Diet-Induced Changes in Functional Disability among People with Multiple Sclerosis: A Secondary Pooled Analysis of Two Randomized Controlled Pilot Trials †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1
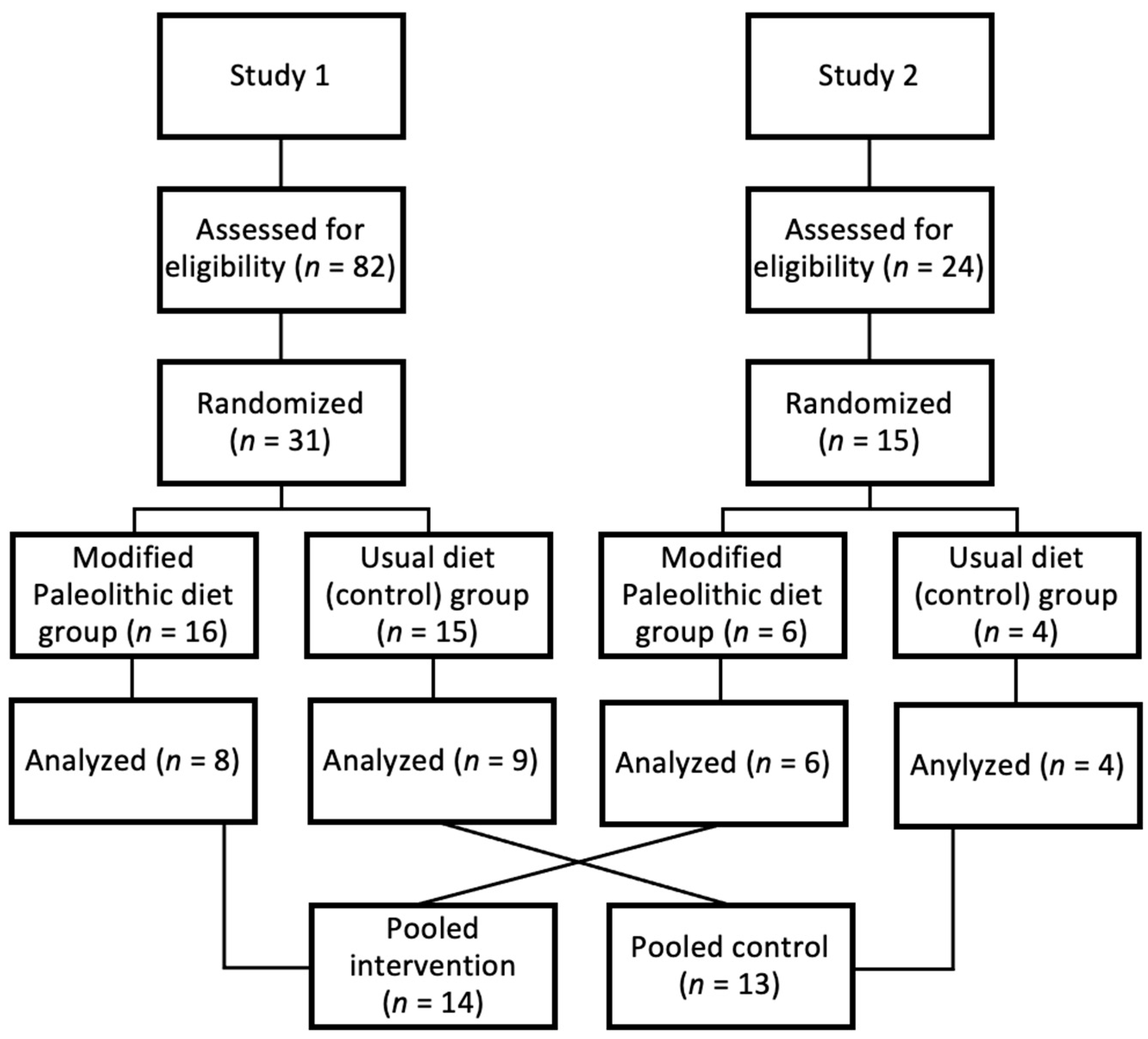
2.1.1. Overview
2.1.2. Participants
2.1.3. Intervention
2.2. Study 2
2.2.1. Overview
2.2.2. Participants
2.2.3. Intervention
2.3. Modified Paleolithic Diet
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, e1029–e1040. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.; Bhargava, P.; Kalb, R. Your Patients with Multiple Sclerosis have Set Wellness as a High Priority—And the National Multiple Sclerosis Society is Responding. US Neurol. 2015, 11, 80–86. [Google Scholar] [CrossRef]
- Russell, R.D.; Black, L.J.; Begley, A. Navigating dietary advice for multiple sclerosis. Health Expect. 2021, 24, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.D.; Lucas, R.M.; Brennan, V.; Sherriff, J.L.; Begley, A.; Ausimmune Investigator, G.; Black, L.J. Reported Changes in Dietary Behavior Following a First Clinical Diagnosis of Central Nervous System Demyelination. Front. Neurol. 2018, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.J.; Marrie, R.A. A survey of dietary characteristics in a large population of people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 22, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.D.; Black, L.J.; Sherriff, J.L.; Begley, A. Dietary responses to a multiple sclerosis diagnosis: A qualitative study. Eur. J. Clin. Nutr. 2019, 73, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Levasseur, V.; Cross, A.H.; Piccio, L. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 36, 101393. [Google Scholar] [CrossRef] [PubMed]
- Parks, N.E.; Jackson-Tarlton, C.S.; Vacchi, L.; Merdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 5, CD004192. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; Cheek, J.J.; Fox, S.S.; Healy, H.S.; Schweizer, M.L.; Bao, W.; Kamholz, J.; Titcomb, T.J. Efficacy of Diet on Fatigue and Quality of Life in Multiple Sclerosis: A Systematic Review and Network Meta-analysis of Randomized Trials. Neurology 2023, 100, e357–e366. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Yap, S.; Neate, S.L.; Nag, N.; Probst, Y.C.; Yu, M.; Jelinek, G.A.; Reece, J.C. Longitudinal associations between quality of diet and disability over 7.5 years in an international sample of people with multiple sclerosis. Eur. J. Neurol. 2023, 30, 3200–3211. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Yap, S.; Nag, N.; Probst, Y.; Jelinek, G.; Neate, S. Higher-quality diet and non-consumption of meat are associated with less self-determined disability progression in people with multiple sclerosis: A longitudinal cohort study. Eur. J. Neurol. 2022, 29, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I.; Levy, S.; Fitzgerald, K.; Sorets, T.; Sumowski, J.F. Mediterranean diet is linked to less objective disability in multiple sclerosis. Mult. Scler. 2022, 29, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Aznar, M.D.; Villanueva Guerrero, M.D.; Cordero Ramos, J.; Eichau Madueno, S.; Morales Bravo, M.; Lopez Ruiz, R.; Beltran Garcia, M. Efficacy of diet on fatigue, quality of life and disability status in multiple sclerosis patients: Rapid review and meta-analysis of randomized controlled trials. BMC Neurol. 2022, 22, 388. [Google Scholar] [CrossRef] [PubMed]
- Wahls, T.L.; Titcomb, T.J.; Bisht, B.; Eyck, P.T.; Rubenstein, L.M.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; Kamholz, J.; Snetselaar, L.G. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The WAVES randomized parallel-arm clinical trial. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 20552173211035399. [Google Scholar] [CrossRef] [PubMed]
- Crippes, L.J.; Saxby, S.M.; Shemirani, F.; Bisht, B.; Gill, C.; Rubenstein, L.M.; Ten Eyck, P.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; et al. Diet-induced changes in functional disability are mediated by fatigue in relapsing-remitting multiple sclerosis: A secondary analysis of the WAVES randomized parallel-arm trial. Mult. Scler. J. Exp. Transl. Clin. 2023, 9, 20552173231209147. [Google Scholar] [CrossRef] [PubMed]
- Irish, A.K.; Erickson, C.M.; Wahls, T.L.; Snetselaar, L.G.; Darling, W.G. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: A pilot study. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Titcomb, T.J.; Bisht, B.; Rubenstein, L.M.; Louison, R.; Wahls, T.L. A Modified MCT-Based Ketogenic Diet Increases Plasma beta-Hydroxybutyrate but Has Less Effect on Fatigue and Quality of Life in People with Multiple Sclerosis Compared to a Modified Paleolithic Diet: A Waitlist-Controlled, Randomized Pilot Study. J. Am. Coll. Nutr. 2020, 40, 13–25. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of Two Popular Eating Plans within the Multiple Sclerosis Community: Low Saturated Fat and Modified Paleolithic. Nutrients 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.S.; Rudick, R.A.; Cutter, G.R.; Reingold, S.C. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult. Scler. 1999, 5, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Hoogervorst, E.L.; Kalkers, N.F.; Cutter, G.R.; Uitdehaag, B.M.; Polman, C.H. The patient’s perception of a (reliable) change in the Multiple Sclerosis Functional Composite. Mult. Scler. 2004, 10, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kragt, J.J.; van der Linden, F.A.; Nielsen, J.M.; Uitdehaag, B.M.; Polman, C.H. Clinical impact of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. Mult. Scler. 2006, 12, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.L.; Purdy, S.C. Determining the Presence of Reliable Change over Time in Multiple Sclerosis: Evidence from the PASAT, Adjusting-PSAT, and Stroop Test. Int. J. MS Care 2013, 15, 170–178. [Google Scholar] [CrossRef]
- Mcculloch, C.E.; Neuhaus, J.M. Generalized Linear Mixed Models. In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Jung, S.J. Introduction to Mediation Analysis and Examples of Its Application to Real-world Data. J. Prev. Med. Public Health 2021, 54, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Cutter, G.R.; Fischer, J.S.; Goodman, A.D.; Heidenreich, F.R.; Kooijmans, M.F.; Sandrock, A.W.; Rudick, R.A.; Simon, J.H.; Simonian, N.A.; et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002, 59, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Marracci, G.; Kim, E.; Spain, R.; Cameron, M.; Overs, S.; Riddehough, A.; Li, D.K.; McDougall, J.; Lovera, J.; et al. Low-fat, plant-based diet in multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2016, 9, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, T.J.; Giesser, B.S.; Plafker, S.M.; Katz Sand, I.B.; Wahls, T.L. Editorial: Diet and multiple sclerosis. Front. Neurol. 2023, 14, 1347478. [Google Scholar] [CrossRef] [PubMed]
- Spain, R.I.; Piccio, L.; Langer-Gould, A.M. The Role of Diet in Multiple Sclerosis: Food for Thought. Neurology 2023, 100, 167–168. [Google Scholar] [CrossRef]
| Characteristic | Study 1 | Study 2 | |||
|---|---|---|---|---|---|
| Control | Paleo | Control | Paleo | ||
| Sex (female) | 8 (88.9) | 7 (87.5) | 2 (50.0) | 2 (33.3) | |
| Age (years) | 37.1 ± 3.7 | 35.4 ± 5.7 | 54.5 ± 11.8 | 50.3 ± 9.5 | |
| MS type | |||||
| -Relapsing | 9 (100) | 8 (100) | 1 (25) | 1 (16.7) | |
| -Progressive | 0 | 0 | 3 (75) | 5 (83.3) | |
| T25FW (seconds) | 4.7 ± 0.5 | 6.1 ± 2.4 | 12.6 ± 3.2 | 21.0 ± 14.4 | |
| NHPT (dominant; seconds) | 19.6 ± 2.0 | 21.0 ± 3.9 | 34.5 ± 18.2 | 38.4 ± 13.9 | |
| NHPT (non-dominant; seconds) | 20.4 ± 3.6 | 24.4 ± 4.5 | 30.5 ± 6.5 | 39.5 ± 17.1 | |
| PASAT (0 to 60) | 53.2 ± 5.0 | 44.9 ± 4.5 | 41.3 ± 11.1 | 49.3 ± 12.5 | |
| Control | Paleo | ||||||
|---|---|---|---|---|---|---|---|
| Pooled | Baseline | 12-Weeks | Change 3 | Baseline | 12-Weeks | Change 3 | p-Value 4 |
| MSFC | 0.00 ± 0.13 | −0.01 ± 0.14 | −0.01 ± 0.08 | −0.32 ± 0.20 | −0.06 ± 0.20 | 0.26 ± 0.12 * | 0.07 |
| NHPT | −0.01 ± 0.18 | 0.09 ± 0.15 | 0.10 ± 0.07 | −0.42 ± 0.17 | 0.03 ± 0.17 | 0.45 ± 0.12 *** | 0.02 |
| T25FW | −0.03 ± 0.12 | −0.08 ± 0.10 | −0.05 ± 0.04 | −0.34 ± 0.27 | −0.41 ± 0.33 | −0.07 ± 0.17 | 0.92 |
| PASAT | 0.04 ± 0.28 | −0.03 ± 0.36 | −0.07 ± 0.17 | −0.19 ± 0.30 | 0.19 ± 0.31 | 0.39 ± 0.18 * | 0.07 |
| Study 1 | |||||||
| MSFC | 0.31 ± 0.16 | 0.58 ± 0.16 | 0.27 ± 0.13 * | −0.35 ± 0.27 | 0.56 ± 0.18 | 0.91 ± 0.18 *** | 0.005 |
| NHPT | 0.30 ± 0.26 | 0.60 ± 0.20 | 0.30 ± 0.11 ** | −0.34 ± 0.37 | 0.72 ± 0.34 | 1.06 ± 0.19 *** | <0.001 |
| T25FW | 0.01 ± 0.27 | 0.25 ± 0.29 | 0.23 ± 0.21 | −0.02 ± 0.39 | 0.90 ± 0.29 | 0.91 ± 0.29 ** | 0.06 |
| PASAT | 0.62 ± 0.25 | 0.88 ± 0.18 | 0.26 ± 0.17 | −0.70 ± 0.24 | 0.07 ± 0.37 | 0.77 ± 0.30 ** | 0.14 |
| Study 2 | |||||||
| MSFC | 0.15 ± 0.27 | −0.14 ± 0.24 | −0.29 ± 0.12 * | −0.10 ± 0.33 | −0.08 ± 0.37 | 0.02 ± 0.18 | 0.16 |
| NHPT | 0.43 ± 0.55 | 0.19 ± 0.46 | −0.24 ± 0.13 | −0.29 ± 0.28 | −0.16 ± 0.33 | 0.13 ± 0.13 | 0.05 |
| T25FW | 0.43 ± 0.12 | 0.27 ± 0.10 | −0.15 ± 0.09 | −0.29 ± 0.46 | −0.47 ± 0.56 | −0.19 ± 0.32 | 0.92 |
| PASAT | −0.40 ± 0.40 | −0.88 ± 0.50 | −0.48 ± 0.25 | 0.27 ± 0.39 | 0.39 ± 0.39 | 0.12 ± 0.22 | 0.07 |
| Control | Paleo | |||||||
|---|---|---|---|---|---|---|---|---|
| Threshold 2 | Worsened | No Change | Improved | Worsened | No Change | Improved | p-Value 3 | |
| MSFC | 0.5 | 1 (7.69) | 12 (92.3) | 0 | 1 (7.14) | 8 (57.1) | 5 (35.7) | 0.04 |
| NHPT | 20% | 0 | 13 (100) | 0 | 0 | 11 (78.6) | 3 (21.4) | 0.23 |
| T25FW | 20% | 2 (15.4) | 11 (84.6) | 0 | 2 (14.3) | 9 (64.3) | 3 (21.4) | 0.30 |
| PASAT | 1 SD | 1 (7.69) | 12 (92.3) | 0 | 0 | 11 (78.6) | 3 (21.4) | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groux, A.R.; Walker, E.S.; Shemirani, F.; Lee, J.E.; Irish, A.K.; Rubenstein, L.M.; Snetselaar, L.G.; Darling, W.G.; Wahls, T.L.; Titcomb, T.J. Diet-Induced Changes in Functional Disability among People with Multiple Sclerosis: A Secondary Pooled Analysis of Two Randomized Controlled Pilot Trials. Sclerosis 2024, 2, 156-165. https://doi.org/10.3390/sclerosis2030011
Groux AR, Walker ES, Shemirani F, Lee JE, Irish AK, Rubenstein LM, Snetselaar LG, Darling WG, Wahls TL, Titcomb TJ. Diet-Induced Changes in Functional Disability among People with Multiple Sclerosis: A Secondary Pooled Analysis of Two Randomized Controlled Pilot Trials. Sclerosis. 2024; 2(3):156-165. https://doi.org/10.3390/sclerosis2030011
Chicago/Turabian StyleGroux, Allison R., Elizabeth S. Walker, Farnoosh Shemirani, Jennifer E. Lee, Amanda K. Irish, Linda M. Rubenstein, Linda G. Snetselaar, Warren G. Darling, Terry L. Wahls, and Tyler J. Titcomb. 2024. "Diet-Induced Changes in Functional Disability among People with Multiple Sclerosis: A Secondary Pooled Analysis of Two Randomized Controlled Pilot Trials" Sclerosis 2, no. 3: 156-165. https://doi.org/10.3390/sclerosis2030011
APA StyleGroux, A. R., Walker, E. S., Shemirani, F., Lee, J. E., Irish, A. K., Rubenstein, L. M., Snetselaar, L. G., Darling, W. G., Wahls, T. L., & Titcomb, T. J. (2024). Diet-Induced Changes in Functional Disability among People with Multiple Sclerosis: A Secondary Pooled Analysis of Two Randomized Controlled Pilot Trials. Sclerosis, 2(3), 156-165. https://doi.org/10.3390/sclerosis2030011







