Abstract
Multiple sclerosis is an autoimmune disease that affects the central nervous system. In Brazil, there are currently several therapeutic options for the treatment of this condition, with some being distributed free of charge, while others are not included in the list of free medications. The objective of this article is to provide a pharmacoepidemiological analysis of the available medications in the country, covering their mechanisms of action, the historical context of approval and free distribution within the healthcare system, and their geographical distribution of application. Additionally, we discuss the impact of the inclusion of these medications on hospitalization and mortality rates in the country. We hope that this work serves as a resource for healthcare professionals to better understand pharmacoepidemiology and for health policymakers seeking data for the planning of public policies aimed at the treatment of multiple sclerosis.
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) characterized by chronic inflammation, demyelination, and axonal damage [1]. It predominantly affects young adults, typically with onset between the ages of 20 and 40, and demonstrates significant clinical variability, making it a diagnostic and therapeutic challenge [2]. MS manifests in episodes of acute neurological symptoms and gradual progression of disability over time [3].
It can present in various clinical forms [4]. Relapsing-remitting multiple sclerosis (RRMS) is the most common form. Symptoms occur in relapses or flare-ups, during which patients experience a worsening of symptoms for a period, followed by remission, during which symptoms improve or completely disappear [5]. In primary progressive multiple sclerosis (PPMS), the disease symptoms progress continuously from the onset without distinct periods of relapses and remissions [6]. Over time, some patients with RRMS may develop a progressive form of the disease known as secondary progressive multiple sclerosis (SPMS). At this stage, the disease evolves into a constant and gradual progression of symptoms, with or without overlapping relapses [7]. Primary progressive multiple sclerosis with activity (PPMS-A) is a less common form of MS, characterized by a constant progression of symptoms from the onset but with occasional bursts of inflammatory activity [8]. There is a benign form of the disease known as benign multiple sclerosis (BMS), in which symptoms are mild and do not lead to significant disability over time. These patients may enjoy a relatively normal quality of life [9].
One of the key events in the pathophysiology of MS is the activation of self-reactive T lymphocytes [10]. These CD4+ and CD8+ T lymphocytes infiltrate the central nervous system, crossing the blood–brain barrier (BBB). They recognize proteins present in myelin, a substance that coats and insulates neurons in the CNS, as foreign antigens, and this event leads to an inflammation of Th1 and Th17 cells that destroy the myelin in white and gray matter areas of the CNS and spinal cord [11]. The central nervous system attempts to repair the damage through the process of remyelination. However, in many cases of MS, this repair process is ineffective, resulting in the formation of scars or sclerosis plaques, a typical hallmark of the disease [12].
The treatment of MS has significantly advanced over recent decades, offering patients increased prospects for managing symptoms, minimizing relapses, and adopting more efficient strategies to decelerate disease progression [11]. Within Brazil, the Ministry of Health establishes the treatment criteria for MS through “Clinical Protocols and Therapeutic Guidelines”. These guidelines cover disease classification, a range of cost-free therapeutic options available within the healthcare system, and specific recommendations tailored to each patient’s condition. Serving as the governing document for treatments within the Brazilian Unified Health System (SUS), it standardizes and oversees the approach to MS management [13].
The therapeutic approach to MS is based on two main strategies: one of them involves the use of medications and the other basically involves techniques. Within behavioral approaches, the most common practices to manage cognitive impairment in MS are cognitive rehabilitation and physical training. Physical activity is recommended as a form of prevention and is also recommended to alleviate cognitive and movement problems. There are studies that show that using exercises with less impact, such as recovery exercises, improves strength, muscular endurance, balance, concentration, as well as quality of life in general [14,15].
This article explores the various classes of therapies available for MS, with a special focus on disease-modifying therapies (DMTs), which stand out as one of the cornerstones in managing MS. The treatment of MS aims to address distinct aspects of the disease, including reducing the inflammatory activity of the immune system, alleviating acute symptoms, and preventing disability progression [16]. This multifaceted approach requires the use of different classes of therapies, each with specific mechanisms of action. One of the classes of drugs commonly used in the treatment of MS is corticosteroids [17]. They work by reducing inflammation in the central nervous system, relieving symptoms, and accelerating neurological recovery during relapses. Nevertheless, it’s important to note that corticosteroids do not modify the long-term course of the disease; they are more directed towards relieving acute symptoms [18]. The diversity also extends to the mechanisms of action of available therapies. From immunomodulatory agents that regulate the autoimmune response to medications targeting neuroprotection and remyelination, the range of approaches reflects the complexity of the disease and the ongoing quest for innovative therapeutic strategies. This multitude of mechanisms provides doctors and patients with diversified options to address multiple sclerosis in a personalized manner [19].
Currently, the primary focus in the treatment of MS lies in DMTs. These therapies are designed to alter the course of the disease, reducing both the frequency and severity of relapses, as well as long-term disability progression [20]. For instance, glatiramer acetate establishes significant binding with molecules of the major histocompatibility complex, thereby inhibiting T cell responses to myelin antigens. Additionally, it acts as an antagonist to the T cell receptor for the 82–100 maltose binding protein (MBP) epitope, inducing alterations in glatiramer-specific T cell function [21]. On the other hand, dimethyl fumarate constitutes a treatment for relapsing-remitting MS. Apart from potential neuroprotective effects, it demonstrates anti-inflammatory properties and stands out for its convenient oral administration [22]. Teriflunomide, also categorized as a DMT, is orally administered and exhibits consistent efficacy, safety, and tolerability in patients with relapsing forms of multiple sclerosis. It functions as a reversible inhibitor of dihydroorotate dehydrogenase, a mitochondrial enzyme crucial for de novo pyrimidine synthesis, essential for the expansion of antigen-activated lymphocytes. Furthermore, it acts as an immunomodulator, diminishing the proliferation activity of T and B cells [23]. Interferons-beta are an example of a DMT; they have the ability to reduce inflammation and disease activity, making them a common choice as a first-line treatment [24]. Another class of DMTs comprises fingolimod, which modulates the immune response by preventing T lymphocytes from entering the central nervous system and causing inflammation [25]. Monoclonal antibodies, such as ocrelizumab and natalizumab, also belong to the group of DMTs, as they target specific immune processes involved in MS, reducing disease activity [26].
In the given context, with well-established guidelines in Brazil for the treatment of MS, our work aims to provide clarification and awareness about MS, emphasizing the available drug therapies distributed by the SUS, highlighting their indications, regulatory approval, and mechanism of action through a pharmacoepidemiological approach.
2. Materials and Methods
2.1. Study Type
This study takes the form of descriptive pharmacoepidemiological research. Pharmacoepidemiology is a discipline that focuses on the analysis of medication usage patterns in specific populations. In this study, our analysis is centered on the use of medications for the treatment of multiple sclerosis in Brazil, with particular attention to medications distributed by the SUS. The central objective is to investigate these medications in detail, including their prescription, geographic distribution, indications as stated in their labels, registration with the Brazilian National Health Surveillance Agency (ANVISA), as well as the reasons that determine their inclusion or exclusion from the SUS distribution list. Additionally, a simplified description of the mechanism of action of each medication will be provided.
2.2. Data Sources
2.2.1. Medication Distribution Data by State
The data related to the distribution of medications for the treatment of multiple sclerosis in Brazil were obtained from the Department of Informatics of the Unified Health System (DATASUS). Information regarding all medications distributed free of charge by SUS was collected. To calculate the medication distribution rate per state, we normalized the population to 10,000 inhabitants in each federative unit, based on population data provided by the Brazilian Institute of Geography and Statistics. Additionally, we calculated the rates of deaths and hospitalizations per 100,000 inhabitants in each federative unit.
2.2.2. Medication Registration Data
The data regarding medication registrations were obtained from the website of the ANVISA, the regulatory agency equivalent to the United States Food and Drug Administration (FDA) and the European Medicines Agency. These data include information about the approval of the medication, including the date and trade name.
2.2.3. Technical and Scientific Information and Indication for Use in the Medication Leaflet
The technical, scientific, and indications information for each medication was obtained from the available package inserts of the respective medications.
2.2.4. Data on Medications Not Approved by the Brazilian Unified Health System (SUS)
Information regarding medications approved by ANVISA but not incorporated into the list of free distribution by SUS was researched on the website of the National Commission for the Incorporation of Technologies in the Unified Health System (CONITEC). CONITEC is the body that advises the Ministry of Health of Brazil in making decisions related to the incorporation, exclusion, or modification of health technologies within SUS, as well as in creating or modifying clinical protocols and therapeutic guidelines.
2.2.5. Mechanisms of Action of Medications
The simplified descriptions of the mechanisms of action of the medications was developed through a literature review conducted on the PUBMED database. Articles providing historical information and details about the mechanism of action of each medication were sought.
2.3. Software
All data were tabulated using Microsoft® Excel® (Version 2307 Build 16.0.16626.20170). In the creation of figures with cartographic bases, QGIS (Version 3.30.2) was employed, with geographic coordinates referenced to SIRGAS 2000 (version), and analysis based on the Optimization of Jenks Natural Breaks Intervals. The remaining figures were generated using Adobe Photoshop CC (Version 14.0) and Microsoft® Excel®. The Brazilian population for the year 2022, which was not available in the consulted database, was estimated using a linear regression model. The available data from 2011 to 2021 were utilized, and this estimation was performed using GraphPad Prism software (Version 8.0.2).
3. Pharmacoepidemiological Analysis
3.1. Disease-Modifying Therapies
In the realm of multiple sclerosis treatment, DMTs have been made accessible. These therapies primarily target the reduction of the immune response, focusing on immune pathways. These therapeutic approaches vary in terms of efficacy levels. The treatment of multiple sclerosis is characterized by a remarkable diversity of therapeutic strategies. Ranging from DMTs to supportive and rehabilitative interventions, the management of this intricate condition encompasses a spectrum of approaches tailored to the specific needs of individual patients. This diversity underscores the multifaceted nature of multiple sclerosis and emphasizes the significance of a personalized approach to enhance the well-being of those afflicted [19,27,28,29].
Moreover, as we delve deeper into the realm of DMTs for multiple sclerosis, one notable advancement that emerges is the utilization of monoclonal antibodies in the treatment of this condition. This innovative approach has introduced a range of highly effective therapeutic options. The use of monoclonal antibodies in multiple sclerosis represents a relatively recent innovation that has exhibited significant efficacy in controlling symptoms and managing disease progression in specific patients. These pharmacological agents, classified as monoclonal antibodies, are biopharmaceuticals meticulously engineered to selectively target specific components of the immune system. Their purpose is to mitigate or suppress the autoimmune response, which plays a pivotal role in the pathophysiology of multiple sclerosis [27,30,31].
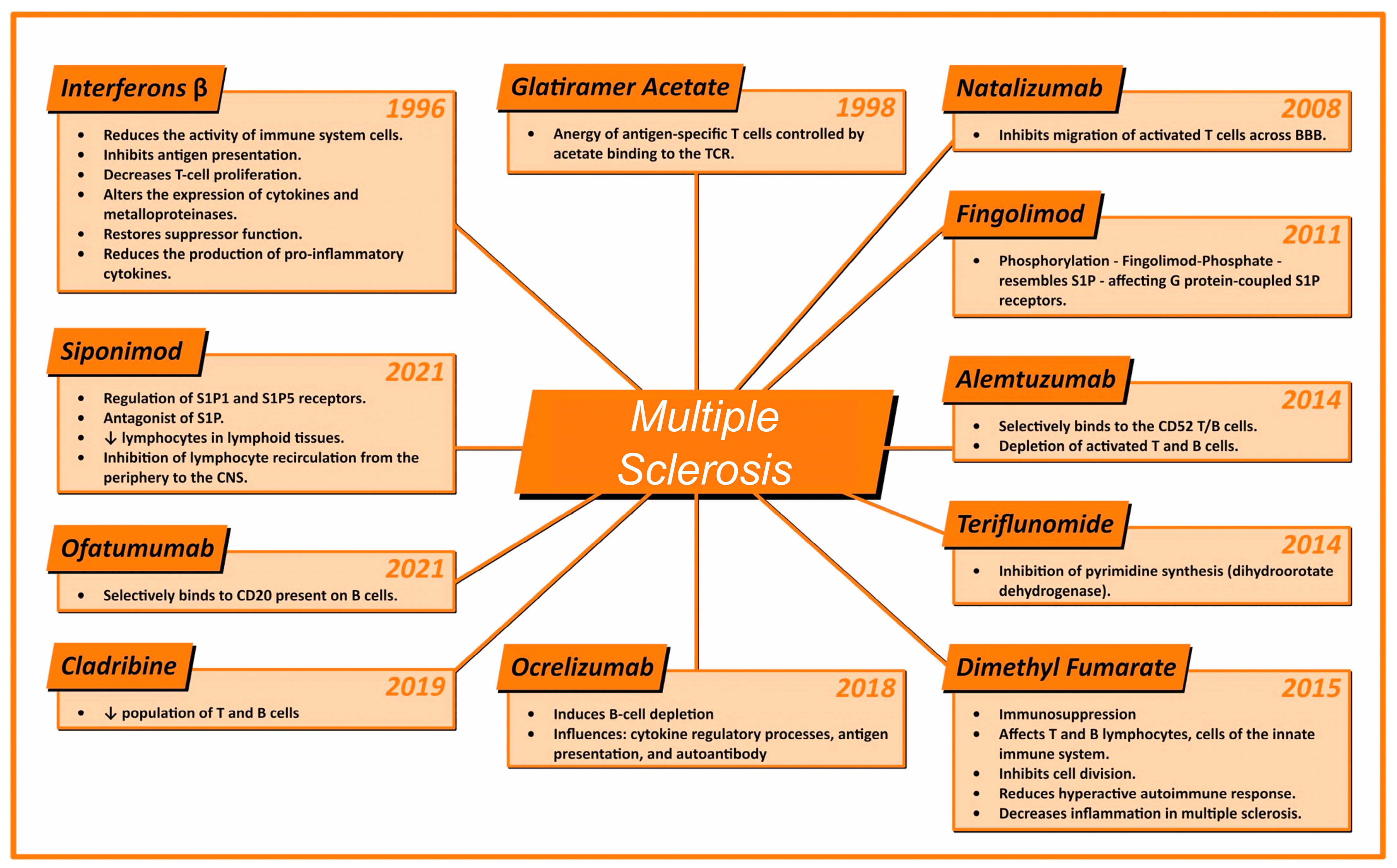
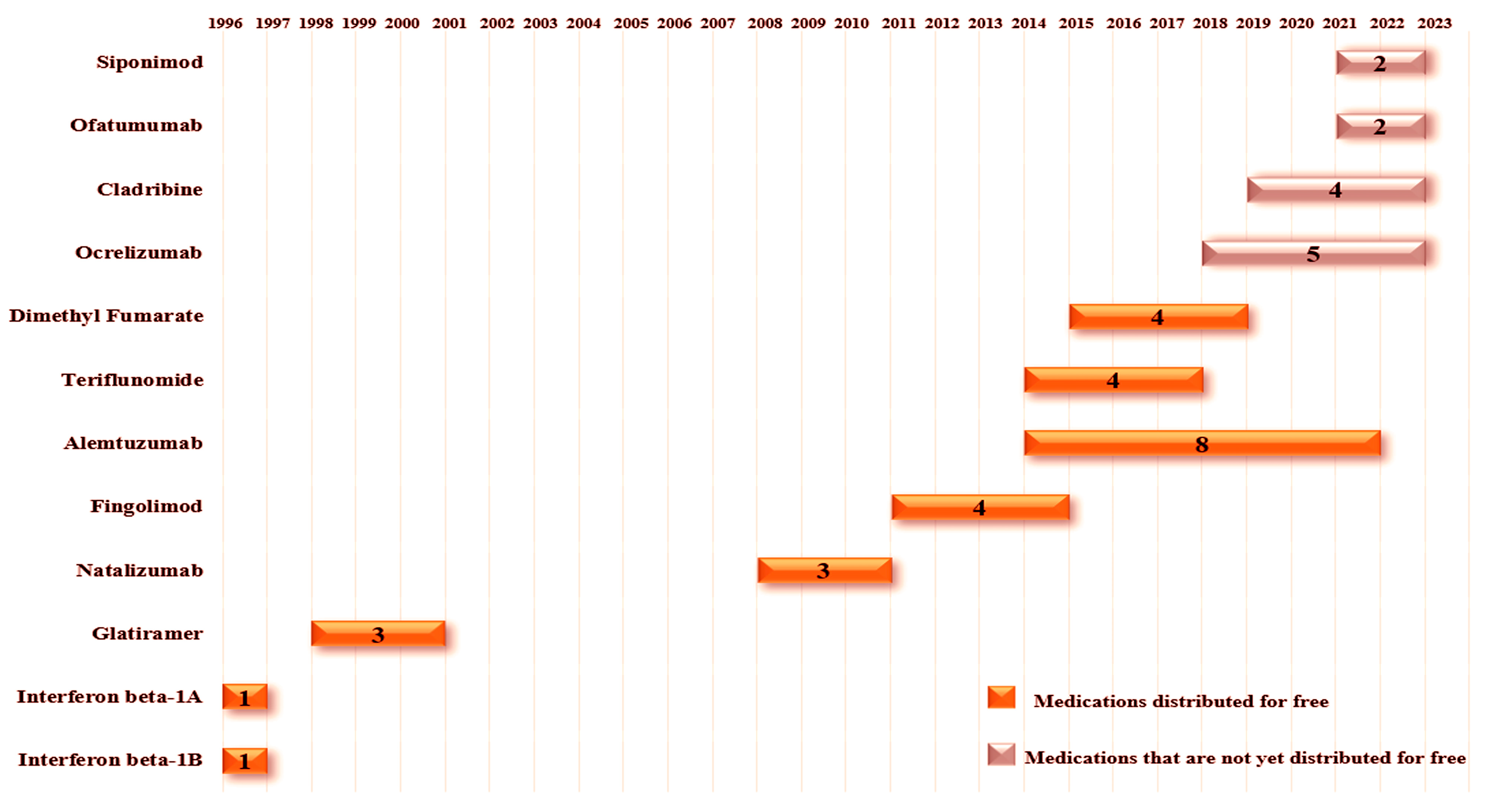
The advent of monoclonal antibodies, or MAbs, has revolutionized the therapeutic landscape of multiple sclerosis, due to their highly targeted mechanism of action, substantial efficacy, and favorable risk profile. Subsequently, the engineering efforts of recent decades have led to the humanization of MAb components, significantly reducing their immunogenicity and, consequently, the risk of anti-drug antibody generation. This modification has also resulted in extensions of pharmacological half-lives. It is worth noting that the first generation of biologic products was entirely murine in structure, which, in some cases, triggered potentially life-threatening immune responses. The second generation of biologic products was designed as chimeric, blending to the human fragment crystallizable (Fc) with murine variable regions. The third generation, on the other hand, consists of entirely human MAbs, although they may still induce the production of anti-human antibodies. The MAbs currently licensed for the treatment of multiple sclerosis have demonstrated high efficacy in phase 3 clinical trials and are therefore recommended for patients with high disease activity. However, it is important to mention that indications and approvals granted by regulatory agencies vary between countries. Notably, in Europe, these agents have not yet been licensed for the treatment of less active forms of the disease, based on a weighted analysis of benefits versus risks [30,31,32,33]. Figure 1 shows the drug, along with its main mechanisms of action and the year of approval of the drug by ANVISA.
Figure 1.
Illustration presenting the various medications approved for the treatment of multiple sclerosis in Brazil, highlighting the year of registration of the medication in the country and their main mechanisms of action. The medications are arranged clockwise in order of approval, starting from the top left corner of the figure. ↓: Indicates a decrease in the related cell population.
3.2. Interferons Beta
Interferons beta are part of a class of polypeptides, typically produced by fibroblasts, that possess antiviral and antiproliferative properties. This class of medications has been widely used in the treatment of multiple sclerosis for over two decades and is considered first-line therapy for patients with relapsing-remitting multiple sclerosis (RRMS). The clinical efficacy of interferons beta is correlated with the reduction in disease activity assessed through magnetic resonance imaging (MRI), and their safety profile is well-established [34,35].
Interferons beta are believed to have pleiotropic mechanisms of action, including the reduction in serum levels of matrix metalloproteinases and/or interleukins, such as IL-17 and IL-10. They play a crucial role in modulating the immune response and reducing inflammation in the central nervous system, which, in turn, contributes to a decrease in the frequency and severity of relapses in patients with multiple sclerosis [34,35].
The primary mechanism of action of interferons beta is based on reducing the activity of immune cells involved in central nervous system inflammation. They inhibit antigen presentation and T-cell proliferation, alter the expression of cytokines and matrix metalloproteinases (MMPs), and restore suppressor function, reducing the production of pro-inflammatory cytokines, which are substances that promote inflammation, and affecting the overall immune response. As a result, they prove effective in reducing the frequency and severity of relapses in patients with relapsing-remitting multiple sclerosis (RRMS), which is the most common form of the disease [35,36]. In some cases, the effectiveness of interferons beta is associated with their continuous use, which may help slow the progression of the disease by preventing the formation of new lesions in the central nervous system. Regarding the mode of administration, they can be administered through subcutaneous (under the skin) or intramuscular injections, with the frequency of injections varying depending on the medication formulation. Some formulations require weekly administration, while others may be daily or every two days [35,36].
Interferons beta were the first treatments authorized for multiple sclerosis in Brazil. In fact, the registrations for beta-interferons date back to 1996 with ANVISA. Beta-interferon 1B received registration in March 1996 under the trade name Betaferon® [37], with indications for the treatment of patients with a single clinical event suggestive of multiple sclerosis, RRMS, and SPMS [38]. Beta-interferon 1A was registered in August 1996 under the trade name Rebif® [39] and was indicated for the treatment of RRMS (adult and pediatric use above 12 years) and single clinical event suggestive of multiple sclerosis (adult use) [40]. Being the first medications with approved registration in Brazil for commercialization and distribution, they were also the first to receive free distribution of the medication. The data indicate that these initial distributions began in 1997, just one year after their approval at the time, the fastest approval among all medications. A significant factor for this rapid approval was the absence, up to that point, of any specific therapy for multiple sclerosis treatment in the Brazilian territory. The total number of interferons beta distributed across Brazil from 1997 to 2022, encompassing all their different dosages, amounts to 13,499,180 doses [41].
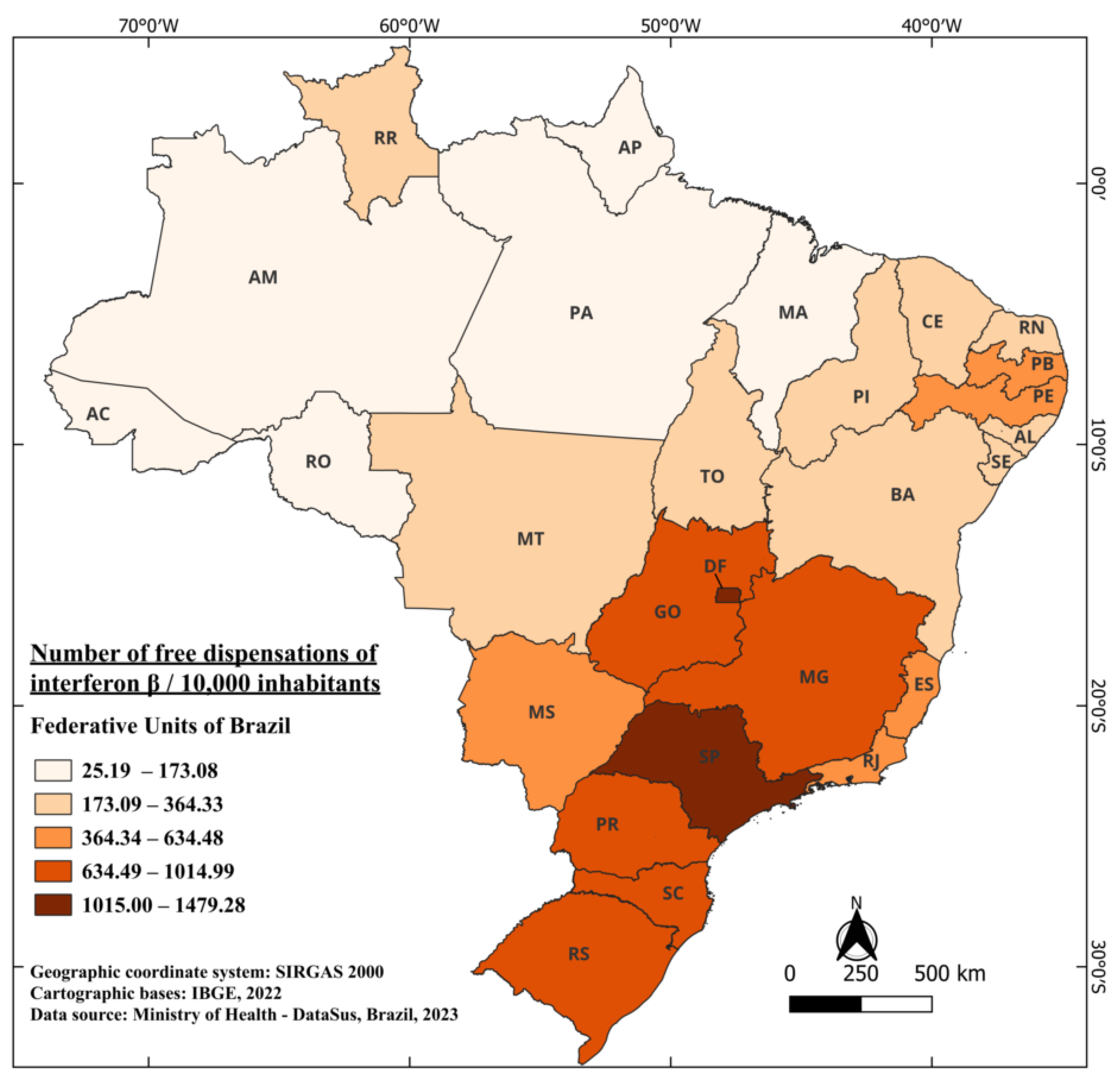
During this same period, considering the country’s average population and the total distributed doses, there is a distribution of 708.61 doses of interferons beta per 10,000 inhabitants. The federal units with the highest distribution of this medication over the years were the Federal District (DF) with 1479.28/10,000 and the state of São Paulo (SP) 1219.90/10,000. On the other hand, the states of Amapá (AP) and Acre (AC) had the lowest distribution of this medication, with 25.19 and 47.73 doses per 10,000 inhabitants, respectively. One noteworthy fact, not only regarding beta-interferons but also in general, is the relatively low distribution in states located near the equator (0°, 0’) as illustrated in Figure 2, when compared to states further south in the country. These findings display a strong correlation with other studies in the literature, showcasing a notable North–South gradient from the equator [42,43]. This gradient reveals an increase in the number of MS cases as the distance from the equator grows. Researchers indicates that these findings may be elucidated by the higher prevalence of ultraviolet light in equatorial regions, which leads to the development of light-induced suppressor cells related to MS antigens [44].
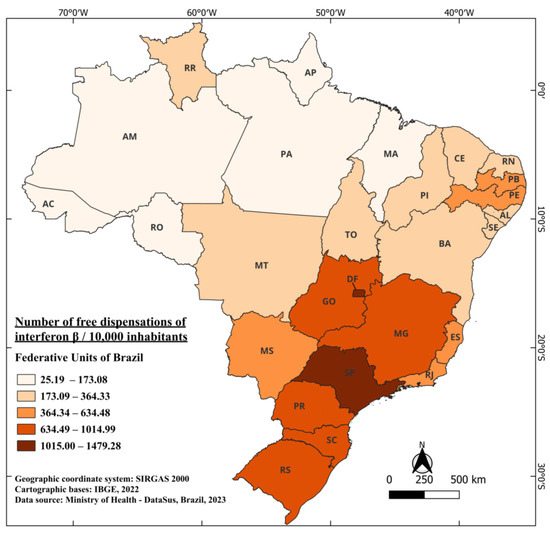
Figure 2.
The number of free dispensations of interferon beta in Brazil between 1997 and 2022, in relation to the population size, was calculated by summing the total number of medication dispensations and dividing it by the average population during the same period. The population was adjusted per 10,000 inhabitants for better data visualization.
3.3. Glatiramer Acetate
Glatiramer acetate is a synthetic substance that resembles the proteins found in myelin, the substance that surrounds nerve fibers in the central nervous system. It was first synthesized over thirty years ago as a research tool to explore the biology of experimental autoimmune encephalomyelitis (EAE) by a group of researchers. This work laid the foundation for its use as a medication against multiple sclerosis, especially the relapsing-remitting clinical form, with its approval in 1996. When administered, it deceives the immune system, causing the immune system not to react against myelin as if it were an invader. This helps prevent or reduce the autoimmune response that characterizes multiple sclerosis, in which the immune system mistakenly attacks myelin [45].
Glatiramer acetate also acts as an anti-inflammatory agent. It reduces the inflammatory response in the central nervous system, which is important for controlling the symptoms of multiple sclerosis and slowing the progression of the disease. By modifying the immune response and reducing inflammation, glatiramer acetate helps prevent the formation of new lesions in the central nervous system. This can help protect the nerves and maintain neurological function over time. Glatiramer acetate is typically administered through subcutaneous injections (under the skin), and the frequency of injections varies depending on the medication formulation (it can be daily or three times a week). Treatment is usually recommended as a long-term regimen for patients with multiple sclerosis. Being more specific, the action of glatiramer in a more complete way is based on three steps, which can occur simultaneously, in a highly orchestrated process, involving peripheral and CNS immunomodulation, and it is also believed that it exerts an essential neuroprotective and cognitive preservation role [46,47,48,49].
The reduction in inflammation by glatiramer acetate is based on a mechanism of action composed of two main constituents. The immune response is modulated through the production of specific suppressor T lymphocytes by glatiramer acetate (Th2 profile). These cells have the capacity to directly and indirectly inhibit inflammation in the CNS caused by multiple sclerosis. At the outset of exposure, these cells predominantly exhibit a Th1 profile. However, with continued treatment, there is a shift in the cell profile, and they become predominantly helper T lymphocytes with a Th2/Treg profile, which inherently possesses anti-inflammatory capabilities [21].
Actually, the negative reduction in inflammation in the CNS (inflammation triggered by antigenic products of demyelination such as myelin basic protein and other myelin antigens) is executed by these cells and not by glatiramer acetate itself. Acetate also has the ability to inhibit self-reactive MBP and other antigen-specific myelin cells that would perpetuate inflammation through the release of pro-inflammatory cytokines, which would be produced by these cells inhibited by acetate. The possible mechanism involves the anergy of these antigen-specific T cells, controlled by the binding of acetate to the T cell receptor (TCR) [21,48].
Glatiramer acetate follows, being the second medication registered in the country in June 1998, under the commercial name Copaxone® [50], with subsequent implementation of its free distribution by the SUS in 2001, an interval three times longer than that found for beta-interferons. This implies that, from 1997 to 2001, the only medication available in the Brazilian healthcare system and distributed free of charge was interferons beta.
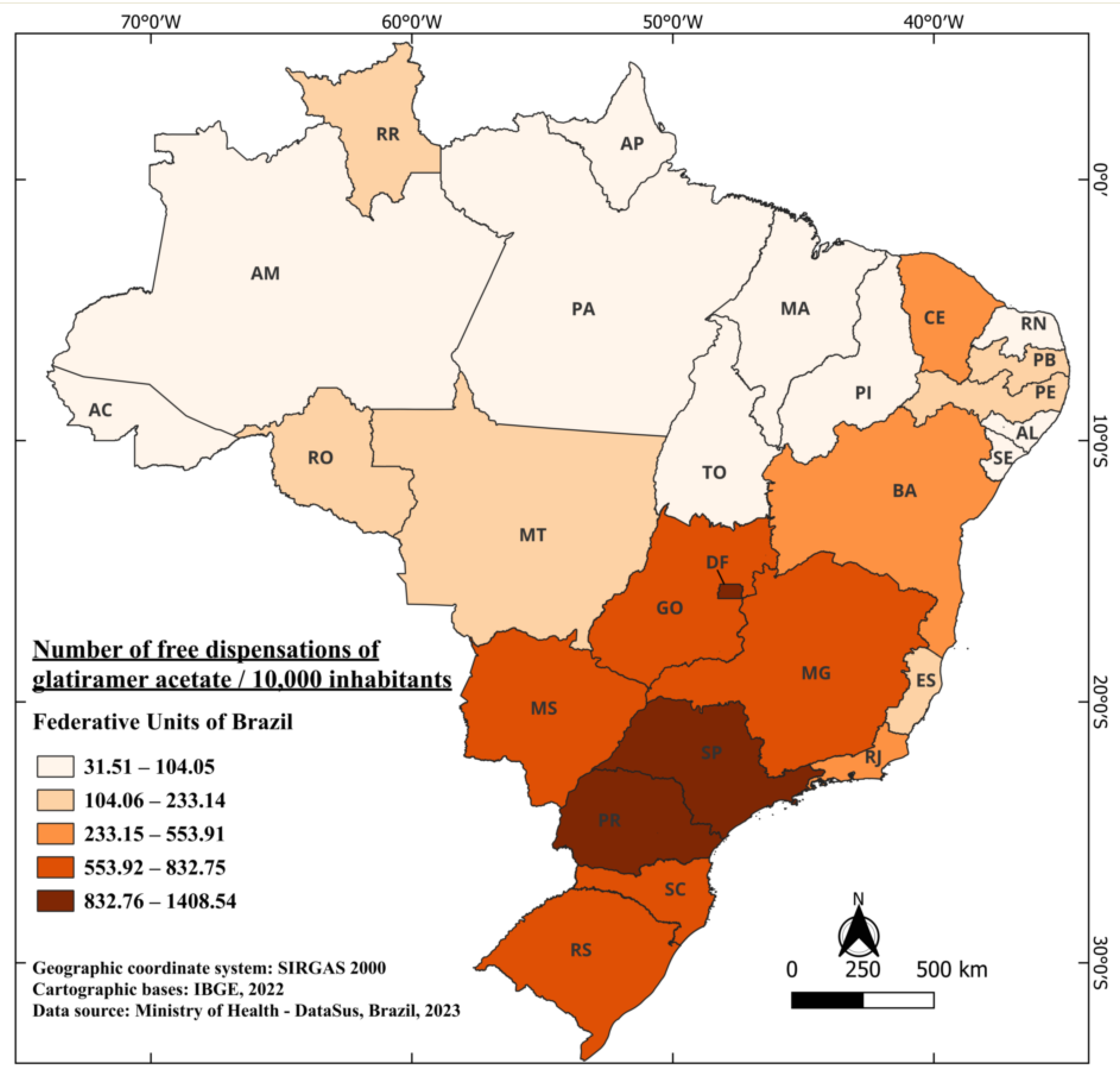
Between 2001 and 2022, a total of 13,136,858 dispensations of glatiramer acetate were carried out, considering all its doses, free of charge [41]. Currently, it is indicated for the treatment of patients who have had a well-defined first clinical episode and who are at high risk of developing clinically definite multiple sclerosis (CDMS), as well as for patients with RRMS [51]. The distribution of medication doses during this period in Brazil averaged 671.88 per 10,000 inhabitants. Unlike interferons beta, the state with the highest dispensation of free doses of glatiramer acetate is Paraná (PR), followed by SP and DF, with 1408.54, 1280.40, and 1265.48 doses per 10,000 inhabitants, respectively. On the other hand, the states with the lowest distribution of the medication are Pará (PA) and Maranhão (MA), with 40.33 and 31.51 doses per 10,000 inhabitants, respectively (Figure 3).
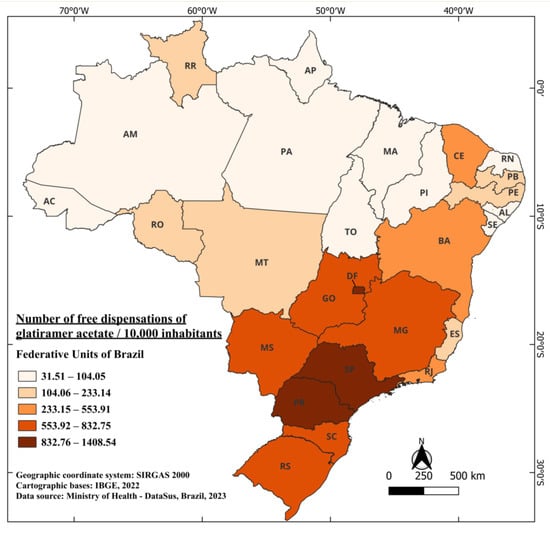
Figure 3.
The number of free dispensations of glatiramer acetate in Brazil between 2001 and 2022, in relation to the population size, was calculated by summing the total number of medication dispensations and dividing it by the average population during the same period. The population was adjusted per 10,000 inhabitants for better data visualization.
3.4. Natalizumab
Natalizumab, a recombinant humanized IgG4κ monoclonal antibody selective for integrin α4, plays a pivotal role in the treatment of MS. It acts as a disease-modifying agent, with the aim of reducing MS activity and preventing relapses in those affected by this condition. Natalizumab is indicated as a monotherapy for treating the relapsing-remitting form of MS, with the goal of decreasing the frequency of clinical exacerbations, reducing the number and volume of active brain lesions observed on magnetic resonance imaging, and slowing the progression of physical disability. Although it was initially withdrawn from the market due to safety concerns in 2005, natalizumab was reintroduced in 2006 and has been available for the treatment of highly active relapsing-remitting MS since 2004. Clinical studies and real-world evidence have confirmed its effectiveness in reducing MS relapses and improving outcomes related to this disease [52,53,54].
The mechanism of action of natalizumab is related to its ability to inhibit the migration of activated T lymphocytes through the blood–brain barrier (BBB) into the CNS. In MS, T lymphocytes and other immune system cells cross the BBB, triggering inflammation, demyelination, and neuronal damage, resulting in the symptoms of the disease. Natalizumab acts by blocking the interaction between T lymphocytes and the vascular cell adhesion molecule (VCAM-1) present on the endothelial cells of the BBB. This blockade prevents the uncontrolled entry of T lymphocytes into the CNS, thereby reducing inflammation and the associated damage in MS. It is worth noting that natalizumab is specifically targeted at the α4 chain of adhesion molecules (CD49), which are part of the very late antigen-4 (VLA4) and integrin α4-β7 (LPAM1) adhesion molecules. These molecules are involved in directing T lymphocytes to the CNS and other regions of the body [54,55,56].
Natalizumab is typically recommended for patients with more active forms of MS, such as relapsing-remitting multiple sclerosis (RRMS), who do not respond well to other therapies or who have rapidly progressing disease. Its use is highly regulated and monitored due to the potential for side effects, including the rare but serious risk of developing progressive multifocal leukoencephalopathy (PML), a condition that affects the brain. Natalizumab is administered via intravenous infusion, usually every four weeks, under medical supervision [54,57].
Natalizumab, marketed under the name Tysabri®, was registered in August 2008, becoming the first medication in the class of MABs to be authorized for commercialization in Brazil [58]. Currently, its label indication in Brazil has several restrictions, being indicated only for two groups of patients: (1) Those who have not responded to a complete and adequate cycle with another medication for RRMS and have had at least one relapse in the previous year during treatment and have at least nine T2 hyperintense lesions on MRI or at least one gadolinium-enhanced lesion. (2) Those with rapidly evolving severe RRMS, defined by two or more disabling relapses within one year and with one or more gadolinium-enhanced lesions on a brain MRI or a significant increase in T2 lesions compared to a recent previous MRI [59].
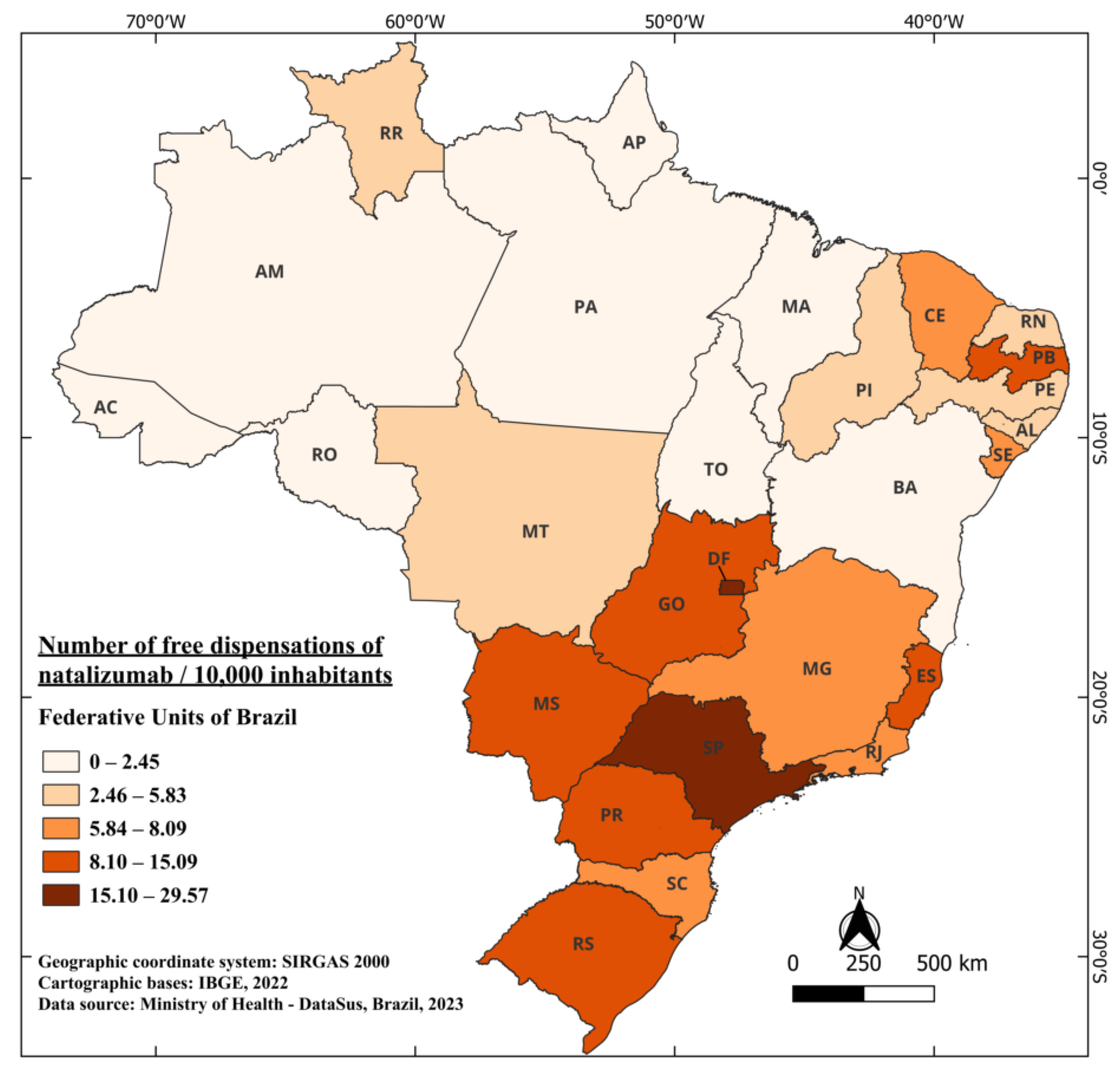
Remarkably, the time interval between its authorization and availability is similar to that of glatiramer, only three years, with the first distribution data recorded in 2011. From 2011 to 2022, a total of 220,548 vials of the medication were dispensed free of charge, highlighting the relevance of this therapy in patient treatment, considering its efficacy and availability through the Brazilian healthcare system. Today, it is one of the most established medications in the class of MAbs in Brazil [41]. Distribution in Brazil over these years averaged 0.89 vials per 10,000 inhabitants. Notably, the two states with the highest distribution are DF and SP, with 29.57 and 23.55 per 10,000 inhabitants, respectively. What stands out in the case of natalizumab is the significant discrepancy among some states compared to the country’s average. For example, DF dispenses 33 times more than the national average, while the state of Acre (AC), for instance, has not had any dispensation of the medication available in the public network over the years (Figure 4). However, when considering interferons Beta and glatiramer, AC dispensed 47.73 and 104.05 doses per 10,000 inhabitants over the years, respectively. One of the factors that may justify this finding is the strict criteria that patients need to meet to have free access to natalizumab, as explained above.
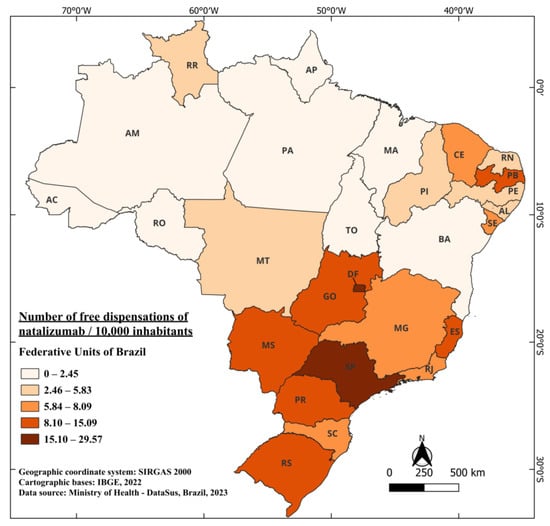
Figure 4.
The number of free dispensations of Natalizumab in Brazil between 2011 and 2022, in relation to the population size, was calculated by summing the total number of medication dispensations and dividing it by the average population during the same period. The population was adjusted per 10,000 inhabitants for better data visualization.
3.5. Fingolimod
Fingolimod (FTY720) is a highly bioavailable compound administered orally and is classified as a first-line drug used in the treatment of multiple sclerosis. Fingolimod is derived from a metabolite of the fungus myriocin and had its origins in early research on organ transplants. Notably, fingolimod is a prodrug that undergoes phosphorylation within the intracellular environment, leading to its conversion to fingolimod phosphate (fingolimod-P) through the action of the enzyme sphingosine kinase-2 (Sphk2). Fingolimod-P exhibits high affinity and a non-selective character, functioning as an agonist at four of the five widely known G protein-coupled phosphate receptors related to sphingosine (S1PRs 1, 3, 4, and 5). This compound, in turn, modulates the expression of these receptors in lymphocytes, cells of the CNS, and cardiovascular system cells, exerting influence over biological systems associated with immunological and cardiovascular conditions [60,61,62].
The primary function of fingolimod is to modulate the immune system, contributing to the control of inflammation in the central nervous system. Concerning its in vivo mechanism of action, fingolimod undergoes phosphorylation, generating fingolimod phosphate, which shares structural similarity with sphingosine-1-phosphate (S1P), an extracellular lipid mediator whose main effects are mediated by G protein-coupled S1P receptors. These S1P receptors include at least five subtypes known as S1P1–5, with four of them interacting with fingolimod phosphate. The expression of these receptors spans a wide range of cells and plays significant roles in various biological processes relevant to multiple sclerosis [60].
With the existence of multiple subtypes of these receptors, fingolimod binds to them, preventing the egress of T lymphocytes from lymph nodes into the bloodstream. As a result, fewer T lymphocytes circulate in the blood, and consequently, fewer immune cells enter the central nervous system, where they would cause inflammation. Consequently, when this restriction of T lymphocyte egress from lymph nodes occurs, fingolimod reduces the immune system’s ability to enter the central nervous system and trigger the autoimmune response that characterizes multiple sclerosis. This helps to decrease inflammation and prevent further nerve damage. It has been shown to be very effective in reducing the frequency of relapses in patients with relapsing-remitting multiple sclerosis (RRMS), which is the most common form of the disease [60,63,64].
One of the advantages of fingolimod compared to some other multiple sclerosis treatments is that it is administered orally, usually in the form of capsules. This can be more convenient for some patients compared to treatments that require injections [60].
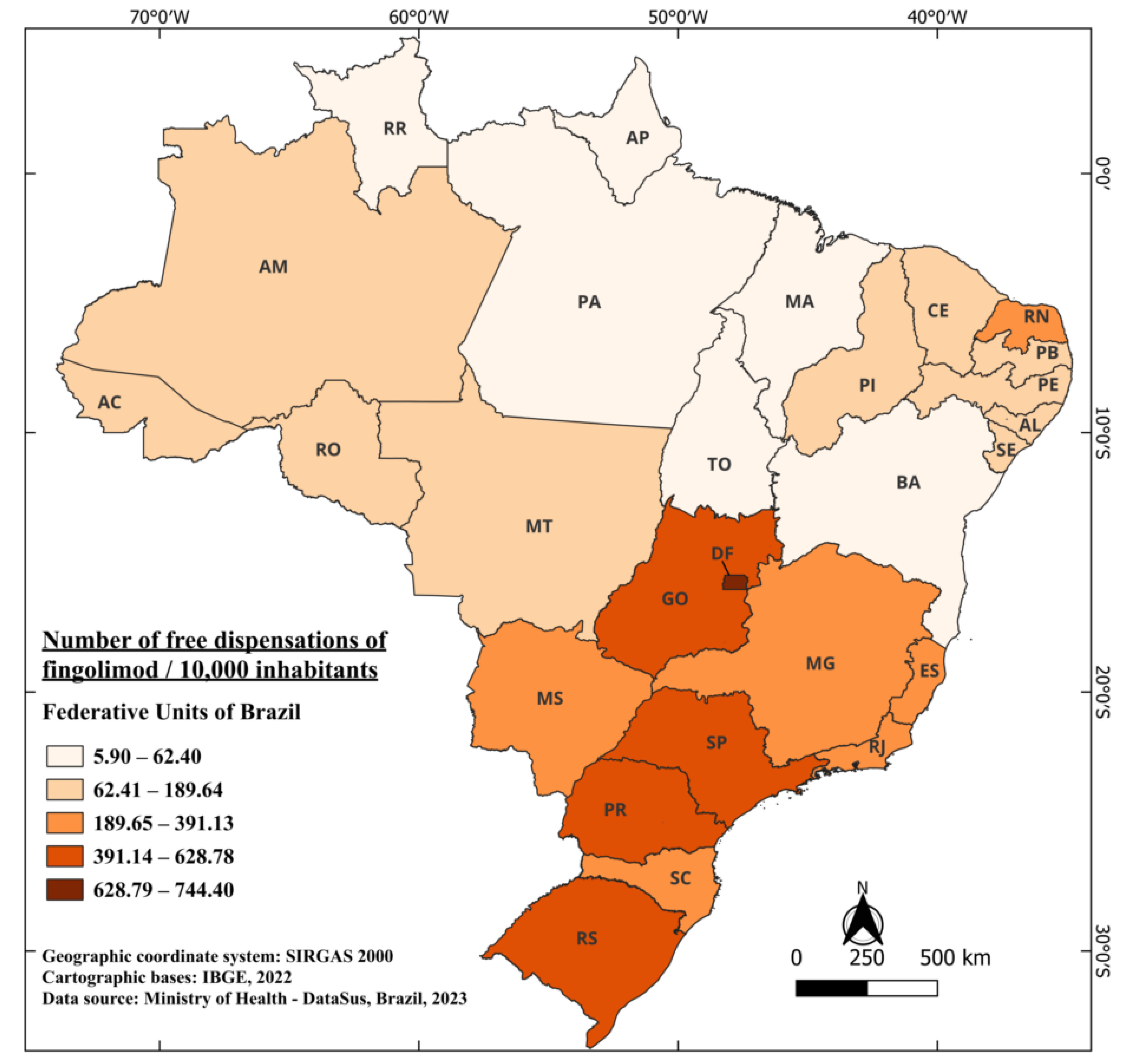
Fingolimod, marketed under the trade name Gilenya®, obtained its registration approval in Brazil in July 2011 [65], marking the introduction of the first orally administered drug in the country. It is currently indicated for the treatment of adults and pediatric patients above 10 years old with RRMS [66]. Its incorporation into the SUS for free distribution occurred in 2015, implying a waiting period of four years between approval and free availability. In the period from 2015 to 2022, a total of 7,309,488 fingolimod capsules were provided free of charge [41]. When considering the population size, Brazil dispensed an average of 43.53 capsules per 10,000 inhabitants. Notably, the states with the highest dispensation were the DF, SP, and Rio Grande do Sul (RS), with 744.40, 628.78, and 588.51 per 10,000 inhabitants, respectively. In contrast, the states of PA and AP had 8.96 and 5.90 per 10,000 inhabitants (Figure 5).
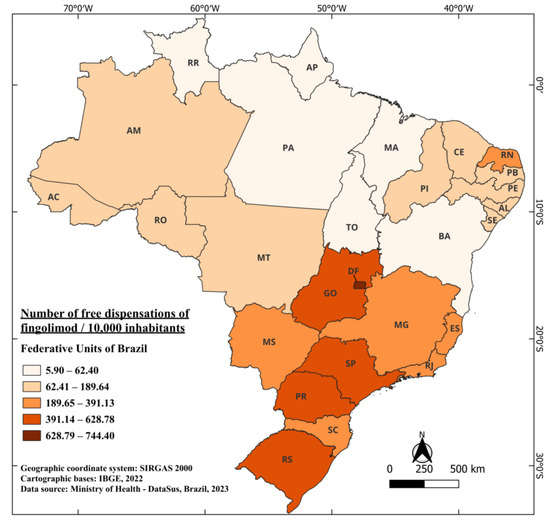
Figure 5.
The number of free dispensations of fingolimod in Brazil between 2015 and 2022, in relation to the population size, was calculated by summing the total number of medication dispensations and dividing it by the average population during the same period. The population was adjusted per 10,000 inhabitants for better data visualization.
3.6. Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody of the IgG1 kappa class, produced from mammalian cell culture in a nutrient medium. This Y-shaped molecule has a molecular weight of 150 kD. Its structure consists of two light polypeptide chains (L-C) of 24 kD each and two heavy polypeptide chains (H-C) of 49 kD each, interconnected by two interdisulfide bridges of (L-C)–(H-C) and two interdisulfide bridges of (H-C)–(H-C). Each alemtuzumab molecule contains 12 intrachain disulfide bridges and one asparagine residue in the heavy chain. It was initially used in transplantation to prevent graft-versus-host disease and later in malignant lymphoid diseases. Subsequently, it was tested in systemic vasculitis. Its application in secondary progressive MS occurred in 1991 with a group of seven patients and later in 29 patients with established progressive MS (SPMS) and significant disability [67,68].
It is a recombinant humanized monoclonal antibody against CD52, of the IgG1 kappa class. CD52 is a cell surface molecule expressed in large quantities on T and B lymphocytes, the primary drivers of inflammation in MS. Its expression is lower in natural killer cells, monocytes, and macrophages, and virtually absent in neutrophils, plasma cells, and bone marrow stem cells. Alemtuzumab induces the depletion of T and B lymphocytes expressing CD52, preserving innate immune cells. After the initial depletion, B cells are restored to baseline levels in approximately 6 months, while T cells do not reach baseline levels until about 12 months. The total lymphocyte count returns to baseline values in most patients (80%) after 12 months. After treatment with alemtuzumab, the proportion of regulatory T cells increases and, while slowly returning to baseline, remains elevated at the end of the first year [69].
The mechanism of action of alemtuzumab involves selective binding to the CD52 protein found on the surface of immune system cells, including T and B lymphocytes. This binding results in the depletion of activated T and B cells, which play a crucial role in the exacerbated autoimmune response seen in MS. In addition to depleting activated T and B cells, alemtuzumab initiates a process of immune system replenishment. Following treatment, T and B cells are regenerated from bone marrow stem cells and precursors. These new immune system cells have a profile that is less prone to autoimmunity and attacking the body’s own tissues [67,70,71].
Given that MS is characterized by chronic inflammation and demyelination in the central nervous system, the reduction in immune system cell activity by alemtuzumab helps to reduce inflammation and preserve myelin, which in turn can decrease disease relapses and symptom progression. Alemtuzumab also modulates the autoimmune response, restoring the balance between regulatory cells, such as regulatory T cells, and effector cells involved in the autoimmune response, contributing to the reduction in the autoimmune response observed in MS. A notable feature of alemtuzumab is its long-term efficacy, providing lasting control over MS activity for several years [67,70,71].
Alemtuzumab, registered with ANVISA in March 2014 under the trade name Lemtrada® [72], was indicated for the treatment of RRMS in patients who have already used another DMT without a therapeutic response or in cases of rapidly evolving severe RRMS [73]. Its first free dispensations recorded by the SUS occurred in 2022. This incorporation made it the most recent medication to be available for free by the SUS and the second in the class of MAbs. The waiting period between ANVISA registration and the first dispensations was eight years: the longest period. This milestone highlights the inclusion of a second MAb class medication in the list of free medications provided by the SUS. In fact, natalizumab was the only MAb available for MS treatment between 2011 and 2021, before the incorporation of alemtuzumab, representing a significant period during which patients had only this therapeutic option in the MAb class [41]. In 2022, only 20 vials of the medication are recorded as dispensed in the database, probably due to the recent addition of the medication to the list.
3.7. Teriflunomide
Teriflunomide, an orally administered medication, plays a significant role in the treatment of MS. Its journey began when it was initially used in the treatment of rheumatoid arthritis, becoming known for its antiproliferative properties, which involve inhibiting cell growth, and its anti-inflammatory characteristics, which counter local inflammatory responses triggered by cellular injuries. Teriflunomide received approval for the treatment of recurrent forms of MS in 2012 from the United States Food and Drug Administration (FDA). This applies to cases where patients experience repeated exacerbations of neurological symptoms. Subsequently, in 2013, the European Medicines Agency also granted its approval for this therapeutic purpose. This medication is classified as a disease-modifying agent, whose purpose is to attenuate MS activity, reduce the frequency of relapses, and delay disease progression [74,75].
The mechanism of action of teriflunomide lies in the selective inhibition of de novo pyrimidine synthesis, a process involving dihydroorotate dehydrogenase (DHODH), a crucial mitochondrial enzyme. This interference leads to the inhibition of de novo pyrimidine synthesis and, consequently, results in a cytostatic effect on the proliferation of T lymphocytes. The precise explanation of the underlying mechanism for therapeutic effects in patients with MS remains unknown; however, evidence suggests that it may involve the reduction in the number of activated lymphocytes in the CNS [74,75,76,77,78,79].
Therefore, teriflunomide exerts its impact on MS treatment by selectively suppressing the proliferation of lymphocytes, with a special focus on T and B lymphocytes, which play a central role in the autoimmune response associated with inflammation and demyelination in the central nervous system. The drug acts by inhibiting an enzyme called dihydroorotate dehydrogenase, which is crucial in DNA production in cells, including lymphocytes. This action reduces these cells’ ability to divide and multiply, thereby reducing the hyperactive autoimmune response. Additionally, teriflunomide also impacts the production of pro-inflammatory cytokines, which are substances involved in promoting inflammation. The medication reduces the production of these cytokines, further amplifying the suppression of inflammation in MS [74,75,76,77,78,79].
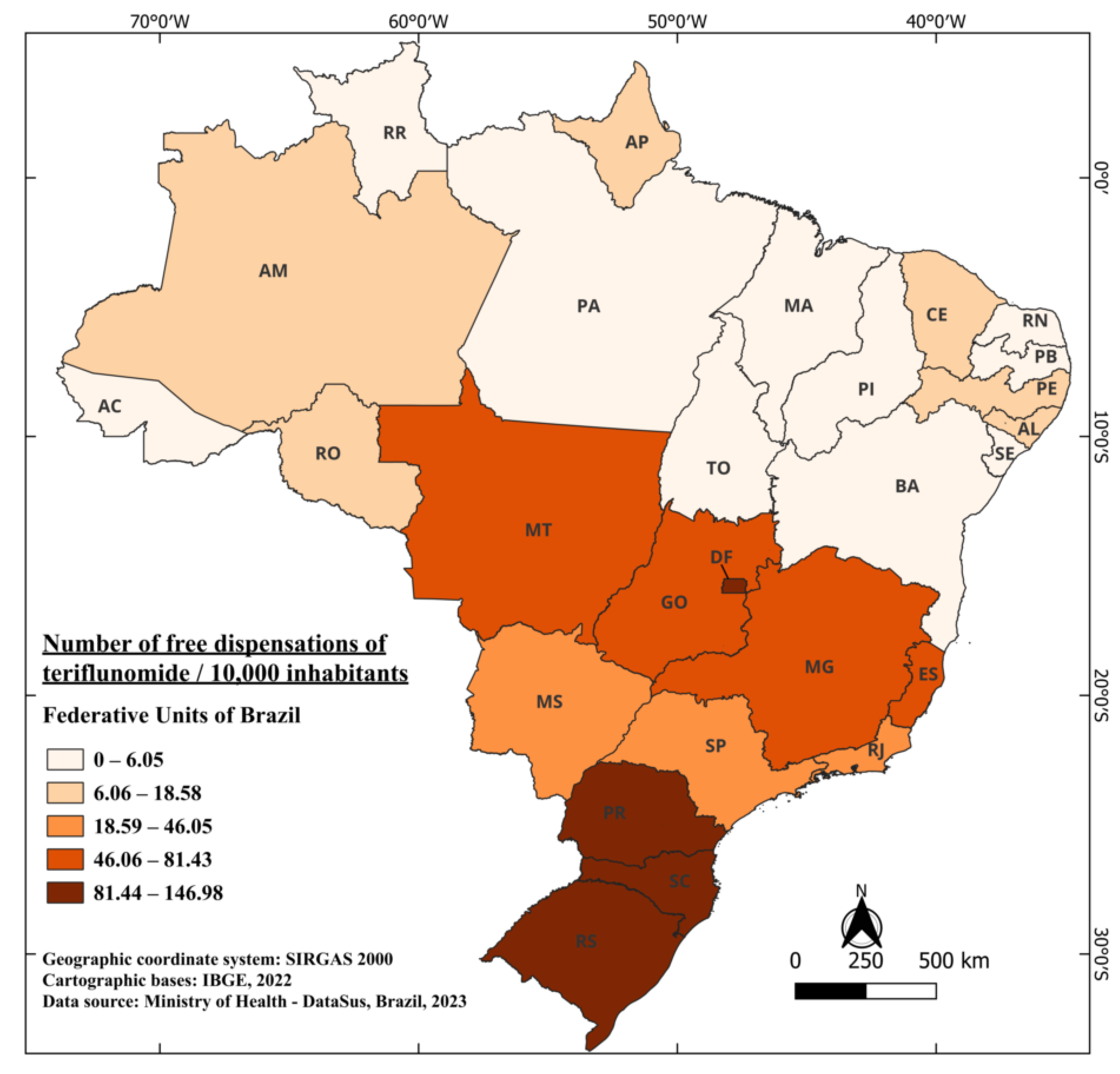
The first registration of teriflunomide dates back to March 2014, under the trade name Aubagio®, becoming the second oral therapy registered and authorized for commercialization in Brazil [80]. In 2018, this medication was included in the list of medications provided free of charge by the healthcare system, representing a four-year interval for its free availability. This implies that from 2015 to 2018, the only orally available medication provided for free in the Brazilian healthcare system was fingolimod. From 2018 to 2021, a total of 1,000,930 tablets of this medication were dispensed, highlighting the significance of teriflunomide’s availability as an additional oral option in the treatment of multiple sclerosis patients [41]. Currently, it is indicated for the treatment of RRMS to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability [81]. When considering the Brazilian population, an average of 18.87 tablets were dispensed for every 10,000 inhabitants. The standout states are RS, DF, and PR, with 146.98, 145.49, and 138.56 tablets dispensed per 10,000 inhabitants, respectively. Meanwhile, four states in the country did not record any dispensations in the years when the medication was available for free: Roraima (RR), Tocantins (TO), MA, and Sergipe (SE). The state, among those with recorded dispensation, that had the lowest recorded dispensation was PA with 2.58 per 10,000 inhabitants (Figure 6).
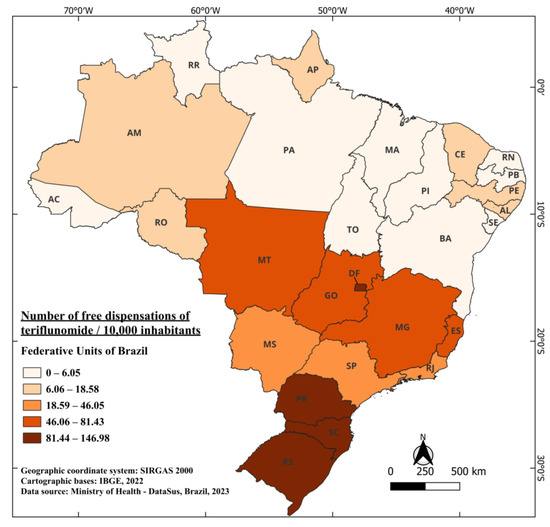
Figure 6.
The number of free dispensations of teriflunomide in Brazil between 2018 and 2022, in relation to the population size, was calculated by summing the total number of medication dispensations and dividing it by the average population during the same period. The population was adjusted per 10,000 inhabitants for better data visualization.
3.8. Dimethyl Fumarate
In the realm of medications combating MS, dimethyl fumarate, an ester of fumaric acid, emerges. This substance is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and Icelandic moss. It is considered a byproduct of purine biosynthesis and an intermediate in the citric acid cycle and urea cycle. Initially, it was employed and regulated as an oral treatment for psoriasis [22]. It undergoes rapid hydrolysis by esterases before reaching a systemic level, and its pharmacological action is actually carried out by its derivative, monomethyl fumarate. In the treatment of multiple sclerosis, it is employed as the most widely prescribed first-line oral medication for the condition [82,83].
Its mechanism of action primarily involves selective immunosuppression. In general, this immunosuppression is caused by phenotypic changes in immune cells and a reduction in inflammatory infiltrates in the CNS. It has the ability to alter all lymphocyte subpopulations, shifting from an inflammatory response to an anti-inflammatory response. This change is of great value, as there is evidence showing a relationship between disease severity and the profile of CD4+ T lymphocytes that produce pro-inflammatory cytokines, such as IFN-γ and IL-17. Therefore, it helps in attenuating relapses and symptoms [83,84,85]. Oxidative stress is also modulated through the use of dimethyl fumarate. It can induce apoptosis in T lymphocytes (via decreased glycolysis in T cells along with an increase in reactive oxygen species), as well as inhibit antigen presentation. This increase in the antioxidant response in the body helps protect central nervous system cells from oxidative damage caused by inflammation [83].
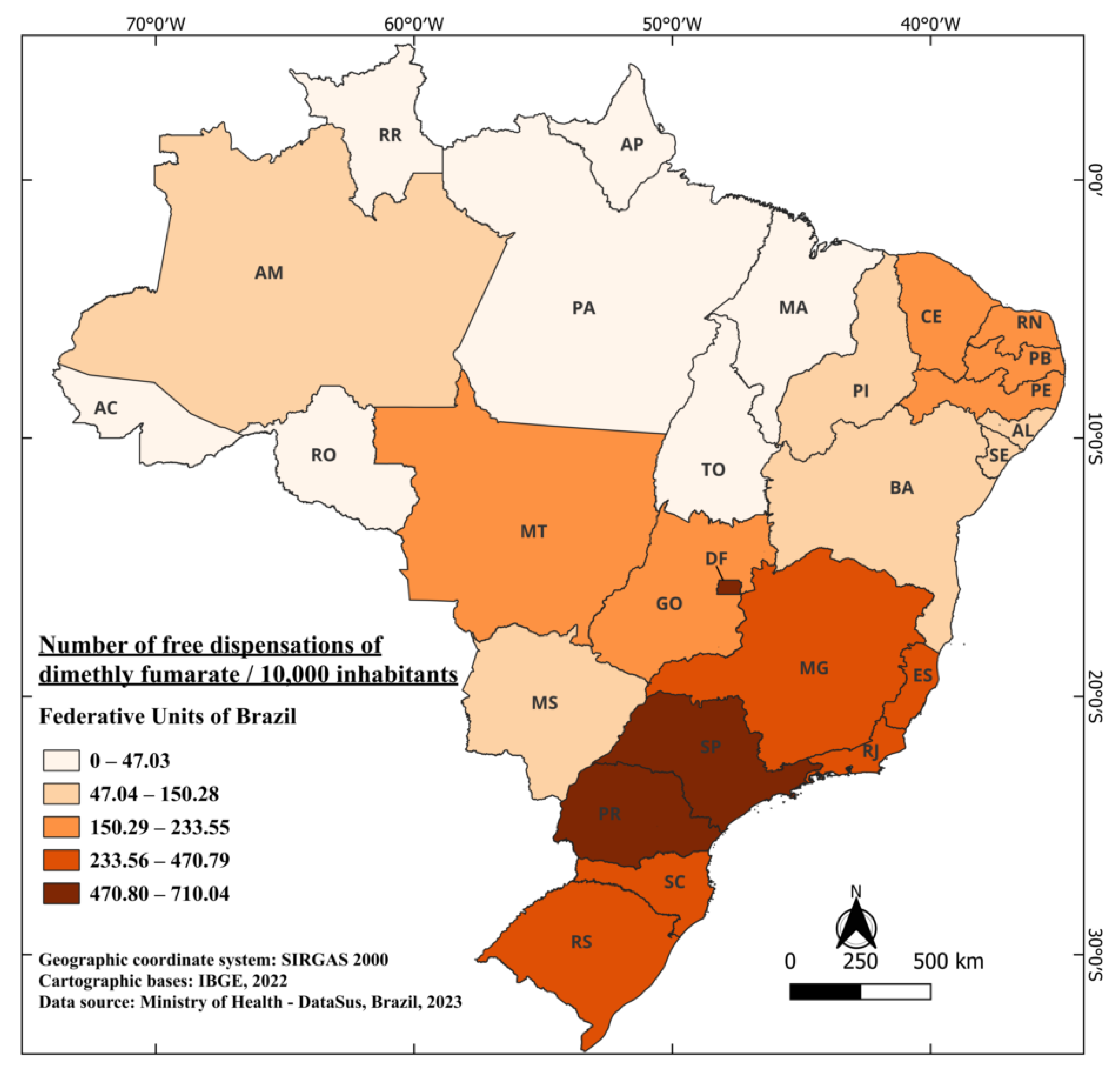
The medication was approved to treat individuals with relapsing-remitting multiple sclerosis. In 2013, it was approved by the FDA and, in 2014, by the European Medicines Agency (EMA). These approvals were based on two phase 3 clinical trials that showed promising and positive results, demonstrating a reduction of up to 50% in relapse cases [86]. Dimethyl fumarate obtained approval from ANVISA in April 2015 [87], and is marketed under the name Tecfidera®. It is indicated for the treatment of adult patients with relapsing-remitting multiple sclerosis [88]. The records of its free distribution began in 2019, resulting in a four-year wait between authorization for commercialization and free availability. From 2019 to 2022, a total of 7,838,443 capsules were distributed, including 46,396 capsules of 120 mg [41]. When considering the Brazilian population during this period, an average of 183.94 capsules were distributed for every 10,000 inhabitants. The standout states are SP, DF, and PR, with 710.04, 671.12, and 657.59 capsules dispensed for free per 10,000 inhabitants, respectively. Meanwhile, the states of RR and AP have recorded no dispensations of the medication to date. The state of Pará (PR), with the lowest number of dispensations, reported 21.47 capsules per 10,000 inhabitants (Figure 7).
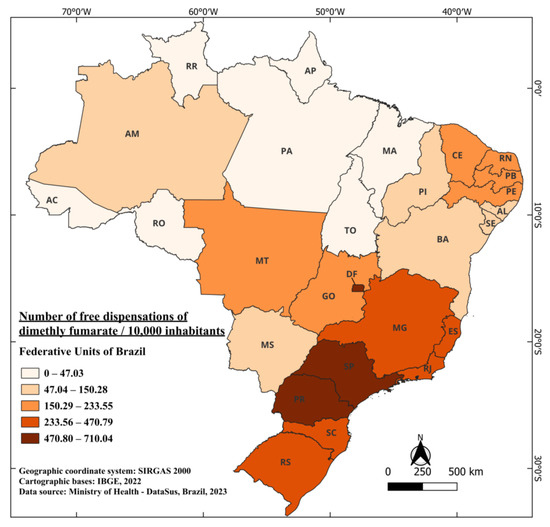
Figure 7.
The number of free dispensations of dimethyl fumarate in Brazil between 2019 and 2022, in relation to the population size, was calculated by summing the total number of medication dispensations and dividing it by the average population during the same period. The population was adjusted per 10,000 inhabitants for better data visualization.
3.9. Ocrelizumab
Ocrelizumab constitutes a pharmacological substance used for the therapy of certain autoimmune diseases, most notably its application in the treatment of multiple sclerosis. This agent represents a second-generation monoclonal antibody, of recombinant configuration, aimed at the neutralization of the CD20 antigen. It has a molecular mass corresponding to 145 kilodaltons (kDa) and features a humanized light and heavy chain structure of the IgG1 class. The adoption of the humanized structure aims to attenuate the intrinsic antigenicity of the compound and optimize its affinity for the CD20 receptor [89,90].
CD20, a phosphoprotein, is expressed in a diverse spectrum of B cells in the human body. However, its presence is not observed in the early stages of the B cell lineage (stem cells and pro-B cells) nor in the advanced stages of this lineage (plasmablasts and plasma cells). Ocrelizumab interacts with the CD20 antigen on B lymphocytic cells, resulting in antibody-dependent cytotoxicity and complement-mediated lysis. Although the precise mechanisms through which ocrelizumab exerts its effect in multiple sclerosis remain unknown, it induces B cell depletion, which is believed to be involved in the pathogenesis of the disease, influencing cytokine regulatory processes, antigen presentation, and autoantibody production [90,91].
The primary target of ocrelizumab is the B cells of the immune system, which play an essential role in the body’s defense by generating antibodies to neutralize exogenous elements, such as pathogenic microorganisms like bacteria and viruses. However, in autoimmune diseases like multiple sclerosis and rheumatoid arthritis, B cells can exhibit hyperactivity, leading to an attack on the host’s own components. The mechanism of action of ocrelizumab involves the selective inhibition of B cell activity, resulting in the attenuation of autoimmune immune responses. This therapeutic approach leads to the reduction in inflammation and disease progression in patients diagnosed with multiple sclerosis, illustrating the beneficial impact that this compound provides in this clinical context [90,91].
Ocrelizumab, marketed under the name Ocrevus®, was registered with ANVISA in February 2018 [92], with indications for the treatment of relapsing forms of multiple sclerosis and patients with primary progressive multiple sclerosis [93]. In 2020, the CONITEC conducted an evaluation regarding the possibility of incorporating this medication for free distribution [94]. As a result of this analysis, the commission chose not to incorporate the medication into the list of drugs provided by SUS due to the existence of equivalence in terms of efficacy between ocrelizumab and natalizumab, a medication already available through SUS. Additionally, the long-term safety of ocrelizumab, which was considered one of its supposed advantages over natalizumab, was not fully clarified at the time of the decision. In summary, due to similar efficacy and proposed prices, the medication did not demonstrate a favorable cost–effectiveness ratio that would justify its inclusion in the list of free medications provided by SUS for the treatment of relapsing-remitting multiple sclerosis. Therefore, ocrelizumab remains outside the list of free medications provided by SUS, totaling five years since its registration.
3.10. Cladribine
Cladribine, an analog of adenosine, selectively targets immune system cells, with a significant impact on lymphocytes. The substance known as 2-chloro-2’-deoxyadenosine (2-CdA or cladribine) was initially synthesized in 1972, following the identification of a variant of severe combined immunodeficiency (SCID). Drs. Ernest Beutler and Dennis A. Carson were investigating this new form of SCID, which was associated with a deficiency of the enzyme adenosine deaminase. Due to this enzymatic deficiency, levels of deoxyadenosine triphosphate increase within the cells, leading to the destruction of T and B lymphocytes and, consequently, resulting in immunodeficiency. These cells play a crucial role in autoimmune responses, including those related to MS [95].
Cladribine can be administered either parenterally or orally, as it has an estimated bioavailability of 40%. It is extensively excreted in the urine without undergoing alteration and demonstrates good penetration into the CNS, with an estimated concentration of approximately 25% of the plasma concentration in healthy individuals. This becomes relevant due to the blood–brain barrier, a common pathological condition found in all stages of MS, which may facilitate greater penetration into the CNS. Its action is preferentially directed towards lymphocytes, exploiting the enzymatic profile of kinases in such cells. This results in moderate and intermittent reductions in T and B cell populations, with relatively minor and transient effects on cells of the innate immune system, such as neutrophils and monocytes [96,97].
Regarding oral administration, cladribine is absorbed through the gastrointestinal tract and enters immune system cells, such as lymphocytes, through specific transporters. Once intracellular, this substance inhibits the activity of the enzyme adenosine deaminase (ADA), which plays an essential role in the degradation of adenosine, a nucleoside crucial for cellular functions. The inhibition of ADA leads to intracellular accumulation of adenosine, which is a critical signaling molecule in the immune system, regulating the activity of T and B cells. Elevated adenosine levels trigger signaling pathways that induce apoptosis (programmed cell death) in activated T and B cells. This results in a reduction in the population of immune system cells involved in the autoimmune response that characterizes MS [98,99].
After treatment with cladribine, a process of immune system repopulation occurs, with the generation of new cells from bone marrow stem cells. These new cells exhibit a more regulated immune response and are less likely to attack the central nervous system, aiding in the reduction in inflammation and the characteristic damage of MS. Cladribine is administered in short treatment cycles, which allows disease control with less frequent dosing. It is typically reserved for cases of highly active MS or when other therapies have been ineffective. Like any immunosuppressive treatment, cladribine can increase the risk of infections, and patients using it should be closely monitored. Treatment with cladribine is often divided into two annual cycles, striking a balance between disease control and minimizing side effects [96,97,98,99].
Cladribine was authorized for commercialization in Brazilian territory by ANVISA in September 2019, under the trade name Mavenclad® [100], and it is indicated for the treatment of adult patients with highly active relapsing multiple sclerosis [101]. Recently, in June 2023, CONITEC made the decision to keep cladribine out of the free medication supply system. This decision was based on the argument that the high cost of the new technology, the accumulated experience with the use of natalizumab in the SUS, and the current outlook for patients do not indicate the need to include the medication in the list of medicines available free of charge [102].
3.11. Ofatumumab
Ofatumumab, belonging to the class of monoclonal antibodies, is an agent used in the treatment of multiple sclerosis with the aim of acting as a disease-modifying therapy, seeking to reduce its activity and prevent relapses in patients affected by MS. It is a fully human immunoglobulin G1 (IgG1k) monoclonal antibody. In vitro, the fragment antigen-binding (Fab) domain of ofatumumab binds to the CD20 molecule, while the Fc domain promotes B cell lysis through its immune effector functions. Similar to other monoclonal antibodies used therapeutically, ofatumumab induces antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. Ofatumumab, marketed under the name Kesimpta®, is a recombinant human immunoglobulin G1 (IgG1) monoclonal antibody, characterized by a considerable molecular weight of 149,000 Daltons. Initially approved for the treatment of chronic lymphocytic leukemia (Arzerra®), ofatumumab has now received approval in various countries, including the United States, member states of the European Union, and Japan, for the treatment of relapsing forms of MS. It is notable for being the first B cell-targeted therapy that allows self-administration by patients after initial training by a healthcare professional [77,103].
The mechanism of action of ofatumumab involves its selective binding to CD20, a transmembrane phosphoprotein present on B cells. Ofatumumab differs from other anti-CD20 antibodies by binding to a distinct region of the CD20 receptor, spanning the smaller loop and the larger loop. This results in high-affinity binding and a slow dissociation rate, favoring the effective lysis of B cells, mediated both by complement-dependent cytotoxicity and, to a lesser extent, antibody-dependent cytotoxicity. It is worth noting that ofatumumab can be administered at lower doses than those studied in other clinical conditions, and its subcutaneous route allows for potential more direct access to lymph nodes. Administering ofatumumab through subcutaneous injections at specified intervals has proven effective in the treatment of relapsing MS. Its efficacy, favorable tolerability profile, less frequent dosing regimen, and the ability for at-home self-administration make it an attractive therapeutic option for MS patients [77,103,104].
Ofatumumab was approved in Brazil in May 2021 under the trade name Kemsimpta®, representing the second most recent addition of medications for the treatment of MS [105]. It is currently indicated for the treatment of adult patients with RRMS (relapsing-remitting multiple sclerosis) [106]. In March 2022, CONITEC conducted an analysis regarding the incorporation of ofatumumab for free distribution as a first-line therapy for the treatment of RRMS. During this evaluation, it was observed that treatment with ofatumumab was likely more effective than other first-line DMTs used in the SUS. However, due to the significant financial impact that the incorporation of ofatumumab would represent for the SUS, with an estimated increase of approximately BRL 231 million over five years, and the assessment of the technological horizon indicating the availability of a large number of technologies for the treatment of RRMS in a short period of time, the recommendation was not to incorporate the medication into the SUS, and this decision remains in effect to the present day [107].
3.12. Siponimod
Another medication belonging to the class of sphingosine-1-phosphate (S1P) receptor modulators is siponimod, which modulates receptors 1 and 5. It is considered a next-generation drug and has been approved in several countries for the treatment of secondary progressive multiple sclerosis, with specific indications varying among individual countries [108].
Its mechanism of action involves the regulation of S1P1 and S1P5 receptors, acting as a functional antagonist of S1P. Consequently, it leads to a reduction in the number of lymphocytes in lymphoid tissues and inhibits the recirculation of lymphocytes from the periphery to the CNS. This occurs following the administration of siponimod, which is orally administered and absorbed in the gastrointestinal tract before entering the bloodstream. Once in the bloodstream, it prevents these cells from leaving the lymph nodes, where they typically accumulate before migrating to peripheral tissues, including the central nervous system. This action reduces the quantity of lymphocytes entering the CNS, resulting in a decrease in inflammation and nerve tissue damage. This helps in controlling relapses of multiple sclerosis and reducing the progression of symptoms [108,109,110].
It is also believed to aid in preserving the integrity of myelin and nerve fibers, contributing to the preservation of neural function, as it has the ability to cross the blood–brain barrier. Studies have shown that it is also capable of promoting remyelination in the central nervous system [110,111,112,113].
Siponimod fumarate is the most recent medication approved by ANVISA for the treatment of multiple sclerosis. In fact, the medication received approval for commercialization in the country in October 2021 under the trade name Kiendra® [114]. It is currently indicated for the treatment of patients with secondary progressive multiple sclerosis (SPMS) with active disease, evidenced by relapses or characteristics of inflammatory activity on imaging [115]. As of the current moment, there is no record of CONITEC’s evaluation regarding the medication and its free availability in the Brazilian healthcare system.
3.13. Other Drug Therapies
There are some other drug therapies for multiple sclerosis that are not provided by the Brazilian public health system or are used off-label. Let us discuss two of them below.
Mitoxantrone is a medication belonging to the class of disease-modifying agents. This compound is part of the anthracycline group, with a molecular weight of 517.4 Da, which consists of chemotherapeutic agents originally approved for primary use in cancer treatment. Anthracyclines act by inserting themselves into DNA through hydrogen bonds, causing cross-links and breaks in the DNA chains. Additionally, they interfere with RNA and inhibit the activity of the enzyme topoisomerase II, which plays a crucial role in the unwinding and repair of damaged DNA. In in vitro experiments, mitoxantrone has demonstrated the ability to inhibit the proliferation of B cells, T cells, and macrophages. Although initially developed for cancer treatment, the use of this medication has proven to be beneficial in improving the quality of life for patients, providing immunosuppression approximately 10 times more potent than widely established immunosuppressants like cyclophosphamide. Furthermore, mitoxantrone has shown efficacy in reducing the activity of multiple sclerosis, contributing to the control of its symptoms [116,117].
Mitoxantrone was approved after studies in the 2000s in Europe and the USA for the treatment of relapsing-remitting, secondary progressive, and progressive relapsing forms of multiple sclerosis. It is administered intravenously every 3 months. However, in recent years, the use of mitoxantrone has decreased due to the risk of serious adverse events such as heart problems and bone marrow damage. Its utilization has been decreasing every year with the emergence of newer therapies that are more modern and have fewer adverse effects [118].
Its mechanism of action is more complex and involves different sites of action. If we think logically, a significant part of the mechanism involves long-term immunosuppression because it has a long-lasting effect. It has an elimination phase with a half-life of nine days, and a substantial portion of it is sequestered in deep tissues for up to a month before being slowly released [119].
First, it suppresses the immune system, specifically affecting T and B lymphocytes, as well as innate immune system cells like macrophages, where the immune system erroneously attacks healthy tissue in the central nervous system. It has the ability to inhibit cell division, disrupting the replication process. This action can affect both immune cells and tumor cells since the medication was originally developed for cancer treatment. Specifically in multiple sclerosis, this inhibition leads to a reduction in the hyperactive autoimmune response. As a result, it promotes a reduction in inflammation due to the suppression of cells. All of this culminates in a decrease in disease relapses and in symptom reduction. Like other medications, it is believed to also act on myelin sheath preservation, as the reduction in inflammation and immune activity is essential for preventing MS progression [117,118,119,120,121].
Rituximab (RTX) stands out as a chimeric monoclonal antibody medication, targeting the CD20 antigen, and, notably, it was the pioneer among anti-CD20 drugs, approved for the treatment of B cell lymphomas, refractory rheumatoid arthritis, and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. In recent years, several observational studies have supported its high efficacy, relatively benign safety profile, convenient administration regimen, and affordable costs, contributing to its growing consideration as an intriguing therapeutic option in the field of multiple sclerosis. Its use has increasingly garnered attention as a primary and escalating therapeutic approach [122,123].
Rituximab is a chimeric monoclonal antibody targeting the CD20 antigen present on pre-B and mature B lymphocytes. The mechanism of action of rituximab involves the elimination of B cells through cellular cytotoxicity, complement activation, and induction of apoptosis. This depletion of B cells modifies the pathological process of MS by preventing B cells from acting as antigen-presenting cells, thus inhibiting the activation of T lymphocytes. Additionally, rituximab inhibits the differentiation of B cells into new plasma cells, reducing the production of self-reactive antibodies and the release of cytokines that can cause damage to the central nervous system [124,125].
It is important to emphasize that although these medications have effects on the management of MS, they are not mentioned in the flowchart recommended by the Brazilian Ministry of Health, which regulates the free distribution of drugs involved in the treatment of MS, along with specific indications for their use in each situation (Table 1).

Table 1.
Main disease-modifying therapies registered in Brazil for the treatment of MS.
3.14. Hospitalizations and Deaths
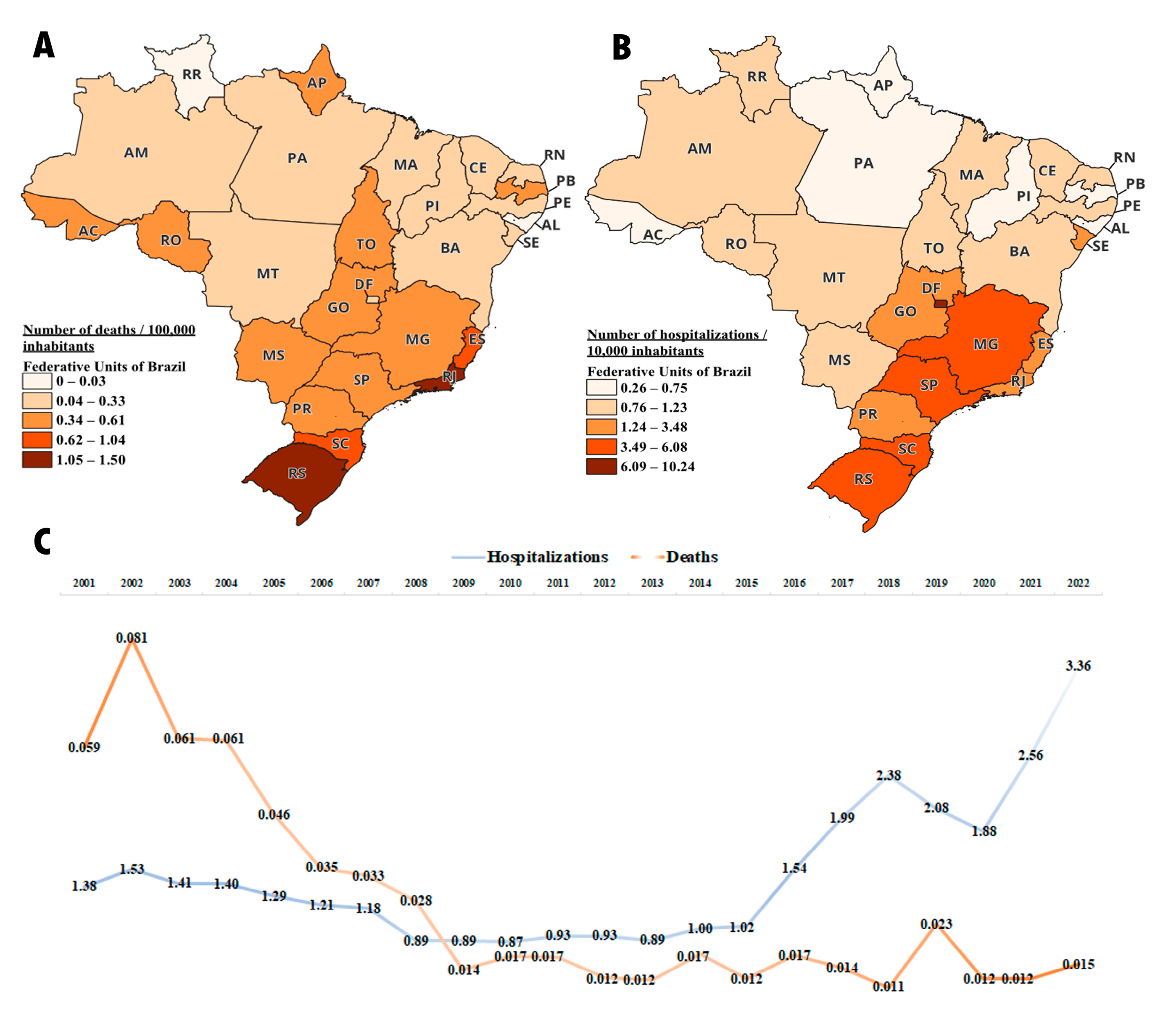
Regarding the number of deaths recorded in Brazil between 2001 and 2022 due to multiple sclerosis, there were an average of 0.58 deaths per 100,000 inhabitants. The states of Rio de Janeiro, Rio Grande do Sul, and Santa Catarina had the highest number of deaths, with 1.5, 1.37, and 1.04 deaths per 100,000 inhabitants, respectively. The state of Roraima did not record any deaths due to multiple sclerosis during this period. The states with the lowest recorded number of deaths were Alagoas and Maranhão, with 0.03 and 0.14 deaths per 100,000 inhabitants, respectively. O Distrito Federal, Paraná, and São Paulo, which were highlighted in medication dispensation, had an average of 0.26, 0.46, and 0.61 deaths per 100,000 inhabitants, respectively (Figure 8A).
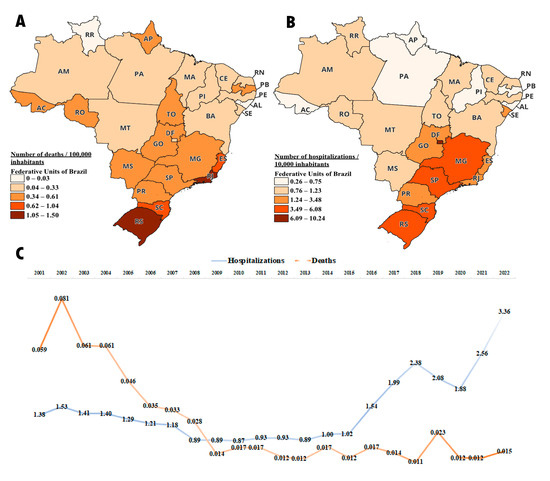
Figure 8.
Number of hospitalizations and deaths due to multiple sclerosis between 2001 and 2022; deaths data are adjusted to 100,000 inhabitants and hospitalizations data are adjusted to 10,000 inhabitants (A) Average number of deaths per federal unit during the period. (B) Average number of hospitalizations per federal unit during the period. (C) Number of deaths and hospitalizations in Brazil over the years.
When evaluating the number of hospitalizations for multiple sclerosis from 2001 to 2022, Brazil had an average of 3.3 hospitalizations per 10,000 inhabitants. It is observed that the Distrito Federal had the highest number of admissions, and it is precisely the one that, as demonstrated in the above sections, has the highest medication distribution per population. The number of hospitalizations in the DF was 10.24 per 10,000 inhabitants, followed by Rio Grande do Sul (RS) with 6.08 per 10,000 inhabitants, and São Paulo (SP) with 5.59 per 100,000 inhabitants. On the other hand, the states with the lowest number of hospitalizations were Alagoas (AL) and Pará (PA) with 0.26 and 0.52 per 10,000 inhabitants, respectively (Figure 8B).
An interesting fact to be observed is that over the years between 2001 and 2022, Brazil has shown a reduction in the number of deaths, while the number of hospitalizations has increased. This observation is noteworthy, as it suggests that the greater availability of medications in the healthcare system leads to a decrease in the mortality rate due to multiple sclerosis. Another noteworthy factor is the increase in hospitalizations in recent years. We believe this may be attributed to the increased longevity of patients due to the improved survival provided by the free distribution of medication, resulting in a higher rate of hospitalizations due to the natural progression of the disease (Figure 8C).
3.15. Lead Time of Free Distribution
Regarding the waiting time between a medication’s registration in Brazil and its implementation in the healthcare system, we found varying results (Figure 9). Currently, the system has eight medications approved for free distribution, with an average lead time of 3 and a half years. Notably, beta interferons had the shortest lead time at 1 year, explained mainly by the fact that, until their approval, they were the only registered medication in the country for multiple sclerosis treatment. On the other hand, alemtuzumab had the longest lead time at 8 years, largely due to the presence of natalizumab already included in the SUS free medication list before the registration of alemtuzumab in Brazil, which made it challenging to include another MAb on the list, especially due to cost–effectiveness studies.
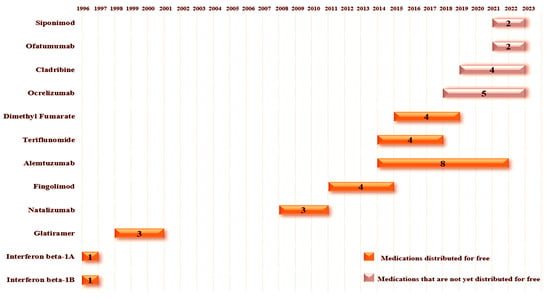
Figure 9.
The figure illustrates the time between a drug’s registration in Brazil and its approval for free distribution. Medications that are not yet available in the public network display the duration between registration and availability outside the free network. Once a drug is approved for free distribution, it remains in that status over the years. For medications distributed free of charge, the year on the left side of the bar represents the year of registration, while the year on the right side of the bar represents the year of approval for free release. The length of the bar reflects the time interval until approval for free distribution or the period off the free drug list if it is not approved. The number within each bar represents, in years, the time from registration to distribution or the duration since registration for medications not yet distributed free of charge.
Furthermore, Brazil currently has four medications that are not included in the list of free medications, with ocrelizumab having the longest waiting time at 5 years and siponimod and ofatumumab having the shortest at 2 years. As highlighted earlier, the reasons for not implementing these medications in the healthcare system, we would like to provoke some reflection based on the data presented. Notably, as observed with the progression of therapeutic options available in the healthcare system, there has been a progressive decline in the number of deaths in the country. We are aware of the various factors that influence a medication being included in the free list, with financial impact playing a crucial role in this decision. However, we would like to leave as a point of reflection: What could be the impact on the quality of life of multiple sclerosis patients if these therapies were being distributed for free in the country?
4. Conclusions
The results of this study have highlighted the complexity of therapeutic management of multiple sclerosis in Brazil. Significant regional variations in prescription and medication access were observed, indicating challenges in the equity of the country’s healthcare system. Despite existing public health policies, including the provision of high-cost medications through the SUS, there is still substantial inequality in medication distribution levels in Brazil. States with a higher human development index (HDI) generally have better access to medical resources (Supplementary Material). These HDI disparities among Brazilian states can impact the ability to provide effective treatments for chronic conditions like multiple sclerosis. States with a higher HDI tend to have more financial resources available for investing in healthcare infrastructure. Additionally, they may have a more qualified medical workforce and a broader healthcare service network. The implementation of new health policies focused on ensuring access and equitable distribution is of utmost necessity.
The analysis of ANVISA records revealed that not all medications used in multiple sclerosis treatment have approval for this indication, raising concerns about the safety and efficacy of these therapies. Furthermore, ANVISA registration of a medication does not guarantee immediate distribution through SUS, as the inclusion or exclusion of medications from the distribution list depends on several factors, including cost–effectiveness considerations and resource availability.
Regarding the mechanisms of action of these medications, understanding these aspects is fundamental for healthcare professionals in the proper management of multiple sclerosis. To this end, this study provided general insights into the complex mechanisms of action of all medications registered with ANVISA and distributed through SUS.
In conclusion, this study provided a comprehensive overview of medications used in the treatment of multiple sclerosis in Brazil, with a critical focus on SUS distribution. The findings underscore the need for a more integrated and equitable approach to medication supply, as well as the importance of continuous updates on information regarding current and new medications and their mechanisms of action. Furthermore, the insights gained from this study demonstrate that, while the data are Brazilian-specific and context-specific, their findings have universal value that can benefit healthcare managers and professionals worldwide, regardless of whether their regions have the same continental scale as Brazil. This information can enrich the global understanding of multiple sclerosis management and serve as a foundation for future research and decision-making in the field of public health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sclerosis2010003/s1, Table S1: Number of medication dispensations in relation to the population size in the Brazilian Federative Unites and their HDI.
Author Contributions
The attributions of each author in the work are pointed out below, highlighting that C.S.D. and Y.M.P.-G. share the authorship of the work because they play an egalitarian role in its development. Conceptualization, C.S.D., Y.M.P.-G., R.O.T., M.A.D.-S., W.G.B. and C.J.F.O.; data curation, C.S.D. and Y.M.P.-G.; formal analysis, C.S.D. and Y.M.P.-G.; funding acquisition, M.A.D.-S., V.R.J. and C.J.F.O.; investigation, C.S.D., Y.M.P.-G., R.O.T., M.A.D.-S., W.G.B. and W.F.R.; methodology, C.S.D., Y.M.P.-G., R.O.T. and W.G.B.; project administration, C.J.F.O.; software, C.S.D. and Y.M.P.-G.; supervision, M.V.d.S., V.R.J. and C.J.F.O.; validation, C.S.D., Y.M.P.-G. and W.G.B.; visualization, C.S.D., Y.M.P.-G., M.A.D.-S., W.G.B., W.F.R., V.R.J. and C.J.F.O.; writing—original draft, C.S.D., Y.M.P.-G., R.O.T. and W.G.B.; writing—review and editing, W.F.R., M.V.d.S., V.R.J. and C.J.F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico. The funding sources were not involved in the study’s design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Institutional Review Board Statement
Approval for the study was not required according to Plataforma Brasil. This study does not involve humans, as it is a descriptive pharmacoepidemiological study. To carry out the study, several databases were used with information about medications for multiple sclerosis in Brazil.
Informed Consent Statement
This study entails the examination of publicly available secondary data. It is not applicable for informed consent, as the research did not involve human subjects.
Data Availability Statement
The data presented in this study are publicly accessible in DATASUS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kornek, B.; Storch, M.K.; Weissert, R.; Wallstroem, E.; Stefferl, A.; Olsson, T.; Linington, C.; Schmidbauer, M.; Lassmann, H.J.T. Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000, 157, 267–276. [Google Scholar] [CrossRef]
- Kamm, C.P.; Uitdehaag, B.M.; Polman, C.H. Multiple sclerosis: Current knowledge and future outlook. Eur. Neurol. 2014, 72, 132–141. [Google Scholar] [CrossRef]
- Arnold, A.C. Evolving management of optic neuritis and multiple sclerosis. Am. J. Ophthalmol. 2005, 139, 1101–1108. [Google Scholar] [CrossRef]
- Ebers, G. Neurosurgery; Psychiatry. Natural history of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2001, 71, ii16–ii19. [Google Scholar]
- Soelberg Sorensen, P. Safety concerns and risk management of multiple sclerosis therapies. Acta Neurol. Scand. 2017, 136, 168–186. [Google Scholar] [CrossRef]
- Thompson, A.J.; Polman, C.H.; Miller, D.H.; McDonald, W.I.; Brochet, B.; Filippi MMontalban, X.; De Sa, J. Primary progressive multiple sclerosis. Brain J. Neurol. 1997, 120, 1085–1096. [Google Scholar] [CrossRef]
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef]
- Milo, R.; Kahana, E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun. Rev. 2010, 9, A387–A394. [Google Scholar] [CrossRef]
- Correale, J.; Ysrraelit, M.C.; Fiol, M.P. Benign multiple sclerosis: Does it exist? Curr. Neurol. Neurosci. Rep. 2012, 12, 601–609. [Google Scholar] [CrossRef]
- Stinissen, P.; Raus, J.; Zhang, J. Autoimmune pathogenesis of multiple sclerosis: Role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit. Rev. Immunol. 1997, 17, 33–75. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Multiple Sclerosis. Multiple Sclerosis: Current Status and Strategies for the Future; Joy, J.E., Johnston, R.B., Jr., Eds.; The National Academies Press: Washington, DC, USA, 2001; p. 456. [Google Scholar]
- Lucchinetti, C.F.; Brück, W.; Rodriguez, M.; Lassmann, H. Distinct Patterns of Multiple Sclerosis Pathology Indicates Heterogeneity in Pathogenesis. Brain Pathol. 1996, 6, 259–274. [Google Scholar] [CrossRef]
- Brazil, Ministério da Saúde. Clinical Protocols and Therapeutic Guidelines for Multiple Sclerosis. 2022. Available online: https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt/arquivos/2022/portal_portaria-conjunta-no-1-pcdt_esclerose-multipla.pdf (accessed on 13 August 2023).
- Lenne, B.; Donze, C.; Massot, C.; Degraeve, B. Impact of physical activity, physical fitness and exercises on cognitive impairment in patients with multiple sclerosis: A review of evidence and underlying mechanisms. Rev. Neurol. 2023. [Google Scholar] [CrossRef]
- Okemuo, A.J.; Gallagher, D.; Dairo, Y.M. Effects of rebound exercises on balance and mobility of people with neurological disorders: A systematic review. PLoS ONE 2023, 18, e0292312. [Google Scholar] [CrossRef]
- Loma, I.; Heyman, R. Multiple sclerosis: Pathogenesis and treatment. Curr. Neuropharmacol. 2011, 9, 409–416. [Google Scholar] [CrossRef]
- Beck, R.W.; Cleary, P.A.; Trobe, J.D.; Kaufman, D.I.; Kupersmith, M.J.; Paty, D.W.; Brown, C.H. The Effect of Corticosteroids for Acute Optic Neuritis on the Subsequent Development of Multiple Sclerosis. N. Engl. J. Med. 1993, 329, 1764–1769. [Google Scholar] [CrossRef]
- Goldenberg, M.M. Therapeutics. Mult. Scler. Rev. 2012, 37, 175. [Google Scholar]
- Callegari, I.; Derfuss, T.; Galli, E. Update on treatment in multiple sclerosis. Presse Med. 2021, 50, 104068. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Weinshenker, B.G. Disease modifying therapies for relapsing multiple sclerosis. BMJ 2016, 354, i3518. [Google Scholar] [CrossRef]
- Schrempf, W.; Ziemssen, T. Glatiramer acetate: Mechanisms of action in multiple sclerosis. Autoimmun. Rev. 2007, 6, 469–475. [Google Scholar] [CrossRef]
- Papadopoulou, A.; D’Souza, M.; Kappos, L.; Yaldizli, O. Dimethyl fumarate for multiple sclerosis. Expert. Opin. Investig. Drugs 2010, 19, 1603–1612. [Google Scholar] [CrossRef]
- Miller, A.E. An updated review of teriflunomide’s use in multiple sclerosis. Neurodegener. Dis. Manag. 2021, 11, 387–409. [Google Scholar] [CrossRef]
- Kappos, L. Interferons in multiple sclerosis. Neurol. Clin. 2005, 23, 189–214. [Google Scholar] [CrossRef]
- Pelletier, D.; Hafler, D.A. Fingolimod for multiple sclerosis. N. Engl. J. Med. 2012, 366, 339–347. [Google Scholar] [CrossRef]
- Voge, N.V.; Alvarez, E. Monoclonal Antibodies in Multiple Sclerosis: Present and Future. Biomedicines 2019, 7, 20. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Stamatellos, V.P.; Papazisis, G. Safety and Monitoring of the Treatment with Disease-Modifying Therapies (DMTs) for Multiple Sclerosis (MS). Curr. Rev. Clin. Exp. Pharmacol. 2023, 18, 39–50. [Google Scholar] [CrossRef]
- Travers, B.S.; Tsang, B.K.; Barton, J.L. Multiple sclerosis: Diagnosis, disease-modifying therapy and prognosis. Aust. J. Gen. Pract. 2022, 51, 199–206. [Google Scholar] [CrossRef]
- Krajnc, N.; Bsteh, G.; Berger, T.; Mares, J.; Hartung, H.P. Monoclonal Antibodies in the Treatment of Relapsing Multiple Sclerosis: An Overview with Emphasis on Pregnancy, Vaccination, and Risk Management. Neurotherapeutics 2022, 19, 753–773. [Google Scholar] [CrossRef]
- McGinley, M.P.; Moss, B.P.; Cohen, J.A. Safety of monoclonal antibodies for the treatment of multiple sclerosis. Expert. Opin. Drug Saf. 2017, 16, 89–100. [Google Scholar] [CrossRef]
- Graf, J.; Aktas, O.; Rejdak, K.; Hartung, H.P. Monoclonal Antibodies for Multiple Sclerosis: An Update. BioDrugs 2019, 33, 61–78. [Google Scholar] [CrossRef]
- Palavra, F. Monoclonal Antibodies for Multiple Sclerosis Treatment. Acta Med. Port. 2015, 28, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Faissner, S.; Pappas, D.; Pula, B.; Akkad, D.A.; Arning, L.; Ruhrmann, S.; Duscha, A.; Gold, R.; Baranzini, S.E.; et al. Interferon-beta affects mitochondrial activity in CD4+ lymphocytes: Implications for mechanism of action in multiple sclerosis. Mult. Scler. 2015, 21, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, C.E. Interferon-beta: Mechanism of action and dosing issues. Neurology 2007, 68, S8–S11. [Google Scholar] [CrossRef]
- Kieseier, B.C. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs 2011, 25, 491–502. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Interferon Beta-1b. Available online: https://consultas.anvisa.gov.br/#/medicamentos/250000045539641/?substancia=23121 (accessed on 19 August 2023).
- REBIF. Prescribing Information. Bayer S.A. São Paulo, Brasil. 2022. Available online: https://consultas.anvisa.gov.br/#/bulario/q/?nomeProduto=Betaferon (accessed on 13 August 2023).
- ANVISA. Medication Registration Search—Interferon Beta-1a. Available online: https://consultas.anvisa.gov.br/#/medicamentos/250000122709411/?substancia=23047 (accessed on 19 August 2023).
- REBIF. Prescribing Information. MERCK S.A. Rio de Janeiro, Brasil. 2021. Available online: https://www.merckgroup.com/br-pt/bulario/Rebif_Bula_Profissional_290421.pdf (accessed on 13 August 2023).
- Saúde, M.d. Access to Brazilian Health System (SUS) Data Information. Available online: https://datasus.saude.gov.br/acesso-a-informacao/producao-ambulatorial-sia-sus/ (accessed on 13 August 2023).
- Astrom, M.E.; Roos, P.M. Geochemistry of multiple sclerosis in Finland. Sci. Total Environ. 2022, 841, 156672. [Google Scholar] [CrossRef]
- Krokki, O.; Bloigu, R.; Reunanen, M.; Remes, A.M. Increasing incidence of multiple sclerosis in women in Northern Finland. Mult. Scler. 2011, 17, 133–138. [Google Scholar] [CrossRef]
- Sharpe, R.J. The low incidence of multiple sclerosis in areas near the equator may be due to ultraviolet light induced suppressor cells to melanocyte antigens. Med. Hypotheses 1986, 19, 319–323. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Meshorer, A.; Hirshfeld, T.; Arnon, R.; Sela, M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur. J. Immunol. 1971, 1, 242–248. [Google Scholar] [CrossRef]
- Blanchette, F.; Neuhaus, O. Glatiramer acetate: Evidence for a dual mechanism of action. J. Neurol. 2008, 255 (Suppl. S1), 26–36. [Google Scholar] [CrossRef]
- Carter, N.J.; Keating, G.M. Glatiramer acetate: A review of its use in relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs 2010, 70, 1545–1577. [Google Scholar] [CrossRef]
- Kasindi, A.; Fuchs, D.T.; Koronyo, Y.; Rentsendorj, A.; Black, K.L.; Koronyo-Hamaoui, M. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells 2022, 11, 1578. [Google Scholar] [CrossRef]
- Simpson, D.; Noble, S.; Perry, C. Glatiramer acetate: A review of its use in relapsing-remitting multiple sclerosis. CNS Drugs 2002, 16, 825–850. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Glatiramer Acetate. Available online: https://consultas.anvisa.gov.br/#/medicamentos/250000267069765/?substancia=20698 (accessed on 19 August 2023).
- Consultas. Prescribing Information. Teva Farmacêutica Ltd.a. São Paulo, Brasil. 2023. Available online: https://consultas.anvisa.gov.br/#/bulario/q/?numeroRegistro=155730001 (accessed on 13 August 2023).
- Morrow, S.A.; Clift, F.; Devonshire, V.; Lapointe, E.; Schneider, R.; Stefanelli, M.; Vosoughi, R. Use of natalizumab in persons with multiple sclerosis: 2022 update. Mult. Scler. Relat. Disord. 2022, 65, 103995. [Google Scholar] [CrossRef]
- Sellner, J.; Rommer, P.S. A review of the evidence for a natalizumab exit strategy for patients with multiple sclerosis. Autoimmun. Rev. 2019, 18, 255–261. [Google Scholar] [CrossRef]
- Outteryck, O. Natalizumab in relapsing-remitting multiple sclerosis. Expert. Rev. Neurother. 2016, 16, 471–481. [Google Scholar] [CrossRef]
- Gandhi, S.; Jakimovski, D.; Ahmed, R.; Hojnacki, D.; Kolb, C.; Weinstock-Guttman, B.; Zivadinov, R. Use of natalizumab in multiple sclerosis: Current perspectives. Expert. Opin. Biol. Ther. 2016, 16, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Shirani, A.; Stuve, O. Natalizumab for Multiple Sclerosis: A Case in Point for the Impact of Translational Neuroimmunology. J. Immunol. 2017, 198, 1381–1386. [Google Scholar] [CrossRef]
- Khoy, K.; Mariotte, D.; Defer, G.; Petit, G.; Toutirais, O.; Le Mauff, B. Natalizumab in Multiple Sclerosis Treatment: From Biological Effects to Immune Monitoring. Front. Immunol. 2020, 11, 549842. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Natalizumab. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351216949200755/?substancia=23613 (accessed on 19 August 2023).
- TYSABRI Natalizumabe. Prescribing Information. Biogen Brasil Produtos Farmacêuticos Ltd.a. São Paulo, Brasil. 2022. Available online: https://br.biogen.com/content/dam/corporate/americas/brazil/pt-br/pdf-medicines/tysabri_natalizumabe_bula_profissional.pdf (accessed on 13 August 2023).
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- Cohen, J.A.; Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 2011, 69, 759–777. [Google Scholar] [CrossRef]
- Tanasescu, R.; Constantinescu, C.S. Pharmacokinetic evaluation of fingolimod for the treatment of multiple sclerosis. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 621–630. [Google Scholar] [CrossRef]
- Yang, T.; Tian, X.; Chen, C.Y.; Ma, L.Y.; Zhou, S.; Li, M.; Wu, Y.; Zhou, Y.; Cui, Y.M. The efficacy and safety of fingolimod in patients with relapsing multiple sclerosis: A meta-analysis. Br. J. Clin. Pharmacol. 2020, 86, 637–645. [Google Scholar] [CrossRef]
- Ziemssen, T.; Medin, J.; Couto, C.A.; Mitchell, C.R. Multiple sclerosis in the real world: A systematic review of fingolimod as a case study. Autoimmun. Rev. 2017, 16, 355–376. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Fingolimod. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351190569201016/?substancia=25273 (accessed on 19 August 2023).
- GILENYA Cloridrato de Fingolimode. Prescribing Information. Novartis Biociências S.A. São Paulo, Brasil. 2023. Available online: https://portal.novartis.com.br/medicamentos/wp-content/uploads/2021/10/Bula-GILENYA-Capsula-Dura-Medico.pdf (accessed on 13 August 2023).
- Katsavos, S.; Coles, A. Alemtuzumab as Treatment for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Evan, J.R.; Bozkurt, S.B.; Thomas, N.C.; Bagnato, F. Alemtuzumab for the treatment of multiple sclerosis. Expert. Opin. Biol. Ther. 2018, 18, 323–334. [Google Scholar] [CrossRef]
- Syed, Y.Y. Alemtuzumab: A Review in Relapsing Remitting Multiple Sclerosis. Drugs 2021, 81, 157–168. [Google Scholar] [CrossRef]
- Riera, R.; Porfirio, G.J.; Torloni, M.R. Alemtuzumab for multiple sclerosis. Cochrane Database Syst. Rev. 2016, 4, CD011203. [Google Scholar] [CrossRef]
- Willis, M.D.; Robertson, N.P. Alemtuzumab for Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2016, 16, 84. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Alemtuzumab. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351730256201318/?substancia=23547 (accessed on 19 August 2023).
- LEMTRADA. Prescribing Information. Sanofi Medley Farmacêutica Ltd.a. São Paulo, Brasil. 2023. Available online: https://sm.far.br/pdfshow/bula_183260349_0437889238_p.pdf (accessed on 13 August 2023).
- Garnock-Jones, K.P. Teriflunomide: A review of its use in relapsing multiple sclerosis. CNS Drugs 2013, 27, 1103–1123. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, C.; Zhao, X.; Zhang, Y.; Dai, Q.; Li, Y.; Chu, L. Teriflunomide for multiple sclerosis. Cochrane Database Syst. Rev. 2016, 3, CD009882. [Google Scholar] [CrossRef]
- Chan, A.; de Seze, J.; Comabella, M. Teriflunomide in Patients with Relapsing-Remitting Forms of Multiple Sclerosis. CNS Drugs 2016, 30, 41–51. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef]
- He, D.; Xu, Z.; Dong, S.; Zhang, H.; Zhou, H.; Wang, L.; Zhang, S. Teriflunomide for multiple sclerosis. Cochrane Database Syst. Rev. 2012, 12, CD009882. [Google Scholar] [CrossRef]
- Scott, L.J. Teriflunomide: A Review in Relapsing-Remitting Multiple Sclerosis. Drugs 2019, 79, 875–886. [Google Scholar] [CrossRef]
- Aubagio® (Teriflunomide))—Prescribing Information. Sanofi Medley Farmacêutica Ltd.a. São Paulo. Prescribing information. Sanofi Medley Farmacêutica Ltd.a. São Paulo, Brasil. 2022. Available online: https://sm.far.br/pdfshow/bula_183260315_0542511223_p.pdf (accessed on 13 August 2023).
- ANVISA. Medication Registration Search—Teriflunomide. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351686962201130/?substancia=24271 (accessed on 19 August 2023).
- Xu, Z.; Zhang, F.; Sun, F.; Gu, K.; Dong, S.; He, D. Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst. Rev. 2015, 2015, CD011076. [Google Scholar] [CrossRef]
- Suneetha, A.; Raja Rajeswari, K. Role of dimethyl fumarate in oxidative stress of multiple sclerosis: A review. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1019, 15–20. [Google Scholar] [CrossRef]
- Blair, H.A. Dimethyl Fumarate: A Review in Relapsing-Remitting MS. Drugs 2019, 79, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Dias de Sousa, M.A.; Desiderio, C.S.; da Silva Catarino, J.; Trevisan, R.O.; Alves da Silva, D.A.; Rocha, V.F.R.; Bovi, W.G.; Timoteo, R.P.; Bonatti, R.C.F.; da Silva, A.E.; et al. Role of Cytokines, Chemokines and IFN-gamma+ IL-17+ Double-Positive CD4+ T Cells in Patients with Multiple Sclerosis. Biomedicines 2022, 10, 2062. [Google Scholar] [CrossRef]
- Dello Russo, C.; Scott, K.A.; Pirmohamed, M. Dimethyl fumarate induced lymphopenia in multiple sclerosis: A review of the literature. Pharmacol. Ther. 2021, 219, 107710. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Dimethyl Fumarate. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351186118201351/?substancia=25886 (accessed on 17 August 2023).
- TECFIDERA® Fumarato de Dimetila. Prescribing Information. Biogen Brasil Produtos Farmacêuticos Ltd.a. São Paulo, Brasil. 2022. Available online: https://br.biogen.com/content/dam/corporate/americas/brazil/pt-br/pdf-medicines/tecfidera_fumarato_de_dimetila_bula_profissional_de_saude.pdf (accessed on 13 August 2023).
- Bigaut, K.; De Seze, J.; Collongues, N. Ocrelizumab for the treatment of multiple sclerosis. Expert. Rev. Neurother. 2019, 19, 97–108. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Blinkenberg, M. The potential role for ocrelizumab in the treatment of multiple sclerosis: Current evidence and future prospects. Ther. Adv. Neurol. Disord. 2016, 9, 44–52. [Google Scholar] [CrossRef]
- Mancinelli, C.R.; Rossi, N.; Capra, R. Ocrelizumab for the Treatment of Multiple Sclerosis: Safety, Efficacy, and Pharmacology. Ther. Clin. Risk Manag. 2021, 17, 765–776. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Ocrelizumab. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351195147201723/?substancia=26228 (accessed on 19 August 2023).
- Ocrevus. Prescribing Information. Produtos Roche Químicos e Farmacêuticos S.A. São Paulo, Brasil. 2023. Available online: https://dialogoroche.com.br/content/dam/roche-dialogo/dialogo-brazil-assets/downloadable-assets/produtos/bulas/ocrevus/Ocrevus_Bula_do_Profissional.pdf (accessed on 13 August 2023).
- CONITEC., M.d.S. Ocrelizumabe Para Tratamento de Pacientes Adultos com Esclerose Múltipla Remitente-Recorrente (EMRR) Como Alternativa ou Contraindicação ao Natalizumabe. Available online: https://www.gov.br/conitec/pt-br/midias/consultas/relatorios/2020/relatorio_inicial_ocrelizumabe_emrr_cp36_2020.pdf (accessed on 13 August 2023).
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vecsei, L. Medicinal Chemistry of Multiple Sclerosis: Focus on Cladribine. Mini Rev. Med. Chem. 2020, 20, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Soelberg Sorensen, P.; Cook, S.; Rammohan, K.; Rieckmann, P.; Comi, G.; Dangond, F.; Adeniji, A.K.; Vermersch, P. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult. Scler. 2018, 24, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.M.; Ammoscato, F.; Giovannoni, G.; Baker, D.; Schmierer, K. Cladribine: Mechanisms and mysteries in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1266–1271. [Google Scholar] [CrossRef]
- Comi, G.; Cook, S.; Giovannoni, G.; Rieckmann, P.; Sorensen, P.S.; Vermersch, P.; Galazka, A.; Nolting, A.; Hicking, C.; Dangond, F. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 29, 168–174. [Google Scholar] [CrossRef]
- Giovannoni, G.; Mathews, J. Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician’s Review. Neurol. Ther. 2022, 11, 571–595. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Cladribine. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351497138201717/?substancia=2166 (accessed on 23 August 2023).
- MAVENCLAD® Cladribina. Prescribing Information. MERCK S.A. Rio de Janeiro, Brasil. 2019. Available online: https://www.merckgroup.com/br-pt/bulario/Mavenclad_Bula%20Profissional_pr%C3%A9-lan%C3%A7amento.pdf (accessed on 13 August 2023).
- Ministério da Saúde—CONITEC. Cladribina Oral No Tratamento de Pacientes Com Esclerose Múltipla Remitente-Recorrente Altamente Ativa. Available online: https://www.gov.br/conitec/pt-br/midias/consultas/relatorios/2023/20230725_relatorio_cp_cladribina_emrr.pdf/view (accessed on 13 August 2023).
- Sanford, M.; McCormack, P.L. Ofatumumab. Drugs 2010, 70, 1013–1019. [Google Scholar] [CrossRef]
- Kang, C.; Blair, H.A. Ofatumumab: A Review in Relapsing Forms of Multiple Sclerosis. Drugs 2022, 82, 55–62. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Ofatumumab. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351481485202023/?substancia=25320 (accessed on 19 August 2023).
- KESIMPTA. Prescribing Information. Novartis Biociências S.A. São Paulo, Brasil. 2023. Available online: https://portal.novartis.com.br/medicamentos/wp-content/uploads/2021/10/Bula-KESIMPTA-Solucao-injetavel-Medico.pdf (accessed on 13 August 2023).
- Ministério da Saúde—CONITEC. Ofatumumabe em Primeira Linha de Terapia Modificadora do Curso da Doença Para o Tratamento da Esclerose Múltipla Recorrente. Available online: http://antigo-conitec.saude.gov.br/images/Consultas/Relatorios/2022/20220401_Relatorio_CP_12_Ofatumumabe_esclerose_multipla_recorrente.pdf (accessed on 13 August 2023).
- Scott, L.J. Siponimod: A Review in Secondary Progressive Multiple Sclerosis. CNS Drugs 2020, 34, 1191–1200. [Google Scholar] [CrossRef]
- Goodman, A.D.; Anadani, N.; Gerwitz, L. Siponimod in the treatment of multiple sclerosis. Expert. Opin. Investig. Drugs 2019, 28, 1051–1057. [Google Scholar] [CrossRef]
- Kappos, L.; Bar-Or, A.; Cree, B.A.C.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Tomic, D.; Cree, B.A.; Fox, R.; Giovannoni, G.; Bar-Or, A.; Gold, R.; Vermersch, P.; Pohlmann, H.; Wright, I.; et al. Siponimod and Cognition in Secondary Progressive Multiple Sclerosis: EXPAND Secondary Analyses. Neurology 2021, 96, e376–e386. [Google Scholar] [CrossRef]
- Cao, L.; Li, M.; Yao, L.; Yan, P.; Wang, X.; Yang, Z.; Lao, Y.; Li, H.; Yang, K.; Li, K. Siponimod for multiple sclerosis. Cochrane Database Syst. Rev. 2021, 11, CD013647. [Google Scholar] [CrossRef]
- Gajofatto, A.; Turatti, M. Siponimod to treat secondary progressive multiple sclerosis. Drugs Today 2020, 56, 37–46. [Google Scholar] [CrossRef]
- ANVISA. Medication Registration Search—Siponimod. Available online: https://consultas.anvisa.gov.br/#/medicamentos/25351780309202071/?substancia=26514 (accessed on 2 August 2023).
- K.S. Prescribing Information. Novartis Biociências S.A. São Paulo, Brasil. 2023. Available online: https://portal.novartis.com.br/medicamentos/wp-content/uploads/2022/10/Bula-KIENDRA-Comprimido-Revestido-Medico.pdf (accessed on 13 August 2023).
- Fox, E.J. Management of worsening multiple sclerosis with mitoxantrone: A review. Clin. Ther. 2006, 28, 461–474. [Google Scholar] [CrossRef]
- Gonsette, R.E. Mitoxantrone immunotherapy in multiple sclerosis. Mult. Scler. 1996, 1, 329–332. [Google Scholar] [CrossRef]
- Cocco, E.; Marrosu, M.G. The current role of mitoxantrone in the treatment of multiple sclerosis. Expert. Rev. Neurother. 2014, 14, 607–616. [Google Scholar] [CrossRef]
- Martinelli Boneschi, F.; Vacchi, L.; Rovaris, M.; Capra, R.; Comi, G. Mitoxantrone for multiple sclerosis. Cochrane Database Syst. Rev. 2013, 2013, CD002127. [Google Scholar] [CrossRef]
- Buttmann, M. Where mitoxantrone for multiple sclerosis is still valuable in 2018. Eur. J. Neurol. 2018, 25, 1400–1401. [Google Scholar] [CrossRef]
- Gonsette, R.E. Mitoxantrone in progressive multiple sclerosis: When and how to treat? J. Neurol. Sci. 2003, 206, 203–208. [Google Scholar] [CrossRef]
- Chisari, C.G.; Sgarlata, E.; Arena, S.; Toscano, S.; Luca, M.; Patti, F. Rituximab for the treatment of multiple sclerosis: A review. J. Neurol. 2022, 269, 159–183. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Moridi, T.; Granberg, T.; Piehl, F. Rituximab treatment for multiple sclerosis. Mult. Scler. 2020, 26, 137–152. [Google Scholar] [CrossRef]
- Kitsos, D.K.; Tsiodras, S.; Stamboulis, E.; Voumvourakis, K.I. Rituximab and multiple sclerosis. Clin. Neuropharmacol. 2012, 35, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Midaglia Fernández, L.; Mora, L.; Mulero Carrillo, P.; Sastre Garriga, J.; Montalban Gairin, X. Rituximab: Eficacia, efectividad y seguridad en el tratamiento de la esclerosis múltiple. Rev. Neurol. 2018, 66, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).