Abstract
Background/Objectives: Uveal melanoma (UM) is a rare but deadly ocular cancer. This review summarizes the characteristics of uveal melanoma and current therapeutic options for primary uveal melanoma and metastatic uveal melanoma, and introduces recent development of therapeutic strategies in preclinical animal studies and clinical trials. Methods: The literature search was conducted to identify relevant articles for UM studies. It was performed using PubMed for articles in English until March 2025. Information on clinical trials was also obtained from ClinicalTrial.gov. Results: Uveal melanoma originates from melanocytes, similar to skin melanoma. However, uveal melanoma has different mutations from skin melanoma. Thus, chemotherapy and immunotherapy, which are effective for skin melanoma, are ineffective for uveal melanoma. Current therapies for UM include radiation therapy, surgical resection, liver-directed therapies, and recently FDA-approved tebentafusp. Although a wide variety of available and newly developed therapies have been tested in clinical trials for UM, tebentafusp is the only FDA-approved therapy for metastatic UM. Given the excessive expression of vascular endothelial growth factor (VEGF) in UM patients with metastatic diseases, anti-VEGF strategies are being tested in clinical trials and pre-clinical animal models. Conclusions: This review summarizes an overview of current therapies and the development of therapeutic strategies in clinical trials and pre-clinical animal models. Despite successful control of primary tumors, 50% of UM patients still experience metastasis in the liver. Although tebentafusp improves the overall survival (OS) of a certain population of UM patients, robust strategies for preventing UM metastasis represent a critical unmet need. Further investigations of the heterogeneity of UM cells and mechanisms of UM metastasis are needed in the future.
1. Introduction
Uveal melanoma (UM) is the most common intraocular malignancy in adults, with the majority of cases originating in the choroid. Despite advances in early detection and treatment, UM has a high rate of metastasis. Approximately 50% of patients with UM develop metastatic disease, which is associated with poor prognosis [1]. Following diagnosis of their UM metastasis, patients have a median survival period of 3.9 months and a three-year survival rate of 4.3% [2].
The liver is the predominant site of UM metastasis, affecting approximately 89% of patients, followed by the lungs (29%) and the bones (17%) [2]. Early detection and effective treatment can mitigate the possibility of metastasis, significantly improving clinical outcomes. Although the mechanism through which UM metastasis occurs is still largely unknown, there are ongoing promising studies. Metastatic UM is highly vascularized with leaky blood vessels [3,4] due to an excess of vascular endothelial growth factor (VEGF) [5,6,7]. We recently showed that some UM lines rely on VEGF to permeate through the endothelial barrier in order to increase UM cell migration across the endothelium and into the circulatory system [8].
This review aims to provide a comprehensive overview of the current understanding of the biology of UM and possible mechanisms facilitating UM metastasis. Furthermore, this review will discuss emerging strategies to treat and manage UM and UM metastasis.
2. Differences Between Skin Melanoma and Uveal Melanoma
Melanoma originates from melanocytes, the pigment-producing cells responsible for melanin synthesis. Melanocytes transfer these melatonin-containing melanosomes to nearby keratinocytes. This process controls skin pigmentation and color and protects against harmful ultraviolet radiation [9]. Skin melanocytes compose the bottom layer of the skin’s epidermis, and ocular melanocytes are located in the conjunctiva and all areas of the uvea, including the iris, ciliary body, and choroid. UM typically arises in these three locations. UM represents 3–5% of all melanomas and 79–81% of ocular melanomas [10]. While both cutaneous (skin) melanoma and UM have the same origin, they differ in their biological behavior, genetic alterations, and clinical presentation, as discussed below.
Although skin and uveal melanoma both originate from melanocytes, they differ through their associated mutations. Skin melanoma primarily has mutations in proteins associated with the mitogen-activated protein kinase (MAPK) pathway [9]. The MAPK is a pathway responsible for cell growth, differentiation, and survival [9]. A majority of skin melanomas have a mutation in the BRAF kinase, which regulates the MAPK pathway [11,12,13,14]. BRAF mutation is present in 40–60% of skin melanoma patients and is associated with a shorter OS [9]. Other commonly found mutations, such as NRAS [15,16] and KIT [17] (Table 1), all regulate the MAPK pathway [9].
For skin melanoma with BRAF mutation, selective inhibitors of the BRAF kinase can be used, including vemurafenib (Zelboraf, Genentech, South San Francisco, CA, USA), dabrafenib (Tafinlar, Novartis, Basel, Switzerland), and encorafenib (Braftovi, Pfizer, New York, NY, USA). Inhibitors of the downstream MEK kinase are also used, including trametinib (Mekinist, Novartis), cobimetinib (Cotellic, Genentech), and binimetinib (Mektovi, Pfizer). A combination of BRAF inhibitor (dabrafenib) and MEK inhibitor (trametinib) improved OS of skin melanoma patients with BRAF mutation, compared to BRAF inhibitor (dabrafenib) alone (NCT01584648, NCT01597908, NCT01584648, NCT01072175) [18,19,20,21]. Other combinations (vemurafenib and cobimetinib) also showed that the combined inhibition of BRAF and MEK is better than BRAF inhibition alone (NCT01689519) [22].
KIT inhibitors such as imatinib (Gleevec, Novartis), sunitinib (Sutent, Pfizer), dasatinib (Sprycel, Bristol Myers Squibb, New York, NY, USA), and nilotinib (Tasigna, Novartis) are used for skin melanoma with KIT mutation (NCT00470470, NCT01028222, NCT0042515, NCT01099514) [23,24,25,26,27].
In contrast to skin melanoma, UM does not typically have mutations in these genes. UM primarily has mutations in the GNA11, GNAQ, BAP1, EIF1AX, and SF3B1 genes (Table 1). UM is typically initiated by a mutation in GNA11 [28] and GNAQ [29], with greater than 90% of mutations found in GNA11 and GNAQ [9]. Genes GNA11 and GNAQ encode Guanine Nucleotide-Binding Protein Alpha Subunit (GNA, or G protein), which is responsible for the activation signaling between G protein-coupled receptors (GPCR) and downstream effectors. Activation of GNA11 or GNAQ leads to activation of downstream pathways, including the protein kinase C (PKC) pathway [protein lipase C (PLC)-PKC], MAPK pathway (BRAF-MEK1/2-ERK1/2), and phosphatidylinositol 3 kinase (PI3K) pathway (PI3K-Akt-mTOR) [9,30]. However, less than 10% of UM patients responded to the PKC inhibitor (NCT02601378, NCT01801358) [31,32,33]. Unlike skin melanoma patients, MEK inhibition did not improve the OS of UM patients (NCT01143402, NCT01974752) [34,35]. Potential reasons for resistance to MEK inhibitors in UM patients can be persisting YAP/TAZ signaling [36], overexpression of DDX43-RAS [37], paracrine effects of neuregulin 1 (NRG1) and hepatocyte growth factor (HGF) [38], or monosomy 3 and mutations in BRCA1 Associated Protein-1 (BAP1) [39].
Inactivating somatic mutations in BAP1 were found in 18–45% of all primary UM and more than 80% of metastasizing UM [40,41,42,43]. BAP1 encodes a nuclear ubiquitin carboxy-terminal hydrolase, one of the deubiquitinating enzymes [44]. BAP1 usually functions as a tumor suppressor, and this mutation has been found to correlate strongly with the development of metastatic disease in UM [40,45].
Eukaryotic translation initiation factor 1A (EIF1AX) is another mutation found in 14-21% of UM [43], although it is not commonly linked to metastatic disease. EIF1AX encodes a eukaryotic translation initiation factor essential for the translation and transfer of tRNA to the small ribosomal unit. This mutation is typically found in nonmetastatic cases of UM [45]. EIF1AX mutations are the most prognostically favorable to common UM mutations [46].
Lastly, splicing factor 3b subunit 1 (SF3B1) is another common mutation found in UM tumors. The SF3B1 mutation is associated with a good prognosis if diagnosed early on and a significantly worse prognosis and development of late metastasis in patients five years after diagnosis [45].
In summary, uveal melanoma has mutations that are different from skin melanoma. Thus, commonly used therapies for skin melanoma, such as inhibitions of MEK [34,35], do not work well for UM patients. In addition, unlike skin melanoma, chemotherapy and immune therapy (except recently approved tebentafusp) are not effective for uveal melanoma [47], although the precise mechanisms of how different mutations cause unresponsiveness to chemotherapy and immune therapy are not fully understood.

Table 1.
Mutations in skin melanoma and uveal melanoma.
Table 1.
Mutations in skin melanoma and uveal melanoma.
| Mutations Found in Skin Melanoma | Functions | References |
| BRAF | B-Raf kinase regulating MAPK pathway | [11,12,13,14] |
| NRAS | Small GTPase | [15,16] |
| KIT | Receptor tyrosine kinase | [17] |
| Mutations Found in Uveal Melanoma | Functions | References |
| GNA11 | The alpha subunit of Guanine nucleotide-binding proteins | [28] |
| GNAQ | The alpha subunit of Guanine nucleotide-binding proteins | [29] |
| BAP1 | Deubiquitinating enzyme | [40] |
| EIF1AX | Eukaryotic translation initiation | [48] |
| SF3B1 | Essential for pre-mRNA splicing | [48] |
3. Current Therapies for Uveal Melanoma
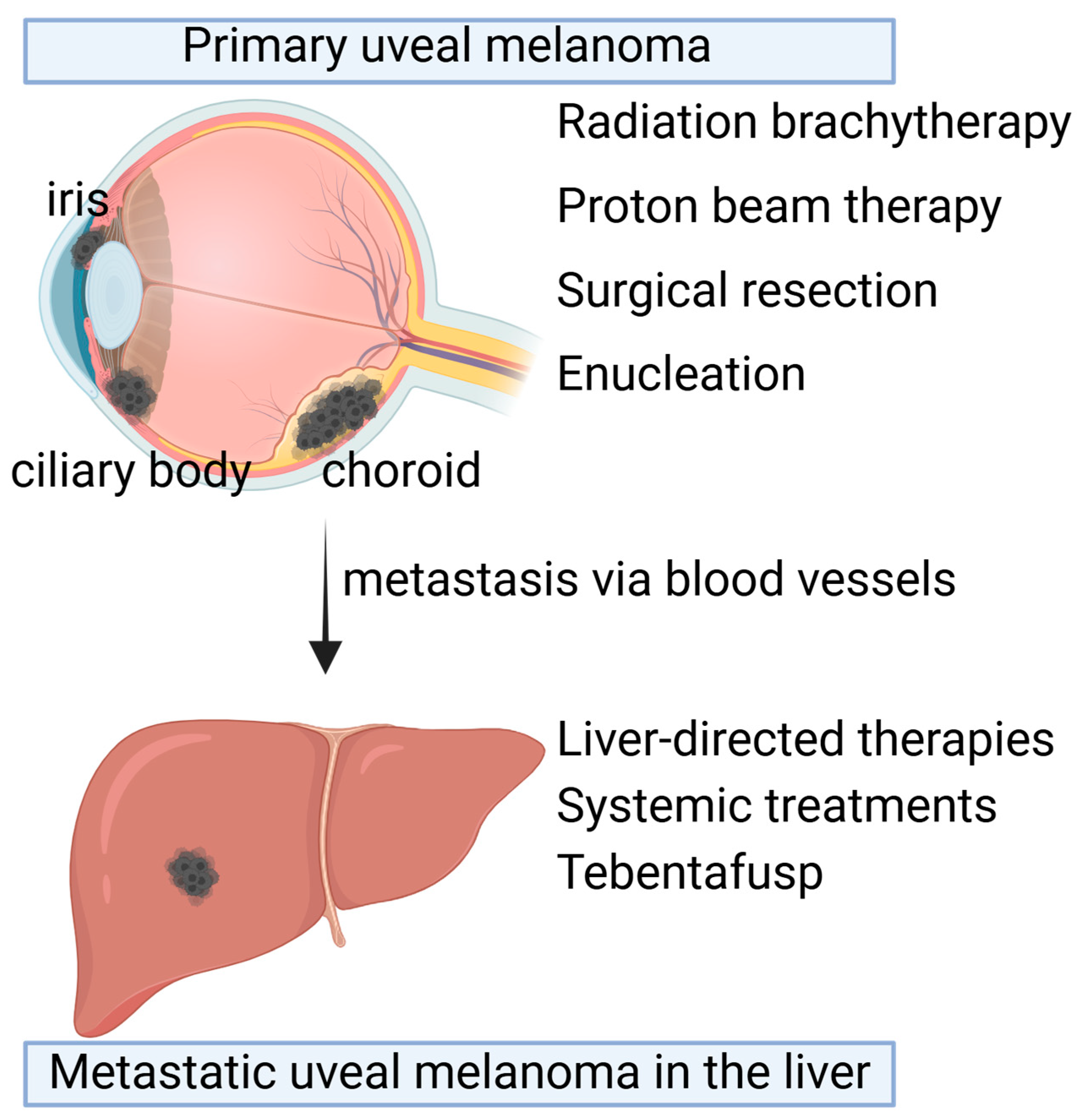
There are various therapies currently approved for the treatment of uveal melanoma tumors, including radiation therapies, surgery, liver-directed therapies, and recently FDA-approved immunotherapy (Figure 1).
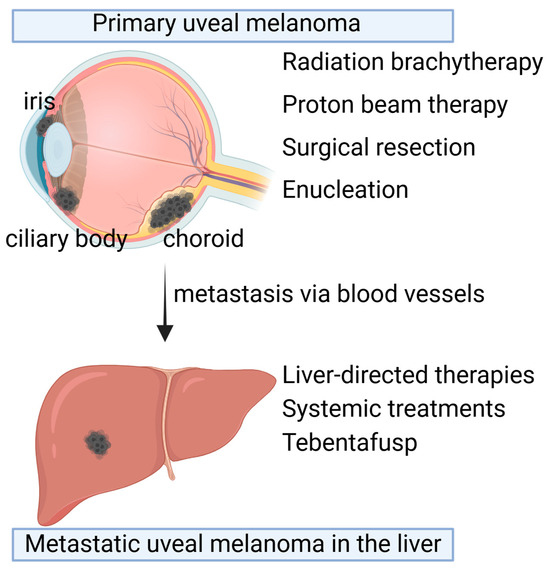
Figure 1.
Therapeutic options for primary uveal melanoma and metastatic uveal melanoma. Primary uveal melanoma (UM) occurs in the choroid, ciliary body, and iris in the eyes. Depending on the size and location, primary UM can be treated with radiation brachytherapy, proton beam therapy, surgical resection, or enucleation. Despite the primary tumor treatments, 50% of patients develop metastasis in the liver a long time after primary tumor treatments. Liver-directed therapies or systemic treatments such as Tebentafusp are the available therapeutic options. Created with BioRender.com.
3.1. Radiation Brachytherapy
Radiation brachytherapy is a commonly used treatment modality for UM [49,50]. This approach involves surgically placing a small radioactive plaque on the sclera adjacent to the tumor, allowing for targeted radiation delivery while minimizing damage to surrounding ocular structures and tissue [1,51]. Plaque brachytherapy has become a standard treatment for localized UM, demonstrating high rates of tumor control and vision preservation [50]. There was no survival difference between patients who underwent enucleation and those treated with iodine-125 plaque brachytherapy [52].
Radiation-induced complications include poor visual outcome, radiation-induced cataracts, vitreous hemorrhage, neovascular glaucoma, secondary glaucoma, retinal detachment, macular edema, and radiation retinopathy [53]. Among them, macular edema and radiation retinopathy, which cause poor visual outcomes, can be dissolved by anti-VEGF therapy. In the randomized Phase IIB trial (NCT0222610), a monthly injection of ranibizumab (anti-VEGF therapy) significantly improved visual outcomes [54]. Corticosteroids, such as dexamethasone (also known as Dexasone, Hexadrol, and Baycadron) and triamcinolone acetonide (Kanalog, Bristol-Myers Squibb), are also used for radiation-induced macular edema and maculopathy [55,56,57,58,59,60]. Both bevacizumab and corticosteroid injections reduced central foveal thickness and improved some patients’ visual improvements without showing differences or advantages [59]. A combination of bevacizumab and corticosteroid is also beneficial for UM patients in treating severe radiation maculopathy [58].
After plaque radiotherapy, 29 (8.5%) of 43 patients experienced local recurrence [61]. Recurrence occurred with significantly higher frequency when the anterior tumor edge involved the ciliary body [61]. Local recurrence increases the risk of metastasis [62].
3.2. Proton Beam Therapy
Proton beam therapy is another form of radiation therapy that is commonly used to treat UM [1,51]. Common indications for the use of proton treatment in UM include small tumors in the posterior pole poorly accessible to plaque treatment, tumors at the posterior pole affecting the fovea, and large anterior tumors traditionally too large for brachytherapy [63]. This treatment option delivers precise, high-energy proton beams to the tumor with the goal of minimizing unwanted radiation exposure to surrounding healthy tissues. While proton beam therapy and plaque brachytherapy are similar, they have key differences with important clinical considerations. Proton beam therapy is more precise and is particularly useful in cases where the tumor is near critical ocular structures, such as the macula and optic nerve, where preserving nearby healthy tissue is vital for maintaining visual function [64]. However, for tumors further from these structures, radiation brachytherapy delivers a dosage closer to the prescribed dose [64]. Additionally, the location of the tumor (temporally vs. nasally) can also impact which radiation treatment option would be more effective.
The downside of this therapeutic option is that most patients become blind after proton therapy [65]. And small populations (1.5%) of patients still experience local recurrence [66,67].
3.3. Surgical Resection
Surgical resection of UM tumors may be considered in select cases of UM, particularly when the tumor is small and far from critical ocular structures [68]. This treatment option used to be more common; however, it has been slowly phased out with the advent and success of radiation therapy options [68].
3.4. Enucleation
This method was more commonly used prior to plaque brachytherapy and involved the removal of the eye globe—despite the consequences of extremely poor vision and decreased quality of life [1]. Enucleation is still used in cases of very large tumors, where the risk of metastasis is high and it is not worth the time it takes for radiation therapies to take effect [69]. Even after radiation therapies, some patients (7.3~10.3%) experienced local recurrence [62,70,71] and required secondary enucleation [72]. After enucleation, local recurrence in the orbital is rare (<1%); however, it still occurs even without signs of optic nerve invasion or extrascleral extension [73].
3.5. Liver-Directed Therapies
Despite the success of primary tumor treatments above, 50% of UM patients still experience metastasis [49]. Given the frequent occurrence of liver metastasis, liver-directed therapies are often used, such as microwave ablation, radio-frequency ablation, and surgical resection [51]. Transarterial chemoembolization (TACE) is another option. During TACE, a combination of chemotherapy drugs and embolic agents that block blood flow is injected directly into the artery supplying blood to the tumor. In addition, selective internal radiotherapy (SIRT), isolated hepatic perfusion (IHP), and percutaneous hepatic perfusion (PHP) are also utilized [51].
A melphalan/hepatic delivery system (HDS) (HEPZATO KIT, Delcath Systems, Inc., New York, NY, USA) provided clinically meaningful response rates and demonstrated a favorable benefit–risk profile in patients with unresectable metastatic UM (NCT02678572) [74]. Melphalan/HDS was approved by the FDA in August 2023 for UM patients with liver metastasis.
3.6. Immunotherapy
Recently, the immunotherapy tebentafusp showed an improvement in the OS of UM patients with metastasis (NCT02570308 and NCT03070392) and was approved by the FDA [75,76,77,78]. Tebentafusp is a bispecific molecule that targets the gp100 peptide presented by HLA-A*02:01 molecules on tumor cells and engages CD3 on T cells, thus connecting tumor cells with T cells to help the immune system target and destroy melanoma cells [79]. A follow-up study for UM patients who received tebentafusp treatment showed promising survival [80]. One drawback may be skin rashes, as tebentafusp induces T-cell recruitment to skin melanocytes [81]. A recent study further showed that tebentafusp treatment leads to M2-to-M1 macrophage reprogramming, and a combination of tebentafusp with interleukin-2 (IL-2) may enhance benefit in UM patients with high levels of tumor-associated macrophages [82]. Although tebentafusp is very promising, the treatment is limited to patients with HLA-A*02:01. HLA-A*02:01 is a human leukocyte antigen (HLA) class I molecule involved in the presentation of antigenic peptides to CD8+ cytotoxic T lymphocytes. HLA-A*02:01 allele is common in Caucasians (96%) and Native Americans (94%) but less common in Asians [83]. For example, only 47% of Japanese, 23% of Singapore Chinese, and 4% of North Indians have HLA-A*02:01 [83]. For other patients, effective systemic treatment is still needed, as discussed below.
Overall, treatment options for uveal melanoma are greatly dependent on the tumor’s location and size. Patients with very large tumors may have a better prognosis with enucleation, especially if the UM has not metastasized yet. Whereas, if a patient has a tumor closer to the macula and optic nerves, their treatment options are limited due to its sensitive location. In the majority of cases, patients are also given the option of having radiation or enucleation treatment instead of other methods. There are various approved treatment methods for UM, and several novel therapies are being developed and tested.
4. Therapies Undergoing Testing in Clinical Trials
In clinical trials, a variety of therapies have been tested for UM, including the aforementioned inhibitions of MEK (NCT01143402, NCT01974752, NCT02768766) [34,35] and PKC (NCT02601378, NCT01801358) [31,32,33]. In this section, we summarize other therapies for UM in clinical trials. Clinical trials with results in published papers are summarized in Table 2.
4.1. Kinase Inhibitors
Tyrosine kinase inhibitors have been tested or are in ongoing tests in clinical trials for UM patients. These tyrosine kinase inhibitors include FDA-approved drugs for other solid cancers, such as crizotinib (Xalkori, Pfizer), sunitinib (Sutent, Pfizer), entrectinib (Rozlytrek, Roche, Basel, Switzerland), cabozantinib (Cabometyx, Exelixis, Alameda, CA, USA), and axitinib (Inlyta, Pfizer) [84]. The use of adjuvant crizotinib did not improve recurrence-free survival (RFS) of patients with high-risk UM (NCT02223819) [85]. However, the other two tyrosine kinase inhibitors show some hope. In a retrospective study comparing high-risk UM patients who received adjuvant sunitinib with institutional controls, the sunitinib group had better OS [86]. A combination of entrectinib with apoptosis inducer PAC-1 was tolerated with no treatment-related grade >3 toxicities, and stable disease was observed in four out of six patients, with a median progression-free survival (PFS) of 3.38 months (95% CI at 1.6–6.5 months) (NCT04589832) [87]. These promising results warrant further clinical investigation of tyrosine kinase inhibitors. Another tyrosine kinase inhibitor, cabozantinib, showed clinical activity in patients with metastatic melanoma, including UM (NCT00940225) [88]. However, in a randomized Phase II trial, cabozantinib did not improve PFS but increased toxicity relative to temozolomide/dacarbazine in metastatic UM (NCT01835145) [89].
Investigational tyrosine kinase inhibitors, including sitravatinib (MGCD516, Mirati Therapeutics, San Diego, CA, USA), cediranib (AZD2171, AstraZeneca, Cambridge, UK), and NN3201 (Novelty Nobility, Seongnam-si, Republic of Korea), are also undergoing testing in ongoing clinical trials.
FDA-approved MEK inhibitors trametinib (Mekinist, Novartis), selumetinib (Koselugo, AstraZeneca), and binimetinib (Mektovi, Pfizer) are also being tested for UM, but no improvements have been observed in PFS [34,35]. Investigational PKC inhibitors darovasertib (IDEAYA Biosciences, South San Francisco, CA, USA) and sotrastaurin (AEB071, Novartis) showed modest clinical activity in metastatic UM [31,32,33].
An inhibitor for downstream ERK1/ERK2, ulixertinib (BVD-523, Biomed Valley Discoveries, Kansas City, MO, USA), did not show clinical activity in UM patients (NCT03417739) [90]. Focal adhesion kinase (FAK) inhibitors defactinib (Verastem Oncology, Needham, MA, USA), ifebemtinib (IN10018, InxMed, Beijing, China), and PI3K inhibitor roginolisib (IOA0244, iOnctura, Geneva, Switzerland) have been developed and are in ongoing clinical trials for UM.
FDA-approved multi-kinase inhibitor sorafenib (Nexavar, Bayer, Leverkusen, Germany) showed non-progression at 24 weeks in 31.2% of UM patients; however, 41.4% of patients required dose modifications due to toxicity and no improvement in health-related quality of life was shown [91]. Sorafenib was also tested in combination with carboplatin and paclitaxel in metastatic UM, but only minor tumor responses and stable disease were observed in some patients (NCT00329641) [92].
FDA-approved mTOR inhibitor everolimus (Afinitor, Novartis) was tested as a combination with pasireotide, a synthetic somatostatin, in metastatic UM but showed limited clinical benefit (NCT01252251) [93]. The addition of everolimus to carboplatin, paclitaxel, and bevacizumab failed to improve outcomes, with increased toxicity in metastatic melanoma (NCT00976573) [94].
In short, kinase inhibitors have shown varying degrees of efficacy and safety in clinical trials for UM, with some promising results warranting further investigation.
4.2. Immunotherapies
In addition to tebentafusp, mentioned above, immunotherapies targeting programmed death receptor-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have been tested in clinical trials [95]. Humanized monoclonal antibodies targeting PD-1 include nivolumab (Opdivo, Bristol-Myers Squibb Co.), pembrolizumab (Keytruda, Merck & Co., Inc., Rahway, NJ, USA), durvalumab (Imfinzi, AstraZeneca), and tislelizumab (Tevimbra, BeiGene, Ltd., Beijing, China). These are FDA-approved drugs for other cancers. The combination of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) for metastatic UM showed a modest improvement in OS over historical benchmarks of chemotherapy (NCT02626962, NCT01585194) [96,97]. The combination of nivolumab and ipilimumab was further tested in combination with percutaneous hepatic perfusion with melphalan for safety (NCT04283890) [98]. Neoadjuvant nivolumab and ipilimumab showed higher response rates but substantial toxicity, whereas treatment with nivolumab monotherapy yielded a modest response and low toxicity in metastatic melanoma patients, including for ocular melanoma (NCT02519322) [99].
Pembrolizumab showed clinical benefit in UM patients without liver metastasis or small metastasis volume (NCT02359851) [100]. The combination of pembrolizumab and multi-kinase inhibitor lenvatinib (Lenvima, Eisai Co., Ltd., Tokyo, Japan) showed tolerability and favorable anti-tumor activity in UM (NCT03006887) [101].
Pembrolizumab was also tested as a combination with entinostat (SNDX-275, MS-275, Syndax Pharmaceuticals, Waltham, MA, USA), an inhibitor of histone deacetylase (HDAC) (NCT02697630) [102,103]. The idea behind it is that BAP1 promotes the expression of key developmental genes regulating the switch from pluripotency to differentiation by preventing the deacetylation of histone H3K27 at gene regulatory regions [104,105]. Thus, the inhibition of HCAC activity can rescue the phenotypes associated with BAP1 deficiency by restoring normal expression of genes [104,105]. As BAP1 deficiency in UM is associated with a metastatic phenotype with poor prognosis [40], HCAC inhibitors, such as vorinostat (Zolinza, Merck & Co., Inc.) and entinostat, have been tested as a monotherapy for uveal melanoma (NCT00121225, NCT03022565, NCT01587352, and NCT00020579), although the results have not yet been published. The combination of pembrolizumab and entinostat in patients with metastatic UM showed an objective response rate of 14%, a clinical benefit rate at 18 weeks of 28%, a median PFS of 2.1 months, and a median OS of 13.4 months (NCT02697630) [102].
Durvalumab was tested together with anti-CTLA-4 tremelimumab in combination with tebentafusp (NCT02535078). Tebentafusp with durvalumab demonstrated promising efficacy for metastatic skin melanoma patients [106].
There are more ongoing clinical trials testing investigational anti-PD-1 drugs for metastatic UM. Anti-PD-1 spartalizumab (PDR001, Novartis) is under development for metastatic melanoma [107]. REGN10597 (Regeneron Pharmaceuticals, Tarrytown, NY, USA) is an investigational, PD-1-targeted and receptor-masked IL-2 drug [108]. This drug is designed to enhance the immune response against cancer cells by targeting the PD-1 receptor on T cells while minimizing systemic toxicity [108]. XmAb23104 (Xencor, Pasadena, CA, USA) is an investigational bispecific antibody targeting PD-1 and ICOS (an immune co-stimulatory receptor) [109,110]. XmAb23104 aims to enhance T-cell activation specifically within the tumor microenvironment by simultaneously targeting these receptors, potentially improving anti-tumor responses [109,110]. Another bispecific antibody, XmAb808 (Xencor), targeting the tumor antigen B7-H3 and CD28 co-receptor on T cells [111], is also in an ongoing clinical trial.
Monoclonal antibodies targeting CTLA-4 include ipilimumab (Yervoy, Bristol-Myers Squibb Co.) and tremelimumab (Imjudo, AstraZeneca) [95]. Ipilimumab was tested in combination with nivolumab, as described above [96,97,98,99]. Tremelimumab monotherapy for advanced UM showed manageable toxicity but modest PFS and a lack of responses (NCT01034787) [112].
T-cell engaging agents are a class of immunotherapies designed to enhance the ability of the immune system to target and destroy cancer cells. TYRP1-TCB (RO7293583, RG6232, Roche) targets tyrosinase-related protein 1 (TYRP1) on the surface of melanoma and CD3 on T cells, facilitating the interaction between melanoma and T cells [113]. The safety, tolerability, maximum tolerated dose/optimal biological dose, and pharmacokinetics (PK) of TYRP1-TCB were tested for patients with metastatic melanoma (NCT04551352) [114].
Cancer vaccines are also promising strategies for solid tumors, including melanoma [115]. Six melanoma helper peptides (6MHP), MELITAC 12.1, gp100 antigen, MART-1 antigen, tyrosinase peptide, NA17-A antigen, MAGE-12, multi-epitope melanoma peptide vaccine, and tyrosinase DNA vaccine have been tested or are in ongoing clinical trials for metastatic melanoma patients. Skin and uveal melanoma patients received vaccination of 6MHP and successfully developed antibodies against cancer peptides (NCT00089219) [116]. Gp100 antigen [also known as premelanosome protein (PMEL)], MART-1 (also known as Melan-A), and tyrosinase peptide were tested as a combination with ipilimumab (anti-CTLA-4) in melanoma (NCT00032045). Although vaccination (gp100 antigen/MART-1/tyrosinase) failed to induce a measurable response, a higher change in Th-17 inducible cells and higher baseline C-reactive protein (CRP) levels were positively associated with freedom from relapse [117]. A multi-epitope melanoma peptide vaccine with incomplete Freund’s adjuvant induced certain types of immune cells; however, optimized vaccine regimens need to be determined (NCT00705640) [118]. A tyrosinase DNA vaccine (pINGmuTyr, Ichor Medical Systems, San Diego, CA, USA) administered by electroporation in malignant melanoma patients was found to be safe and resulted in Tyr-reactive immune responses (NCT00471133) [119].
Interferon is also used as an immunotherapy as it stimulates the immune system to fight cancer more effectively. However, adjuvant treatment of interferon-α-2b (IFN-α-2b, Merck & Co.) and low-dose dacarbazine (DTIC-Dome, a chemotherapy medication, Bayer) in metastatic UM patients failed to show a significant difference compared to untreated patients (NCT0110528) [120].
Granulocyte-macrophage colony-stimulating factor (GM-CSF, sargramostim, Sanofi, Paris, France) boosts whole white blood cell counts. Immunoembolization with GM-CSF for UM with liver metastasis was safe and showed efficacy in UM patients (NCT00661622) [121]. High doses of immunoembolization with GM-CSF prolonged the survival of UM patients and possibly delayed the progression of extrahepatic metastases [122,123].
Aldesleukin (Proleukin, Iovance Biotherapeutics, San Carlos, CA, USA) is a synthetic version of IL-2, which helps regulate the immune system. The combination of aldesleukin with ipilimumab showed a 17% complete response rate, compared with 7% and 6% with respect to the combination of ipilimumab and gp100 treatment (NCT00058279) [124].
Other immunotherapy drugs are being tested for UM in ongoing clinical trials, including Obinutuzumab (Gazyva, Roche), SEA-CD40 (Seagen Inc., Bothell, WA, USA), tocilizumab (Actemra, Roche), and nelitolimod (SD-101, TriSalus Life Sciences, Westminster, CO, USA). Obinutuzumab is a humanized monoclonal antibody targeting CD20 on the surface of B-lymphocyte [125]. SEA-CD40 is a non-fucosylated, humanized monoclonal antibody targeting CD40, a co-stimulatory receptor on antigen-presenting cells [126]. Tocilizumab is an immunosuppressive, monoclonal antibody targeting interleukin-6 (IL-6) [127]. Nelitolimod is designed to activate Toll-like receptor 9 (TLR9). This activation induces the production of type I interferons, which play a crucial role in the immune response against cancer [128].
In sum, immunotherapies targeting PD-1 and CTLA-4, including combinations of nivolumab and ipilimumab, have shown varying degrees of efficacy and safety in clinical trials for metastatic UM, with ongoing research exploring additional treatments and combinations.
4.3. Chemotherapies
Alkylating agents, such as melphalan and fotemustine (LKT Labs, Saint Paul, MN, USA), lomustine (Gleostine, NextSource Biotechnology Miami, FL, USA), carmustine (BiCNU, AVET Lifesciences, Maharashtra, India) (Gliadel, Azurity Pharmaceuticals, Woburn, MA, USA), and cyclophosphamide (Cytoxan, Baxter Healthcare, Deerfield, IL, USA), are widely used chemotherapies for cancers and are being tested for UM patients in ongoing clinical trials. Melphalan/HDS showed efficacy and was approved by the FDA for UM patients with liver metastasis (NCT02678572) [74]. However, another alkylating agent, fotemustine, for intrahepatic treatment for metastatic UM did not improve OS [129].
Paclitaxel (Pfizer) is also a widely used chemotherapy for cancer treatment. Taxoprexin (DHA-paclitaxel, Protarga, King of Prussia, PA, USA), docosahexaenoic acid (DHA)-conjugated paclitaxel, is less toxic and more effective than paclitaxel [130]. Taxoprexin (i.v.) was safe and well-tolerated in metastatic UM patients and showed efficacy, with 32% of patients achieving stable disease (NCT00244816) [131]. Paclitaxel is a microtubule-stabilizing agent, whereas vincristine is a microtubule-destabilizing agent [132]. Marquibo (vincristine sulfate liposome injection) was well tolerated in melanoma patients with impaired liver function (NCT00506142) [133].
In short, alkylating agents and microtubule regulating agents are widely used chemotherapies for various cancers. Although most chemotherapies do not show efficacy for UM patients, there is some hope in the ongoing clinical trials of chemotherapies for UM.
4.4. Cell Therapies
Tumor-infiltrating lymphocytes (TIL), autologous T cells, and autologous dendritic cells (DC) are used as cell therapies for cancers, along with autologous TIL therapy for metastatic UM-mediated tumor regression (NCT01814046) [134]. The personalized IKKβ-matured RNA-transfected DC vaccine, which primes T cells and activates natural killer (NK) cells, has been tested for metastatic UM (NCT04335890) [135]. Lifileucel (Amtagvi, Iovance Biotherapeutics), AloCelyvir (Viralgen Vector Core, San Sebastian, Spain), TBio-4101 (Turnstone Biologics, San Diego, CA, USA), BPX-701 (Bellicum Pharmaceuticals, Houston, TX, USA), and ACTengine (Immatics, Stafford, TX, USA) are in ongoing clinical trials for metastatic UM [136]. Although single intravenous administration of oncolytic adenovirus ICOVIR-5 failed to induce tumor regressions in skin and uveal melanoma patients [137], ICOVIR-5 was further used to modify allogenic bone marrow-derived mesenchymal stem cells and as a cell therapy, in the form of AloCelyvir, in ongoing clinical trials [138].
4.5. Viral Therapies
Oncolytic virus therapy uses viruses that selectively replicate within cancer cells, causing them to burst and die. This process also helps stimulate the immune system to recognize and attach to the cancer. Coxsackievirus A21 (CVA21) (Cavatak, Merck & Co.) is an oncolytic virus therapy, targeting intracellular adhesion molecule 1 (ICAM-1) and decay-accelerating factor (DAF), which are abundant on the surface of cancer cells [139]. CVA21 was tested in combination with ipilimumab in UM patients (NCT03408587), but a meaningful clinical benefit was not observed [140]. Other viral therapies tested in clinical trials for UM include RP2 (Replimune, Woburn, MA, USA) and ADV/HSV-tk (Candel Therapeutics, Needham, MA, USA). ADV/HSV-tk uses a replication-deficient adenovirus vector to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene into cancer cells. Once inside the cells, the HSV-tk gene makes the cells sensitive to antiviral drugs like ganciclovir or valacyclovir, which kill the cancer cells [141].
4.6. Targeted Cancer Therapy
BAP1 mutation in UM causes dysfunction in the DNA damage response [142]. The enzyme poly ADP-ribose polymerase (PARP) helps repair damaged DNA in the cells; thus, inhibition of PARP prevents cancer cells from repairing their DNA, leading to cancer cell death [143]. PARP inhibitors include olaparib (Lynparza, AstraZeneca) and niraparib (Zejula, GlaxoSmithKline, London, UK). However, niraparib treatment for cancer patients with mutant BAP1 failed to meet the prespecified efficacy end point for response (NCT03207347) [144].
ADI-PEG20 (pegargiminase) works by depleting arginine, an amino acid essential for the growth and proliferation of cancer cells, and has been tested in combination with pemetrexed (antimetabolites) and cisplatin chemotherapy for argininosuccinate synthetase (ASS1)-deficient metastatic UM (NCT02029690). Seven out of ten patients had stable disease with a median PFS of 3.0 months and a median OS of 11.5 months [145].
Targeted delivery of drugs using the cell surface proteins of melanoma has been used to develop novel therapies for UM. DYP688 (Novartis Pharmaceuticals) is an antibody–drug conjugate targeting gp100 [146]. 225Ac-MTI-201 (Modulation Therapeutics, Tampa, FL, USA) targets the melanocortin-1 receptor (MC1R) and uses the alpha-emitting radionuclide actinium-225 [147]. VMT01 and VMT02 (Perspective Therapeutics, Richland, WA, USA) are also targeted radiation for MC1R [148].
Belzupacap Sarotalocan (AU-011, Aura Biosciences, Boston, MA, USA) is a nanoparticle conjugate that selectively binds to cancer cells in the eyes and is activated by light [149,150]. Glembatumumab vedotin (CDX-011, CR011-vcMMAE) is an antibody–drug conjugate targeting GPNMB [151]. Among 35 metastatic UM patients who received glembatumumab vedotin treatment, two patients had confirmed partial responses and 18 had stable disease as the best objective response (NCT02363283) [151].
Alrizomadlin (APG-115, Ascentage Pharma, Suzhou, China) is an orally administrated, selective, small molecular inhibitor of the MDM2 protein, designed to reactivate the p53 tumor suppressor pathway [152], and is in an ongoing clinical trial for skin and uveal melanoma.
Melatonin is an indolamine hormone that has improved survival in previous trials with patients with various cancers and is in ongoing clinical trials for UM patients (NCT05502900) [153].
In summary, various FDA-approved therapies for other cancers or investigational drugs have been tested for UM patients in clinical trials. Although some trials showed moderate efficacy that warrants further investigations, tebentafusp is the only FDA-approved therapy for metastatic UM.

Table 2.
Clinical trials for UM with results.
Table 2.
Clinical trials for UM with results.
| Drugs | Mode of Action | Phase | References |
|---|---|---|---|
| Darovasertib (LXS196, IDE196) | PKC inhibitor | Phase I (NCT02601378) | [31] |
| Sotrastaurin (AEB071) | PKC inhibitor | Phase I/II (NCT01801358) | [33] |
| Selumetinib (Koselugo) | MEK1/2 inhibitor | Phase II (NCT01143402, NCT02768766), Phase III (NCT01974752) | [34,35] |
| Ranibizumab | Anti-VEGF | Phase II (NCT02222610) | [54] |
| Crizotinib (Zalkori) | Tyrosine kinase inhibitor | Phase II (NCT0222819) | [85] |
| Entrectinib | Tyrosine kinase inhibitor | Phase I/II (NCT04589832) | [87] |
| Cabozantinib | Tyrosine kinase inhibitor | Phase II (NCT01835145, NCT00940225) | [88,154] |
| Ulixertinib (BVD-523) | ERK inhibitor | Phase II (NCT03417739) | [90] |
| Sorafenib (Nexavar) | Multi-kinase inhibitor | Phase II (NCT00329642) | [92] |
| Everolimus (Afinitor, Zortress, Votubia) | mTOR inhibitor | Phase II (NCT01252251, NCT00976573) | [93,94] |
| Nivolumab (Opdivo) | Anti-PD-1 | Phase II (NCT02626962), Phase I/II (NCT04283890), Phase II (NCT02519322, NCT01585194) | [96,97,98,99] |
| Pembrolizumab (Keytruda) | Anti-PD-1 | Phase I (NCT03006887), Phase II (NCT02359851, NCT02697630) | [100,101,102] |
| Tremelimumab (Imjudo) | Anti-CTLA-4 | Phase II (NCT01034787) | [112] |
| RO7293583 | TYRP-1 targeting CD3 T cell engager | Phase I (NCT04551352) | [114] |
| 6MHP | Peptide vaccine | Phase I/II (NCT00089219) | [116] |
| Gp100 antigen | Peptide vaccine | Phase II (NCT00032045, NCT00084656) | [117,155] |
| Multi-epitope melanoma peptide vaccine | Peptide vaccine | Phase I (NCT00705640) | [118] |
| Tyrosinase DNA vaccine | Vaccine | Phase I (NCT00471133) | [119] |
| Interferon | Interferon | Phase II (NCT01100528) | [120] |
| GM-CSF | Growth factor | Phase II (NCT00661622) | [121] |
| Aldesleukin (Proleukin) | A synthetic IL-2 | Phase I/II (NCT00058279) | [117] |
| Melphalan (Alkeran, Evomela) | Alkylating agent | Phase III (NCT02678572) | [74] |
| Fotemustine | Alkylating agent | Phase III (NCT00110123) | [129] |
| Taxoprexin (DHA-paclitaxel) | Chemotherapy | Phase II (NCT00244816) | [131] |
| Marqibo | Vincristine sulfate liposome injection | Phase II (NCT00506142) | [133] |
| Tumor-infiltrating lymphocytes | Cell therapy | Phase II (NCT01814046) | [134] |
| Autologous dendritic cells | Cell therapy | Phase I (NCT04335890) | [135] |
| CVA21 (Cavatak) | Oncolytic virus targeting ICAM1 and decay-accelerating factor (DAF) | Phase I (NCT03408687) | [140] |
| Niraparib (Zejula) | PARP inhibitor | Phase II (NCT03207347) | [144] |
| ADE-PEG20 | Depleting arginine | Phase I (NCT02029690) | [145] |
| Glembatumumab vedotin (CDX-011, CR011-vcMMAE) | Antibody–drug conjugate targeting GPNMB | Phase II (NCT02363283) | [151] |
| Melatonin | Hormone | Phase III (NCT05502900) | [153] |
| Aflibercept | Anti-VEGF | Phase II (NCT00450255) | [156,157] |
5. Metastasis of Uveal Melanoma
Primary UM has a high rate of metastasis, with approximately 32% of cases metastasizing by 5 years, 50% by 15 years, 56% by 25 years, and 62% by 35 years [45]. Despite this, the mechanism by which UM escapes the eye remains largely an area of active study.
In addition to the mutations described above in Section 2, upregulated expressions of genes in UM have been studied [158,159,160,161,162]. High expression of preferentially expressed antigen of melanoma (PRAME) in UM is associated with poor outcomes and correlated with extraocular extension and chromosome 8q alterations [158,163]. The first identified differentially expressed genes (DEGs) in high-risk UM compared to low-risk UM are CDH1 (up), ECM1 (up), HTR2B (up), RAB31 (up), EIF1B (down), FXR1 (down), ID2 (down), LMCD1 (down), LTA4H (down), MTUS1 (down), ROBO1 (down), and SATB1 (down) [164,165]. Similar approaches identified different sets of genes; for example, three upregulated genes, HTR2B, AHNAK2, and CALHM2, and six downregulated genes, SLC25A38, EDNRB, TLR1, RNF43, IL12RB2, and MEGF10 [166]. Proteomics analysis of UM further suggests upregulated proteins in metastatic UM [167]. These upregulated expressions can be prognostic biomarker and therapeutic targets; however, further investigations are needed to develop therapeutic approaches.
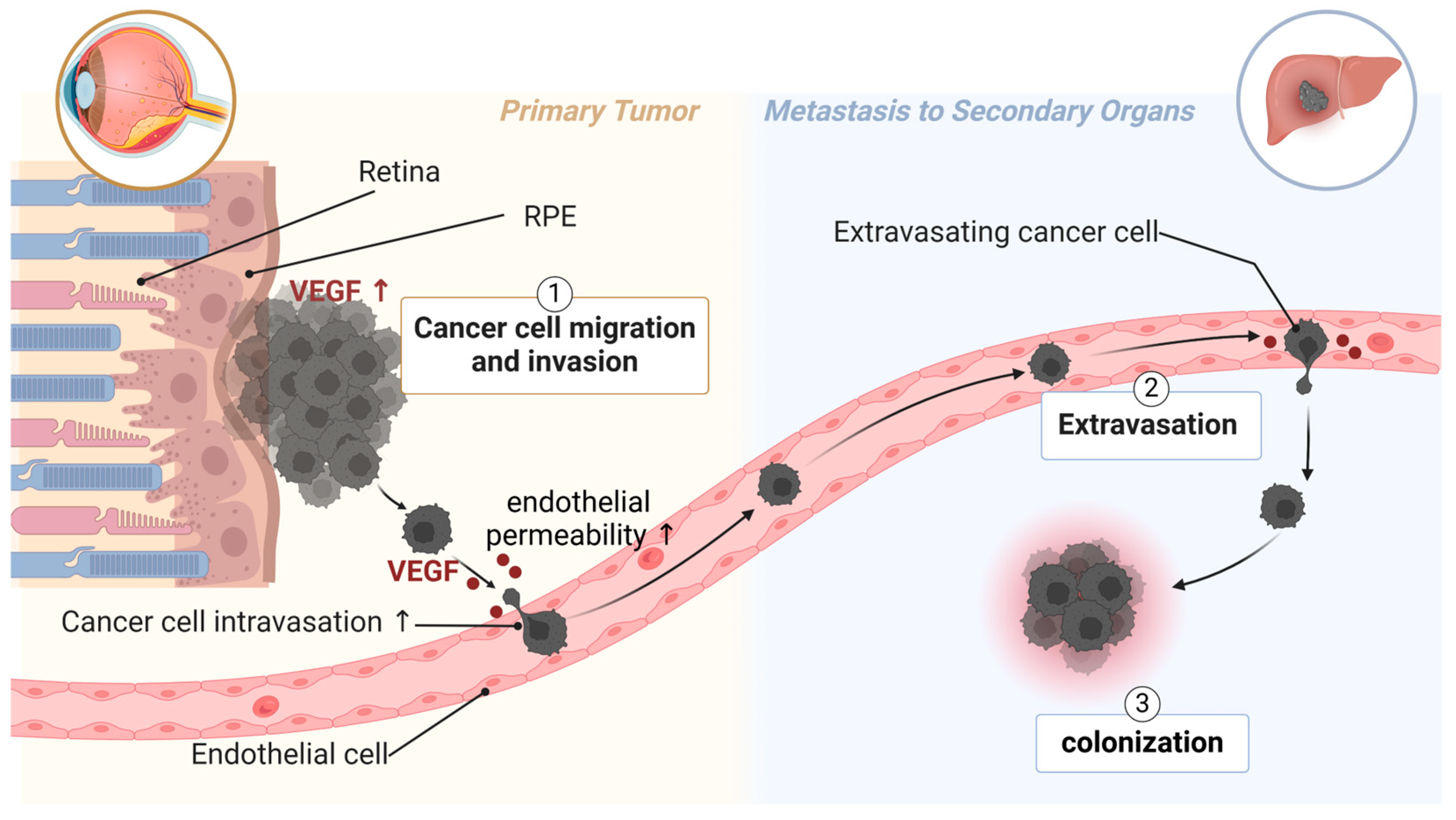
UM metastasizes primarily through the bloodstream, as lymphatic vessels do not exist in the eyes [168]. Poor patient survival is associated with high microvascular density in primary tumors from UM patients [3,4]. UM secretes many factors that facilitate endothelial permeability, the adhesion of cancer cells to endothelium, and the digestion of extracellular matrix [53,169,170]. Such factors include VEGF (Figure 2) [167]. UM patients with higher VEGF-A levels (in the aqueous humor [5] or serum [6,7]) and more activated VEGF receptor 2 (VEGFR2) in primary UM tissue [171] have a higher risk of metastasis and poorer survival. VEGF is known to induce endothelial permeability, facilitating the transmigration of UM cells across the endothelium and into distant organs. This underscores the importance of targeting angiogenic factors to limit the spread of UM and improve clinical outcomes.
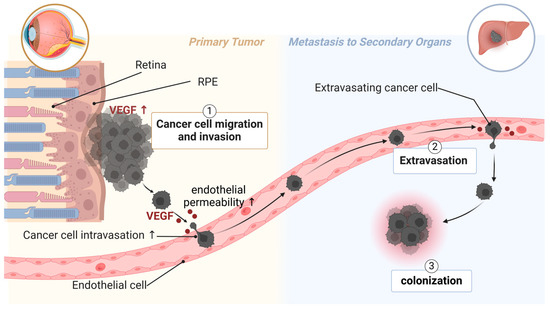
Figure 2.
The role of VEGF in UM metastasis. Highly metastatic UM has excessive expression of VEGF in primary tumors. UM secretes VEGF to induce endothelial permeability, thus facilitating intravasation of UM cells into circulation. UM patients with metastasis also have higher levels of VEGF in circulation, which can be from circulating UM cells. Clinical trial studies show that systemic treatment with aflibercept is beneficial for metastatic UM patients. Created with BioRender.com.
In clinical trials, systemic treatment of an anti-VEGF drug (aflibercept) showed beneficial effects (e.g., PFS) in patients with stage III and stage IV melanoma of cutaneous or uveal origin NCT00450255) [156]. In a randomized Phase II study, a combination of systemic aflibercept treatment and IL-2 improved PFS compared with IL-2 alone for patients with metastatic UM [157]. In an animal model of orthotopically implanted UM, systemic (i.p.) treatment of anti-VEGF (bevacizumab) inhibited tumor growth, tumor angiogenesis, and metastasis [172]. However, intravitreal injection (i.v.t.) of bevacizumab promoted primary tumor growth in mice [173] and humans [174], which is unexpected and paradoxical. The reasons why bevacizumab (i.v.t.) is ineffective remain unclear. Nonetheless, systemic treatment with anti-VEGF drugs seems to be effective for UM patients with metastasis [156,157], and combinations with stereotactic body radiation therapy (NCT03712904), Nab-Paclitaxel (Abraxane, Bristol-Myers Squibb) (NCT02158520), and cemiplimab (Libtayo, anti-PD-1, Regeneron Pharmaceuticals) (NCT06121180) are in ongoing clinical trials.
In addition to the secreted factors, tumor-derived extracellular vesicles (TEVs) are known to bridge the communication between tumor cells and their microenvironment. UM TEVs contain proteins involved in several cell signaling pathways, including VEGF. UM-derived EVs have been shown to upregulate the expression of VEGF and contribute to increased vascularization through capillary-like networks in endothelial cells [53]. UM-derived EVs increased cell proliferation, migration, invasion, angiogenesis, and metastases compared to EVs from normal choroidal melanocytes [175]. As tumor cells escape into the circulatory system, these TEVs can transfer their material to neighboring cells, which favors tumorigenesis. In addition to UM dissemination, TEV use has been proposed for early detection of UM [53]. TEVs contain various biomarkers in other cancers and other types of clinical information [175]. There is hope that a non-invasive method of early diagnosis can be developed using EVs, which would significantly improve the prognosis of patients if detected earlier in the cancer’s progression.
6. Potential Therapies to Prevent Uveal Melanoma Metastases
In preclinical animal models, more strategies to prevent UM metastasis have been tested. In a study by Nhàn et al., we determined that UM cells secrete VEGF to induce endothelial permeability, which facilitated UM cell transmigration across the endothelium [8]. Among the UM cell lines tested, transendothelial migration of MP41 (GNA11Q209L) and 92.1 (GNAQQ209L with EIF1AXG6D) were inhibited by anti-VEGF treatment, whereas Mel202 (GNAQQ209L/R210K, CDKN2AL65R, SF3B1R625G) was not inhibited, suggesting reliance on other pathways. Previously, we developed a novel peptide, KAI, that inhibits the trafficking of the receptor for VEGF, VEGFR2, to the cell surface, thereby blocking VEGF/VEGFR2 signaling [176,177]. Systemic treatment with KAI was found to effectively inhibit tumor angiogenesis and extravasation of skin melanoma in mice [177]. In the animal model of orthotopically injected UM, treatment of tumor-bearing mice with daily eyedrops of KAI reduced the number of mice with circulating tumor cells (CTCs) compared to the no-treatment group, indicating inhibition of UM cells escaping from the eyes by KAI treatment [8]. As the intravasation of UM cells into circulation is an initial step of metastasis, its inhibition can be one of the effective strategies for inhibiting metastasis in distal organs. Other effective strategies include the inhibition of the transition of dormant cancer cells into colonization and the inhibition of cancer growth in the distal organs.
7. Methods
We used PubMed to search for the literature describing “uveal melanoma” and “therapy”. The search provided us with 3738 results from 1956 to March 2025. Among them, we read the literature written in English and cited the relevant literature in this review. For recent clinical trials which have not been published yet, we obtained information from ClinicalTrials.gov by searching “uveal melanoma” as a condition/disease and retrieved 238 trials.
8. Conclusions
Current therapies for UM include the treatment of primary tumors, mainly by radiotherapy and surgical resection. Despite successful control of primary tumors, 50% of UM patients still experience metastasis in the liver. Once metastasized, liver-directed therapies and/or recently FDA-approved tebentafusp can be employed, which is beneficial for a certain population of UM patients. However, robust strategies for preventing UM metastasis represent a critical unmet need. Although recent clinical trials and preclinical studies showed potential for targeting the VEGF pathway, further investigation of heterogeneity of UM cells with different mutations and different responses to anti-VEGF therapy is needed in the future.
Author Contributions
Conceptualization, K.H.Y.; writing—original draft preparation, S.S. and S.G.; writing—review and editing, K.H.Y.; funding acquisition, K.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by American Cancer Society AHEAD-DBG-23-1156429-01-MM to K.H.Y. and National Institute of Health (NIH) R01EY029339 to K.H.Y.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 6MHP | 6 melanoma helper peptides |
| ASS | Argininosuccinate synthetase |
| BAP1 | BRCA1 Associated Protein-1 |
| CRP | C-reactive protein |
| CTC | Circulating tumor cells |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CVA21 | Coxsackievirus A21 |
| DAF | Decay-accelerating factor |
| DC | Dendritic cells |
| EIF1AX | Eukaryotic translation initiation factor 1A |
| FAK | Focal adhesion kinase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GNA | Guanine Nucleotide-Binding Protein Alpha Subunit |
| GPCR | G protein-coupled receptors |
| HDAC | Histone deacetylase |
| HDS | Hepatic delivery system |
| HLA | Human leukocyte antigen |
| HSV-tk | Herpes simplex virus thymidine kinase |
| IFN | Interferon |
| IHP | Isolated hepatic perfusion |
| IL | Interleukin |
| i.v.t | Intravitreal injection |
| MAPK | Mitogen-activated protein kinase |
| MC1R | Melanocortin-1 receptor |
| NK | Natural killer |
| OS | Overall survival |
| PARP | Poly ADP-ribose polymerase |
| PD-1 | Programmed death receptor-1 |
| PFS | Progression-free survival |
| PHP | Percutaneous hepatic perfusion |
| PK | Pharmacokinetics |
| PMEL | Premelanosome protein |
| RFS | Recurrence-free survival |
| SF3B1 | Splicing factor 3b subunit 1 |
| SIRT | Selective internal radiotherapy |
| TACE | Transarterial chemoembolization |
| TEV | Tumor-derived extracellular vesicle |
| TIL | Tumor-infiltrating lymphocytes |
| TLR | Toll-like receptor |
| TYRP1 | Tyrosinase-related protein 1 |
| UM | Uveal melanoma |
| VEGF | Vascular endothelial growth factor |
References
- Toro, M.D.; Gozzo, L.; Tracia, L.; Cicciù, M.; Drago, F.; Bucolo, C.; Avitabile, T.; Rejdak, R.; Nowomiejska, K.; Zweifel, S.; et al. New Therapeutic Perspectives in the Treatment of Uveal Melanoma: A Systematic Review. Biomedicines 2021, 9, 1311. [Google Scholar] [CrossRef]
- Lane, A.M.; Kim, I.K.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986. [Google Scholar] [CrossRef]
- Foss, A.J.; Alexander, R.A.; Jefferies, L.W.; Hungerford, J.L.; Harris, A.L.; Lightman, S. Microvessel Count Predicts Survival in Uveal Melanoma. Cancer Res. 1996, 56, 2900–2903. [Google Scholar] [PubMed]
- Mäkitie, T.; Summanen, P.; Tarkkanen, A.; Kivelä, T. Microvascular Density in Predicting Survival of Patients with Choroidal and Ciliary Body Melanoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2471–2480. [Google Scholar]
- Missotten, G.S.O.; Notting, I.C.; Schlingemann, R.O.; Zijlmans, H.J.; Lau, C.; Eilers, P.H.C.; Keunen, J.E.E.; Jager, M.J. Vascular Endothelial Growth Factor A in Eyes with Uveal Melanoma. Arch. Ophthalmol. 2006, 124, 1428–1434. [Google Scholar] [CrossRef]
- el Filali, M.; Missotten, G.S.; Maat, W.; Ly, L.V.; Luyten, G.P.; van der Velden, P.A.; Jager, M.J. Regulation of VEGF-A in Uveal Melanoma. Investig. Opthalmology. Vis. Sci. 2010, 51, 2329–2337. [Google Scholar] [CrossRef]
- Barak, V.; Pe’er, J.; Kalickman, I.; Frenkel, S. VEGF as a Biomarker for Metastatic Uveal Melanoma in Humans. Curr. Eye Res. 2011, 36, 386–390. [Google Scholar] [CrossRef]
- Nhàn, N.T.T.; Ganesh, S.; Maidana, D.E.; Heiferman, M.J.; Yamada, K.H. Uveal Melanoma with a GNA11/GNAQ Mutation Secretes VEGF for Systemic Spread. Signal Transduct. Target. Ther. 2025, 10, 51. [Google Scholar] [CrossRef]
- van der Kooij, M.K.; Speetjens, F.M.; van der Burg, S.H.; Kapiteijn, E. Uveal Versus Cutaneous Melanoma; Same Origin, Very Distinct Tumor Types. Cancers 2019, 11, 845. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal Melanoma: Estimating Prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef]
- Ny, L.; Hernberg, M.; Nyakas, M.; Koivunen, J.; Oddershede, L.; Yoon, M.; Wang, X.; Guyot, P.; Geisler, J. BRAF Mutational Status as a Prognostic Marker for Survival in Malignant Melanoma: A Systematic Review and Meta-Analysis. Acta Oncol. 2020, 59, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Spathis, A.; Katoulis, A.C.; Damaskou, V.; Liakou, A.I.; Kottaridi, C.; Leventakou, D.; Sgouros, D.; Mamantopoulos, A.; Rigopoulos, D.; Karakitsos, P.; et al. BRAF Mutation Status in Primary, Recurrent, and Metastatic Malignant Melanoma and Its Relation to Histopathological Parameters. Dermatol. Pract. Concept. 2019, 9, 54–62. [Google Scholar] [CrossRef]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic Significance of BRAF and NRAS Mutations in Melanoma: A German Study from Routine Care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-Genome Landscapes of Major Melanoma Subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Colombino, M.; Capone, M.; Lissia, A.; Cossu, A.; Rubino, C.; Giorgi, V.D.; Massi, D.; Fonsatti, E.; Staibano, S.; Nappi, O.; et al. BRAF/NRAS Mutation Frequencies Among Primary Tumors and Metastases in Patients with Melanoma. J. Clin. Oncol. 2012, 30, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Carvajal, R.D. KIT as an Oncogenic Driver in Melanoma: An Update on Clinical Development. Am. J. Clin. Dermatol. 2019, 20, 315–323. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and Trametinib versus Dabrafenib and Placebo for Val600 BRAF-Mutant Melanoma: A Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Caroline, R.; Boguslawa, K.; Jacob, S.; Piotr, R.; Andrzej, M.; Daniil, S.; Michael, L.; Reinhard, D.; Florent, G.; Laurent, M.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Long, G.V.; Daniil, S.; Helen, G.; Evgeny, L.; de Filippo, B.; James, L.; Claus, G.; Thomas, J.; Axel, H.; Jacques, G.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Infante, J.R.; Adil, D.; Rene, G.; Kefford, R.F.; Jeffrey, S.; Omid, H.; Lynn, S.; Jonathan, C.; Nageatte, I.; et al. Combined BRAF and MEK Inhibition in Melanoma with BRAF V600 Mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- James, L.; Ascierto, P.A.; Brigitte, D.; Victoria, A.; Gabriella, L.; Michele, M.; Mario, M.; Lev, D.; Daniil, S.; Luc, T.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.-A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and Safety of Nilotinib in Patients with KIT-Mutated Metastatic or Inoperable Melanoma: Final Results from the Global, Single-Arm, Phase II TEAM Trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Si, L.; Kong, Y.; Flaherty, K.T.; Xu, X.; Zhu, Y.; Corless, C.L.; Li, L.; Li, H.; Sheng, X.; et al. Phase II, Open-Label, Single-Arm Trial of Imatinib Mesylate in Patients with Metastatic Melanoma Harboring c-Kit Mutation or Amplification. J. Clin. Oncol. 2011, 29, 2904–2909. [Google Scholar] [CrossRef]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for Melanomas Harboring Mutationally Activated or Amplified KIT Arising on Mucosal, Acral, and Chronically Sun-Damaged Skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, T.M.; Kim, Y.J.; Jang, K.; Lee, H.J.; Lee, S.N.; Ahn, M.S.; Hwang, I.G.; Lee, S.; Lee, M.; et al. Phase II Trial of Nilotinib in Patients with Metastatic Malignant Melanoma Harboring KIT Gene Aberration: A Multicenter Trial of Korean Cancer Study Group (UN10-06). Oncologist 2015, 20, 1312–1319. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in Uveal Melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Raamsdonk, C.D.V.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent Somatic Mutations of GNAQ in Uveal Melanoma and Blue Naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef]
- Arang, N.; Lubrano, S.; Ceribelli, M.; Rigiracciolo, D.C.; Saddawi-Konefka, R.; Faraji, F.; Ramirez, S.I.; Kim, D.; Tosto, F.A.; Stevenson, E.; et al. High-Throughput Chemogenetic Drug Screening Reveals PKC-RhoA/PKN as a Targetable Signaling Vulnerability in GNAQ-Driven Uveal Melanoma. Cell Rep. Med. 2023, 4, 101244. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Carlino, M.S.; Boni, V.; Loirat, D.; Speetjens, F.M.; Park, J.J.; Calvo, E.; Carvajal, R.D.; Nyakas, M.; Gonzalez-Maffe, J.; et al. A Phase I Trial of LXS196, a Protein Kinase C (PKC) Inhibitor, for Metastatic Uveal Melanoma. Br. J. Cancer 2023, 128, 1040–1051. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Khan, S.; Komatsubara, K.; Feun, L.; Acquavella, N.; Singh-Kandah, S.; Negri, T.; Nesson, A.; Abbate, K.; Cremers, S.; et al. A Phase Ib Study of Sotrastaurin, a PKC Inhibitor, and Alpelisib, a PI3Kα Inhibitor, in Patients with Metastatic Uveal Melanoma. Cancers 2021, 13, 5504. [Google Scholar] [CrossRef]
- Bauer, S.; Larkin, J.; Hodi, F.S.; Stephen, F.; Kapiteijn, E.H.W.; Schwartz, G.K.; Calvo, E.; Yerramilli-Rao, P.; Piperno-Neumann, S.; Carvajal, R.D. A Phase Ib Trial of Combined PKC and MEK Inhibition with Sotrastaurin and Binimetinib in Patients with Metastatic Uveal Melanoma. Front. Oncol. 2023, 12, 975642. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of Selumetinib vs Chemotherapy on Progression-Free Survival in Uveal Melanoma: A Randomized Clinical Trial. JAMA 2014, 311, 2397–2405. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination with Dacarbazine in Patients with Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef]
- Amaro, A.A.; Gangemi, R.; Emionite, L.; Castagnola, P.; Filaci, G.; Jager, M.J.; Tanda, E.T.; Spagnolo, F.; Mascherini, M.; Pfeffer, U.; et al. Cerivastatin Synergizes with Trametinib and Enhances Its Efficacy in the Therapy of Uveal Melanoma. Cancers 2023, 15, 886. [Google Scholar] [CrossRef]
- Ambrosini, G.; Khanin, R.; Carvajal, R.D.; Schwartz, G.K. Overexpression of DDX43 Mediates MEK Inhibitor Resistance through RAS Upregulation in Uveal Melanoma Cells. Mol. Cancer Ther. 2014, 13, 2073–2080. [Google Scholar] [CrossRef]
- Cheng, H.; Terai, M.; Kageyama, K.; Ozaki, S.; McCue, P.A.; Sato, T.; Aplin, A.E. Paracrine Effect of NRG1 and HGF Drives Resistance to MEK Inhibitors in Metastatic Uveal Melanoma. Cancer Res. 2015, 75, 2737–2748. [Google Scholar] [CrossRef]
- Mergener, S.; Siveke, J.T.; Peña-Llopis, S. Monosomy 3 Is Linked to Resistance to MEK Inhibitors in Uveal Melanoma. Int. J. Mol. Sci. 2021, 22, 6727. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent Mutation of BAP1 in Metastasizing Uveal Melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Karlsson, J.; Nilsson, L.M.; Mitra, S.; Alsén, S.; Shelke, G.V.; Sah, V.R.; Forsberg, E.M.V.; Stierner, U.; All-Eriksson, C.; Einarsdottir, B.; et al. Molecular Profiling of Driver Events in Metastatic Uveal Melanoma. Nat. Commun. 2020, 11, 1894. [Google Scholar] [CrossRef]
- Uner, O.E.; See, T.R.O.; Szalai, E.; Grossniklaus, H.E.; Stålhammar, G. Estimation of the Timing of BAP1 Mutation in Uveal Melanoma Progression. Sci. Rep. 2021, 11, 8923. [Google Scholar] [CrossRef]
- Helgadottir, H.; Höiom, V. The Genetics of Uveal Melanoma: Current Insights. Appl. Clin. Genet. 2016, 9, 147–155. [Google Scholar] [CrossRef]
- Jensen, D.E.; Proctor, M.; Marquis, S.T.; Gardner, H.P.; Ha, S.I.; Chodosh, L.A.; Ishov, A.M.; Tommerup, N.; Vissing, H.; Sekido, Y.; et al. BAP1: A Novel Ubiquitin Hydrolase Which Binds to the BRCA1 RING Finger and Enhances BRCA1-Mediated Cell Growth Suppression. Oncogene 1998, 16, 1097–1112. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of Uveal Melanoma Millimeter-by-Millimeter in 8033 Consecutive Eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Gelmi, M.C.; Jager, M.J. Uveal Melanoma: Current Evidence on Prognosis, Treatment and Potential Developments. Asia-Pac. J. Ophthalmol. 2024, 13, 100060. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Yin, H. The Future of Checkpoint Inhibitors in Uveal Melanoma: A Narrative Review. Ophthalmol. Ther. 2024, 13, 1103–1123. [Google Scholar] [CrossRef]
- Martin, M.; Maßhöfer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; van de Nes, J.; Klein-Hitpass, L.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome Sequencing Identifies Recurrent Somatic Mutations in EIF1AX and SF3B1 in Uveal Melanoma with Disomy. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal Melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of Uveal Melanoma: Where Are We Now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Heppt, M.V.; Berking, C. The Current State of Systemic Therapy of Metastatic Uveal Melanoma. Am. J. Clin. Dermatol. 2024, 25, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: V. Twelve-Year Mortality Rates and Prognostic Factors: COMS Report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Kulbay, M.; Marcotte, E.; Remtulla, R.; Lau, T.H.A.; Paez-Escamilla, M.; Wu, K.Y.; Burnier, M.N. Uveal Melanoma: Comprehensive Review of Its Pathophysiology, Diagnosis, Treatment, and Future Perspectives. Biomedicines 2024, 12, 1758. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Fuller, D.; Anand, R.; Fuller, T.; Munoz, J.; Moore, C.; Kim, R.S.; Schefler, A.C.; Bretana, M.E.; Diugnan, K. Two-Year Results for Ranibizumab for Radiation Retinopathy (RRR): A Randomized, Prospective Trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 47–54. [Google Scholar] [CrossRef]
- Horgan, N.; Shields, C.L.; Mashayekhi, A.; Teixeira, L.F.; Materin, M.A.; O’Regan, M.; Shields, J.A. Periocular Triamcinolone for Prevention of Macular Edema After Iodine 125 Plaque Radiotherapy of Uveal Melanoma. Retina 2008, 28, 987–995. [Google Scholar] [CrossRef]
- Horgan, N.; Shields, C.L.; Mashayekhi, A.; Salazar, P.F.; Materin, M.A.; O’Regan, M.; Shields, J.A. Periocular Triamcinolone for Prevention of Macular Edema after Plaque Radiotherapy of Uveal Melanoma A Randomized Controlled Trial. Ophthalmology 2009, 116, 1383–1390. [Google Scholar] [CrossRef]
- Parrozzani, R.; Pilotto, E.; Dario, A.; Miglionico, G.; Midena, E. Intravitreal Triamcinolone Versus Intravitreal Bevacizumab in the Treatment of Exudative Retinal Detachment Secondary to Posterior Uveal Melanoma. Am. J. Ophthalmol. 2013, 155, 127–133.e2. [Google Scholar] [CrossRef]
- Shah, N.V.; Houston, S.K.; Markoe, A.; Murray, T.G. Combination Therapy with Triamcinolone Acetonide and Bevacizumab for the Treatment of Severe Radiation Maculopathy in Patients with Posterior Uveal Melanoma. Clin. Ophthalmol. 2013, 7, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Hager, A.; Riechardt, A.I.; Davids, A.M.; Böker, A.; Joussen, A.M. Antiangiogenic or Corticosteroid Treatment in Patients with Radiation Maculopathy After Proton Beam Therapy for Uveal Melanoma. Am. J. Ophthalmol. 2016, 168, 31–39. [Google Scholar] [CrossRef]
- Koc, I.; Kadayifcilar, S.; Kiratli, H.; Eldem, B. Intravitreal dexamethasone (ozurdex) implant for radiation maculopathy secondary to stereotactic radiotherapy for posterior uveal melanoma. Retin. Cases Brief Rep. 2017, 13, 352–356. [Google Scholar] [CrossRef]
- Kowal, J.; Markiewicz, A.; Dębicka-Kumela, M.; Bogdali, A.; Jakubowska, B.; Karska-Basta, I.; Romanowska-Dixon, B. Analysis of Local Recurrence Causes in Uveal Melanoma Patients Treated with 125I Brachytherapy—A Single Institution Study. J. Contemp. Brachyther. 2019, 11, 554–562. [Google Scholar] [CrossRef]
- Force, T.O.O.T.; Gallie, B.L.; Simpson, E.R.; Saakyan, S.; Amiryan, A.; Valskiy, V.; Finger, P.T.; Chin, K.J.; Semenova, E.; Seregard, S.; et al. Local Recurrence Significantly Increases the Risk of Metastatic Uveal Melanoma. Ophthalmology 2016, 123, 86–91. [Google Scholar] [CrossRef]
- Hussain, R.N.; Chiu, A.; Pittam, B.; Taktak, A.; Damato, B.E.; Kacperek, A.; Errington, D.; Cauchi, P.; Chadha, V.; Connolly, J.; et al. Proton Beam Radiotherapy for Choroidal and Ciliary Body Melanoma in the UK—National Audit of Referral Patterns of 1084 Cases. Eye 2022, 37, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.V.; Aronow, M.E.; Gragoudas, E.S.; Keane, F.K.; Kim, I.K.; Shih, H.A.; Bhagwat, M.S. A Systematic Comparison of Dose Distributions Delivered in 125I Plaque Brachytherapy and Proton Radiation Therapy for Ocular Melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 501–510. [Google Scholar] [CrossRef]
- Marinkovic, M.; Pors, L.J.; van den Berg, V.; Peters, F.P.; Schalenbourg, A.; Zografos, L.; Pica, A.; Hrbacek, J.; Duinen, S.G.V.; Vu, T.H.K.; et al. Clinical Outcomes after International Referral of Uveal Melanoma Patients for Proton Therapy. Cancers 2021, 13, 6241. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.M.; Hartley, C.; Go, A.K.; Wu, F.; Gragoudas, E.S.; Kim, I.K. Survival of Patients with Recurrent Uveal Melanoma after Treatment with Radiation Therapy. Br. J. Ophthalmol. 2024, 108, 729–734. [Google Scholar] [CrossRef]
- Lane, A.M.; Oxenreiter, M.M.; Hashmi, M.; Aronow, M.E.; Trofimov, A.V.; Shih, H.A.; Gragoudas, E.S.; Kim, I.K. A Comparison of Treatment Outcomes after Standard Dose (70 Gy) versus Reduced Dose (50 Gy) Proton Radiation in Patients with Uveal Melanoma. Ophthalmol. Retin. 2022, 6, 1089–1097. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L. Management of Posterior Uveal Melanoma: Past, Present, and Future The 2014 Charles L. Schepens Lecture. Ophthalmology 2015, 122, 414–428. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A. Albert and Jakobiec’s Principles and Practice of Ophthalmology. Springer: Cham, Switzerland, 2022; pp. 7717–7727. [Google Scholar] [CrossRef]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R.; Group, C.O.M.S. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma, III: Initial Mortality Findings: COMS Report No. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef]
- Chang, M.Y.; McCannel, T.A. Local Treatment Failure after Globe-Conserving Therapy for Choroidal Melanoma. Br. J. Ophthalmol. 2013, 97, 804. [Google Scholar] [CrossRef]
- Sarode, D.; McClay, T.; Roberts, F.; Connolly, J.; Cauchi, P.; Chadha, V. Post-Enucleation Outcomes of Patients with Uveal Melanoma in Scotland. Eye 2022, 37, 988–994. [Google Scholar] [CrossRef]
- Heng, J.S.; Perzia, B.M.; Sinard, J.H.; Pointdujour-Lim, R. Local Recurrence of Uveal Melanoma and Concomitant Brain Metastases Associated with an Activating Telomerase Promoter Mutation Seven Years after Secondary Enucleation. Am. J. Ophthalmol. Case Rep. 2022, 27, 101607. [Google Scholar] [CrossRef]
- Zager, J.S.; Orloff, M.; Ferrucci, P.F.; Choi, J.; Eschelman, D.J.; Glazer, E.S.; Ejaz, A.; Howard, J.H.; Richtig, E.; Ochsenreither, S.; et al. Efficacy and Safety of the Melphalan/Hepatic Delivery System in Patients with Unresectable Metastatic Uveal Melanoma: Results from an Open-Label, Single-Arm, Multicenter Phase 3 Study. Ann. Surg. Oncol. 2024, 31, 5340–5351. [Google Scholar] [CrossRef]
- Schank, T.E.; Hassel, J.C. Immunotherapies for the Treatment of Uveal Melanoma—History and Future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef]
- Schank, T.E.; Hassel, J.C. Tebentafusp for the Treatment of Metastatic Uveal Melanoma. Future Oncol. 2022, 18, 1303–1311. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Butler, M.O.; Shoushtari, A.N.; Hassel, J.C.; Ikeguchi, A.; Hernandez-Aya, L.; Nathan, P.; Hamid, O.; Piulats, J.M.; Rioth, M.; et al. Clinical and Molecular Response to Tebentafusp in Previously Treated Patients with Metastatic Uveal Melanoma: A Phase 2 Trial. Nat. Med. 2022, 28, 2364–2373. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Montazeri, K.; Pattanayak, V.; Sullivan, R.J. Tebentafusp in the Treatment of Metastatic Uveal Melanoma: Patient Selection and Special Considerations. Drug Des. Dev. Ther. 2023, 17, 333–339. [Google Scholar] [CrossRef]
- Sacco, J.J.; Carvajal, R.D.; Butler, M.O.; Shoushtari, A.N.; Hassel, J.C.; Ikeguchi, A.; Hernandez-Aya, L.; Nathan, P.; Hamid, O.; Piulats, J.M.; et al. Long-Term Survival Follow-up for Tebentafusp in Previously Treated Metastatic Uveal Melanoma. J. Immunother. Cancer 2024, 12, e009028. [Google Scholar] [CrossRef]
- Hassel, J.C.; Stanhope, S.; Greenshields-Watson, A.; Machiraju, D.; Enk, A.; Holland, C.; Abdullah, S.E.; Benlahrech, A.; Orloff, M.; Nathan, P.; et al. Tebentafusp Induces a T-Cell–Driven Rash in Melanocyte-Bearing Skin as an Adverse Event Consistent with the Mechanism of Action. J. Investig. Dermatol. 2025, 145, 559–572.e9. [Google Scholar] [CrossRef]
- Güç, E.; Treveil, A.; Leach, E.; Broomfield, A.; Camera, A.; Clubley, J.; Garcia, P.N.; Kazachenka, A.; Khanolkar, R.; del Carpio, L.; et al. Tebentafusp, a T Cell Engager, Promotes Macrophage Reprogramming and in Combination with IL-2 Overcomes Macrophage Immunosuppression in Cancer. Nat. Commun. 2025, 16, 2374. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Liu, J.; Ren, E.C. Structural and Functional Distinctiveness of HLA-A2 Allelic Variants. Immunol. Res. 2012, 53, 182–190. [Google Scholar] [CrossRef]
- Sobczuk, P.; Cholewiński, M.; Rutkowski, P. Recent Advances in Tyrosine Kinase Inhibitors VEGFR 1-3 for the Treatment of Advanced Metastatic Melanoma. Expert Opin. Pharmacother. 2024, 25, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lutzky, J.; Shoushtari, A.N.; Jeter, J.; Marr, B.; Olencki, T.E.; Cebulla, C.M.; Abdel-Rahman, M.; Harbour, J.W.; Sender, N.; et al. Adjuvant Crizotinib in High-Risk Uveal Melanoma Following Definitive Therapy. Front. Oncol. 2022, 12, 976837. [Google Scholar] [CrossRef]
- Valsecchi, M.E.; Orloff, M.; Sato, R.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; Mastrangelo, M.J.; Sato, T. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma Comparison with Institutional Controls. Ophthalmology 2018, 125, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.W.; Tonogai, E.J.; Schane, C.P.; Xi, M.X.; Fischer, J.H.; Vijayakumar, J.; Ji, Y.; Tarasow, T.M.; Fan, T.M.; Hergenrother, P.J.; et al. The Combination of PAC-1 and Entrectinib for the Treatment of Metastatic Uveal Melanoma. Melanoma Res. 2023, 33, 514–524. [Google Scholar] [CrossRef]
- Daud, A.; Kluger, H.M.; Kurzrock, R.; Schimmoller, F.; Weitzman, A.L.; Samuel, T.A.; Moussa, A.H.; Gordon, M.S.; Shapiro, G.I. Phase II Randomised Discontinuation Trial of the MET/VEGF Receptor Inhibitor Cabozantinib in Metastatic Melanoma. Br. J. Cancer 2017, 116, 432–440. [Google Scholar] [CrossRef]
- Luke, J.J.; Olson, D.J.; Allred, J.B.; Strand, C.A.; Bao, R.; Zha, Y.; Carll, T.; Labadie, B.W.; Bastos, B.R.; Butler, M.O.; et al. Randomized Phase II Trial and Tumor Mutational Spectrum Analysis from Cabozantinib versus Chemotherapy in Metastatic Uveal Melanoma (Alliance A091201). Clin. Cancer Res. 2020, 26, 804–811. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Cohen, J.V.; Tarantino, G.; Lian, C.G.; Liu, D.; Haq, R.; Hodi, F.S.; Lawrence, D.P.; Giobbie-Hurder, A.; Knoerzer, D.; et al. A Phase II Study of ERK Inhibition by Ulixertinib (BVD-523) in Metastatic Uveal Melanoma. Cancer Res. Commun. 2024, 4, 1321–1327. [Google Scholar] [CrossRef]
- Mouriaux, F.; Servois, V.; Parienti, J.J.; Lesimple, T.; Thyss, A.; Dutriaux, C.; Neidhart-Berard, E.M.; Penel, N.; Delcambre, C.; Paul, L.P.S.; et al. Sorafenib in Metastatic Uveal Melanoma: Efficacy, Toxicity and Health-Related Quality of Life in a Multicentre Phase II Study. Br. J. Cancer 2016, 115, 20–24. [Google Scholar] [CrossRef]
- Bhatia, S.; Moon, J.; Margolin, K.A.; Weber, J.S.; Lao, C.D.; Othus, M.; Aparicio, A.M.; Ribas, A.; Sondak, V.K. Phase II Trial of Sorafenib in Combination with Carboplatin and Paclitaxel in Patients with Metastatic Uveal Melanoma: SWOG S0512. PLoS ONE 2012, 7, e48787. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Ong, L.T.; Schoder, H.; Singh-Kandah, S.; Abbate, K.T.; Postow, M.A.; Callahan, M.K.; Wolchok, J.; Chapman, P.B.; Panageas, K.S.; et al. A Phase 2 Trial of Everolimus and Pasireotide Long-Acting Release in Patients with Metastatic Uveal Melanoma. Melanoma Res. 2016, 26, 272–277. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Allred, J.B.; Slostad, J.A.; Katipamula, R.; Dronca, R.S.; Rumilla, K.M.; Erickson, L.A.; Bryce, A.H.; Joseph, R.W.; Kottschade, L.A.; et al. NCCTG N0879 (Alliance): A Randomized Phase 2 Cooperative Group Trial of Carboplatin, Paclitaxel, and Bevacizumab ± Everolimus for Metastatic Melanoma. Cancer 2018, 124, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cui, L.; Zhao, X.; Bai, H.; Cai, S.; Wang, G.; Zhao, Z.; Zhao, J.; Chen, S.; Song, J.; et al. Use of Immunotherapy with Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients with Cancer. JAMA Oncol. 2020, 6, 375–384. [Google Scholar] [CrossRef]
- Piulats, J.M.; Espinosa, E.; De la Cruz Merino, L.; Varela, M.; Carrión, L.A.; Martín-Algarra, S.; Castro, R.L.; Curiel, T.; Rodríguez-Abreu, D.; Redrado, M.; et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J. Clin. Oncol. 2021, 39, 586–598. [Google Scholar] [CrossRef]
- Pelster, M.S.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.S.; Simien, R.; Diab, A.; Hwu, P.; et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results from a Single-Arm Phase II Study. J. Clin. Oncol. 2021, 39, 599–607. [Google Scholar] [CrossRef]
- Tong, T.M.L.; Burgmans, M.C.; Speetjens, F.M.; van Erkel, A.R.; van der Meer, R.W.; van Rijswijk, C.S.P.; Jonker-Bos, M.A.; Roozen, C.F.M.; Sporrel-Blokland, M.; Lutjeboer, J.; et al. Combining Melphalan Percutaneous Hepatic Perfusion with Ipilimumab Plus Nivolumab in Advanced Uveal Melanoma: First Safety and Efficacy Data from the Phase Ib Part of the Chopin Trial. Cardiovasc. Interv. Radiol. 2023, 46, 350–359. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant Immune Checkpoint Blockade in High-Risk Resectable Melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef]
- Johnson, D.B.; Bao, R.; Ancell, K.K.; Daniels, A.B.; Wallace, D.; Sosman, J.A.; Luke, J.J. Response to Anti–PD-1 in Uveal Melanoma Without High-Volume Liver Metastasis. J. Natl. Compr. Cancer Netw. 2019, 17, 114–117. [Google Scholar] [CrossRef]
- Kitano, S.; Fujiwara, Y.; Shimizu, T.; Iwasa, S.; Yonemori, K.; Kondo, S.; Shimomura, A.; Koyama, T.; Ebata, T.; Ikezawa, H.; et al. A Feasibility Study of Lenvatinib plus Pembrolizumab in Japanese Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2022, 90, 523–529. [Google Scholar] [CrossRef]
- Ny, L.; Jespersen, H.; Karlsson, J.; Alsén, S.; Filges, S.; All-Eriksson, C.; Andersson, B.; Carneiro, A.; Helgadottir, H.; Levin, M.; et al. The PEMDAC Phase 2 Study of Pembrolizumab and Entinostat in Patients with Metastatic Uveal Melanoma. Nat. Commun. 2021, 12, 5155. [Google Scholar] [CrossRef]
- Jespersen, H.; Bagge, R.O.; Ullenhag, G.; Carneiro, A.; Helgadottir, H.; Ljuslinder, I.; Levin, M.; All-Eriksson, C.; Andersson, B.; Stierner, U.; et al. Concomitant Use of Pembrolizumab and Entinostat in Adult Patients with Metastatic Uveal Melanoma (PEMDAC Study): Protocol for a Multicenter Phase II Open Label Study. BMC Cancer 2019, 19, 415. [Google Scholar] [CrossRef]
- Kuznetsov, J.N.; Aguero, T.H.; Owens, D.A.; Kurtenbach, S.; Field, M.G.; Durante, M.A.; Rodriguez, D.A.; King, M.L.; Harbour, J.W. BAP1 Regulates Epigenetic Switch from Pluripotency to Differentiation in Developmental Lineages Giving Rise to BAP1-Mutant Cancers. Sci. Adv. 2019, 5, eaax1738. [Google Scholar] [CrossRef]
- Masclef, L.; Ahmed, O.; Estavoyer, B.; Larrivée, B.; Labrecque, N.; Nijnik, A.; Affar, E.B. Roles and Mechanisms of BAP1 Deubiquitinase in Tumor Suppression. Cell Death Differ. 2021, 28, 606–625. [Google Scholar] [CrossRef]
- Hamid, O.; Hassel, J.C.; Shoushtari, A.N.; Meier, F.; Bauer, T.M.; Salama, A.K.S.; Kirkwood, J.M.; Ascierto, P.A.; Lorigan, P.C.; Mauch, C.; et al. Tebentafusp in Combination with Durvalumab and/or Tremelimumab in Patients with Metastatic Cutaneous Melanoma: A Phase 1 Study. J. Immunother. Cancer 2023, 11, e006747. [Google Scholar] [CrossRef]
- Dummer, R.; Long, G.V.; Robert, C.; Tawbi, H.A.; Flaherty, K.T.; Ascierto, P.A.; Nathan, P.D.; Rutkowski, P.; Leonov, O.; Dutriaux, C.; et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022, 40, 1428–1438. [Google Scholar] [CrossRef]
- Wu, J.; Bloch, N.; Chang, A.Y.; Bhavsar, R.; Wang, Q.; Crawford, A.; DiLillo, D.J.; Vazzana, K.; Mohrs, K.; Dudgeon, D.; et al. A PD-1-Targeted, Receptor-Masked IL-2 Immunocytokine That Engages IL-2Rα Strengthens T Cell-Mediated Anti-Tumor Therapies. Cell Rep. Med. 2024, 5, 101747. [Google Scholar] [CrossRef]
- Akce, M.; Hu-Lieskovan, S.; Reilley, M.; Strauss, J.F.; Specht, J.M.; Stein, M.N.; Wang, J.S.; Choe, J.H.; Leidner, R.; Davar, D.; et al. A Phase 1 Multiple-Ascending Dose Study to Evaluate the Safety and Tolerability of XmAb23104 (PD-1 x ICOS) in Subjects with Selected Advanced Solid Tumors (DUET-3). J. Clin. Oncol. 2022, 40, 2604. [Google Scholar] [CrossRef]
- Jacob, S.; Daud, A. Phase Ib/II Study of XmAb23104 (PD1 X ICOS) and XmAb22841 (CTLA-4 X LAG3) Combination in Metastatic Melanoma Refractory to Prior Immune Checkpoint Inhibitor Therapy with and without CNS Disease. J. Clin. Oncol. 2023, 41, TPS9595. [Google Scholar] [CrossRef]
- Moore, G.L.; Zeng, V.G.; Diaz, J.E.; Bonzon, C.; Avery, K.N.; Rashid, R.; Qi, J.; Nam, D.H.; Jacinto, J.; Dragovich, M.A.; et al. A B7-H3-Targeted CD28 Bispecific Antibody Enhances the Activity of Anti-PD1 and CD3 T-Cell Engager Immunotherapies. Mol. Cancer Ther. 2024, 24, 331–344. [Google Scholar] [CrossRef]
- Joshua, A.M.; Monzon, J.G.; Mihalcioiu, C.; Hogg, D.; Smylie, M.; Cheng, T. A Phase 2 Study of Tremelimumab in Patients with Advanced Uveal Melanoma. Melanoma Res. 2015, 25, 342–347. [Google Scholar] [CrossRef]
- Sánchez, J.; Nicolini, V.; Fahrni, L.; Waldhauer, I.; Walz, A.-C.; Jamois, C.; Fowler, S.; Simon, S.; Klein, C.; Umaña, P.; et al. Preclinical InVivo Data Integrated in a Modeling Network Informs a Refined Clinical Strategy for a CD3 T-Cell Bispecific in Combination with Anti-PD-L1. AAPS J. 2022, 24, 106. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, A.; Couselo, E.M.; Irmisch, A.; Bessa, J.; Au-Yeung, G.; Bechter, O.; Svane, I.M.; Sanmamed, M.F.; Gambardella, V.; McKean, M.; et al. Phase 1, First-in-Human Study of TYRP1-TCB (RO7293583), a Novel TYRP1-Targeting CD3 T-Cell Engager, in Metastatic Melanoma: Active Drug Monitoring to Assess the Impact of Immune Response on Drug Exposure. Front. Oncol. 2024, 14, 1346502. [Google Scholar] [CrossRef]
- Ninmer, E.K.; Xu, F.; Slingluff, C.L. The Landmark Series: Cancer Vaccines for Solid Tumors. Ann. Surg. Oncol. 2025, 32, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.S.; Olson, W.C.; Pero, S.C.; Sun, Y.; Carman, C.L.; Slingluff, C.L.; Krag, D.N. Vaccine-Draining Lymph Nodes of Cancer Patients for Generating Anti-Cancer Antibodies. J. Transl. Med. 2017, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.-T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data from Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef]
- Schaefer, J.T.; Patterson, J.W.; Deacon, D.H.; Smolkin, M.E.; Petroni, G.R.; Jackson, E.M.; Slingluff, C.L. Dynamic Changes in Cellular Infiltrates with Repeated Cutaneous Vaccination: A Histologic and Immunophenotypic Analysis. J. Transl. Med. 2010, 8, 79. [Google Scholar] [CrossRef]
- Yuan, J.; Ku, G.Y.; Adamow, M.; Mu, Z.; Tandon, S.; Hannaman, D.; Chapman, P.; Schwartz, G.; Carvajal, R.; Panageas, K.S.; et al. Immunologic Responses to Xenogeneic Tyrosinase DNA Vaccine Administered by Electroporation in Patients with Malignant Melanoma. J. Immunother. Cancer 2013, 1, 20. [Google Scholar] [CrossRef]
- Binkley, E.; Triozzi, P.L.; Rybicki, L.; Achberger, S.; Aldrich, W.; Singh, A. A Prospective Trial of Adjuvant Therapy for High-Risk Uveal Melanoma: Assessing 5-Year Survival Outcomes. Br. J. Ophthalmol. 2020, 104, 524. [Google Scholar] [CrossRef]
- Sato, T.; Eschelman, D.J.; Gonsalves, C.F.; Terai, M.; Chervoneva, I.; McCue, P.A.; Shields, J.A.; Shields, C.L.; Yamamoto, A.; Berd, D.; et al. Immunoembolization of Malignant Liver Tumors, Including Uveal Melanoma, Using Granulocyte-Macrophage Colony-Stimulating Factor. J. Clin. Oncol. 2008, 26, 5436–5442. [Google Scholar] [CrossRef]
- Yamamoto, A.; Chervoneva, I.; Sullivan, K.L.; Eschelman, D.J.; Gonsalves, C.F.; Mastrangelo, M.J.; Berd, D.; Shields, J.A.; Shields, C.L.; Terai, M.; et al. High-Dose Immunoembolization: Survival Benefit in Patients with Hepatic Metastases from Uveal Melanoma. Radiology 2009, 252, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Terai, M.; Eschelman, D.J.; Gonsalves, C.F.; Chervoneva, I.; Shields, J.A.; Shields, C.L.; Yamamoto, A.; Sullivan, K.L.; Laudadio, M.; et al. Double-Blinded, Randomized Phase II Study Using Embolization with or without Granulocyte–Macrophage Colony-Stimulating Factor in Uveal Melanoma with Hepatic Metastases. J. Vasc. Interv. Radiol. 2015, 26, 523–532.e2. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.A.; Yang, J.C.; Sherry, R.M.; Hughes, M.S.; Kammula, U.S.; White, D.E.; Levy, C.L.; Rosenberg, S.A.; Phan, G.Q. CTLA-4 Blockade with Ipilimumab: Long-Term Follow-up of 177 Patients with Metastatic Melanoma. Clin. Cancer Res. 2012, 18, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Thompson, P.A.; Burger, J.A.; Yilmaz, M.; Jain, N.; Kadia, T.M.; Masarova, L.; Valero, Y.A.; Bose, P.; Ferrajoli, A.; et al. Early Obinutuzumab Significantly Increases Bone Marrow Undetectable MRD (10-4 Sensitivity) (UMRD4) Rate When Combined with Acalabrutinib and Venetoclax in a Randomized Phase II Trial for Treatment Naïve CLL. Blood 2024, 144, 1855. [Google Scholar] [CrossRef]
- Coveler, A.L.; Smith, D.C.; Phillips, T.; Curti, B.D.; Goel, S.; Mehta, A.N.; Kuzel, T.M.; Markovic, S.N.; Rixe, O.; Bajor, D.L.; et al. Phase 1 Dose-Escalation Study of SEA-CD40: A Non-Fucosylated CD40 Agonist, in Advanced Solid Tumors and Lymphomas. J. Immunother. Cancer 2023, 11, e005584. [Google Scholar] [CrossRef]
- Fa’ak, F.; Buni, M.; Falohun, A.; Lu, H.; Song, J.; Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Awiwi, M.O.; Tahon, N.H.; et al. Selective Immune Suppression Using Interleukin-6 Receptor Inhibitors for Management of Immune-Related Adverse Events. J. Immunother. Cancer 2023, 11, e006814. [Google Scholar] [CrossRef]
- Ghosh, C.C.; Cournoyer, L.; Liu, Y.; Ballarin, A.; Layman, I.B.; LaPorte, J.; Morrissey, M.; Fraser, K.; Perati, S.; Cox, B.F.; et al. Subcutaneous Checkpoint Inhibition Is Equivalent to Systemic Delivery When Combined with Nelitolimod Delivered via Pressure-Enabled Drug Delivery for Depletion of Intrahepatic Myeloid-Derived Suppressor Cells and Control of Liver Metastases. J. Immunother. Cancer 2024, 12, e008837. [Google Scholar] [CrossRef]
- Leyvraz, S.; Piperno-Neumann, S.; Suciu, S.; Baurain, J.F.; Zdzienicki, M.; Testori, A.; Marshall, E.; Scheulen, M.; Jouary, T.; Negrier, S.; et al. Hepatic Intra-Arterial versus Intravenous Fotemustine in Patients with Liver Metastases from Uveal Melanoma (EORTC 18021): A Multicentric Randomized Trial. Ann. Oncol. 2014, 25, 742–746. [Google Scholar] [CrossRef]
- Bradley, M.O.; Swindell, C.S.; Anthony, F.H.; Witman, P.A.; Devanesan, P.; Webb, N.L.; Baker, S.D.; Wolff, A.C.; Donehower, R.C. Tumor Targeting by Conjugation of DHA to Paclitaxel. J. Control. Release 2001, 74, 233–236. [Google Scholar] [CrossRef]
- Homsi, J.; Bedikian, A.Y.; Papadopoulos, N.E.; Kim, K.B.; Hwu, W.-J.; Mahoney, S.L.; Hwu, P. Phase 2 Open-Label Study of Weekly Docosahexaenoic Acid–Paclitaxel in Patients with Metastatic Uveal Melanoma. Melanoma Res. 2010, 20, 507–510. [Google Scholar] [CrossRef]
- Blajeski, A.L.; Phan, V.A.; Kottke, T.J.; Kaufmann, S.H. G1 and G2 Cell-Cycle Arrest Following Microtubule Depolymerization in Human Breast Cancer Cells. J. Clin. Investig. 2002, 110, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Silverman, J.A.; Papadopoulos, N.E.; Kim, K.B.; Hagey, A.E.; Vardeleon, A.; Hwu, W.; Homsi, J.; Davies, M.; Hwu, P. Pharmacokinetics and Safety of Marqibo (Vincristine Sulfate Liposomes Injection) in Cancer Patients with Impaired Liver Function. J. Clin. Pharmacol. 2011, 51, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of Metastatic Uveal Melanoma with Adoptive Transfer of Tumour-Infiltrating Lymphocytes: A Single-Centre, Two-Stage, Single-Arm, Phase 2 Study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- Koch, E.A.T.; Schaft, N.; Kummer, M.; Berking, C.; Schuler, G.; Hasumi, K.; Dörrie, J.; Schuler-Thurner, B. A One-Armed Phase I Dose Escalation Trial Design: Personalized Vaccination with IKKβ-Matured, RNA-Loaded Dendritic Cells for Metastatic Uveal Melanoma. Front. Immunol. 2022, 13, 785231. [Google Scholar] [CrossRef]
- Leonard-Murali, S.; Kammula, U.S. Optimizing TIL Therapy for Uveal Melanoma: Lessons Learned and Unlearned from Cutaneous Melanoma. Immunotherapy 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Moreno, R.; Gil-Martin, M.; Cascallò, M.; de Olza, M.O.; Cuadra, C.; Piulats, J.M.; Navarro, V.; Domenech, M.; Alemany, R.; et al. A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients. Hum. Gene Ther. 2019, 30, 352–364. [Google Scholar] [CrossRef]
- Ghajar-Rahimi, G.; Kang, K.-D.; Totsch, S.K.; Gary, S.; Rocco, A.; Blitz, S.; Kachurak, K.; Chambers, M.R.; Li, R.; Beierle, E.A.; et al. Clinical Advances in Oncolytic Virotherapy for Pediatric Brain Tumors. Pharmacol. Ther. 2022, 239, 108193. [Google Scholar] [CrossRef]
- Au, G.; Lindberg, A.; Barry, R.; Shafren, D. Oncolysis of Vascular Malignant Human Melanoma Tumors by Coxsackievirus A21. Int. J. Oncol. 2005, 26, 1471–1476. [Google Scholar] [CrossRef]
- Lutzky, J.; Sullivan, R.J.; Cohen, J.V.; Ren, Y.; Li, A.; Haq, R. Phase 1b Study of Intravenous Coxsackievirus A21 (V937) and Ipilimumab for Patients with Metastatic Uveal Melanoma. J. Cancer Res. Clin. Oncol. 2023, 149, 6059–6066. [Google Scholar] [CrossRef]
- Hasenburg, A.; Tong, X.W.; Rojas-Martinez, A.; Nyberg-Hoffman, C.; Kieback, C.C.; Kaplan, A.L.; Kaufman, R.H.; Ramzy, I.; Aguilar-Cordova, E.; Kieback, D.G. Thymidine Kinase (TK) Gene Therapy of Solid Tumors: Valacyclovir Facilitates Outpatient Treatment. Anticancer Res. 1999, 19, 2163–2165. [Google Scholar]
- Ismail, I.H.; Davidson, R.; Gagné, J.-P.; Xu, Z.Z.; Poirier, G.G.; Hendzel, M.J. Germline Mutations in BAP1 Impair Its Function in DNA Double-Strand Break Repair. Cancer Res. 2014, 74, 4282–4294. [Google Scholar] [CrossRef] [PubMed]
- Muzzana, M.; Broggini, M.; Damia, G. The Landscape of PARP Inhibitors in Solid Cancers. OncoTargets Ther. 2025, 18, 297–317. [Google Scholar] [CrossRef]
- George, T.J.; Lee, J.-H.; DeRemer, D.L.; Hosein, P.J.; Staal, S.; Markham, M.J.; Jones, D.; Daily, K.C.; Chatzkel, J.A.; Ramnaraign, B.H.; et al. Phase II Trial of the PARP Inhibitor, Niraparib, in BAP1 and Other DNA Damage Response Pathway-Deficient Neoplasms. JCO Precis. Oncol. 2024, 8, e2400406. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.Y.; Phillips, M.M.; Ellis, S.; Johnston, A.; Feng, X.; Arora, A.; Hay, G.; Cohen, V.M.L.; Sagoo, M.S.; Bomalaski, J.S.; et al. A Phase 1 Study of ADI-PEG20 (Pegargiminase) Combined with Cisplatin and Pemetrexed in ASS1-negative Metastatic Uveal Melanoma. Pigment Cell Melanoma Res. 2022, 35, 461–470. [Google Scholar] [CrossRef]
- Khera, E.; Dharmarajan, L.; Hainzl, D.; Engelhardt, V.; Vostiarova, H.; Davis, J.; Ebel, N.; Wuersch, K.; Romanet, V.; Sharaby, S.; et al. QSP Modeling of a Transiently Inactivating Antibody-Drug Conjugate Highlights Benefit of Short Antibody Half Life. J. Pharmacokinet. Pharmacodyn. 2025, 52, 7. [Google Scholar] [CrossRef]
- Khushalani, N.I.; El-Haddad, G.; Gage, K.L.; Budzevich, M.; Schell, M.J.; Moros, E.G.; Tichacek, C.; Gibbons, W.R.; Rogers, R.E.; Nickels, M.L.; et al. First-in-Human Study of 225actinium Mti-201 (225Ac-MTI-201) in Metastatic Uveal Melanoma (UM). J. Clin. Oncol. 2024, 42, TPS9612. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Lee, D.; Cheng, Y.; Baumhover, N.J.; Marks, B.M.; Sagastume, E.A.; Ballas, Z.K.; Johnson, F.L.; Morris, Z.S.; et al. Targeted Alpha-Particle Radiotherapy and Immune Checkpoint Inhibitors Induces Cooperative Inhibition on Tumor Growth of Malignant Melanoma. Cancers 2021, 13, 3676. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Veld, R.V.H.I.; de los Pinos, E.; Ossendorp, F.A.; Jager, M.J. Targeting Ocular Malignancies Using a Novel Light-Activated Virus-like Drug Conjugate. Adv. Ophthalmol. Pract. Res. 2025, 5, 49–57. [Google Scholar] [CrossRef]
- Ma, S.; Veld, R.V.H.I.; Houy, A.; Stern, M.-H.; Rich, C.; Ossendorp, F.A.; Jager, M.J. In Vitro Testing of the Virus-Like Drug Conjugate Belzupacap Sarotalocan (AU-011) on Uveal Melanoma Suggests BAP1-Related Immunostimulatory Capacity. Investig. Ophthalmol. Vis. Sci. 2023, 64, 10. [Google Scholar] [CrossRef]
- Hasanov, M.; Rioth, M.J.; Kendra, K.; Hernandez-Aya, L.; Joseph, R.W.; Williamson, S.; Chandra, S.; Shirai, K.; Turner, C.D.; Lewis, K.; et al. A Phase II Study of Glembatumumab Vedotin for Metastatic Uveal Melanoma. Cancers 2020, 12, 2270. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, X.; Peng, R.; Pan, Q.; Weng, D.; Ma, Y.; Zhang, Y.; Yang, J.; Men, L.; Wang, H.; et al. A First-in-Human Phase I Study of a Novel MDM2/P53 Inhibitor Alrizomadlin in Advanced Solid Tumors. ESMO Open 2024, 9, 103636. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.K.; Hagström, A.; Stålhammar, G. Adjuvant Melatonin for Uveal Melanoma (AMUM): Protocol for a Randomized Open-Label Phase III Study. Trials 2023, 24, 230. [Google Scholar] [CrossRef]
- Bao, R.; Surriga, O.; Olson, D.J.; Allred, J.B.; Strand, C.A.; Zha, Y.; Carll, T.; Labadie, B.W.; Bastos, B.R.; Butler, M.; et al. Transcriptional Analysis of Metastatic Uveal Melanoma Survival Nominates NRP1 as a Therapeutic Target. Melanoma Res. 2021, 31, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sarnaik, A.A.; Yu, B.; Yu, D.; Morelli, D.; Hall, M.; Bogle, D.; Yan, L.; Targan, S.; Solomon, J.; Nichol, G.; et al. Extended Dose Ipilimumab with a Peptide Vaccine: Immune Correlates Associated with Clinical Benefit in Patients with Resected High-Risk Stage IIIc/IV Melanoma. Clin. Cancer Res. 2011, 17, 896–906. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Frankel, P.; Margolin, K.A.; Christensen, S.; Ruel, C.; Shipe-Spotloe, J.; Gandara, D.R.; Chen, A.; Kirkwood, J.M. Aflibercept (VEGF Trap) in Inoperable Stage III or Stage IV Melanoma of Cutaneous or Uveal Origin. Clin. Cancer Res. 2011, 17, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Frankel, P.; Ruel, C.; Ernstoff, M.S.; Kuzel, T.M.; Logan, T.F.; Khushalani, N.I.; Tawbi, H.A.; Margolin, K.A.; Awasthi, S.; et al. NCI 8628: A Randomized Phase 2 Study of Ziv-Aflibercept and High-Dose Interleukin 2 or High-Dose Interleukin 2 Alone for Inoperable Stage III or IV Melanoma. Cancer 2018, 124, 4332–4341. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Tambuwala, M.M.; El-Tanani, M.; Hassan, S.S.; Lundstrom, K.; Mishra, V.; Mishra, Y.; Hromić-Jahjefendić, A.; Redwan, E.M.; Uversky, V.N. A Comprehensive Review of PRAME and BAP1 in Melanoma: Genomic Instability and Immunotherapy Targets. Cell. Signal. 2024, 124, 111434. [Google Scholar] [CrossRef]
- Barbagallo, C.; Stella, M.; Broggi, G.; Russo, A.; Caltabiano, R.; Ragusa, M. Genetics and RNA Regulation of Uveal Melanoma. Cancers 2023, 15, 775. [Google Scholar] [CrossRef]
- Seider, M.I.; Mruthyunjaya, P. Molecular prognostics for uveal melanoma. Retina 2018, 38, 211–219. [Google Scholar] [CrossRef]
- Cai, L.; Paez-Escamilla, M.; Walter, S.D.; Tarlan, B.; Decatur, C.L.; Perez, B.M.; Harbour, J.W. Gene Expression Profiling and PRAME Status Versus Tumor-Node-Metastasis Staging for Prognostication in Uveal Melanoma. Am. J. Ophthalmol. 2018, 195, 154–160. [Google Scholar] [CrossRef]
- Gallenga, C.E.; Franco, E.; Adamo, G.G.; Violanti, S.S.; Tassinari, P.; Tognon, M.; Perri, P. Genetic Basis and Molecular Mechanisms of Uveal Melanoma Metastasis: A Focus on Prognosis. Front. Oncol. 2022, 12, 828112. [Google Scholar] [CrossRef]
- Field, M.G.; Decatur, C.L.; Kurtenbach, S.; Gezgin, G.; van der Velden, P.A.; Jager, M.J.; Kozak, K.N.; Harbour, J.W. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin. Cancer Res. 2016, 22, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group Report Number 1: Prospective Validation of a Multi-Gene Prognostic Assay in Uveal Melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Tuscan, M.D.; Harbour, J.W. An Accurate, Clinically Feasible Multi-Gene Expression Assay for Predicting Metastasis in Uveal Melanoma. J. Mol. Diagn. 2010, 12, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Soltysova, A.; Dvorska, D.; Kajabova, V.H.; Pecimonova, M.; Cepcekova, K.; Ficek, A.; Demkova, L.; Buocikova, V.; Babal, P.; Juras, I.; et al. Uncovering Accurate Prognostic Markers for High-risk Uveal Melanoma through DNA Methylation Profiling. Clin. Transl. Med. 2023, 13, e1317. [Google Scholar] [CrossRef]
- Heiferman, M.J.; Mahajan, V.B.; Mruthyunjaya, P. Proteomics in Uveal Melanoma. Curr. Opin. Ophthalmol. 2022, 33, 202–210. [Google Scholar] [CrossRef]
- Nakao, S.; Hafezi-Moghadam, A.; Ishibashi, T. Lymphatics and Lymphangiogenesis in the Eye. J. Ophthalmol. 2012, 2012, 783163. [Google Scholar] [CrossRef]
- Boyd, S.R.; Tan, D.S.W.; de Souza, L.; Neale, M.H.; Myatt, N.E.; Alexander, R.A.; Robb, M.; Hungerford, J.L.; Cree, I.A. Uveal Melanomas Express Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor and Support Endothelial Cell Growth. Br. J. Ophthalmol. 2002, 86, 440. [Google Scholar] [CrossRef]
- Velez, G.; Wolf, J.; Dufour, A.; Mruthyunjaya, P.; Mahajan, V.B. Cross-Platform Identification and Validation of Uveal Melanoma Vitreous Protein Biomarkers. Investig. Ophthalmol. Vis. Sci. 2023, 64, 14. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Bechrakis, N.E.; Willerding, G.; Charitoudis, G.S.; Foerster, M.H.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Phosphorylated PVEGFR2/KDR Receptor Expression in Uveal Melanomas: Relation with HIF2α and Survival. Clin. Exp. Metastasis 2012, 29, 11–17. [Google Scholar] [CrossRef]
- Yang, H.; Jager, M.J.; Grossniklaus, H.E. Bevacizumab Suppression of Establishment of Micrometastases in Experimental Ocular Melanoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2835–2842. [Google Scholar] [CrossRef]
- el Filali, M.; Ly, L.V.; Luyten, G.P.M.; Versluis, M.; Grossniklaus, H.E.; van der Velden, P.A.; Jager, M.J. Bevacizumab and Intraocular Tumors: An Intriguing Paradox. Mol. Vis. 2012, 18, 2454–2467. [Google Scholar] [PubMed]
- Francis, J.H.; Kim, J.; Lin, A.; Folberg, R.; Iyer, S.; Abramson, D.H. Growth of Uveal Melanoma Following Intravitreal Bevacizumab. Ocul. Oncol. Pathol. 2017, 3, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Tsering, T.; Laskaris, A.; Abdouh, M.; Bustamante, P.; Parent, S.; Jin, E.; Ferrier, S.T.; Arena, G.; Burnier, J.V. Uveal Melanoma-Derived Extracellular Vesicles Display Transforming Potential and Carry Protein Cargo Involved in Metastatic Niche Preparation. Cancers 2020, 12, 2923. [Google Scholar] [CrossRef]
- Yamada, K.H.; Kang, H.; Malik, A.B. Antiangiogenic Therapeutic Potential of Peptides Derived from the Molecular Motor KIF13B That Transports VEGFR2 to Plasmalemma in Endothelial Cells. Am. J. Pathol. 2017, 187, 214–224. [Google Scholar] [CrossRef]
- Waters, S.B.; Dominguez, J.R.; Cho, H.D.; Sarich, N.A.; Malik, A.B.; Yamada, K.H. KIF13B-Mediated VEGFR2 Trafficking Is Essential for Vascular Leakage and Metastasis in Vivo. Life Sci. Alliance 2022, 5, e202101170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).