; Kuchar, M.; Sustkova-Fiserova, M. Cannabinoid-Induced Conditioned Place Preference, Intravenous Self-Administration, and Behavioral Stimulation Influenced by Ghrelin Receptor Antagonism in Rats. Int. J. Mol. Sci. 2021, 22, 2397. [Google Scholar] [CrossRef]
- Barattini, A.E.; Montanari, C.; Edwards, K.N.; Edwards, S.; Gilpin, N.W.; Pahng, A.R. Chronic inflammatory pain promotes place preference for fentanyl in male rats but does not change fentanyl self-administration in male and female rats. Neuropharmacology 2023, 231, 109512. [Google Scholar] [CrossRef]
- Zhang, J.; Deji, C.; Fan, J.; Chang, L.; Miao, X.; Xiao, Y.; Zhu, Y.; Li, S. Differential alteration in gut microbiome profiles during acquisition, extinction and reinstatement of morphine-induced CPP. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110058. [Google Scholar] [CrossRef]
- Amohashemi, E.; Reisi, P.; Alaei, H. Lateral habenula electrical stimulation with different intensities in combination with GABAB receptor antagonist reduces acquisition and expression phases of morphine-induced CPP. Neurosci. Lett. 2021, 759, 135996. [Google Scholar] [CrossRef]
- Khezri, A.; Mohsenzadeh, M.S.; Mirzayan, E.; Bagherpasand, N.; Fathi, M.; Abnous, K.; Imenshahidi, M.; Mehri, S.; Hosseinzadeh, H. Quetiapine attenuates the acquisition of morphine-induced conditioned place preference and reduces ERK phosphorylation in the hippocampus and cerebral cortex. Am. J. Drug Alcohol Abus. 2022, 48, 422–432. [Google Scholar] [CrossRef]
- Mohaddeseh, A.; Hossein, H.; Ali, S.; Ali, R. The effect of O-1602, an atypical cannabinoid, on morphine-induced conditioned place preference and physical dependence. Pharmacol. Rep. 2016, 68, 592–597. [Google Scholar]
- Amohashemi, E.; Reisi, P.; Alaei, H. Involvement of GABAA receptors of lateral habenula in the acquisition and expression phases of morphine-induced place preference in male rats. Behav. Pharmacol. 2022, 33, 452–465. [Google Scholar] [CrossRef]
- Shirazy, M.; RayatSanati, K.; Jamali, S.; Motamedi, F.; Haghparast, A. Role of orexinergic receptors in the dentate gyrus of the hippocampus in the acquisition and expression of morphine-induced conditioned place preference in rats. Behav. Brain Res. 2020, 379, 112349. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Bojar, H.; Góralczyk, A.; Skalicka-Woźniak, K. Antiseizure Effects of Scoparone, Borneol and Their Impact on the Anticonvulsant Potency of Four Classic Antiseizure Medications in the Mouse MES Model—An Isobolographic Transformation. Int. J. Mol. Sci. 2023, 24, 1395. [Google Scholar] [CrossRef] [PubMed]
- Lucindo, J.; Adriana, G.; Bruno, A.; Geovana, O.; Marília, S.; Flávia, M.; Márcio, S.; Cavalcanti, S.C.; Júnior, W.D.L.; Botelho, M.A.; et al. Carvacrol, borneol and citral reduce convulsant activity in rodents. Afr. J. Biotechnol. 2010, 9, 6566–6572. [Google Scholar]
- Sahraei, H.; Amiri, Y.A.; Haeri-Rohani, A.; Sepehri, H.; Salimi, S.H.; Pourmotabbed, A.; Ghoshooni, H.; Zahirodin, A.; Zardooz, H. Different effects of GABAergic receptors located in the ventral tegmental area on the expression of morphine-induced conditioned place preference in rat. Eur. J. Pharmacol. 2005, 524, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Siivonen, M.S.; de Miguel, E.; Aaltio, J.; Manner, A.K.; Vahermo, M.; Yli-Kauhaluoma, J.; Linden, A.M.; Aitta-Aho, T.; Korpi, E.R. Conditioned Reward of Opioids, but not Psychostimulants, is Impaired in GABA-A Receptor δ Subunit Knockout Mice. Basic Clin. Pharmacol. Toxicol. 2018, 123, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef]
- Steidl, S.; Wasserman, D.I.; Blaha, C.D.; Yeomans, J.S. Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons. Neurosci. Biobehav. Rev. 2017, 83, 72–82. [Google Scholar] [CrossRef]
- Andrews, N.; Loomis, S.; Blake, R.; Ferrigan, L.; Singh, L.; McKnight, A.T. Effect of gabapentin-like compounds on development and maintenance of morphine-induced conditioned place preference. Psychopharmacology 2001, 157, 381–387. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsuda, M.; Funada, M.; Misawa, M. Blockade of morphine-induced place preference by diazepam in mice. Eur. J. Pharmacol. 1995, 280, 327–330. [Google Scholar] [CrossRef]
- Shukla, P.K.; Tang, L.; Wang, Z.J. Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci. Lett. 2006, 404, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Zhou, Y.; Li, C.; Chen, Z.; Li, H.; Fang, M.; Zhu, C.; Huo, C.; Yung, K.K.; Li, J.; et al. Sinomenine Protects Against Morphine Dependence through the NMDAR1/CAMKII/CREB Pathway: A Possible Role of Astrocyte-Derived Exosomes. Molecules 2018, 23, 2370. [Google Scholar] [CrossRef] [PubMed]
- Hayama, T.; Noguchi, J.; Watanabe, S.; Takahashi, N.; Hayashi-Takagi, A.; Ellis-Davies, G.C.; Matsuzaki, M.; Kasai, H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat. Neurosci. 2013, 16, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Kim, N.S.; Lee, Y.K.; Choi, J.S. N-Methyl-D-Aspartate (NMDA) Receptors in the Prelimbic Cortex Are Required for Short- and Long-Term Memory Formation in Trace Fear Conditioning. Life 2022, 12, 672. [Google Scholar] [CrossRef]
- Higley, J. Localized GABAergic inhibition of dendritic calcium signaling. Nat. Rev. Neurosci. 2016, 8, 583–592. [Google Scholar]
- Zhang, J.J.; Song, C.G.; Dai, J.M.; Li, L.; Yang, X.M.; Chen, Z.N. Mechanism of opioid addiction and its intervention therapy: Focusing on the reward circuitry and mu-opioid receptor. MedComm 2022, 3, e148. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Wang, G.J.; Volkow, N.D. Naloxone precipitated withdrawal increases dopamine release in the dorsal striatum of opioid dependent men. Transl. Psychiatry 2021, 11, 445. [Google Scholar] [CrossRef]
- Cheaha, D.; Reakkamnuan, C.; Nukitram, J.; Chittrakarn, S.; Phukpattaranont, P.; Keawpradub, N.; Kumarnsit, E. Effects of alkaloid-rich extract from Mitragyna speciosa (Korth.) Havil. on naloxone-precipitated morphine withdrawal symptoms and local field potential in the nucleus accumbens of mice. J. Ethnopharmacol. 2017, 208, 129–137. [Google Scholar] [CrossRef]
- Ramesh, D.; Ross, R.; Schlosburg, E.; Owens, A.; Abdullah, A.; Kinsey, G.; Long, Z.; Nomura, K.; Sim-Selley, J.; Cravatt, F.; et al. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J. Pharmacol. Exp. Ther. 2011, 339, 173–185. [Google Scholar] [CrossRef]
- Majid, M.; Mohammad, B.; Pantea, H.; Seyed, K.; Ozra, M. Attenuation of morphine withdrawal syndrome by various dosages of curcumin in comparison with clonidine in mouse: Possible mechanism. Iran. J. Med. Sci. 2015, 40, 125–132. [Google Scholar]
- Glei, M.; Schneider, T.; Schlörmann, W. Comet assay: An essential tool in toxicological research. Arch. Toxicol. 2016, 90, 2315–2336. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Ravlić, S.; Godschalk, R.; Collins, A.; Dusinska, M.; Brunborg, G. Application of the comet assay for the evaluation of DNA damage in mature sperm. Mutat. Res.-Rev. Mutat. Res. 2021, 788, 108398. [Google Scholar] [CrossRef] [PubMed]
- Beach, H.D. Morphine addiction in rats. Can. J. Psychol. 1957, 11, 104–112. [Google Scholar] [CrossRef]
- Marshall, I.; Grahame-Smith, D.G. Evidence against a role of brain 5-hydroxytryptamine in the development of physical dependence upon morphine in mice. J. Pharmacol. Exp. Ther. 1971, 179, 634–641. [Google Scholar] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Milic, M.; Bonassi, S.; Dusinska, M. The comet assay in human biomonitoring: Technical and epidemiological perspectives. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 843, 1–2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).