Abstract
Rheumatoid arthritis (RA) is a chronic and debilitating condition that affects a significant number of individuals worldwide. Unfortunately, the currently available therapeutic approaches often yield unsatisfactory results and may be accompanied by harmful side effects. A medicinal plant called Psidium glaziovianum Kiaersk has potential benefits in the treatment of this condition due to its anti-inflammatory and analgesic properties. In this study, our objective was to investigate the potential therapeutic effects of P. glaziovianum essential oil (PgEO) in alleviating arthritis symptoms in mice induced by Complete Freund’s Adjuvant (CFA). The effect of P. glaziovianum essential oil was evaluated in mice with Complete Freund’s Adjuvant (CFA)-induced arthritis. Edema sizes, macroscopic and radiographic images, cytokine levels, and oxidative stress were evaluated. Administration of PgEO at dosages of 50 and 100 mg/kg effectively prevented CFA-induced osteoarticular changes in arthritic mice, resulting in a significant reduction in joint damage. Additionally, the PgEO treatment exhibited the ability to minimize edema, a common symptom associated with arthritis. Furthermore, PgEO can modulate the levels of pro-inflammatory cytokines and oxidative stress, both of which play crucial roles in the progression of the disease. In conclusion, our study suggests that PgEO holds great potential as a natural therapeutic agent for rheumatoid arthritis.
1. Introduction
Rheumatoid arthritis (RA) affects millions of individuals worldwide and it can affect people of any age, although it is more common in women and individuals over 40 years of age [1]. RA is caused by genetic and environmental factors such as stress, repetitive strain, and smoking [2]. In the later stages, joint stiffness and bone deformities develop. Additionally, pro-inflammatory mediators such as cytokines and oxidative stress are responsible for bone destruction in the synovial membrane, leading to joint dysfunction [3].
The therapeutic approach for RA is based on the use of nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and disease-modifying antirheumatic drugs (DMARDs). Disease-modifying antirheumatic drugs (DMARDs) are a key class of medications in the management of chronic inflammatory rheumatic diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and psoriasis, among others. These medications can alter the course of these diseases by reducing their activity, slowing the progression of structural damage, and ultimately improving the quality of life for patients [4,5].
DMARDs are divided into two main subclasses: conventional synthetics and biologics, each with unique mechanisms of action that target the pathogenic pathways of rheumatic diseases. Conventional drugs, like methotrexate and sulfasalazine, have been long-standing pillars in treatment, acting to modulate the immune system in a more generalized and accessible manner. Biologics, produced through recombinant DNA technology, focus on specific targets within the immune system, such as cytokines or cells, offering a more directed and effective treatment for cases that are refractory to conventional therapies [5,6].
However, some patients do not respond effectively to treatment and long-term use of these therapies can lead to serious gastrointestinal and renal adverse effects [7,8]. Therefore, there is a need for the search for new compounds with potential antiarthritic action that are affordable and safe. As an alternative, medicinal plants have emerged as a promising strategy since some plants are used to treat various types of diseases, including inflammation [9,10].
Psidium is a genus in the family Myrtaceae and includes 118 accepted species worldwide [11]. With distribution in tropical and subtropical regions of the world, traditional populations mention the genus Psidium as a resource in folk medicine to treat various diseases related to the digestive system, such as diarrhea, stomach pains, abdominal pain, and dysentery [12,13,14,15,16,17]. In addition, this genus is also used in the treatment of disorders of the genitourinary system [18] and the respiratory system, such as sore throat [16,19].
This genus is known to possess various biological activities, such as analgesic [20,21,22], anti-inflammatory [22,23,24], gastroprotective [25,26], and antimicrobial effects [27,28,29], among others. Among these species, we have Psidium glaziovianum, commonly known as araçá-pitanga, which grows in the northeastern and southeastern regions of Brazil; this species is known for its edible fruits, which are similar to guavas, and it is also used in traditional medicine for various purposes [30].
Previously, the essential oil from P. glaziovianum resulted in a decrease in nociception in both chemical and thermal stimulus tests. Investigating the mechanisms of action behind this effect, with the aid of specific pain pathway blockers, it was observed that the opioidergic and muscarinic pathways work together in mediating this effect. Furthermore, the anti-inflammatory potential of P. glaziovianum essential oil was demonstrated by its ability to reduce edema, decrease the migration of inflammatory cells, lower the levels of pro-inflammatory cytokines, and attenuate the formation of granulomas in chronic conditions [31].
Therefore, the aim of this study was to evaluate the antiarthritic potential (chronic inflammation) of the essential oil obtained from Psidium glaziovianum leaves in mice with Complete Freund’s Adjuvant (CFA)-induced arthritis.
2. Results and Discussion
Traditional approaches to arthritis management primarily focus on pain management and inflammation reduction using nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs). While these medications can alleviate symptoms for some patients, they are not universally effective and may cause adverse side effects, ranging from gastrointestinal issues to increased risk of infections. Moreover, as arthritis encompasses a heterogeneous group of conditions, including rheumatoid arthritis, osteoarthritis, and juvenile arthritis, there is no one-size-fits-all treatment approach. The complexity of arthritis pathophysiology, involving intricate interactions between genetic, environmental, and immunological factors, further complicates treatment strategies [4,5].
In a previous study, PgEO showed efficacy in the treatment of acute inflammation and in the chronic inflammation assay in the granuloma model [22]. Therefore, using the same sample, we evaluated the treatment effect of PgEO on a model of chronic inflammation induced by Complete Freund’s Adjuvant (CFA). The mouse model with CFA-induced arthritis is a well-known example of a painful arthritic joint that results in inflammation-related behaviors, such as increased edema, pro-inflammatory cytokines, reduced weight, and decreased use of the arthritic joint [32,33].
Joint edema is a common and significant manifestation in the context of arthritis, a chronic inflammatory condition that affects millions of people worldwide. This abnormal accumulation of synovial fluid in the joints plays a crucial role in the underlying inflammatory process of arthritis, directly influencing the progression of the disease. This inflammation is triggered by the dysregulated immune response, resulting in increased permeability of blood vessels in the synovium, allowing fluid extravasation into the joint cavity. This fluid accumulation not only amplifies local inflammation but can also cause structural damage to the affected joints. Joint edema exerts pressure on surrounding tissues and pain receptors, triggering sensations of discomfort and pain. Additionally, the increased synovial volume can limit the range of motion of the joints, contributing to stiffness and functional impairment [3,5].
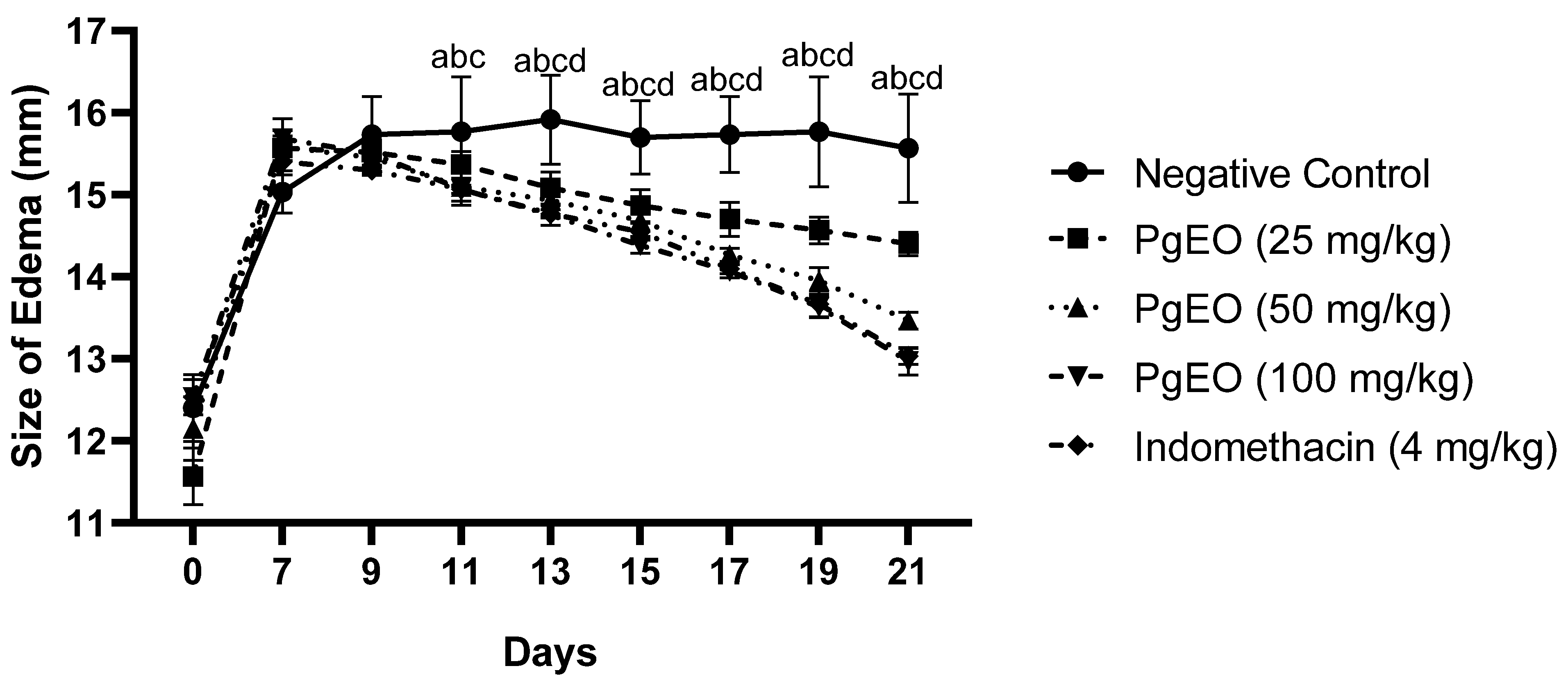
Repeated intra-articular injection of CFA induced long-lasting joint inflammation with significantly swollen joints and the cumulative changes were consistent with chronic inflammation. Treatment for 14 days with PgEO at concentrations of 25, 50, and 100 mg/kg led to a significant decrease (p < 0.001) compared to the negative control in inflammatory signs, measured as the size of arthritic edema every two days, as observed in Figure 1.
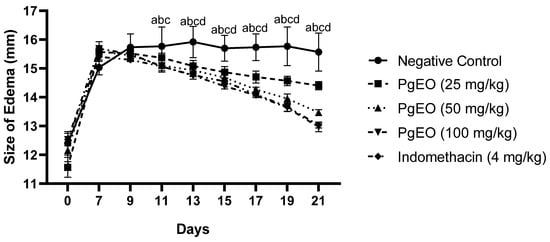
Figure 1.
Effect of PgEO administration for 14 days on CFA-induced arthritic edema. Results are expressed as mean ± SEM of 6 animals per group (p < 0.001). a: Indomethacin vs. control group; b: 100 mg/kg vs. control group; c: 50 mg/kg vs. control group; and d: 25 mg/kg vs. control group.
After two applications of CFA, the control group animals exhibited an average edema size of 15.03 ± 0.22 and after 14 days, the group had an average size of 15.63 ± 0.56, maintaining the joint edema. On the other hand, the groups treated with PgEO at concentrations of 25, 50, and 100 mg/kg showed edema sizes on day 7 as follows: 15.56 ± 0.13, 15.58 ± 0.25, and 15.68 ± 0.06, respectively. After 14 days of treatment, compared to the negative control group, the edemas significantly reduced (p < 0.001) to average sizes of 14.40 ± 0.10 (25 mg/kg), 13.45 ± 0.06 (50 mg/kg), and 12.96 ± 0.13 (100 mg/kg). Indomethacin (4 mg/kg) had an initial size of 15.40 ± 0.06 on day 7 and reduced the edema size to 13.03 ± 0.07.
A previous study demonstrated that other species of Psidium spp. are effective in preventing paw edema induced by different inflammatory agents other than CFA. For example, oral administration of PgEO is effective in reducing paw edema induced by carrageenan in mice, with an 89.23% reduction in edema size at a concentration of 100 mg/kg [22]. When evaluated at doses of 30, 100, and 300 mg/kg, the essential oil from the leaves of P. guineense showed a reduction in edema size by 48.48%, 51.03%, and 59.46%, respectively [24].
The anti-edematogenic activity of PgEO may be associated with the major compounds present in the oil. Santos and Rao [34] described that treatment with 400 mg/kg of 1,8-cineole reduced edema by 46%. Martins et al. [35] also reported that 1,8-cineole strongly suppresses the generation of cytokines, which are responsible for the edematogenic effect. Additionally, Khoshnazar et al. [36] demonstrated that α-pinene (50 and 100 mg/kg) caused a significant decrease in edema size. Moreover, β-pinene (50 mg/kg) significantly reduced paw edema by 36% at the fourth hour of evaluation [37].
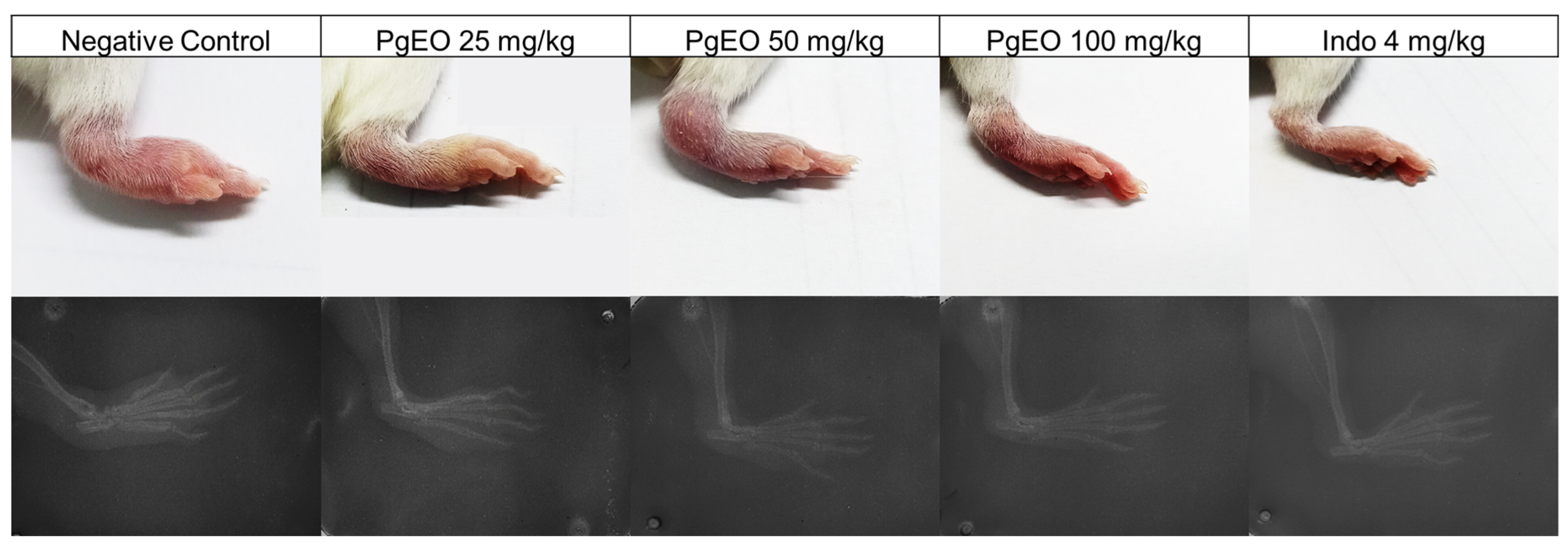
The evaluation of edema and radiographic imaging are methods used to assess the severity and progression of chronic joint diseases. These methodologies are considered the main approaches used in clinical practice for the diagnosis and prognosis of individuals affected by certain osteoarticular diseases [38]. Therefore, to correlate with clinical practice in the diagnosis of rheumatoid arthritis (RA), at the end of the treatment in the experimental model of CFA-induced arthritis, the paw edemas were photographed, followed by radiological examinations to assess whether the inducing agent caused osteoarticular alterations and whether treatment with PgEO reduced the damage in the animals (Figure 2).
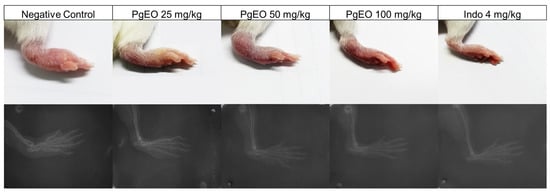
Figure 2.
Macroscopic images and radiographic images of the effect of PgEO administration for 14 days on CFA-induced arthritic edema. Effect of treatment with PgEO 25, 50, and 100 mg/kg, orally, on the joints of mice with chronic CFA-induced arthritis.
The animals in the control group and those treated with PgEO at a dose of 25 mg/kg showed a significant increase in soft tissues. However, animals treated with PgEO at concentrations of 50 and 100 mg/kg, as well as the group treated with indomethacin (4 mg/kg), showed smaller increases in soft tissues compared to the control and PgEO 25 mg/kg treated groups. When compared to the control group and those receiving a dose of 25 mg/kg, the therapy was able to prevent osteoarticular alterations caused by the inducing drug and minimize edema (Figure 2).
The PgEO 25 mg/kg and control groups show a space between the joints, as interpreted from the provided radiographic images, indicating that CFA inoculation caused an increase in the size of the edema. These alterations were observed in the present study, as well as in previous research using the same inflammatory agent [10,39,40]. The irritant impact of CFA is what causes the initial increase in edema, a characteristic observed in all animals, along with the thickening of the soft tissue [32].
A study by Kany et al. [41] described that the pathological state of arthritis is directly linked to an increase in pro-inflammatory mediators, as they are crucial for the initiation and progression of arthritis. Additionally, various inflammatory cells present in the synovial fluid produce pro-inflammatory cytokines, playing an important role in the destruction of articular cartilage and adjacent bone tissue, such as TNF-α and other interleukins, namely IL-1β and IL-6 [1,42,43]. Therefore, we evaluated the influence of PgEO treatment on the inflammatory profile of mice subjected to the anti-arthritic assay.
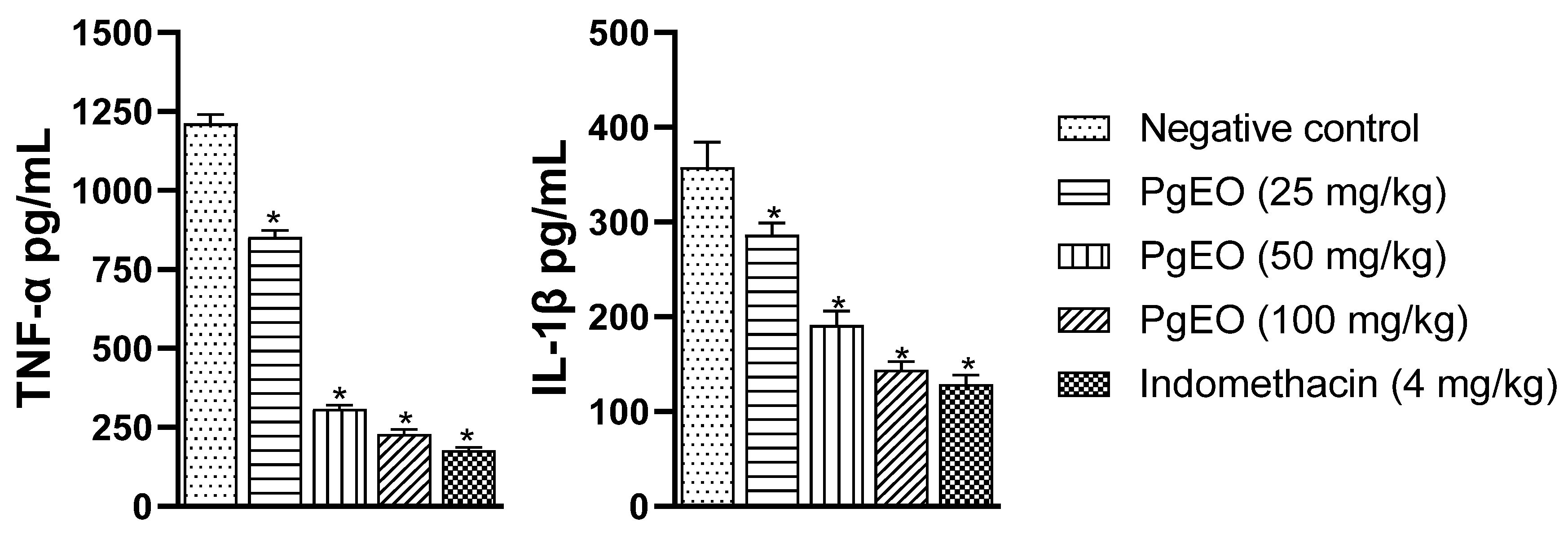
The results presented in Figure 3 demonstrate that treatment with PgEO at different concentrations (25, 50, and 100 mg/kg) reduced the levels of pro-inflammatory cytokines compared to the control group (p < 0.001). TNF-α showed reductions of 29.72%, 74.52%, and 81.19% at concentrations of 25, 50, and 100 mg/kg, respectively. IL-1β showed reductions of 19.96% (25 mg/kg), 46.64% (50 mg/kg), and 59.81% (100 mg/kg). Indomethacin reduced TNF-α and IL-1β by 85.41% and 63.98%, respectively.
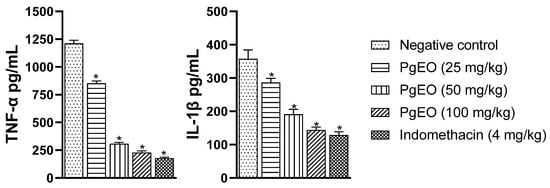
Figure 3.
Effect of 14-day administration of PgEO on TNF-α and IL-1β levels in CFA-induced arthritis. The values represent mean ± SEM. * p < 0.001 compared to the control and one-way analysis of variance (ANOVA) followed by Dunnett’s test.
These results are in line with what was observed by Aiyalu et al. [44], who stated that an increase in serum levels of TNF-α, IL-1α, and IL-1β is a characteristic of arthritis. Therefore, a reduction in these mediators decreases inflammatory damage. Previously, PgEO has been shown to be effective in reducing levels of pro-inflammatory cytokines in acute inflammation assays [22]. Additionally, in an animal model of LPS-induced lung inflammation, 1,8-cineole inhibited TNF-α and IL-1β and increased the anti-inflammatory cytokine IL-10 in lung tissues [45].
In addition to the inflammatory profile, oxidative stress is a key factor in the worsening of chronic inflammatory joint diseases [46,47]. Both reactive oxygen species (ROS), lipid peroxidation, and a decrease in antioxidant defenses are involved in the body’s metabolic processes and alterations in these compounds cause damage to cellular and tissue components [48,49].
Table 1 presents data showing that treatment with PgEO attenuates oxidative stress in arthritic animals. The control group animals showed elevated levels of MDA, while the levels of endogenous antioxidants SOD and CAT decreased. On the other hand, the results demonstrate that arthritic animals treated with PgEO at concentrations of 50 and 100 mg/kg showed a decrease in MDA levels by 42.03% and 47.25%, respectively, compared to the control group. SOD levels increased by 253.19% and 288.95%, while CAT levels increased by 382.60% and 386.95%, respectively. Animals treated with PgEO at a concentration of 25 mg/kg did not show a significant difference from the control group.

Table 1.
Effect of Psidium glaziovianum essential oil (PgEO) on the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) in mice with arthritis.
Oxidative stress is closely linked to the chronic inflammation observed in RA. During inflammation, there is an increase in the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are components of oxidative stress. These species can damage cells and tissues, contributing to the inflammatory cycle in RA. ROS and RNS can directly harm joint tissues, including cartilage, bones, and synovial membranes. This can lead to progressive joint degradation and deterioration of joint function [42,43].
Moreover, oxidative stress can induce the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which are known to play a central role in the pathogenesis of RA. Additionally, oxidative stress can activate transcription factors such as nuclear factor kappa B (NF-κB), which regulates the expression of genes involved in the inflammatory response and joint destruction in RA [47].
Recent studies on the in vivo antioxidant activity of essential oils have reported activity on antioxidant enzymes such as catalases, SOD, glutathione reductase (GR), heme oxygenase 1 (HO1), and glutathione peroxidase (GPx) [50,51,52]. Additionally, when 1,8-cineole was applied to stimulated normal human monocytes with fetal calf serum (FCS) in vitro, it inhibited by O2 (−53%) and H2O2 (−48%) compounds involved in oxidative stress [53]. Furthermore, α-pinene (100 mg/kg) restored the function of superoxide dismutase, catalase, and glutathione peroxidase and reduced the concentration of MDA [36].
Given the relevance of oxidative stress in the pathogenesis of inflammatory diseases, we evaluated the antioxidant properties of PgEO using different in vitro methods: DPPH, ABTS, and TAC (Table 2). The oil inhibited DPPH and ABTS radicals with IC50 values of 39.47 and 122.10 µg/mL, respectively. In the total antioxidant capacity assay, PgEO showed high total antioxidant potential, with an inhibition of 87.32% compared to ascorbic acid.

Table 2.
Antioxidant activities using the ABTS, DPPH, and TAC methods.
An approach for the treatment of RA is to employ antioxidants, considering that free radicals are chemical species that possess an unpaired electron capable of damaging membrane lipids, proteins, DNA, and even cartilaginous and bony tissues [54]. Therefore, treatments that reduce oxidant levels and/or increase antioxidant levels have great potential as anti-inflammatory drugs for the treatment of various disorders related to oxidative stress [48]. In this sense, antioxidant therapy with medicinal plants such as P. glaziovianum may potentially offer new options for adjuvant/complementary treatment, aiming at better disease control.
3. Materials and Methods
3.1. Plant Material and Essential Oil Extraction
The leaves of Psidium glaziovianum Kiaersk (Myrtaceae) were gathered in December 2019 (dry season) in the Caatinga district of the municipality of Exu in Pernambuco, Brazil (07°30′43″ S 39°43′27″ W). The Agronomic Institute of Pernambuco’s (IPA) Dárdano de Andrade Lima Herbarium now houses a voucher specimen with sample number 93,728. Under the designation A08E18B, the plant material was recorded in the SisGen database, which is part of the National System for the Management of Genetic Heritage and Associated Traditional Knowledge.
As previously stated by [22], to extract the oil, meticulous care was taken in the preparation of P. glaziovianum Kiaersk leaves. Initially, the leaves underwent thorough sanitization using copious amounts of distilled water, ensuring the removal of any contaminants. Subsequently, they were delicately dried under controlled conditions at a temperature of 25 °C to preserve the integrity of their bioactive compounds. Once dried, the leaves were carefully partially broken to facilitate the release of their aromatic essence. These prepared leaves were then subjected to a hydrodistillation process using a specialized apparatus (Clevenger method) and were then identified using gas chromatography-mass spectrometry (GC/MS). In total, 48 compounds were identified by the study, accounting for 98.22% of the makeup, with 1,8-cineole (24.29%), -pinene (19.73%), and -pinene (17.31%) serving as the main constituents.
3.2. In Vivo Studies
The trials employed female Swiss mice that were 8 to 10 weeks old and weighed between 30 and 35g. The animals were kept in temperature-controlled rooms (22–25 °C) with a 12-h light/dark cycle and had unlimited access to food and drink. The study received clearance from the Animal Ethics Committee of the Federal University of Pernambuco (approval number 122/2019) and was carried out in line with all applicable Brazilian legislation regarding the use of animals in research.
3.3. Complete Freund’s Adjuvant (CFA)-Induced Chronic Inflammation Model
Female Swiss mice had the size of their right hind paws measured and were randomly divided into 5 groups (n = 6/group). On days one and four, 20 μL of Complete Freund’s Adjuvant (CFA) suspension (containing Mycobacterium tuberculosis in oil) was injected intraplantarly into the right hind paw. From the seventh to the twentieth day after the initial arthritis induction, the animals received the following treatments: Group I: PgEO 25 mg/kg orally; Group II: PgEO 50 mg/kg orally; Group III: PgEO 100 mg/kg orally; Group IV: Indomethacin 4 mg/kg intraperitoneally (positive control); Group V: 0.9% NaCl orally (negative control). On the 21st day after the first arthritis induction, all mice were sacrificed under anesthesia for paw and serum collection by cardiac puncture.
3.4. Measurement of Edema Size
The size of the paw was measured using an electronic caliper on days 0, 7, 9, 11, 13, 15, 17, 19, and 21 after the initial administration of CFA. Briefly, the mice were restrained and the paw diameter was measured from the space between all the pads of the hind paw’s dorsal surface to the front (plantar surface) of the paw. All evaluations were conducted by the same trained evaluator, proficient in the specific measurement technique to ensure uniformity. Additionally, measures were taken to reduce potential random errors, including regular calibration of measurement instruments to ensure their continuous accuracy over time.
3.5. Macroscopic and Radiographic Images
On the 21st day after the initial CFA-induced arthritis induction, macroscopic and radiographic images of the mice’s paws were captured. Initially, the animals were anesthetized with 2% xylazine (1 mg/kg) and 10% ketamine (50 mg/kg, via intraperitoneal injection) and positioned in ventral decubitus. Subsequently, the paws were photographed to document the presence of edema. The animals were then transferred to obtain radiographs in the plantar projection. A Kodak X-ray machine with a resolution of 2000/40×, a diaphragm aperture of 2.6 cm, and an exposure time of 60 s was used for the radiographs.
3.6. Measurement of Serum Cytokine Levels
On the 21st day after arthritis induction with CFA and after anesthesia, blood was collected by cardiac puncture in the mice. Subsequently, serum was obtained by centrifugation at 3000 rpm for 15 min. The levels of TNF-α (E-HSEL-M0009) and IL-1β (E-HSEL-R0002) in the serum were measured using ELISA kits according to the manufacturer’s instructions (Elabscience®, Houston, TX, USA). The results are expressed as cytokine picograms per milliliter of serum (pg/mL).
3.7. Analysis of Oxidative Stress
The determination of total protein content in the blood serum was performed as described by Bradford (1976). Lipid peroxidation was assessed by estimating the level of thiobarbituric acid reactive substances (TBARS), with the results expressed in nanomoles of malondialdehyde (MDA) per milligram of protein [55]. The activity of superoxide dismutase (SOD) was measured by evaluating the kinetics of epinephrine autoxidation inhibition based on absorbance at 480 nm, with the enzymatic activity of tissue SOD expressed in U/mg of protein [56]. The activity of catalase (CAT) was measured at 240 nm to monitor the change in absorbance between the first and sixth minute, with the enzymatic activity of tissue CAT expressed in mU/mg of protein [57].
3.8. Evaluation of In Vitro Antioxidant Activity
The antioxidant potential of PgEO was evaluated using various methods at concentrations ranging from 31.25 to 1000 μg/mL. The radical inhibition methods used were DPPH (2,2-Diphenyl-1-picrylhydrazyl) [58] and ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) [59]. Ascorbic acid was used as the positive control. The percentage inhibition (I%) was calculated using the following equation: I% = [(Ac − As)/(Ac)] × 100, where Ac is the absorbance of the control and As is the absorbance of the sample. The concentrations of the samples responsible for a 50% reduction in the initial free radical activity (IC50) were calculated using linear regression.
The Folin ≠ Ciocalteu method was used to determine the total antioxidant capacity (TAC). The antioxidant capacity of the samples was calculated as relative antioxidant activity compared to ascorbic acid, using the formula CAT% = [(Aa − Ac)/(Aaa − Ac)] × 100, where Aa is the absorbance of the sample, Ac is the absorbance of the control, and Aaa is the absorbance of ascorbic acid [44].
3.9. Statistical Analysis
The GraphPad Prism program, version 8.0, was used to conduct the statistical analysis. The data containing a single independent variable were analyzed using one-way ANOVA and Tukey’s post hoc test. A statistically significant value of p < 0.001 was used to portray the data as the mean SEM.
4. Conclusions
The observed results regarding the effect of essential oil of P. glaziovianum (PgEO) hold significant clinical relevance in the context of rheumatoid arthritis (RA) treatment. PgEO’s ability to modulate the inflammatory response and reduce oxidative stress may represent a promising therapeutic approach for patients with inflammatory joint diseases. By reducing the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), PgEO may help alleviate the joint inflammation characteristic of RA. Additionally, its ability to neutralize the harmful effects of reactive oxygen species (ROS) and reactive nitrogen species (RNS) can protect joint cells and tissues from progressive degeneration. As a result, PgEO may not only alleviate RA symptoms but also help preserve joint integrity and improve patients’ quality of life. These findings underscore the importance of PgEO as a potential complementary or alternative therapy in the RA treatment arsenal and suggest the need for further clinical studies to validate its therapeutic use.
Author Contributions
W.K.C.: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, and Writing—original draft; J.V.d.O.A.: Conceptualization, Formal Analysis, Investigation, and Methodology; B.M.B.D.F.: Data curation and Investigation; V.B.G.S.: Data curation and Investigation; R.J.F.: Formal Analysis, Investigation, and Methodology; T.H.N.: Funding acquisition; P.M.G.P.: Funding acquisition and Writing—review and editing; M.T.d.S.C.: Data curation, Funding acquisition, and Writing—review and editing; A.M.d.O.: Conceptualization, Funding acquisition, and Writing—review and editing; M.V.d.S.: Conceptualization, Funding acquisition, and Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Fuding was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001) and the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE).
Institutional Review Board Statement
All the experimental procedures are in accordance with the Brazilian laws for animal experimentation and were submitted to the Animal Ethics Committee of the Universidade Federal de Pernambuco and received a favorable opinion in accordance with protocol no 122/2019.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); CAT: catalase; CAT: total antioxidant capacity; CFA: Complete Freund’s Adjuvant; DPPH: 2,2-diphenyl-1-picrylhydrazyl; GC-MS: gas chromatography-mass spectrometry; IL-1β: interleukin-1 beta; MDA: malondialdehyde; PgEO: Psidium glaziovianum essential oil; RA: rheumatoid arthritis; SOD: superoxide dismutase; TBARS: thiobarbituric acid reactive species; TNF-α: alpha tumor necrosis factor.
References
- Alabi, A.O.; Ajayi, A.M.; Omorogbe, O.; Umukoro, S. Anti-nociceptive and anti-inflammatory effects of an aqueous extract of blended leaves of Ocimum gratissimum and Psidium guajava. Clin. Phytosci. 2019, 5, 34. [Google Scholar] [CrossRef]
- Aiyalu, R.; Subramaniam, I.; Govindarajan, A.; Ramasamy, A. Formulation and Evaluation of Novel Herbal Aerosol for Arthritis. J. Rheumatol. Arthritic Dis. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2019, 309, 125566. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Auclair, C.; Voisin, E. Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Baptista, M.M.; Ramos, M.A.; de Albuquerque, U.P.; Coelho-De-Souza, G.; Ritter, M.R. Traditional botanical knowledge of artisanal fishers in southern Brazil. J. Ethnobiol. Ethnomed. 2013, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef] [PubMed]
- Birmann, P.T.; Casaril, A.M.; Zugno, G.P.; Acosta, G.G.; Sousa, F.S.S.; Collares, T.; Seixas, F.K.; Jacob, R.G.; Brüning, C.A.; Savegnago, L.; et al. Flower essential oil of Tagetes minuta mitigates oxidative stress and restores BDNF-Akt/ERK2 signaling attenuating inflammation- and stress-induced depressive-like behavior in mice. Brain Res. 2022, 1784, 147845. [Google Scholar] [CrossRef] [PubMed]
- Bieski, I.G.C.; Leonti, M.; Arnason, J.T.; Ferrier, J.; Rapinski, M.; Violante, I.M.P.; Balogun, S.O.; Pereira, J.F.C.A.; Figueiredo, R.d.C.F.; Lopes, C.R.A.S.; et al. Ethnobotanical study of medicinal plants by population of Valley of Juruena Region, Legal Amazon, Mato Grosso, Brazil. J. Ethnopharmacol. 2015, 173, 383–423. [Google Scholar] [CrossRef]
- Bolson, M.; Hefler, S.R.; Dall, E.I.; Chaves, O.; Junior, A.G.; Junior, E.L.C. Ethno-medicinal study of plants used for treatment of human ailments, with residents of the surrounding region of forest fragments of Paraná, Brazil. J. Ethnopharmacol. 2015, 161, 1–10. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Brito, M.F.; Marín, E.A.; Cruz DD, D. Medicinal plants in rural settlements of a protected area in the littoral of Northeast Brazil. Ambiente Soc. 2017, 20, 83–104. [Google Scholar] [CrossRef]
- Castro, J.A.; Brasileiro, B.P.; Lyra, D.H.; Pereira, D.D.A.; Chaves, J.L.; Amaral, C.L. F Ethnobotanical study of traditional uses of medicinal plants: The flora of caatinga in the community of Cravolândia-BA, Brazil. J. Med. Plants Res. 2011, 5, 1905–1917. [Google Scholar]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2021, 35, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chae, S.-J.; Kang, S.W.; Cheong, Y.; Hong, S.-J.; Park, H.-K. Non-invasive screening of progressive joint defects in the Type II collagen-induced arthritis animal model using radiographic paw images. Inflamm. Res. 2011, 60, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.K.; Nascimento, M.F.D.; Barbosa, L.S.; Souza, T.G.d.S.; Chagas, C.A.; Napoleão, T.H.; Correia, M.T.d.S.; Brayner, F.A.; de Oliveira, A.M.; da Silva, M.V. Cytotoxicity, oral toxicity, genotoxicity, and mutagenicity evaluation of essential oil from Psidium glaziovianum Kiaersk leaves. J. Ethnopharmacol. 2023, 303, 115955. [Google Scholar] [CrossRef]
- Costa, W.K.; do Nascimento, M.F.; dos Santos, C.R.B.; do Amaral Ferraz Navarro, D.M.; Napoleão, T.H.; Dos Santos Correia, M.T.; Brayner, F.A.; de Oliveira, A.M.; da Silva, M.V. Oral administration of essential oil from Psidium glaziovianum Kiaersk leaves alleviates pain and inflammation in mice. Inflammopharmacology 2022, 30, 599–607. [Google Scholar] [CrossRef]
- da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative stress in rheumatoid arthritis: What the future might hold regarding novel biomarkers and add-on therapies. Oxidative Med. Cell. Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef]
- Greiner, J.F.W.; Müller, J.; Zeuner, M.T.; Hauser, S.; Seidel, T.; Klenke, C.; Grunwald, L.-M.; Schomann, T.; Widera, D.; Sudhoff, H.; et al. 1,8-Cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 2866–2878. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, L.; Liu, B.; Lu, P.; Cao, Z.; Ji, X.; Ouyang, G.; Nie, Z.; Lyu, A.; Lu, C. Inappropriate treatment response to DMARDs: A pathway to difficult-to-treat rheumatoid arthritis. Int. Immunopharmacol. 2023, 122, 110655. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Kumar, B.; Singh, S.B.; Ganju, L. Andrographolide attenuates complete freund’s adjuvant induced arthritis via suppression of inflammatory mediators and pro-inflammatory cytokines. J. Ethnopharmacol. 2020, 261, 113022. [Google Scholar] [CrossRef] [PubMed]
- Heimfarth, L.; Rezende, M.M.; Pereira, E.W.M.; Passos, F.R.S.; Monteiro, B.S.; Santos, T.K.B.; Lima, N.T.; Souza, I.C.L.; de Albuquerque Junior, R.L.C.; de Souza Siqueira Lima, P.; et al. Pharmacological effects of a complex α-bisabolol/β-cyclodextrin in a mice arthritis model with involvement of IL-1β, IL-6 and MAPK. Biomed. Pharmacother. 2022, 151, 113142. [Google Scholar] [CrossRef] [PubMed]
- Ibeh, L.N.; Ijioma, S.N.; Emmanuel, O.; Timothy, C.O.; Ugbogu, E.A. Psidium guajava leaf extract improves gastrointestinal functions in rats and rabbits: An implication for ulcer and diarrhoea management. Biomarkers 2021, 26, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Juergens, L.J.; Tuleta, I.; Stoeber, M.; Racké, K.; Juergens, U.R. Regulation of monocyte redox balance by 1,8-cineole (eucalyptol) controls oxidative stress and pro-inflammatory responses in vitro: A new option to increase the antioxidant effects of combined respiratory therapy with budesonide and formoterol? Synergy 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Khoshnazar, M.; Bigdeli, M.R.; Parvardeh, S.; Pouriran, R. Attenuating effect of α-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. J. Pharm. Pharmacol. 2019, 71, 1725–1733. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Khiari, M.; Klibet, F.; Kechrid, Z. Oxidative stress toxicity effect of potential metal nanoparticles on human cells. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; pp. 107–117. [Google Scholar]
- Landrum, L.R. The Genus Psidium (Myrtaceae) in the State of Bahia, Brazil. Canotia 2017, 13, 1–101. [Google Scholar]
- Lee, J.; Bae, Y.; Kim, N.J.; Lim, S.; Kim, Y.-M.; Kim, J.; Chin, Y.-W. Anti-rheumatic, and analgesic effects by the parent tuberous roots of Aconitum jaluense in adjuvant induced arthritis rats. J. Ethnopharmacol. 2022, 289, 114518. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, S.-M.; Park, E.-J.; Lee, H.-J. Anti-arthritic effects of Schisandra chinensis extract in monosodium iodoacetate-induced osteoarthritis rats. Inflammopharmacology 2022, 30, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, Y.; Gao, Y.; Qi, D.; Zhao, L.; Zhao, L.; Liu, C.; Tao, T.; Zhou, C.; Sun, X.; et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020, 11, 129. [Google Scholar] [CrossRef]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; de Oliveira, M.R.C.; Tintino, C.D.M.; e Castro, F.F.; Alcântara, I.S.; Fernandes, M.N.M.; de Albuquerque, T.R.; da Silva, M.S.A.; et al. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef]
- Meng, R.; Wu, S.; Chen, J.; Cao, J.; Li, L.; Feng, C.; Liu, J.; Luo, Y.; Huang, Z. Alleviating effects of essential oil from Artemisia vulgaris on enteritis in zebrafish via modulating oxidative stress and inflammatory response. Fish Shellfish. Immunol. 2022, 131, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Montilla-García, Á.; Tejada, M.Á.; Perazzoli, G.; Entrena, J.M.; Portillo-Salido, E.; Fernández-Segura, E.; Cañizares, F.J.; Cobos, E.J. Grip strength in mice with joint inflammation: A rheumatology function test sensitive to pain and analgesia. Neuropharmacology 2017, 125, 231–242. [Google Scholar] [CrossRef]
- Nascimento, K.F.D.; Kassuya, C.A.L.; Cabral, M.R.P.; Souza, R.I.C.; Marangoni, J.A.; Silva, R.M.M.F.; Canella, D.A.d.C.; Formagio, A.S.N. Chemical analysis and antioxidant, anti-inflammatory and toxicological evaluations of the hydromethanolic extract of Psidium guineense Swartz leaves. J. Ethnopharmacol. 2021, 281, 114492. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytoscience 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Abreu, D.B.d.O.; Santoro, F.R.; de Albuquerque, U.P.; Ladio, A.H.; de Medeiros, P.M. Medicinal plant knowledge in a context of cultural pluralism: A case study in Northeastern Brazil. J. Ethnopharmacol. 2015, 175, 124–130. [Google Scholar] [CrossRef]
- Ou, Z.; Zhao, J.; Zhu, L.; Huang, L.; Ma, Y.; Ma, C.; Luo, C.; Zhu, Z.; Yuan, Z.; Wu, J.; et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed. Pharmacother. 2019, 118, 109347. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sallam, M.F.; Ahmed, H.M.; Diab, K.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Sharaf, H.A.; Abdel-Wahhab, M.A. Improvement of the antioxidant activity of thyme essential oil against biosynthesized titanium dioxide nanoparticles-induced oxidative stress, DNA damage, and disturbances in gene expression in vivo. J. Trace Elements Med. Biol. 2022, 73, 127024. [Google Scholar] [CrossRef]
- Santos, E.S.; Coelho, G.L.A.; Loula, Y.K.S.F.; Landim, B.L.S.; Lima, C.N.F.; Machado, S.T.d.S.; Lopes, M.J.P.; Gomes, A.D.S.; da Costa, J.G.M.; de Menezes, I.R.A.; et al. Hypoglycemic, Hypolipidemic, and Anti-Inflammatory Effects of Beta-Pinene in Diabetic Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 8173307. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Shady, N.H.; Abdullah, H.S.; Maher, S.A.; Albohy, A.; Elrehany, M.A.; Mokhtar, F.A.; Oraby, H.F.; Shawky, A.M.; Abdelmohsen, U.R. Antiulcer Potential of Psidium guajava Seed Extract Supported by Metabolic Profiling and Molecular Docking. Antioxidants 2022, 11, 1230. [Google Scholar] [CrossRef]
- Shamlan, G.; Al-Nouri, D.M.; Alathbah, A.A.; Arzoo, S.; Habibullah, M.M. Antiarthritic, anti-inflammatory activity of Moringa peregrina seed oil and leaves in Freund’s complete adjuvant-induced arthritis in rats. J. King Saud Univ.-Sci. 2021, 33, 101350. [Google Scholar] [CrossRef]
- Siebert, S.; Tsoukas, A.; Robertson, J.; McInnes, I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol. Rev. 2015, 67, 280–309. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Sleen, Y.; van der Geest, K.S.M.; Huckriede, A.L.W.; van Baarle, D.; Brouwer, E. Effect of DMARDs on the immunogenicity of vaccines. Nat. Rev. Rheumatol. 2023, 19, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, R.d.S.; Nascimento, E.P.D.; de Menezes, I.R.A.; Sales, V.d.S.; Pereira, A.O.B.; de Lacerda, G.M.; Santos, E.S.; Lopes, M.J.P.; da Silva, L.G.; Delmondes, G.d.A.; et al. Antinociceptive activity of the Psidium brownianum Mart ex DC. leaf essential oil in mice. Food Chem. Toxicol. 2020, 135, 111053. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Psidium L. Published on the Internet. 2022. Available online: http://www.theplantlist.org/1.1/browse/A/Myrtaceae/Psidium/ (accessed on 6 November 2022).
- Tomazi, L.B.; Aguiar, P.A.; Citadini-Zanette, V.; Rossato, A.E. Estudo etnobotânico das árvores medicinais do Parque Ecológico Municipal José Milanese, Criciúma, Santa Catarina, Brasil. Rev. Bras. Plantas Med. 2014, 16, 450–461. [Google Scholar] [CrossRef]
- Tseuguem, P.P.; Ngangoum, D.A.M.; Pouadjeu, J.M.; Piégang, B.N.; Sando, Z.; Kolber, B.J.; Tidgewell, K.J.; Nguelefack, T.B. Aqueous and methanol extracts of Paullinia pinnata L. (Sapindaceae) improve inflammation, pain and histological features in CFA-induced mono-arthritis: Evidence from in vivo and in vitro studies. J. Ethnopharmacol. 2019, 236, 183–195. [Google Scholar] [CrossRef]
- Yarwood, A.; Huizinga, T.W.J.; Worthington, J. The genetics of rheumatoid arthritis: Risk and protection in different stages of the evolution of RA: Table 1. Rheumatology 2016, 55, 199–209. [Google Scholar] [CrossRef]
- Zandoná, G.P.; Bagatini, L.; Woloszyn, N.; de Souza Cardoso, J.; Hoffmann, J.F.; Moroni, L.S.; Stefanello, F.M.; Junges, A.; Rombaldi, C.V. Extraction and characterization of phytochemical compounds from araçazeiro (Psidium cattleianum) leaf: Putative antioxidant and antimicrobial properties. Food Res. Int. 2020, 137, 109573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).