A Review on Revolutionizing Healthcare Technologies with AI and ML Applications in Pharmaceutical Sciences
Abstract
1. Introduction
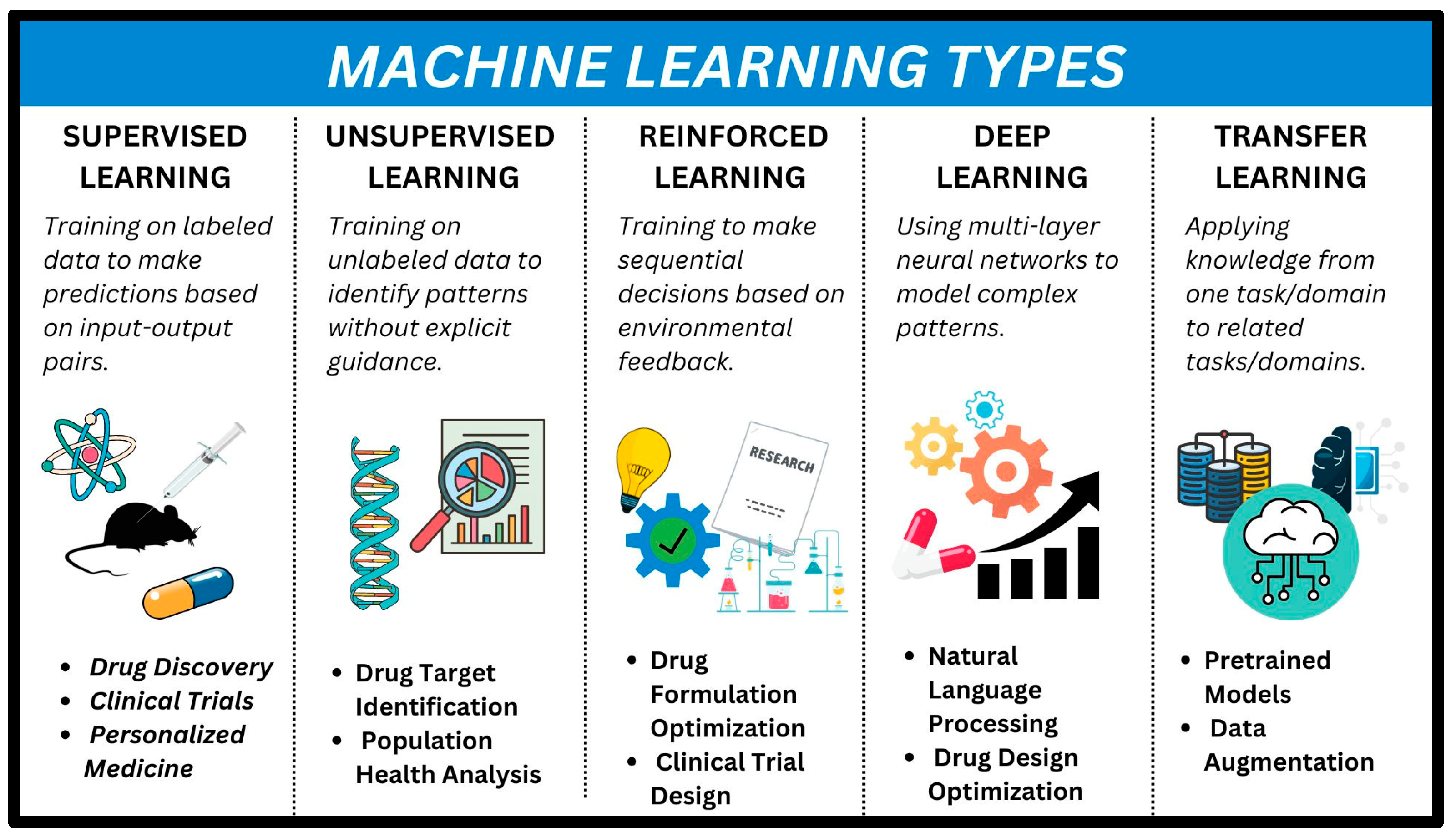
- Supervised Machine Learning: In supervised ML, both the data and the problem is known. When given a set of features (x), we can predict the value of y. It includes both classification (assigning data to categories) and regression (predicting numerical values).
- Unsupervised Machine Learning: The data provided are unlabeled. Clustering groups these data points together (useful for detecting anomalies or creating new categories). Dimension reduction can help in visualize complex datasets [2].
| Parameters | Description | AI/ML Models Used | Working | References |
|---|---|---|---|---|
| Data Collection | Electronic Health Records (EHRs), Genomic Data, Imaging Data, Clinical Data, Lifestyle Data, Environmental Data | N/A | The collection of data from various sources is the first step in precision medicine. These data are used to develop personalized treatment plans for individual patients. | [3,4,5,6] |
| Data Preprocessing | Data Cleaning, Data Integration, Data Transformation, Data Reduction | N/A | The preprocessing of data is an essential step in precision medicine. This involves cleaning and transforming the data to make them suitable for analysis. | [3,4,5,6] |
| Machine Learning Model Selection | Supervised Learning (Classification, Regression), Unsupervised Learning (Clustering), Reinforcement Learning | Random Forest, Support Vector Machine, Neural Networks, Decision Trees, Naive Bayes, K-Nearest Neighbors, Gradient Boosting, Deep Learning, Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN) | The selection of the appropriate machine learning model is crucial in precision medicine. Supervised learning models are used for classification and regression tasks, while unsupervised learning models are used for clustering tasks. Reinforcement learning models are used for decision-making tasks. | [7] |
| Model Training | Model Selection, Model Training, Model Evaluation, Model Optimization | Random Forest, Support Vector Machine, Neural Networks, Decision Trees, Naive Bayes, K-Nearest Neighbors, Gradient Boosting, Deep Learning, Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN) | The training of the machine learning model is an essential step in precision medicine. This involves selecting the appropriate model, training it on the pre-processed data, and evaluating its performance. The model is then optimized to improve its accuracy. | [8] |
| Model Deployment | Model Integration with EHRs, Model Integration with Clinical Workflows, Model Integration with Imaging Systems | Random Forest, Support Vector Machine, Neural Networks, Decision Trees, Naive Bayes, K-Nearest Neighbors, Gradient Boosting, Deep Learning, Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN) | The deployment of the machine learning model is the final step in precision medicine. This involves integrating the model with EHRs, clinical workflows, and imaging systems to make it accessible to healthcare providers. | [9] |
| Model Monitoring and Updating | Model Performance Monitoring, Model Updating with New Data, Model Retraining with New Data | Random Forest, Support Vector Machine, Neural Networks, Decision Trees, Naive Bayes, K-Nearest Neighbors, Gradient Boosting, Deep Learning, Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN) | The monitoring and updating of the machine learning model are essential in precision medicine. This involves monitoring the model’s performance, updating it with new data, and retraining it with new data to ensure that it remains accurate and up-to-date. | [10,11] |
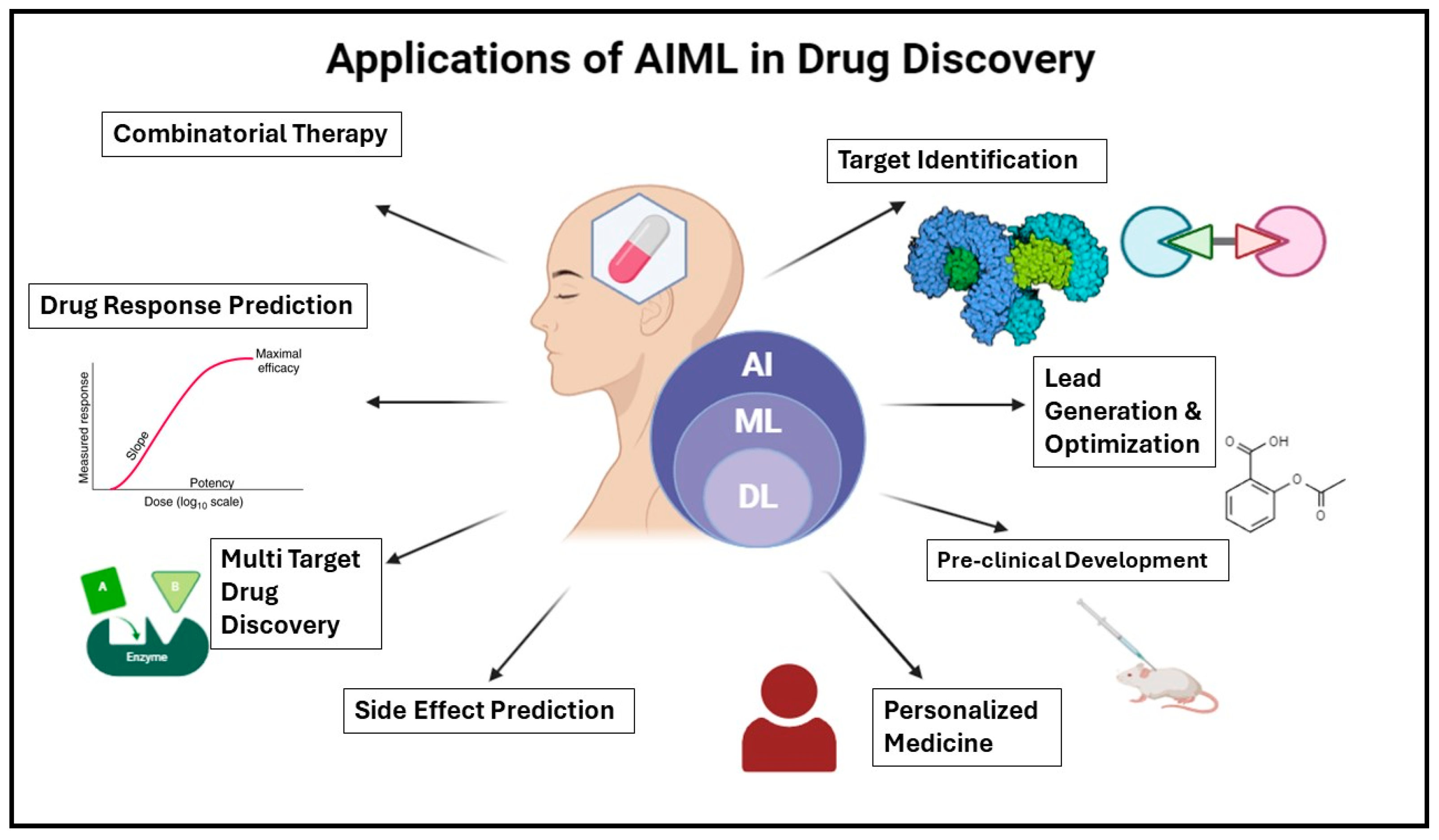
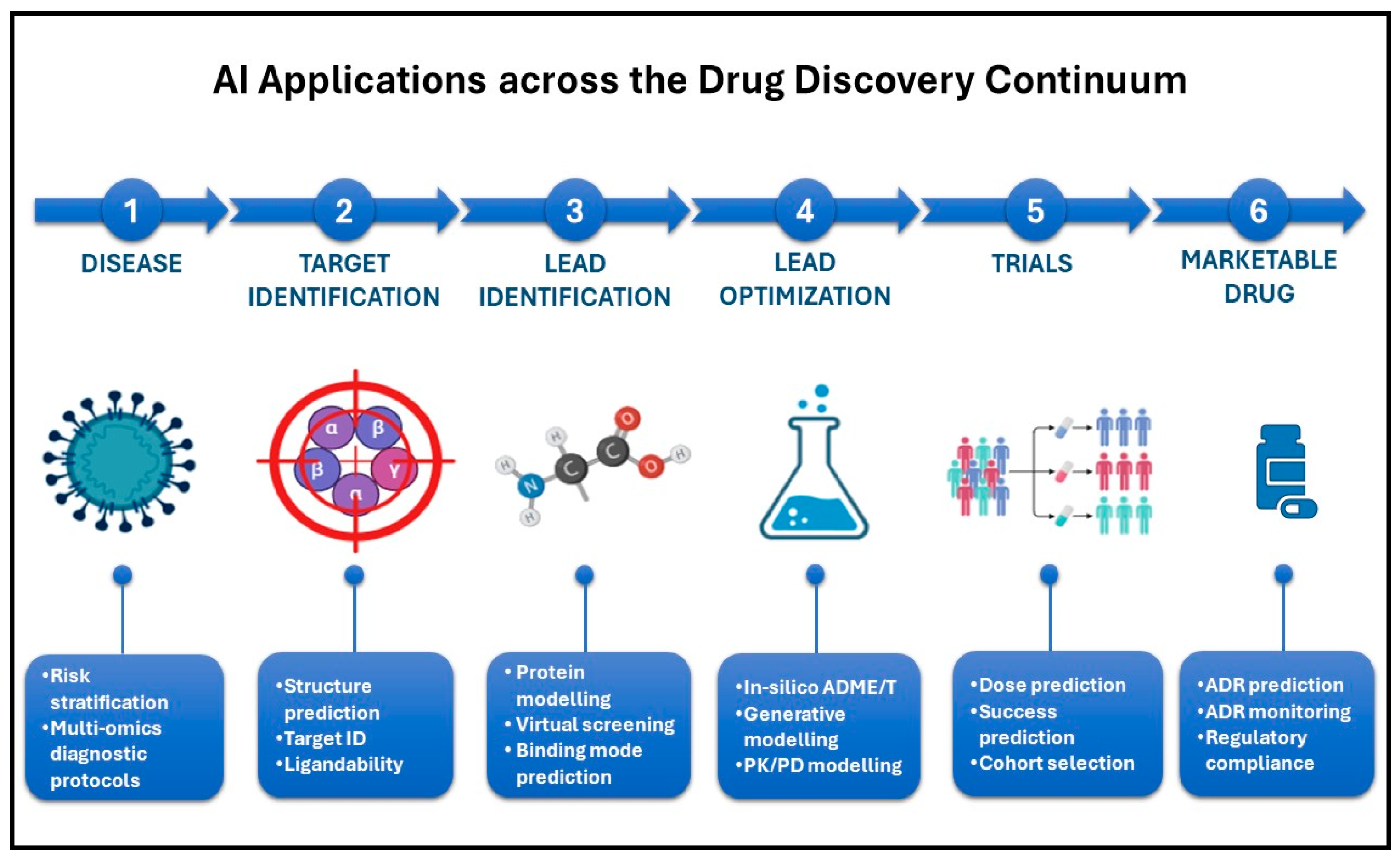
2. Drug Discovery and Development
2.1. Predictive Modeling for Target Identification
2.2. Drug Repurposing Using AI and ML Techniques
2.3. Lead Optimization Through Machine Learning Algorithms
- Structural Alert and Toxicity Analysis: ML can be used to predict the toxicity of a compound based on its structure [42].
- High-Throughput Virtual Screening: ML algorithms can quickly screen large databases of compounds to identify potential leads [43].
- 3D Quantitative Structure–Activity Relationships (QSAR): ML can be used to predict the biological activity of a compound based on its 3D structure [43].
- Multi-Parameter Optimization: ML can optimize multiple parameters simultaneously to find the best lead compounds [41].
- Graph Neural Networks: These can be used to predict the properties of a compound based on its molecular graph [10].
2.4. De Novo Drug Design with Generative Models
3. Cheminformatics and Computational Chemistry
3.1. Virtual Screening of Compound Libraries
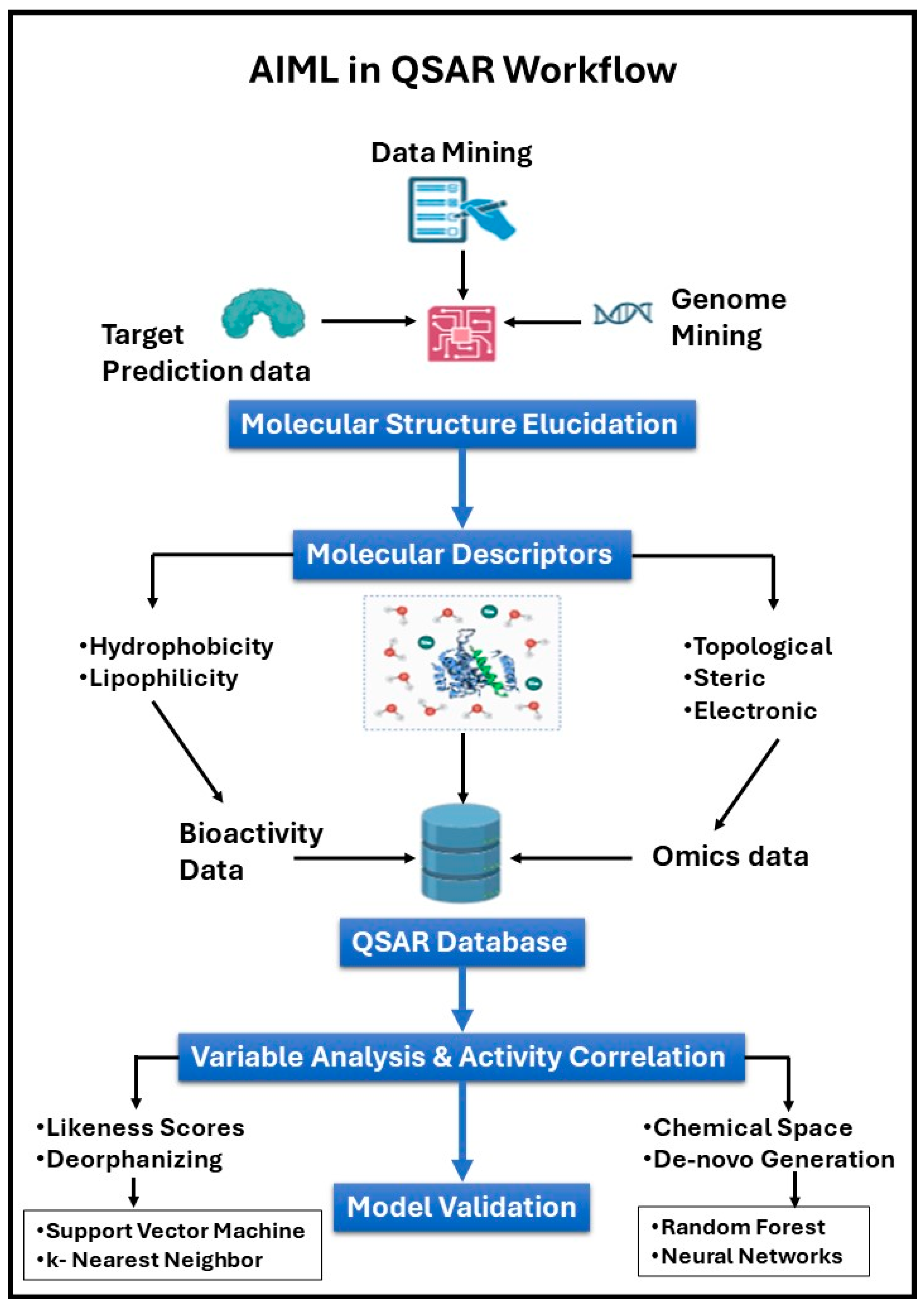
3.2. QSAR Modeling for Predicting Compound Properties
3.3. Molecular Docking and Dynamics Simulations
3.4. Structure-Based Drug Design Aided by AI
4. Clinical Trials Optimization
4.1. Patient Recruitment and Eligibility Assessment Using AI Algorithms
4.2. Predictive Analytics for Trial Outcome Prediction
4.3. Real-Time Monitoring of Patient Data for Safety and Efficacy Analysis
4.4. Personalized Medicine and Treatment Response Prediction
5. Fundamentals of Perturbation-Theory Machine Learning (PTML)
- Multi-Target Learning: Unlike conventional AI models that focus on a single target (e.g., a protein or a specific disease pathway), PTML simultaneously predicts interactions across multiple biological targets, making it more suitable for complex, multi-genetic diseases [83].
- Physicochemical and Structural Interpretability: PTML allows for a deeper understanding of molecular features that contribute to biological activity, reducing the black-box nature of AI models [82].
- Multi-Objective Optimization: Most pharmaceutical applications involve optimizing multiple properties (e.g., efficacy, toxicity, and pharmacokinetics). PTML achieves this by considering multiple endpoints simultaneously [84].
6. Regulatory Compliance and Drug Safety
6.1. AI Applications in Pharmacovigilance for Adverse Event Detection
6.2. Automated Compliance Monitoring and Reporting
6.3. Risk Assessment and Mitigation Strategies Using ML Techniques
6.4. Enhancing Drug Safety Profiles Through AI-Driven Approaches
7. Manufacturing and Supply Chain Management
7.1. Predictive Maintenance of Manufacturing Equipment
7.2. Optimization of Production Processes with Machine Learning
7.3. Demand Forecasting and Inventory Management Using AI
7.4. Supply Chain Optimization for Timely Delivery of Pharmaceutical Products
8. Precision Medicine and Healthcare
8.1. Genomic Data Analysis for Personalized Treatment Strategies
8.2. AI-Driven Diagnostics and Biomarker Discovery
8.3. Drug Response Prediction Based on Patient Genetics and Biomarkers
8.4. Integration of AI and ML in Patient Care for Better Treatment Outcomes
9. Ethical and Regulatory Considerations
9.1. Ethical Implications of AI and ML in Pharmaceutical Research
9.2. Regulatory Challenges and Guidelines for AI-Driven Drug Development
9.3. Ensuring Transparency, Fairness, and Accountability in AI Algorithms
9.4. Addressing Data Privacy Concerns in Healthcare AI Applications
10. Future Perspectives and Challenges
10.1. Emerging Trends in AI and ML for Pharmaceutical Innovation
10.2. Potential Impact of AI on the Future of Drug Discovery and Healthcare
10.3. Addressing Challenges Such as Data Quality, Interpretability, and Scalability
10.4. Collaborative Efforts to Advance AI Technology in the Pharmaceutical Sector
11. Disadvantages of AI Integration in Pharmacy
Effects on the Environment
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Abbreviations
| Sr. No. | Abbreviation | Full Form |
| 1 | AI | Artificial Intelligence |
| 2 | ML | Machine Learning |
| 3 | RWD | Real World Data |
| 4 | NLP | Natural Language Processing |
| 5 | DL | Deep Learning |
| 6 | Ro5 | Rule of Five |
| 7 | GEO | Gene Expression Omnibus |
| 8 | TCGA | The Cancer Genome Atlas |
| 9 | GWAS | Genome-Wide Association Studies |
| 10 | QSAR | Quantitative Structure-Activity Relationship |
| 11 | SMILES | Simplified Molecular Input Line Entry System |
| 12 | L-Net | Ligand Neural Network |
| 13 | DFT | Density Functional Theory |
| 14 | V-SYNTHES | Virtual Synthon Hierarchical Enumeration Screening |
| 15 | ADMET | Absorption Distribution Metabolism Elimination Toxicity |
| 16 | GCNN | Graph Convolutional Neural Network |
| 17 | MRL | Molecular Representation Learning |
| 18 | CP | Conformal Prediction |
| 19 | AC | Activity Cliff |
| 20 | ROC | Receiver Operating Characteristic |
| 21 | MDS | Molecular Dynamics Simulation |
| 22 | ACE | Angiotensin Converting Enzyme |
| 23 | mPro | Main Protease |
| 24 | AIDD | Artificial Intelligence-Driven Drug Design |
| 25 | HINT | Hierarchical Interaction Network |
| 26 | SPOT | Sequential Prediction Modeling of Clinical Trial Outcome |
| 27 | RBM | Risk-Based Management |
| 28 | ADE | Adverse Drug Event |
| 29 | ADR | Adverse Drug Reaction |
| 30 | EHR | Electronic Health Record |
| 31 | FDA | Food and Drug Administration |
| 32 | PTML | Perturbation-Theory Machine Learning |
References
- Olczak, J.; Pavlopoulos, J.; Prijs, J.; Ijpma, F.F.A.; Doornberg, J.N.; Lundström, C.; Hedlund, J.; Gordon, M. Presenting artificial intelligence, deep learning, and machine learning studies to clinicians and healthcare stakeholders: An introductory reference with a guideline and a Clinical AI Research (CAIR) checklist proposal. Acta Orthop. 2021, 92, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Gkouvas, N.; Gkouvas, N. Precision Medicine & Pharmacogenomics: Personalized Medication in Neuropsychiatric Disorders using AI and telepsychiatry. Eur. Psychiatry 2022, 65, S678. [Google Scholar] [CrossRef]
- Bello, B.K.; Bundey, Y.; Bhave, R.; Khotimchenko, M.; Baran, S.W.; Chakravarty, K.; Varshney, J. Integrating AI/ML Models for Patient Stratification Leveraging Omics Dataset and Clinical Biomarkers from COVID-19 Patients: A Promising Approach to Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6250. [Google Scholar] [CrossRef]
- Andrews, S.M. Emerging Role of Artificial Intelligence and Machine learning in precision medicine. Int. J. Eng. Technol. Manag. Sci. 2023, 7, 622–626. [Google Scholar] [CrossRef]
- Sanchez, P.; Sánchez, P.A.; Voisey, J.P.; Voisey, J.P.; Xia, T.; Xia, T.; Watson, H.I.; Watson, H.; O’Neil, A.Q.; O’Neil, A.Q.; et al. Causal machine learning for healthcare and precision medicine. R. Soc. Open Sci. 2022, 9, 220638. [Google Scholar] [CrossRef]
- Xu, Z.; Biswas, B.; Liu, L.; Amzal, B. AI/ML in Precision Medicine: A Look Beyond the Hype. Ther. Innov. Regul. Sci. 2023. [Google Scholar] [CrossRef]
- Han, Y.; Tao, J. Revolutionizing Pharma: Unveiling the AI and LLM Trends in the Pharmaceutical Industry. arXiv 2024, arXiv:2401.10273. [Google Scholar] [CrossRef]
- Terranova, N.; Renard, D.; Shahin, M.H.; Menon, S.; Cao, Y.; Hop, C.E.C.A.; Hayes, S.T.; Madrasi, K.; Stodtmann, S.; Tensfeldt, T.G.; et al. Artificial Intelligence for Quantitative Modeling in Drug Discovery and Development: An Innovation & Quality (IQ) Consortium Perspective on Use Cases and Best Practices. Clin. Pharmacol. Ther. 2023, 115, 658–672. [Google Scholar] [CrossRef]
- Knutson, C.; Bontha, M.; Bilbrey, J.A.; Kumar, N. Decoding the protein–ligand interactions using parallel graph neural networks. Sci. Rep. 2022, 12, 7624. [Google Scholar] [CrossRef]
- Shahzad, M.; Tahir, M.A.; Alhussein, M.; Mobin, A.; Malick, R.A.S.; Anwar, M.S. NeuPD—A Neural Network-Based Approach to Predict Antineoplastic Drug Response. Diagnostics 2023, 13, 2043. [Google Scholar] [CrossRef]
- Kolluri, S.; Lin, J.; Liu, R.; Zhang, Y.; Zhang, W. Machine Learning and Artificial Intelligence in Pharmaceutical Research and Development: A Review. AAPS J. 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Yelne, S.; Chaudhary, M.; Dod, K.; Sayyad, A.; Sharma, R. Harnessing the Power of AI: A Comprehensive Review of Its Impact and Challenges in Nursing Science and Healthcare. Cureus 2023, 15, e49252. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, L.; Du, Z.; Yan, Z.; Wang, W. Mechanism Design and Optimization of a Haptic Master Manipulator for Laparoscopic Surgical Robots. IEEE Access 2019, 7, 147808–147824. [Google Scholar] [CrossRef]
- Kompa, B.; Hakim, J.B.; Palepu, A.; Kompa, K.G.; Smith, M.; Bain, P.A.; Woloszynek, S.; Painter, J.L.; Bate, A.; Beam, A.L. Artificial Intelligence Based on Machine Learning in Pharmacovigilance: A Scoping Review. Drug Saf. 2022, 45, 477–491. [Google Scholar] [CrossRef]
- FDA. Artificial Intelligence and Machine Learning (AI/ML) for Drug Development|FDA. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/artificial-intelligence-and-machine-learning-aiml-drug-development (accessed on 4 January 2025).
- Bender, A.; Cortes-Ciriano, I. Artificial intelligence in drug discovery: What is realistic, what are illusions? Part 2: A discussion of chemical and biological data. Drug Discov. Today 2021, 26, 1040–1052. [Google Scholar] [CrossRef]
- Sarkar, C.; Das, B.; Rawat, V.S.; Wahlang, J.B.; Nongpiur, A.; Tiewsoh, I.; Lyngdoh, N.M.; Das, D.; Bidarolli, M.; Sony, H.T. Artificial Intelligence and Machine Learning Technology Driven Modern Drug Discovery and Development. Int. J. Mol. Sci. 2023, 24, 2026. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Kalyane, D.; Sanap, G.; Paul, D.; Shenoy, S.; Anup, N.; Polaka, S.; Tambe, V.; Tekade, R.K. Artificial intelligence in the pharmaceutical sector: Current scene and future prospect. In The Future of Pharmaceutical Product Development and Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–107. ISBN 978-0-12-814455-8. [Google Scholar]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, S.; Schlessinger, A.; Wacker, D.; Kaniskan, H.Ü.; Jin, J.; Zhou, M.; Zhang, B. Artificial intelligence and machine learning-aided drug discovery in central nervous system diseases: State-of-the-arts and future directions. Med. Res. Rev. 2021, 41, 1427–1473. [Google Scholar] [CrossRef]
- Athar, M.; Lone, M.Y.; Jha, P.C. First protein drug target’s appraisal of lead-likeness descriptors to unfold the intervening chemical space. J. Mol. Graph. Model. 2017, 72, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lalhmangaihzuala, S.; Vanlaldinpuia, K.; Khiangte, V.; Laldinpuii, Z.; Liana, T.; Lalhriatpuia, C.; Pachuau, Z. Therapeutic applications of carbohydrate-based compounds: A sweet solution for medical advancement. Mol. Divers. 2024, 28, 4553–4579. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Aponick, A.; Zimmermann, E.M.; Dahan, A. Lipids and Lipid-Processing Pathways in Drug Delivery and Therapeutics. Int. J. Mol. Sci. 2020, 21, 3248. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Ren, G.; Honda, H.; Davis, K.L. Artificial Intelligence-Based Drug Design and Discovery. In Cheminformatics and Its Applications; Stefaniu, A., Rasul, A., Hussain, G., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-067-3. [Google Scholar]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Lau, A.; So, H.-C. Turning genome-wide association study findings into opportunities for drug repositioning. Comput. Struct. Biotechnol. J. 2020, 18, 1639–1650. [Google Scholar] [CrossRef]
- Valentini, G.; Paccanaro, A.; Caniza, H.; Romero, A.E.; Re, M. An extensive analysis of disease-gene associations using network integration and fast kernel-based gene prioritization methods. Artif. Intell. Med. 2014, 61, 63–78. [Google Scholar] [CrossRef]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef]
- Desai, D.; Kantliwala, S.V.; Vybhavi, J.; Ravi, R.; Patel, H.; Patel, J. Review of AlphaFold 3: Transformative Advances in Drug Design and Therapeutics. Cureus 2024, 16, e63646. [Google Scholar] [CrossRef] [PubMed]
- Kamya, P.; Ozerov, I.V.; Pun, F.W.; Tretina, K.; Fokina, T.; Chen, S.; Naumov, V.; Long, X.; Lin, S.; Korzinkin, M.; et al. PandaOmics: An AI-Driven Platform for Therapeutic Target and Biomarker Discovery. J. Chem. Inf. Model. 2024, 64, 3961–3969. [Google Scholar] [CrossRef]
- Chen, Y.; Elenee Argentinis, J.; Weber, G. IBM Watson: How Cognitive Computing Can Be Applied to Big Data Challenges in Life Sciences Research. Clin. Ther. 2016, 38, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Makhouri, F.R.; Ghasemi, J.B. Combating Diseases with Computational Strategies Used for Drug Design and Discovery. Curr. Top. Med. Chem. 2019, 18, 2743–2773. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, S.; Lu, W.; Liu, Z.; Huang, J.; Zhou, Y.; Fang, J.; Huang, Y.; Guo, H.; Li, L.; et al. Target identification among known drugs by deep learning from heterogeneous networks. Chem. Sci. 2020, 11, 1775–1797. [Google Scholar] [CrossRef]
- Varikoti, R.A.; Schultz, K.J.; Kombala, C.J.; Kruel, A.; Brandvold, K.R.; Zhou, M.; Kumar, N. Integrated data-driven and experimental approaches to accelerate lead optimization targeting SARS-CoV-2 main protease. J. Comput. Aided Mol. Des. 2023, 37, 339–355. [Google Scholar] [CrossRef]
- Joshi, R.P.; Kumar, N. Artificial Intelligence for Autonomous Molecular Design: A Perspective. Molecules 2021, 26, 6761. [Google Scholar] [CrossRef]
- Arul Murugan, N.; Ruba Priya, G.; Narahari Sastry, G.; Markidis, S. Artificial intelligence in virtual screening: Models versus experiments. Drug Discov. Today 2022, 27, 1913–1923. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, Z. Multi-objective de novo drug design with conditional graph generative model. J. Cheminform. 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Xie, X.-Q. Generative chemistry: Drug discovery with deep learning generative models. J. Mol. Model. 2021, 27, 71. [Google Scholar] [CrossRef]
- Li, Y.; Pei, J.; Lai, L. Structure-based de novo drug design using 3D deep generative models. Chem. Sci. 2021, 12, 13664–13675. [Google Scholar] [CrossRef] [PubMed]
- Atance, S.R.; Diez, J.V.; Engkvist, O.; Olsson, S.; Mercado, R. De Novo Drug Design Using Reinforcement Learning with Graph-Based Deep Generative Models. J. Chem. Inf. Model. 2022, 62, 4863–4872. [Google Scholar] [CrossRef]
- Keith, J.A.; Vassilev-Galindo, V.; Cheng, B.; Chmiela, S.; Gastegger, M.; Müller, K.-R.; Tkatchenko, A. Combining Machine Learning and Computational Chemistry for Predictive Insights Into Chemical Systems. Chem. Rev. 2021, 121, 9816–9872. [Google Scholar] [CrossRef]
- Shi, Y.-F.; Yang, Z.-X.; Ma, S.; Kang, P.-L.; Shang, C.; Hu, P.; Liu, Z.-P. Machine Learning for Chemistry: Basics and Applications. Engineering 2023, 27, 70–83. [Google Scholar] [CrossRef]
- Glen, R.C. Computational chemistry and cheminformatics: An essay on the future. J. Comput. Aided Mol. Des. 2012, 26, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Gryniukova, A.; Kaiser, F.; Myziuk, I.; Alieksieieva, D.; Leberecht, C.; Heym, P.P.; Tarkhanova, O.O.; Moroz, Y.S.; Borysko, P.; Haupt, V.J. AI-Powered Virtual Screening of Large Compound Libraries Leads to the Discovery of Novel Inhibitors of Sirtuin-1. J. Med. Chem. 2023, 66, 10241–10251. [Google Scholar] [CrossRef]
- Sotriffer, C. (Ed.) Virtual Screening: Principles, Challenges, and Practical Guidelines, 1st ed.; Methods and Principles in Medicinal Chemistry; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-3-527-32636-5. [Google Scholar]
- Murugan, N.A.; Podobas, A.; Gadioli, D.; Vitali, E.; Palermo, G.; Markidis, S. A Review on Parallel Virtual Screening Softwares for High-Performance Computers. Pharmaceuticals 2022, 15, 63. [Google Scholar] [CrossRef]
- Lavecchia, A.; Giovanni, C. Virtual Screening Strategies in Drug Discovery: A Critical Review. Curr. Med. Chem. 2013, 20, 2839–2860. [Google Scholar] [CrossRef]
- Deane, C.; Mokaya, M. A virtual drug-screening approach to conquer huge chemical libraries. Nature 2022, 601, 322–323. [Google Scholar] [CrossRef]
- Jung, S.; Vatheuer, H.; Czodrowski, P. VSFlow: An open-source ligand-based virtual screening tool. J. Cheminform. 2023, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Rusnac, D.-V.; Park, H.; Canzani, D.; Nguyen, H.M.; Stewart, L.; Bush, M.F.; Nguyen, P.T.; Wulff, H.; Yarov-Yarovoy, V.; et al. An artificial intelligence accelerated virtual screening platform for drug discovery. Nat. Commun. 2024, 15, 7761. [Google Scholar] [CrossRef] [PubMed]
- Buvailo, A. AI Takes on DNA-encoded Chemical Libraries. Available online: https://enamine.net/blog/ai-takes-on-dna-encoded-chemical-libraries (accessed on 4 January 2025).
- Golbraikh, A.; Wang, X.S.; Zhu, H.; Tropsha, A. Predictive QSAR Modeling: Methods and Applications in Drug Discovery and Chemical Risk Assessment. In Handbook of Computational Chemistry; Leszczynski, J., Kaczmarek-Kedziera, A., Puzyn, T.G., Papadopoulos, M., Reis, H., Shukla, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2303–2340. ISBN 978-3-319-27281-8. [Google Scholar]
- Xu, Y.; Liaw, A.; Sheridan, R.P.; Svetnik, V. Development and Evaluation of Conformal Prediction Methods for QSAR. arXiv 2023, arXiv:2304.00970. [Google Scholar]
- Kwon, S.; Bae, H.; Jo, J.; Yoon, S. Comprehensive ensemble in QSAR prediction for drug discovery. BMC Bioinform. 2019, 20, 521. [Google Scholar] [CrossRef]
- Gao, Z.; Ji, X.; Zhao, G.; Wang, H.; Zheng, H.; Ke, G.; Zhang, L. Uni-QSAR: An Auto-ML Tool for Molecular Property Prediction. arXiv 2023, arXiv:2304.12239. [Google Scholar]
- Ambure, P.; Halder, A.K.; González Díaz, H.; Cordeiro, M.N.D.S. QSAR-Co: An Open Source Software for Developing Robust Multitasking or Multitarget Classification-Based QSAR Models. J. Chem. Inf. Model. 2019, 59, 2538–2544. [Google Scholar] [CrossRef]
- Vukovic, K.; Gadaleta, D.; Benfenati, E. Methodology of aiQSAR: A group-specific approach to QSAR modelling. J. Cheminform. 2019, 11, 27. [Google Scholar] [CrossRef]
- Dablander, M.; Hanser, T.; Lambiotte, R.; Morris, G.M. Exploring QSAR models for activity-cliff prediction. J. Cheminform. 2023, 15, 47. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef]
- Crampon, K.; Giorkallos, A.; Deldossi, M.; Baud, S.; Steffenel, L.A. Machine-learning methods for ligand–protein molecular docking. Drug Discov. Today 2022, 27, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of Molecular Docking Analysis and Molecular Dynamics Simulations for Studying Food Proteins and Bioactive Peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef]
- Niazi, S. The Coming of Age of AI/ML in Drug Discovery, Development, Clinical Testing, and Manufacturing: The FDA Perspectives. Drug Des. Devel. Ther. 2023, 17, 2691–2725. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, H.; Hu, J.; Hakami, M.A.; Hazazi, A.; Alamri, M.A.; Alkhatabi, H.A.; Mahmood, A.; Alotaibi, B.S.; Wadood, A.; Huang, X. Identification of novel STAT3 inhibitors for liver fibrosis, using pharmacophore-based virtual screening, molecular docking, and biomolecular dynamics simulations. Sci. Rep. 2023, 13, 20147. [Google Scholar] [CrossRef]
- Mustali, J.; Yasuda, I.; Hirano, Y.; Yasuoka, K.; Gautieri, A.; Arai, N. Unsupervised deep learning for molecular dynamics simulations: A novel analysis of protein–ligand interactions in SARS-CoV-2 M pro. RSC Adv. 2023, 13, 34249–34261. [Google Scholar] [CrossRef]
- Zhang, Y. An In-depth Summary of Recent Artificial Intelligence Applications in Drug Design. arXiv 2021, arXiv:2110.05478. [Google Scholar]
- Jones, J.; Clark, R.D.; Lawless, M.S.; Miller, D.W.; Waldman, M. The AI-driven Drug Design (AIDD) platform: An interactive multi-parameter optimization system integrating molecular evolution with physiologically based pharmacokinetic simulations. J. Comput. Aided Mol. Des. 2024, 38, 14. [Google Scholar] [CrossRef]
- Batool, M.; Ahmad, B.; Choi, S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019, 20, 2783. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, S.; Tian, Y.; Xu, T.; Banegas-Luna, A.J.; Pérez-Sánchez, H.; Huang, J.; Liu, H.; Yao, X. Application advances of deep learning methods for de novo drug design and molecular dynamics simulation. WIREs Comput. Mol. Sci. 2022, 12, e1581. [Google Scholar] [CrossRef]
- Ismail, A.; Al-Zoubi, T.; El Naqa, I.; Saeed, H. The role of artificial intelligence in hastening time to recruitment in clinical trials. BJR|Open 2023, 5, 20220023. [Google Scholar] [CrossRef]
- Hutson, M. How AI is being used to accelerate clinical trials. Nature 2024, 627, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Askin, S.; Burkhalter, D.; Calado, G.; El Dakrouni, S. Artificial Intelligence Applied to clinical trials: Opportunities and challenges. Health Technol. 2023, 13, 203–213. [Google Scholar] [CrossRef]
- Fu, T.; Huang, K.; Xiao, C.; Glass, L.M.; Sun, J. HINT: Hierarchical interaction network for clinical-trial-outcome predictions. Patterns 2022, 3, 100445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.B.; Wei, W.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. ISBN 978-0-12-818438-7. [Google Scholar]
- Herrera-Ibatá, D.M. Machine Learning and Perturbation Theory Machine Learning (PTML) in Medicinal Chemistry, Biotechnology, and Nanotechnology. Curr. Top. Med. Chem. 2021, 21, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Kleandrova, V.V.; Scotti, M.T.; Scotti, L.; Speck-Planche, A. Multi-target Drug Discovery via PTML Modeling: Applications to the Design of Virtual Dual Inhibitors of CDK4 and HER2. Curr. Top. Med. Chem. 2021, 21, 661–675. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Kleandrova, V.V. Multi-Condition QSAR Model for the Virtual Design of Chemicals with Dual Pan-Antiviral and Anti-Cytokine Storm Profiles. ACS Omega 2022, 7, 32119–32130. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Cordeiro, M.N.D.; Speck-Planche, A. Perturbation Theory Machine Learning Model for Phenotypic Early Antineoplastic Drug Discovery: Design of Virtual Anti-Lung-Cancer Agents. Appl. Sci. 2024, 14, 9344. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Speck-Planche, A. PTML Modeling for Pancreatic Cancer Research: In Silico Design of Simultaneous Multi-Protein and Multi-Cell Inhibitors. Biomedicines 2022, 10, 491. [Google Scholar] [CrossRef]
- Cabrera-Andrade, A.; López-Cortés, A.; Munteanu, C.R.; Pazos, A.; Pérez-Castillo, Y.; Tejera, E.; Arrasate, S.; Gonzalez-Diaz, H. Perturbation-Theory Machine Learning (PTML) Multilabel Model of the ChEMBL Dataset of Preclinical Assays for Antisarcoma Compounds. ACS Omega 2020, 5, 27211–27220. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Scotti, M.T.; Scotti, L.; Nayarisseri, A.; Speck-Planche, A. Cell-based multi-target QSAR model for design of virtual versatile inhibitors of liver cancer cell lines. Sar Qsar Environ. Res. 2020, 31, 815–836. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Cordeiro, M.N.D.; Speck-Planche, A. In Silico Approach for Antibacterial Discovery: PTML Modeling of Virtual Multi-Strain Inhibitors Against Staphylococcus aureus. Pharmaceuticals 2025, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-López, Y.; Ruiz-Escudero, A.; Arrasate, S.; González-Díaz, H. Implementation of IFPTML Computational Models in Drug Discovery Against Flaviviridae Family. J. Chem. Inf. Model. 2024, 64, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Kleandrova, V.V.; Scotti, M.T.; Speck-Planche, A. Computational Drug Repurposing for Antituberculosis Therapy: Discovery of Multi-Strain Inhibitors. Antibiotics 2021, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Ortega-Tenezaca, B.; Barbolla, I.; Fundora-Ortiz, B.; Arrasate, S.; Dea-Ayuela, M.A.; González-Díaz, H.; Sotomayor, N.; Lete, E. Prediction of Antileishmanial Compounds: General Model, Preparation, and Evaluation of 2-Acylpyrrole Derivatives. J. Chem. Inf. Model. 2022, 62, 3928–3940. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Scotti, L.; Bezerra Mendonça, F.J., Jr.; Muratov, E.; Scotti, M.T.; Speck-Planche, A. QSAR Modeling for Multi-Target Drug Discovery: Designing Simultaneous Inhibitors of Proteins in Diverse Pathogenic Parasites. Front. Chem. 2021, 9, 634663. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Kleandrova, V.V. Chapter 16: Demystifying Artificial Neural Networks as Generators of New Chemical Knowledge: Antimalarial Drug Discovery as a Case Study. In Machine Learning in Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2020; pp. 398–423. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Cordeiro, M.N.D.; Speck-Planche, A. Perturbation-theory machine learning for mood disorders: Virtual design of dual inhibitors of NET and SERT proteins. BMC Chem. 2025, 19, 2. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Kleandrova, V.V.; Scotti, M.T. In Silico Drug Repurposing for Anti-Inflammatory Therapy: Virtual Search for Dual Inhibitors of Caspase-1 and TNF-Alpha. Biomolecules 2021, 11, 1832. [Google Scholar] [CrossRef]
- Kleandrova, V.V.; Speck-Planche, A. PTML Modeling for Alzheimer’s Disease: Design and Prediction of Virtual Multi-Target Inhibitors of GSK3B, HDAC1, and HDAC6. Curr. Top. Med. Chem. 2020, 20, 1661–1676. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Scotti, M.T. BET bromodomain inhibitors: Fragment-based in silico design using multi-target QSAR models. Mol. Divers. 2019, 23, 555–572. [Google Scholar] [CrossRef]
- Salas, M.; Petracek, J.; Yalamanchili, P.; Aimer, O.; Kasthuril, D.; Dhingra, S.; Junaid, T.; Bostic, T. The Use of Artificial Intelligence in Pharmacovigilance: A Systematic Review of the Literature. Pharm. Med. 2022, 36, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Huysentruyt, K.; Kjoersvik, O.; Dobracki, P.; Savage, E.; Mishalov, E.; Cherry, M.; Leonard, E.; Taylor, R.; Patel, B.; Abatemarco, D. Validating Intelligent Automation Systems in Pharmacovigilance: Insights from Good Manufacturing Practices. Drug Saf. 2021, 44, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C. Governing the safety of artificial intelligence in healthcare. BMJ Qual. Saf. 2019, 28, 495–498. [Google Scholar] [CrossRef]
- Basile, A.O.; Yahi, A.; Tatonetti, N.P. Artificial Intelligence for Drug Toxicity and Safety. Trends Pharmacol. Sci. 2019, 40, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.W.; Levine, D.; Syrowatka, A.; Kuznetsova, M.; Craig, K.J.T.; Rui, A.; Jackson, G.P.; Rhee, K. The potential of artificial intelligence to improve patient safety: A scoping review. Npj Digit. Med. 2021, 4, 54. [Google Scholar] [CrossRef]
- Shamayleh, A.; Shamayleh, A.; Awad, M.; Awad, M.; Farhat, J.; Farhat, J.; Farhat, J. IoT Based Predictive Maintenance Management of Medical Equipment. J. Med. Syst. 2020, 44, 72. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Krishnamurthy, R.; Mummudi, N.; Mummudi, N.; Goda, J.S.; Goda, J.S.; Chopra, S.; Chopra, S.; Heijmen, B.; Heijmen, B.; et al. Using Artificial Intelligence for Optimization of the Processes and Resource Utilization in Radiotherapy. JCO Glob. Oncol. 2022, 8, e2100393. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, H.; Han, X.; Yao, F. Vaccine supply chain coordination using blockchain and artificial intelligence technologies. Comput. Ind. Eng. 2023, 175, 108885. [Google Scholar] [CrossRef]
- Duarte, J.M.; Veiga, F.; Mascarenhas-Melo, F. Exploiting Pharma 4.0 Technologies in the Non-Biological Complex Drugs Manufacturing: Innovations and Implications. Pharmaceutics 2023, 15, 2545. [Google Scholar] [CrossRef]
- Abisha, D.; Varshini, S.; Thejaswini, A.; Sineka, P. Blockchain-Enabled Pharmacy Supply Chain Management: Ensuring Transparency and Efficiency. In Proceedings of the 2023 International Conference on Sustainable Communication Networks and Application (ICSCNA), Theni, India, 15–17 November 2023. [Google Scholar] [CrossRef]
- Santosh, K.C.; Gaur, L. AI in Precision Medicine. Artif. Intell. Mach. Learn. Public Healthc. 2021, 41–47. [Google Scholar] [CrossRef]
- DeGroat, W.; Abdelhalim, H.; Patel, K.; Mendhe, D.; Zeeshan, S.; Ahmed, Z. Discovering biomarkers associated and predicting cardiovascular disease with high accuracy using a novel nexus of machine learning techniques for precision medicine. Sci. Rep. 2024, 14, 1. [Google Scholar] [CrossRef]
- Restrepo, J.C.; Dueñas, D.; Corredor, Z.; Liscano, Y. Advances in Genomic Data and Biomarkers: Revolutionizing NSCLC Diagnosis and Treatment. Cancers 2023, 15, 3474. [Google Scholar] [CrossRef] [PubMed]
- Terranova, N.; Venkatakrishnan, K. Machine Learning in Modeling Disease Trajectory and Treatment Outcomes: An Emerging Enabler for Model-Informed Precision Medicine. Clin. Pharmacol. Ther. 2024, 115, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Napitupulu, P.A. Ethical Dilemmas in the Use of Artificial Intelligence in Breast Cancer Diagnosis and Treatment (Addressing Issues of Bias, Transferability, and Patient Trust in Breast Cancer AI). West Sci. Law Hum. Rights 2023, 1, 4. [Google Scholar] [CrossRef]
- Sharma, A.K. A study on the applicability of AI in Pharmaceutical Industry. In Proceedings of the 2022 1st International Conference on Computational Science and Technology (ICCST), Chennai, India, 9–10 November 2022. [Google Scholar] [CrossRef]
- Nagaprasad, S.; Padmaja, D.L.; Qureshi, Y.; Bangare, S.L.; Mishra, M.; Mazumdar, B.D. Investigating the Impact of Machine Learning in Pharmaceutical Industry. J. Pharm. Res. 2021, 33, 6–14. [Google Scholar] [CrossRef]
- Pedraza, D.H.; de la Cruz, M.L.; Nettleton, D.F.; Solà, P.A. Integrating Artificial Intelligence and Big Data in Pharmaceutical Development: Ethical Considerations and Legislative Frameworks. Available online: https://www.eternalproject.eu/downloads/publications/ai_and_big_data_in_pharma_dev.pdf (accessed on 3 February 2025).
- Lekadir, K.; Frangi, A.F.; Porras, A.R.; Glocker, B.; Cintas, C.; Langlotz, C.P.; Weicken, E.; Asselbergs, F.W.; Prior, F.; Collins, G.S.; et al. FUTURE-AI: International consensus guideline for trustworthy and deployable artificial intelligence in healthcare. arXiv 2023, arXiv:2309.12325. [Google Scholar] [CrossRef]
- Pashkov, V.; Soloviov, O. Legal implementation of blockchain technology in pharmacy. SHS Web Conf. 2019, 68, 01027. [Google Scholar] [CrossRef]
- Beauxis-Aussalet, E.; Behrisch, M.; Borgo, R.; Chau, D.H.; Collins, C.; Ebert, D.; El-Assady, M.; Endert, A.; Keim, D.A.; Kohlhammer, J.; et al. The Role of Interactive Visualization in Fostering Trust in AI. IEEE Comput. Graph. Appl. 2021, 41, 7–12. [Google Scholar] [CrossRef]
- Busuioc, M. Accountable Artificial Intelligence: Holding Algorithms to Account. Public Adm. Rev. 2020, 81, 825–836. [Google Scholar] [CrossRef]
- Saeed, G.; Kohler, J.C.; Cuomo, R.E.; Mackey, T.K. A systematic review of digital technology and innovation and its potential to address anti-corruption, transparency, and accountability in the pharmaceutical supply chain. Expert Opin. Drug Saf. 2022, 21, 1061–1088. [Google Scholar] [CrossRef]
- Frasca, M.; La Torre, D.; Pravettoni, G.; Cutica, I. Explainable and interpretable artificial intelligence in medicine: A systematic bibliometric review. Discov. Artif. Intell. 2024, 4, 15. [Google Scholar] [CrossRef]
- Goswami, R.; Yadav, S.; Kumar, V. Explainable AI in healthcare: A theoretical overview of interpretable models for medical diagnosis. Pharma Innov. 2019, 8, 29–33. [Google Scholar] [CrossRef]
- Sandeep Reddy Explainability and artificial intelligence in medicine. Lancet Digit. Health 2022, 4, e214–e215. [CrossRef] [PubMed]
- Bhosale, A.; Sawant, A.; Kamble, T.; Chougule, N. Exploring the Role of Artificial Intelligence in Modernizing Quality Assurance and Quality Control in the Pharmaceutical Sector. Int. J. Pharm. Sci. 2024, 2, 522–529. [Google Scholar] [CrossRef]
- Singh, D.; Chaddah, J.K. A Study On Application of Blockchain Technology to Control Counterfeit Drugs, Enhance Data Privacy and Improve Distribution in Online Pharmacy. Asia Pac. J. Health Manag. 2021, 16, 59–66. [Google Scholar] [CrossRef]
- Mahesh, A.B.; Reche, A. A New Era of Dental Care: Harnessing Artificial Intelligence for Better Diagnosis and Treatment. Cureus 2023, 15, e49319. [Google Scholar] [CrossRef]
- Danishuddin; Jamal, M.S.; Song, K.-S.; Lee, K.-W.; Kim, J.-J.; Park, Y.-M. Revolutionizing Drug Targeting Strategies: Integrating Artificial Intelligence and Structure-Based Methods in PROTAC Development. Pharmaceuticals 2023, 16, 1649. [Google Scholar] [CrossRef]
- Frascarelli, C.; Bonizzi, G.; Musico, C.R.; Mane, E.; Cassi, C.; Guerini-Rocco, E.; Farina, A.; Scarpa, A.; Lawlor, R.T.; Bonetti, L.R.; et al. Revolutionizing Cancer Research: The Impact of Artificial Intelligence in Digital Biobanking. J. Pers. Med. 2023, 13, 1390. [Google Scholar] [CrossRef]
- McComb, M.; McComb, M.; Bies, R.R.; Bies, R.R.; Ramanathan, M.; Ramanathan, M. Machine learning in pharmacometrics: Opportunities and challenges. Br. J. Clin. Pharmacol. 2021, 88, 1482–1499. [Google Scholar] [CrossRef]
- Dave, M.; Patel, N. Artificial intelligence in healthcare and education. Br. Dent. J. 2023, 234, 761–764. [Google Scholar] [CrossRef]
- Alshuhri, M.S.; Al-Musawi, S.G.; Al-Alwany, A.A.; Uinarni, H.; Rasulova, I.; Rodrigues, P.; Alkhafaji, A.T.; Alshanberi, A.M.; Alawadi, A.H.; Abbas, A.H. Artificial intelligence in cancer diagnosis: Opportunities and challenges. Pathol. Res. Pract. 2024, 253, 154996. [Google Scholar] [CrossRef] [PubMed]
- Jahn, S.W.; Plass, M.; Moinfar, F. Digital Pathology: Advantages, Limitations and Emerging Perspectives. J. Clin. Med. 2020, 9, 3697. [Google Scholar] [CrossRef]
- Chen, P.; Gao, J.; Ji, Z.; Liang, H.; Peng, Y. Do Artificial Intelligence Applications Affect Carbon Emission Performance?—Evidence from Panel Data Analysis of Chinese Cities. Energies 2022, 15, 5730. [Google Scholar] [CrossRef]
- Farchi, F.; Farchi, C.; Touzi, B.; Mabrouki, C. A Comparative Study on AI-Based Algorithms for Cost Prediction in Pharmaceutical Transport Logistics. Acadlore Trans. AI Mach. Learn. 2023, 2, 129–141. [Google Scholar] [CrossRef]
- Zhong, J.; Zhong, Y.; Han, M.; Yang, T.; Zhang, Q. The impact of AI on carbon emissions: Evidence from 66 countries. Appl. Econ. 2023, 56, 2975–2989. [Google Scholar] [CrossRef]
- Dubey, A.; Yadav, A. New Era’s of Artificial Intelligence in Pharmaceutical Industries. Asian J. Pharm. Res. Dev. 2024, 12, 71–76. [Google Scholar] [CrossRef]
- Sangeetha, M.; Hoti, A.; Bansal, R.; Hasan, M.F.; Gajjar, K.; Srivastava, K. Facilitating artificial intelligence supply chain analytics through finance management during the pandemic crises. Mater. Today Proc. 2021, 56, 2092–2095. [Google Scholar] [CrossRef]
- Paschek, D.; Luminosu, C.T.; Draghici, A. Automated business process management—In times of digital transformation using machine learning or artificial intelligence. MATEC Web Conf. 2017, 121, 04007. [Google Scholar] [CrossRef]
- Sommerfeld, S.; Strube, J. Challenges in biotechnology production—Generic processes and process optimization for monoclonal antibodies. Chem. Eng. Process. 2005, 44, 1123–1137. [Google Scholar] [CrossRef]
- Kumar, R.; Sood, P. Uses of AI in Field of Radiology- What is State of Doctor & Pateints Communication in Different Disease for Diagnosis Purpose. J. Res. Appl. Sci. Biotechnol. 2023, 2, 51–60. [Google Scholar] [CrossRef]
- Sharma, D.K.; Chatterjee, M.; Kaur, G.; Vavilala, S. Deep learning applications for disease diagnosis. In Deep Learning for Medical Applications with Unique Data; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Samala, A.; Rawas, S. Generative AI as Virtual Healthcare Assistant for Enhancing Patient Care Quality. Int. J. Online Biomed. Eng. 2024, 20, 174–187. [Google Scholar] [CrossRef]
- Stypińska, J.; Franke, A. AI revolution in healthcare and medicine and the (re-)emergence of inequalities and disadvantages for ageing population. Front. Sociol. 2023, 7, 1038854. [Google Scholar] [CrossRef] [PubMed]

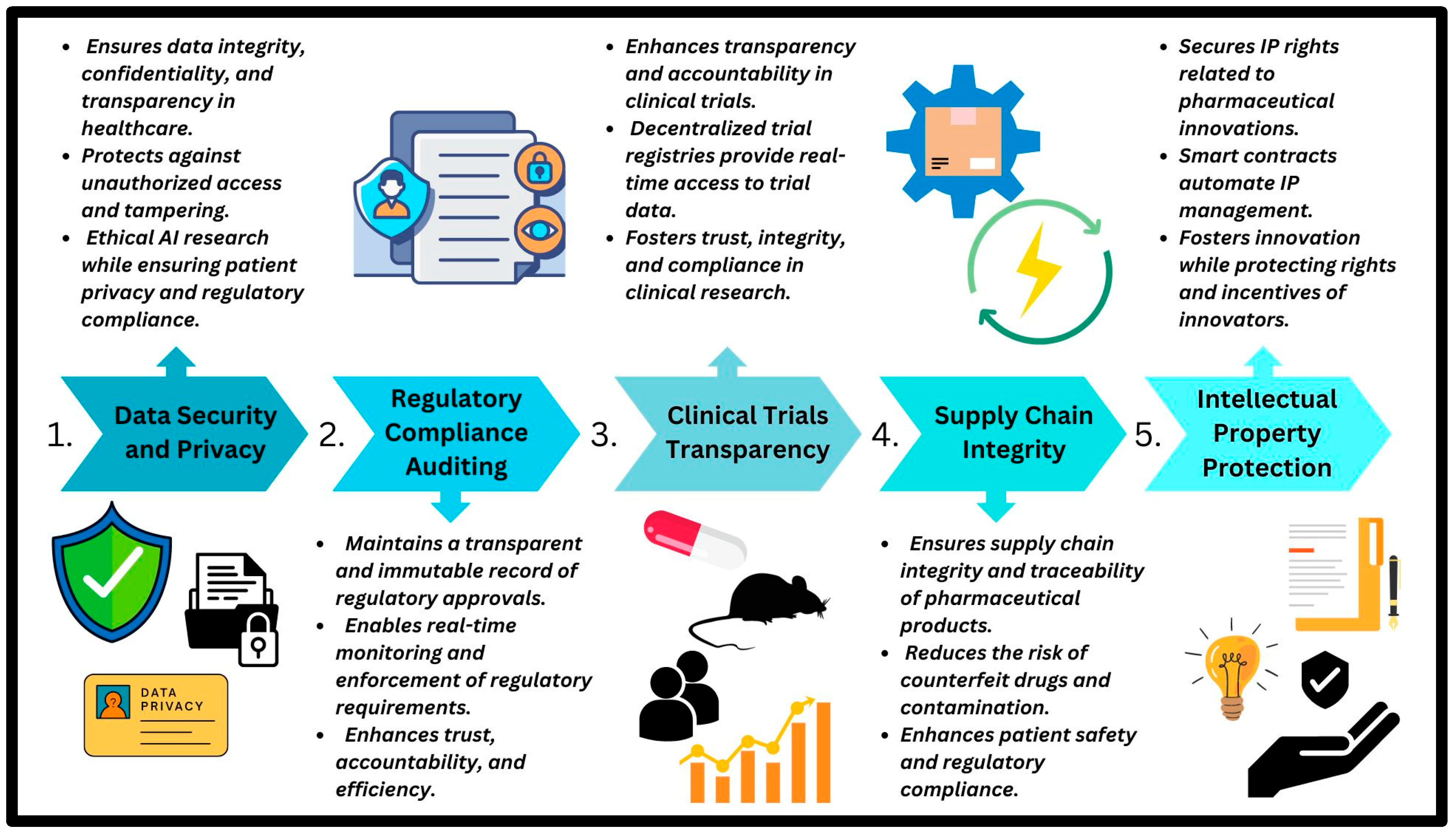
| Application Area | Study Title | Key Findings | References |
|---|---|---|---|
| Anticancer Research | PTML for phenotypic early antineoplastic drug discovery | Designed virtual anti-lung-cancer agents with optimized multi-target activity | [85] |
| PTML modeling for pancreatic cancer research | Identified simultaneous multi-protein and multi-cell inhibitors | [86] | |
| Multilabel model of the ChEMBL dataset of preclinical assays for antisarcoma compounds | Enabled prediction of multi-condition anticancer efficacy | [87] | |
| Cell-based multi-target QSAR model | Designed virtual versatile inhibitors for liver cancer cell lines | [88] | |
| Antimicrobial Agents | In Silico Approach for Antibacterial Discovery | Designed inhibitors against multi-strain S. aureus infections | [89] |
| Implementation of IFPTML computational models | Drug discovery against Flaviviridae family | [90] | |
| Multi-Condition QSAR Model | Designed chemicals with dual pan-antiviral and anti-cytokine storm profiles | [84] | |
| Computational Drug Repurposing for Tuberculosis | Discovered multi-strain inhibitors for tuberculosis therapy | [91] | |
| Prediction of Antileishmanial Compounds | Designed and evaluated 2-acylpyrrole derivatives | [92] | |
| QSAR Modeling for Multi-Target Drug Discovery | Designed inhibitors for diverse pathogenic parasites | [93] | |
| Demystifying Artificial Neural Networks in Drug Discovery | Applied AI for antimalarial compound discovery | [94] | |
| Dual-Target/Multi-Target Inhibitors | PTML for Mood Disorders | Designed inhibitors targeting NET and SERT proteins | [95] |
| In Silico Drug Repurposing for Anti-Inflammatory Therapy | Identified dual inhibitors of caspase-1 and TNF-alpha | [96] | |
| Multi-Target Drug Discovery via PTML | Designed virtual dual inhibitors of CDK4 and HER2 | [83] | |
| PTML Modeling for Alzheimer’s Disease | Designed multi-target inhibitors for GSK3B, HDAC1, and HDAC6 | [97] | |
| BET Bromodomain Inhibitors | Designed inhibitors using fragment-based QSAR modeling | [98] |
| Specific Parameter | Description | Relevance to Precision Medicine | ML Model Used | References |
|---|---|---|---|---|
| Patient demographics | Age, gender, race, ethnicity | Disease risk, treatment response | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Clinical history | Past medical history, family history, lifestyle factors | Disease risk, progression | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Genetic data | Genomic, epigenetic, transcriptomic data | Disease risk, progression, treatment response | Random Forest, Support Vector Machine, Deep Learning | [4,7,62] |
| Imaging data | Radiologic, pathologic images | Disease severity, progression | Convolutional Neural Networks (CNN), Deep Learning | [4,7,62] |
| Laboratory data | Blood tests, urine tests, other laboratory measures | Disease status, treatment response | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Environmental data | Environmental exposures | Disease risk, progression | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Therapeutic index | Ratio of therapeutic to toxic dose | Dosing decisions | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| PK/PD variability | Variability in drug absorption, distribution, metabolism, excretion | Treatment response | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Biomarkers | Measurable biological markers | Guiding individualized dosing | Random Forest, Support Vector Machine, Deep Learning | [4,7,62] |
| Disease severity and progression | Tumor size, stage, other measures of disease severity and progression | Treatment response | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Pharmacoeconomics | Cost of drug therapy | Treatment decisions, resource allocation | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Dose-exposure and exposure-response relationships | Relationship between drug dose, exposure, and response | Informing precision dosing strategies | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Real-world patient gap | Incongruity between study patients and patients in the real world | Generalizability of clinical trial results | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Personalized treatment plans | Tailored treatment plans based on patient-specific factors | Improved patient outcomes, reduced healthcare costs | Decision Trees, Random Forest, Logistic Regression | [4,7,62] |
| Predictive analytics | Predicting patient outcomes based on historical data | Improved patient outcomes, reduced healthcare costs | Random Forest, Support Vector Machine, Deep Learning | [4,7,62] |
| Real-time monitoring | Continuous monitoring of patient health data | Improved patient outcomes, reduced healthcare costs | Deep Learning, Recurrent Neural Networks (RNN), Long Short-Term Memory (LSTM) | [4,7,62] |
| Application | Language Model | Type | Company | Reference |
|---|---|---|---|---|
| Predictive Maintenance | Random Forest | Supervised Learning | GE Healthcare | https://www.ge.com/digital/predix-asset-performance-management, accessed on 4 January 2025 |
| Production Process Optimization | Neural Network | Supervised Learning | Merck | https://www.merckgroup.com/en/research/open-innovation/merck-digital-science.html, accessed on 4 January 2025 |
| Demand Forecasting and Inventory Management | Long Short-Term Memory (LSTM) | Sequence Prediction | Pfizer | https://www.pfizer.com/research/science/ai, accessed on 4 January 2025 |
| Supply Chain Optimization | Support Vector Machine (SVM) | Supervised Learning | Novo Nordisk | https://www.novonordisk.com/about/supply-chain.html, accessed on 4 January 2025 |
| Genomic Data Analysis | Convolutional Neural Network (CNN) | Supervised Learning | Foundation Medicine | https://www.foundationmedicine.com/genomic-testing/foundation-one-cdx, accessed on 4 January 2025 |
| AI-Driven Diagnostics | Random Forest | Supervised Learning | Tempus | https://www.tempus.com/xai/, accessed on 4 January 2025 |
| Drug Response Prediction | Gradient Boosting Machine (GBM) | Supervised Learning | Berg Health | https://www.berghealth.com/ai-driven-drug-discovery/, accessed on 4 January 2025 |
| Patient Care Integration | Recurrent Neural Network (RNN) | Sequence Prediction | Philips | https://www.philips.com/a-w/healthcare/solutions/healthsuite-insights, accessed on 4 January 2025 |
| Machine Learning Model | Application in Precision Medicine | Example | Reference |
|---|---|---|---|
| Support Vector Machines (SVMs) | Classifying patients based on genetic data or identifying biomarkers associated with diseases | Identifying genetic variants associated with breast cancer risk | [5,12,112] |
| Random Forests | Classifying patients based on clinical data or identifying patient clusters | Identifying patient clusters based on gene expression data in lung cancer | [5,12,112] |
| Convolutional Neural Networks (CNNs) | Analyzing medical images or identifying genetic variants associated with diseases | Analyzing brain images to identify biomarkers associated with Alzheimer’s disease | [5,12,112] |
| Generative Adversarial Networks (GANs) | Generating synthetic data or improving the quality of medical images | Generating synthetic CT images to improve the accuracy of liver segmentation | [5,12,112] |
| FINDER | Predicting the risk of developing a disease based on genetic and environmental factors | Predicting disease risk based on genetic and environmental factors | [5,12,112] |
| Recurrent Neural Networks (RNNs) | Analyzing sequential patient data for disease progression prediction | Predicting disease progression in patients with chronic conditions | [5,12,112] |
| Long Short-Term Memory (LSTM) | Forecasting patient outcomes and treatment responses | Predicting treatment responses in cancer patients based on genomic data | [5,12,112] |
| Decision Trees | Identifying key decision points in treatment planning | Guiding treatment decisions for patients with rare genetic disorders | [5,12,112] |
| Gradient Boosting Machines | Optimizing treatment plans based on patient-specific data | Personalizing treatment strategies for patients with autoimmune diseases | [5,12,112] |
| Deep Belief Networks | Discovering complex patterns in multi-omics data | Identifying novel biomarkers | [5,12,112] |
| Innovations | Disadvantages | References |
|---|---|---|
| Predictive Maintenance |
| [137] |
| Production Process Optimization |
| [138] |
| Demand Forecasting and Inventory Management |
| [135] |
| Supply Chain Optimization |
| [139] |
| Genomic Data Analysis for Personalized Treatment Strategies |
| [140] |
| AI-driven Diagnostics and Biomarker Discovery |
| [141,142] |
| Integration of AI and ML in Patient Care |
| [143,144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandhare, P.; Kurlekar, M.; Deshpande, T.; Pawar, A. A Review on Revolutionizing Healthcare Technologies with AI and ML Applications in Pharmaceutical Sciences. Drugs Drug Candidates 2025, 4, 9. https://doi.org/10.3390/ddc4010009
Kandhare P, Kurlekar M, Deshpande T, Pawar A. A Review on Revolutionizing Healthcare Technologies with AI and ML Applications in Pharmaceutical Sciences. Drugs and Drug Candidates. 2025; 4(1):9. https://doi.org/10.3390/ddc4010009
Chicago/Turabian StyleKandhare, Priyanka, Mrunal Kurlekar, Tanvi Deshpande, and Atmaram Pawar. 2025. "A Review on Revolutionizing Healthcare Technologies with AI and ML Applications in Pharmaceutical Sciences" Drugs and Drug Candidates 4, no. 1: 9. https://doi.org/10.3390/ddc4010009
APA StyleKandhare, P., Kurlekar, M., Deshpande, T., & Pawar, A. (2025). A Review on Revolutionizing Healthcare Technologies with AI and ML Applications in Pharmaceutical Sciences. Drugs and Drug Candidates, 4(1), 9. https://doi.org/10.3390/ddc4010009






