The Road Well Traveled: From Inflammasomes to Collagen Export During Fibrosis
Abstract
1. Introduction
2. Systemic Sclerosis
3. Fibroblasts
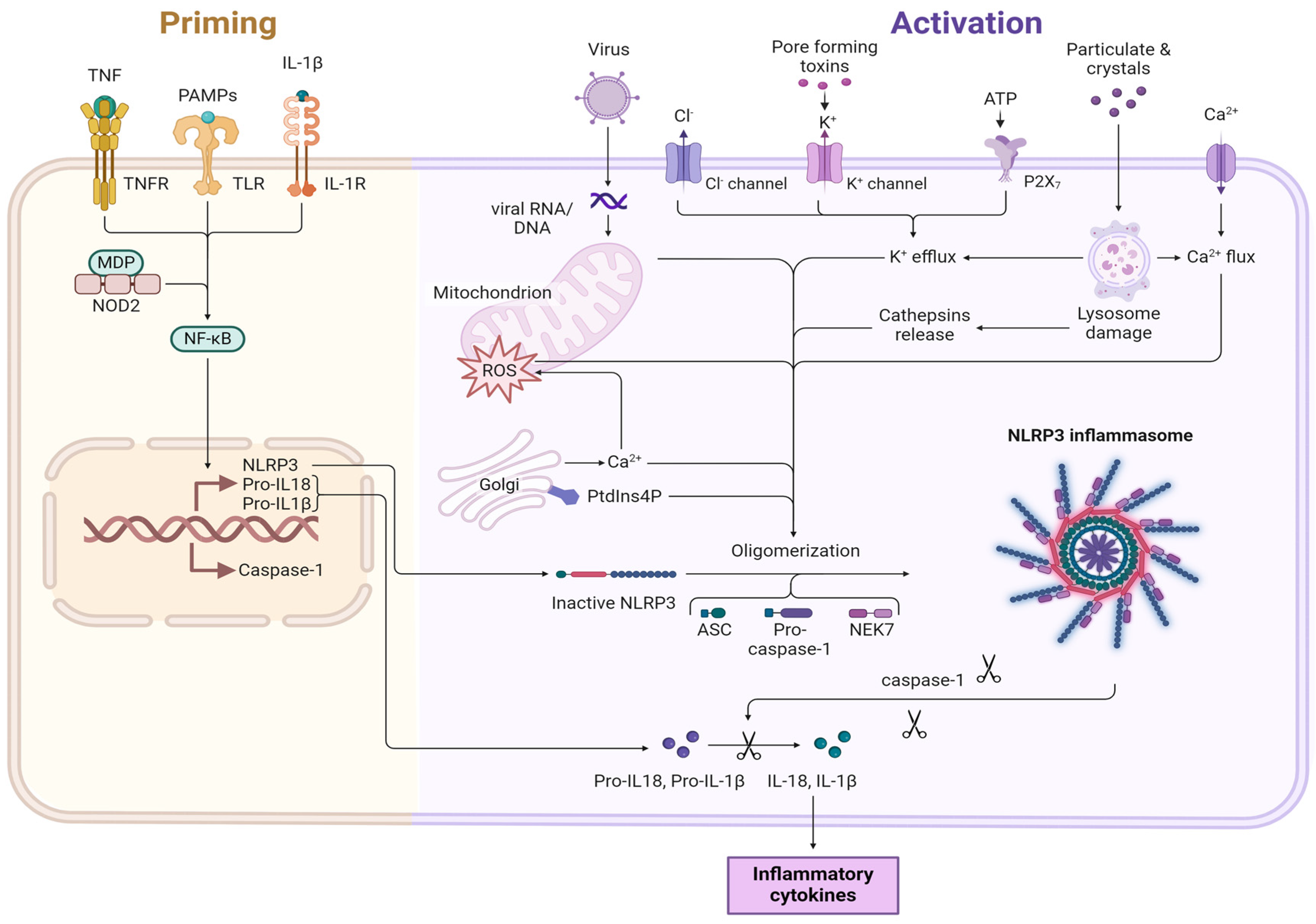
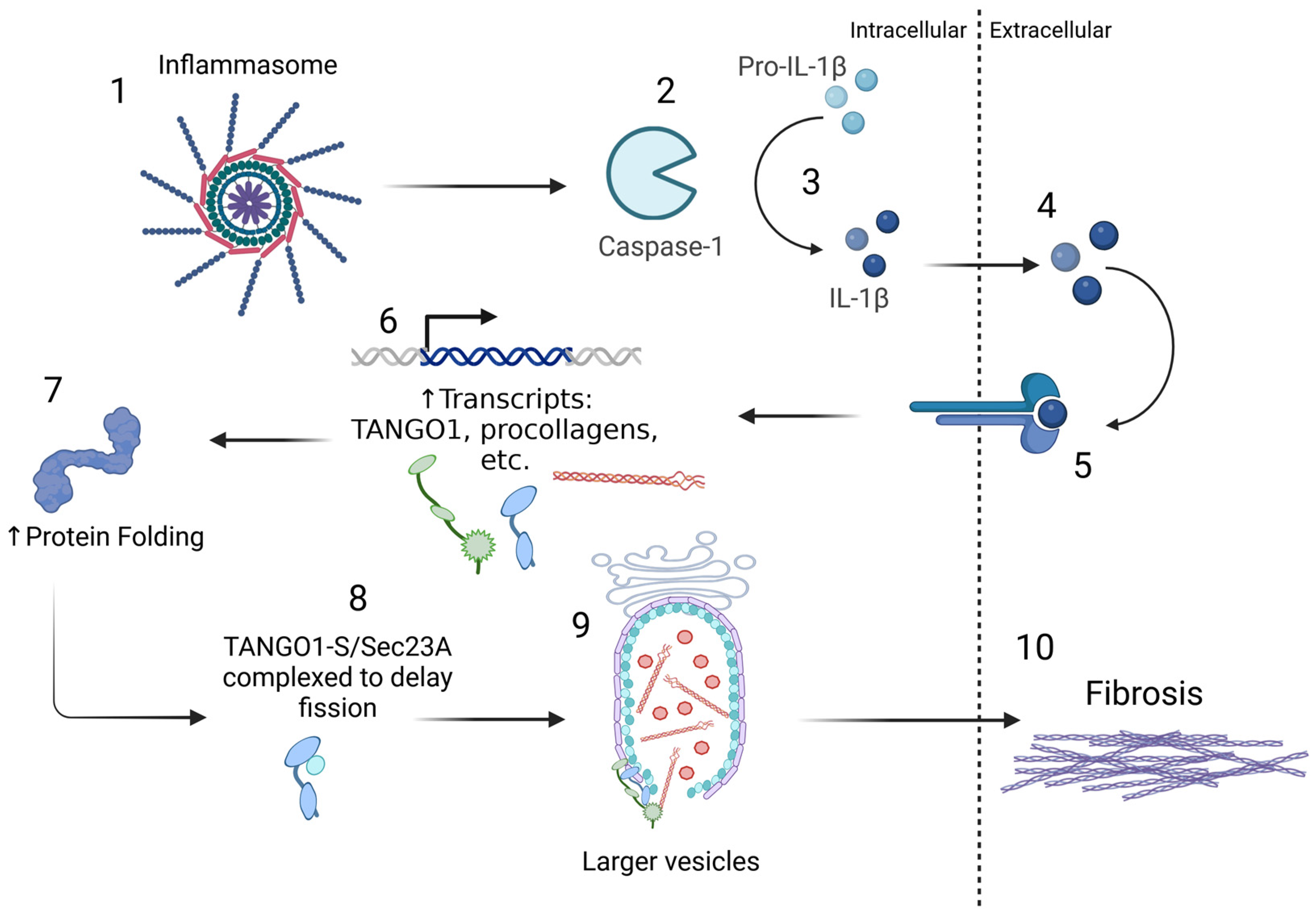
4. NLRP3 Inflammasome Activity in Fibrotic Diseases
5. Post-Translational Modifications of NLRP3
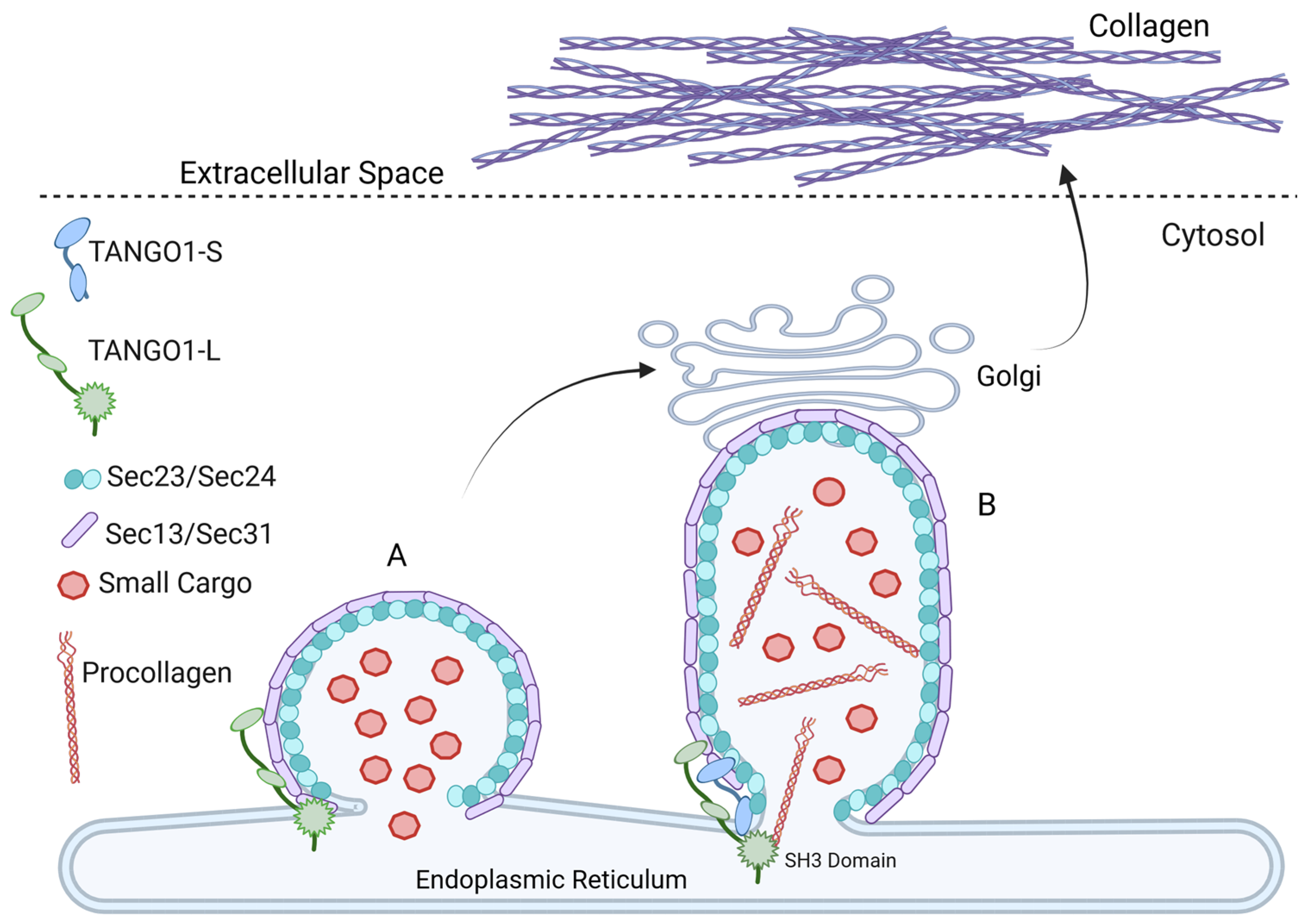
6. TANGO1 Drives ER to Golgi Trafficking of Collagen Export During Fibrosis
7. Activation of TANGO1 by O-Glycosylation
8. TANGO1 Mutations Correlate with Collagenopathies
9. Targeting TANGO1 to Treat Fibrosis
10. Discussion
11. Conclusions
Funding
Conflicts of Interest
References
- Köhler, A.; Mörgelin, M.; Gebauer, J.M.; Öcal, S.; Imhof, T.; Koch, M.; Nagata, K.; Paulsson, M.; Aumailley, M.; Baumann, U.; et al. New specific HSP47 functions in collagen subfamily chaperoning. FASEB J. 2020, 34, 12040–12052. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Ito, S.; Nagata, K.; Sakai, L.Y.; Bachinger, H.P. Intracellular mechanisms of molecular recognition and sorting for transport of large extracellular matrix molecules. Proc. Natl. Acad. Sci. USA 2016, 113, E6036–E6044. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kenny, S.J.; Hemmati, J.; Xu, K.; Schekman, R. TANGO1 and SEC12 are copackaged with procollagen I to facilitate the generation of large COPII carriers. Proc. Natl. Acad. Sci. USA 2018, 115, E12255–E12264. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.T.; Oecal, S.; Mörgelin, M.; Schmid, P.W.N.; Buchner, J.; Baumann, U.; Gebauer, J.M. Collagen’s primary structure determines collagen:HSP47 complex stoichiometry. J. Biol. Chem. 2021, 297, 101169. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nagata, K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017, 62, 142–151. [Google Scholar] [CrossRef]
- Harwood, R.; Grant, M.E.; Jackson, D.S. Studies on the glycosylation of hydroxylysine residues during collagen biosynthesis and the subcellular localization of collagen galactosyltransferase and collagen glucosyltransferase in tendon and cartilage cells. Biochem. J. 1975, 152, 291–302. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Leikin, S. Does the triple helical domain of type I collagen encode molecular recognition and fiber assembly while telopeptides serve as catalytic domains? Effect of proteolytic cleavage on fibrillogenesis and on collagen-collagen interaction in fibers. J. Biol. Chem. 1999, 274, 36083–36088. [Google Scholar] [CrossRef]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef]
- Wight, T.N.; Potter-Perigo, S. The extracellular matrix: An active or passive player in fibrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G950–G955. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, Z.; Zuo, H.; Yang, Z.; Qu, J. Idiopathic Pulmonary Fibrosis: An Update on Pathogenesis. Front. Pharmacol. 2021, 12, 797292. [Google Scholar] [CrossRef]
- Brosius, F.C., 3rd. New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev. Endocr. Metab. Disorders 2008, 9, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Busletta, C.; Novo, E.; di Bonzo, L.V.; Cannito, S.; Paternostro, C.; Parola, M. Liver fibrosis: A dynamic and potentially reversible process. Histol. Histopathol. 2010, 25, 1075–1091. [Google Scholar] [PubMed]
- Bhargava, V.; Singh, K.; Meena, P.; Sanyal, R. Nephrogenic systemic fibrosis: A frivolous entity. World J. Nephrol. 2021, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, H.; Lavalle, L.; Moon, J.C.C.; Hughes, D. Inflammation in Fabry disease: Stages, molecular pathways, and therapeutic implications. Front. Cardiovasc. Med. 2024, 11, 1420067. [Google Scholar] [CrossRef]
- Black, C.M.; Stevens, W.M. Scleroderma. Rheum. Dis. Clin. NA 1989, 15, 193–212. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Beanes, S.R.; Dang, C.; Soo, C.; Ting, K. Skin repair and scar formation: The central role of TGF-beta. Expert. Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef]
- Gibran, N.S.; Boyce, S.; Greenhalgh, D.G. Cutaneous wound healing. J. Burn. Care Res. 2007, 28, 577–579. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Wootton, S.C.; Kim, D.S.; Kondoh, Y.; Chen, E.; Lee, J.S.; Song, J.W.; Huh, J.W.; Taniguchi, H.; Chiu, C.; Boushey, H.; et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 1698–1702. [Google Scholar] [CrossRef]
- Collazos, J.; Carton, J.A.; Asensi, V. Immunological status does not influence hepatitis c virus or liver fibrosis in HIV-hepatitis C virus-coinfected patients. AIDS Res. Hum. Retroviruses 2011, 27, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.D.; Kuechenmeister, L.J.; Anderson, K.L.; Daily, S.; Beenken, K.E.; Roux, C.M.; Reniere, M.L.; Lewis, T.L.; Weiss, W.J.; Pulse, M.; et al. Small molecule inhibitors of Staphylococcus aureus RnpA alter cellular mRNA turnover, exhibit antimicrobial activity, and attenuate pathogenesis. PLoS Pathog. 2011, 7, e1001287. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Daba, M.H.; El-Tahir, K.E.; Al-Arifi, M.N.; Gubara, O.A. Drug-induced pulmonary fibrosis. Saudi Med. J. 2004, 25, 700–706. [Google Scholar] [PubMed]
- Churg, A.; Muller, N.L. Update on Silicosis. Surg. Pathol. Clin. 2024, 17, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Zhou, S.; Wang, X.; Wang, R.; Wright, J.L. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am. J. Respir. cell Mol. Biol. 2009, 40, 482–490. [Google Scholar] [CrossRef]
- Tirelli, C.; Pesenti, C.; Miozzo, M.; Mondoni, M.; Fontana, L.; Centanni, S. The Genetic and Epigenetic Footprint in Idiopathic Pulmonary Fibrosis and Familial Pulmonary Fibrosis: A State-of-the-Art Review. Diagnostics 2022, 12, 3107. [Google Scholar] [CrossRef]
- Mercier, S.; Küry, S.; Barbarot, S. Hereditary Fibrosing Poikiloderma with Tendon Contractures, Myopathy, and Pulmonary Fibrosis. In GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Diesler, R.; Cottin, V. Pulmonary fibrosis associated with rheumatoid arthritis: From pathophysiology to treatment strategies. Expert. Rev. Respir. Med. 2022, 16, 541–553. [Google Scholar] [CrossRef]
- Kelly, C.A.; Saravanan, V.; Nisar, M.; Arthanari, S.; Woodhead, F.A.; Price-Forbes, A.N.; Dawson, J.; Sathi, N.; Ahmad, Y.; Koduri, G.; et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics—A large multicentre UK study. Rheumatology 2014, 53, 1676–1682. [Google Scholar] [CrossRef]
- Sciascia, S.; Cozzi, M.; Barinotti, A.; Radin, M.; Cecchi, I.; Fenoglio, R.; Mancardi, D.; Wilson Jones, G.; Rossi, D.; Roccatello, D. Renal Fibrosis in Lupus Nephritis. Int. J. Mol. Sci. 2022, 23, 14317. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Prendergast, C.M.; Salvatore, M.M.; Capaccione, K.M. TGF-β signaling: Critical nexus of fibrogenesis and cancer. J. Transl. Med. 2024, 22, 594. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Yoshihara, M.; Rodgers, R.; Iyoshi, S.; Mogi, K.; Miyamoto, E.; Hayakawa, S.; Hayashi, M.; Nomura, S.; Kitami, K.; et al. Tumor-associated fibrosis: A unique mechanism promoting ovarian cancer metastasis and peritoneal dissemination. Cancer Metastasis Rev. 2024, 43, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. The role of immunosuppressive myofibroblasts in the aging process and age-related diseases. J. Mol. Med. 2023, 101, 1169–1189. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Fibroageing: An ageing pathological feature driven by dysregulated extracellular matrix-cell mechanobiology. Ageing Res. Rev. 2021, 70, 101393. [Google Scholar] [CrossRef]
- Saito, K.; Chen, M.; Bard, F.; Chen, S.; Zhou, H.; Woodley, D.; Polischuk, R.; Schekman, R.; Malhotra, V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell 2009, 136, 891–902. [Google Scholar] [CrossRef]
- Maeda, M.; Saito, K.; Katada, T. Distinct isoform-specific complexes of TANGO1 cooperatively facilitate collagen secretion from the endoplasmic reticulum. Mol. Biol. Cell 2016, 27, 2688–2696. [Google Scholar] [CrossRef]
- Santos, A.J.; Raote, I.; Scarpa, M.; Brouwers, N.; Malhotra, V. TANGO1 recruits ERGIC membranes to the endoplasmic reticulum for procollagen export. eLife 2015, 4, e10982. [Google Scholar] [CrossRef]
- Sulli, A.; Ruaro, B.; Smith, V.; Pizzorni, C.; Zampogna, G.; Gallo, M.; Cutolo, M. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J. Rheumatol. 2013, 40, 634–639. [Google Scholar] [CrossRef]
- Santiago, T.; Santiago, M.; Ruaro, B.; Salvador, M.J.; Cutolo, M.; da Silva, J.A.P. Ultrasonography for the Assessment of Skin in Systemic Sclerosis: A Systematic Review. Arthritis Care Res. 2019, 71, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Derk, C.T.; Sakkas, L.I.; Rasheed, M.; Artlett, C.; Jimenez, S.A. Autoantibodies in patients with systemic sclerosis and cancer: A case-control study. J. Rheumatol. 2003, 30, 1994–1996. [Google Scholar] [PubMed]
- Shima, Y.; Kuwahara, Y.; Murota, H.; Kitaba, S.; Kawai, M.; Hirano, T.; Arimitsu, J.; Narazaki, M.; Hagihara, K.; Ogata, A.; et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology 2010, 49, 2408–2412. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Lagares, D.; Tager, A.M.; Kapoor, M. Fibrosis--a lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O.C.; Fertig, N.; Lucas, M.; Somogyi, N.; Medsger, T.A., Jr. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J. Rheumatol. 2007, 34, 104–109. [Google Scholar]
- Ng, S.A.; Low, A.H.L. Systemic sclerosis in Asians: Are there racial differences? J. Scleroderma Relat. Disord. 2022, 7, 98–109. [Google Scholar] [CrossRef]
- Wielosz, E.; Wiąk-Walerowicz, K.; Łyś, E.; Lipska, A.; Dryglewska, M.; Majdan, M. Late-age onset systemic sclerosis-clinical and serological characteristics. Clin. Rheumatol. 2024, 43, 2565–2572. [Google Scholar] [CrossRef]
- Selmi, C.; Invernizzi, P.; Gershwin, M.E. The X chromosome and systemic sclerosis. Curr. Opin. Rheumatol. 2006, 18, 601–605. [Google Scholar] [CrossRef]
- Kurteva, E.K.; Boyadzhieva, V.V.; Stoilov, N.R. Systemic sclerosis in mother and daughter with susceptible HLA haplotype and anti-topoisomerase I autoantibodies. Rheumatol. Int. 2020, 40, 1001–1009. [Google Scholar] [CrossRef]
- Tsou, P.S.; Varga, J.; O’Reilly, S. Advances in epigenetics in systemic sclerosis: Molecular mechanisms and therapeutic potential. Nat. Rev. Rheumatol. 2021, 17, 596–607. [Google Scholar] [CrossRef]
- Vijayraghavan, S.; Blouin, T.; McCollum, J.; Porcher, L.; Virard, F.; Zavadil, J.; Feghali-Bostwick, C.; Saini, N. Widespread mutagenesis and chromosomal instability shape somatic genomes in systemic sclerosis. Nat. Commun. 2024, 15, 8889. [Google Scholar] [CrossRef] [PubMed]
- Meijs, J.; de Vries-Bouwstra, J.K.; Cohen Tervaert, J.W.; Hoogenberg, K. A case of late-onset systemic sclerosis with ruptured silicone breast implants. Netherlands J. Med. 2018, 76, 243–248. [Google Scholar]
- Haustein, U.F.; Herrmann, K. Environmental scleroderma. Clin. Dermatol. 1994, 12, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.J.; Hochberg, M.C. Occupational and environmental influences on scleroderma. Rheum. Dis. Clin. NA 1996, 22, 737–749. [Google Scholar] [CrossRef]
- Mayes, M.D. Scleroderma epidemiology. Rheum. Dis. Clin. NA 1996, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.J.; Jannini, S.; Symmons, D.; Bacon, P. An epidemiologic study of scleroderma in the West Midlands. Br. J. Rheumatol. 1988, 27, 286–290. [Google Scholar] [CrossRef]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef]
- Stunova, A.; Vistejnova, L. Dermal fibroblasts-A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef]
- Henderson, J.; Bhattacharyya, S.; Varga, J.; O’Reilly, S. Targeting TLRs and the inflammasome in systemic sclerosis. Pharmacol. Ther. 2018, 192, 163–169. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Dugina, V.; Ballestrem, C.; Wehrle-Haller, B.; Chaponnier, C. à-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell 2003, 14, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Prunotto, M.; Bruschi, M.; Gunning, P.; Gabbiani, G.; Weibel, F.; Ghiggeri, G.M.; Petretto, A.; Scaloni, A.; Bonello, T.; Schevzov, G.; et al. Stable incorporation of α-smooth muscle actin into stress fibers is dependent on specific tropomyosin isoforms. Cytoskeleton 2015, 72, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Goffin, J.M.; Pittet, P.; Csucs, G.; Lussi, J.W.; Meister, J.J.; Hinz, B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J. Cell Biol. 2006, 172, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef]
- Barnes, J.L.; Gorin, Y. Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney Int. 2011, 79, 944–956. [Google Scholar] [CrossRef]
- Hinz, B. Mechanical aspects of lung fibrosis: A spotlight on the myofibroblast. Proc. Am. Thor. Soc. 2012, 9, 137–147. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Kondo, T.; Ohshima, T. The dynamics of inflammatory cytokines in the healing process of mouse skin wound: A preliminary study for possible wound age determination. Int. J. Legal Med. 1996, 108, 231–236. [Google Scholar] [CrossRef]
- Alyaseer, A.A.A.; de Lima, M.H.S.; Braga, T.T. The Role of NLRP3 Inflammasome Activation in the Epithelial to Mesenchymal Transition Process During the Fibrosis. Front. Immunol. 2020, 11, 883. [Google Scholar] [CrossRef]
- Artlett, C.M.; Sassi-Gaha, S.; Rieger, J.L.; Boesteanu, A.C.; Feghali-Bostwick, C.A.; Katsikis, P.D. The inflammasome activating caspase-1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum. 2011, 63, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ravanti, L.; Kähäri, V.M. Matrix metalloproteinases in wound repair (review). Int. J. Mol. Med. 2000, 6, 391–407. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, S.J.; Zhou, S.C.; Wu, S.Z.; Wang, H. Inflammasomes and Fibrosis. Front. Immunol. 2021, 12, 643149. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Feldman, N.; Rotter-Maskowitz, A.; Okun, E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015, 24, 29–39. [Google Scholar] [CrossRef]
- Liston, A.; Masters, S.L. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat. Rev. Immunol. 2017, 17, 208–214. [Google Scholar] [CrossRef]
- Gong, T.; Yang, Y.; Jin, T.; Jiang, W.; Zhou, R. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol. 2018, 39, 393–406. [Google Scholar] [CrossRef]
- Hughes, M.M.; O’Neill, L.A.J. Metabolic regulation of NLRP3. Immunol. Rev. 2018, 281, 88–98. [Google Scholar] [CrossRef]
- Seoane, P.I.; Lee, B.; Hoyle, C.; Yu, S.; Lopez-Castejon, G.; Lowe, M.; Brough, D. The NLRP3-inflammasome as a sensor of organelle dysfunction. J. Cell Biol. 2020, 219, e202006194. [Google Scholar] [CrossRef]
- Arend, W.P.; Palmer, G.; Gabay, C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008, 223, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.J.; Kozak, W.; Leon, L.R.; Soszynski, D.; Conn, C.A. Cytokines and fever. Neuroimmunomodulation 1995, 2, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Kashiwamura, S.; Tsutsui, H.; Yoshimoto, T.; Nakanishi, K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr. Opin. Immunol. 1998, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.P.; Culshaw, S.; Gracie, J.A.; Hunter, D.; Canetti, C.A.; Campbell, C.; Cunha, F.; Liew, F.Y.; McInnes, I.B. A role for IL-18 in neutrophil activation. J. Immunol. 2001, 167, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Ruegg, A.; Werner, S.; Beer, H.D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 2008, 132, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, J.; Falck-Hansen, M.A.; Davies, A.H.; Monaco, C. Innate immunity and monocyte-macrophage activation in atherosclerosis. J. Inflamm. 2011, 8, 9. [Google Scholar] [CrossRef]
- Lalor, S.J.; Dungan, L.S.; Sutton, C.E.; Basdeo, S.A.; Fletcher, J.M.; Mills, K.H. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J. Immunol. 2011, 186, 5738–5748. [Google Scholar] [CrossRef]
- Sellin, M.E.; Müller, A.A.; Hardt, W.D. Consequences of Epithelial Inflammasome Activation by Bacterial Pathogens. J. Mol. Biol. 2018, 430, 193–206. [Google Scholar] [CrossRef]
- Dai, X.; Sayama, K.; Tohyama, M.; Shirakata, Y.; Hanakawa, Y.; Tokumaru, S.; Yang, L.; Hirakawa, S.; Hashimoto, K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J. Allergy Clin. Immunol. 2011, 127, 806–814. [Google Scholar] [CrossRef]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef]
- Watanabe, A.; Sohail, M.A.; Gomes, D.A.; Hashmi, A.; Nagata, J.; Sutterwala, F.S.; Mahmood, S.; Jhandier, M.N.; Shi, Y.; Flavell, R.A.; et al. Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1248–G1257. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.A.; Trinchieri, G.; Beck, J.M.; Simon, P.L.; Sehgal, P.B.; May, L.T.; Kern, J.A. A synergistic interaction of IL-6 and IL-1 mediates the thymocyte-stimulating activity produced by recombinant IL-1-stimulated fibroblasts. J. Immunol. 1989, 142, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.A.; Lentz, V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabllize IL-6 messanger RNA. J. Immunol. 1990, 145, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Saito, F.; Tasaka, S.; Inoue, K.; Miyamoto, K.; Nakano, Y.; Ogawa, Y.; Yamada, W.; Shiraishi, Y.; Hasegawa, N.; Fujishima, S.; et al. Role for interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol. 2007, 38, 566–571. [Google Scholar] [CrossRef]
- Doerner, A.M.; Zuraw, B.L. TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir. Res. 2009, 10, 100. [Google Scholar] [CrossRef]
- Ide, M.; Jinnin, M.; Tomizawa, Y.; Wang, Z.; Kajihara, I.; Fukushima, S.; Hashizume, Y.; Asano, Y.; Ihn, H. Transforming growth factor beta-inhibitor Repsox downregulates collagen expression of scleroderma dermal fibroblasts and prevents bleomycin-induced mice skin fibrosis. Exp. Dermatol. 2017, 26, 1139–1143. [Google Scholar] [CrossRef]
- Dantas, A.T.; Goncalves, S.M.; de Almeida, A.R.; Goncalves, R.S.; Sampaio, M.C.; Vilar, K.M.; Pereira, M.C.; Rego, M.J.; Pitta, I.D.; Marques, C.D.; et al. Reassessing the Role of the Active TGF-beta1 as a Biomarker in Systemic Sclerosis: Association of Serum Levels with Clinical Manifestations. Dis. Markers 2016, 2016, 6064830. [Google Scholar] [CrossRef]
- Liang, R.; Sumova, B.; Cordazzo, C.; Mallano, T.; Zhang, Y.; Wohlfahrt, T.; Dees, C.; Ramming, A.; Krasowska, D.; Michalska-Jakubus, M.; et al. The transcription factor GLI2 as a downstream mediator of transforming growth factor-beta-induced fibroblast activation in SSc. Ann. Rheum. Dis. 2017, 76, 756–764. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Aspects Med. 2018, 65, 70–99. [Google Scholar] [CrossRef]
- Vesey, D.A.; Cheung, C.; Cuttle, L.; Endre, Z.; Gobe, G.; Johnson, D.W. Interleukin-1beta stimulates human renal fibroblast proliferation and matrix protein production by means of a transforming growth factor-beta-dependent mechanism. J. Lab. Clin. Med. 2002, 140, 342–350. [Google Scholar] [CrossRef]
- Lonnemann, G.; Engler-Blum, G.; Muller, G.A.; Koch, K.M.; Dinarello, C.A. Cytokines in human renal interstitial fibrosis. II. Intrinsic interleukin (IL)-1 synthesis and IL-1-dependent production of IL-6 and IL-8 by cultured kidney fibroblasts. Kidney Int. 1995, 47, 845–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knorr, J.; Kaufmann, B.; Inzaugarat, M.E.; Holtmann, T.M.; Geisler, L.; Hundertmark, J.; Kohlhepp, M.S.; Boosheri, L.M.; Chilin-Fuentes, D.R.; Birmingham, A.; et al. Interleukin-18 signaling promotes activation of hepatic stellate cells in mouse liver fibrosis. Hepatology 2023, 77, 1968–1982. [Google Scholar] [CrossRef]
- Luan, J.; Fu, J.; Jiao, C.; Hao, X.; Feng, Z.; Zhu, L.; Zhang, Y.; Zhou, G.; Li, H.; Yang, W.; et al. IL-18 deficiency ameliorates the progression from AKI to CKD. Cell Death Dis. 2022, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Allantaz, F.; Chaussabel, D.; Banchereau, J.; Pascual, V. Microarray-based identification of novel biomarkers in IL-1-mediated diseases. Curr. Opin. Immunol. 2007, 19, 623–632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, D.D.; Fielding, C.; Phillips, A.; Fraser, D. Interleukin-1 beta regulates proximal tubular cell transforming growth factor beta-1 signalling. Nephrol. Dial. Transplant. 2009, 24, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Gairola, S.; Sinha, A.; Kaundal, R.K. Linking NLRP3 inflammasome and pulmonary fibrosis: Mechanistic insights and promising therapeutic avenues. Inflammopharmacology 2024, 32, 287–305. [Google Scholar] [CrossRef]
- Stephens, D.J.; Pepperkok, R. Imaging of procollagen transport reveals COPI-dependent cargo sorting during ER-to-Golgi transport in mammalian cells. J. Cell Sci. 2002, 115, 1149–1160. [Google Scholar] [CrossRef]
- Malhotra, V.; Erlmann, P. The pathway of collagen secretion. Ann. Rev. Cell Dev. Biol. 2015, 31, 109–124. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, H.H.; Nguyen, T.D.; Tran, V.T.; Nguyen, H.A.; Pham, D.V. NLRP3 inflammasome activation contributes to the development of the pro-fibrotic phenotype of lung fibroblasts. Biochem. Pharmacol. 2024, 229, 116496. [Google Scholar] [CrossRef]
- Stout-Delgado, H.W.; Cho, S.J.; Chu, S.G.; Mitzel, D.N.; Villalba, J.; El-Chemaly, S.; Ryter, S.W.; Choi, A.M.; Rosas, I.O. Age-Dependent Susceptibility to Pulmonary Fibrosis is Associated with NLRP3 Inflammasome Activation. Am. J. Respir. Cell Mol. Biol. 2016, 55, 252–263. [Google Scholar] [CrossRef]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Bolla, E.; Semb, A.G.; Kerola, A.M.; Ikdahl, E.; Petri, M.; Pons-Estel, G.J.; Karpouzas, G.A.; Sfikakis, P.P.; Quintana, R.; Misra, D.P.; et al. Prevalence and target attainment of traditional cardiovascular risk factors in patients with systemic lupus erythematosus: A cross-sectional study including 3401 individuals from 24 countries. Lancet Rheumatol 2024, 6, e447–e459. [Google Scholar] [CrossRef] [PubMed]
- Larregue, M.; Cannel, C.; Bazex, J. Systemic sclerosis in children. Ann. Dermatol. Venereol. 1983, 110, 326. [Google Scholar]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 2012, 287, 36617–36622. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lear, T.B.; Jerome, J.A.; Rajbhandari, S.; Snavely, C.A.; Gulick, D.L.; Gibson, K.F.; Zou, C.; Chen, B.B.; Mallampalli, R.K. Lipopolysaccharide Primes the NALP3 Inflammasome by Inhibiting Its Ubiquitination and Degradation Mediated by the SCFFBXL2 E3 Ligase. J. Biol. Chem. 2015, 290, 18124–18133. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef]
- Song, H.; Liu, B.; Huai, W.; Yu, Z.; Wang, W.; Zhao, J.; Han, L.; Jiang, G.; Zhang, L.; Gao, C.; et al. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. Nat. Commun. 2016, 7, 13727. [Google Scholar] [CrossRef]
- Song, C.; He, L.; Zhang, J.; Ma, H.; Yuan, X.; Hu, G.; Tao, L.; Zhang, J.; Meng, J. Fluorofenidone attenuates pulmonary inflammation and fibrosis via inhibiting the activation of NALP3 inflammasome and IL-1β/IL-1R1/MyD88/NF-κB pathway. J. Cell. Mol. Med. 2016, 20, 2064–2077. [Google Scholar] [CrossRef]
- Ramachandran, A.; Kumar, B.; Waris, G.; Everly, D. Deubiquitination and Activation of the NLRP3 Inflammasome by UCHL5 in HCV-Infected Cells. Microbiol. Spectr. 2021, 9, e0075521. [Google Scholar] [CrossRef]
- Barry, R.; John, S.W.; Liccardi, G.; Tenev, T.; Jaco, I.; Chen, C.H.; Choi, J.; Kasperkiewicz, P.; Fernandes-Alnemri, T.; Alnemri, E.; et al. SUMO-mediated regulation of NLRP3 modulates inflammasome activity. Nat. Commun. 2018, 9, 3001. [Google Scholar] [CrossRef]
- Shao, L.; Liu, Y.; Wang, W.; Li, A.; Wan, P.; Liu, W.; Shereen, M.A.; Liu, F.; Zhang, W.; Tan, Q.; et al. SUMO1 SUMOylates and SENP3 deSUMOylates NLRP3 to orchestrate the inflammasome activation. FASEB J. 2020, 34, 1497–1515. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Liu, Z.S.; Xue, W.; Bai, Z.F.; Wang, Q.Y.; Dai, J.; Liu, X.; Huang, Y.J.; Cai, H.; Zhan, X.Y.; et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell 2017, 68, 185–197.e6. [Google Scholar] [CrossRef] [PubMed]
- Stutz, A.; Kolbe, C.C.; Stahl, R.; Horvath, G.L.; Franklin, B.S.; van Ray, O.; Brinkschulte, R.; Geyer, M.; Meissner, F.; Latz, E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017, 214, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Kasper, S.; Gottier, C.; Lang, S.; Atrott, K.; Vavricka, S.R.; Scharl, S.; Raselli, T.; Frey-Wagner, I.; Gutte, P.M.; et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Investig. 2016, 126, 1783–1800. [Google Scholar] [CrossRef]
- Saito, K.; Maeda, M. Not just a cargo receptor for large cargoes; an emerging role of TANGO1 as an organizer of ER exit sites. J. Biochem. 2019, 166, 115–119. [Google Scholar] [CrossRef]
- Maiers, J.L.; Kostallari, E.; Mushref, M.; deAssuncao, T.M.; Li, H.; Jalan-Sakrikar, N.; Huebert, R.C.; Cao, S.; Malhi, H.; Shah, V.H. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice. Hepatology 2017, 65, 983–998. [Google Scholar] [CrossRef]
- Connolly, L.M.; McFalls, C.M.; McMahon, I.G.; Bhat, A.M.; Artlett, C.M. Caspase 1 Enhances Transport and Golgi Organization Protein 1 Expression to Promote Procollagen Export From the Endoplasmic Reticulum in Systemic Sclerosis Contributing to Fibrosis. Arthritis Rheum. 2023, 75, 1831–1841. [Google Scholar] [CrossRef]
- Raote, I.; Ortega Bellido, M.; Pirozzi, M.; Zhang, C.; Melville, D.; Parashuraman, S.; Zimmermann, T.; Malhotra, V. TANGO1 assembles into rings around COPII coats at ER exit sites. J. Cell Biol. 2017, 216, 901–909. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Zhang, L.; Tran, D.T.; Ten Hagen, K.G. Tango1 coordinates the formation of endoplasmic reticulum/Golgi docking sites to mediate secretory granule formation. J. Biol. Chem. 2019, 294, 19498–19510. [Google Scholar] [CrossRef]
- McCaughey, J.; Stephens, D.J. COPII-dependent ER export in animal cells: Adaptation and control for diverse cargo. Histochem. Cell Biol. 2018, 150, 119–131. [Google Scholar] [CrossRef]
- McCaughey, J.; Stevenson, N.L.; Cross, S.; Stephens, D.J. ER-to-Golgi trafficking of procollagen in the absence of large carriers. J. Cell Biol. 2019, 218, 929–948. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nakano, A. Mechanisms of COPII vesicle formation and protein sorting. FEBS Lett. 2007, 581, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Foresti, O.; Liu, A.; Androulaki, S.; Pena Rodriguez, M.; Raote, I.; Aridor, M.; Cui, B.; Malhotra, V. Endoplasmic reticulum exit sites are segregated for secretion based on cargo size. Dev. Cell 2024, 59, 2593–2608.e6. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bard, F.; Casano, L.; Mallabiabarrena, A.; Wallace, E.; Saito, K.; Kitayama, H.; Guizzunti, G.; Hu, Y.; Wendler, F.; Dasgupta, R.; et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 2006, 439, 604–607. [Google Scholar] [CrossRef]
- Zhang, L.; Ten Hagen, K.G. Dissecting the biological role of mucin-type O-glycosylation using RNA interference in Drosophila cell culture. J. Biol. Chem. 2010, 285, 34477–34484. [Google Scholar] [CrossRef]
- Nishihara, S. Functional analysis of glycosylation using Drosophila melanogaster. Glycoconj. J. 2020, 37, 1–14. [Google Scholar] [CrossRef]
- Zang, Y.; Wan, M.; Liu, M.; Ke, H.; Ma, S.; Liu, L.P.; Ni, J.Q.; Pastor-Pareja, J.C. Plasma membrane overgrowth causes fibrotic collagen accumulation and immune activation in Drosophila adipocytes. eLife 2015, 4, e07187. [Google Scholar] [CrossRef]
- Zhang, L.; Syed, Z.A.; van Dijk Hard, I.; Lim, J.M.; Wells, L.; Ten Hagen, K.G. O-glycosylation regulates polarized secretion by modulating Tango1 stability. Proc. Natl. Acad. Sci. USA 2014, 111, 7296–7301. [Google Scholar] [CrossRef]
- Clark, E.M.; Link, B.A. Complementary and divergent functions of zebrafish Tango1 and Ctage5 in tissue development and homeostasis. Mol. Biol. Cell 2021, 32, 391–401. [Google Scholar] [CrossRef]
- Wilson, D.G.; Phamluong, K.; Li, L.; Sun, M.; Cao, T.C.; Liu, P.S.; Modrusan, Z.; Sandoval, W.N.; Rangell, L.; Carano, R.A.; et al. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J. Cell Biol. 2011, 193, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Booij-Vrieling, H.; Oksa-Minalto, J.; de Vries, C.; Kehl, A.; Jagannathan, V.; Leeb, T. MIA3 Splice Defect in Cane Corso Dogs with Dental-Skeletal-Retinal Anomaly (DSRA). Genes 2021, 12, 1497. [Google Scholar] [CrossRef] [PubMed]
- Junkiert-Czarnecka, A.; Pilarska-Deltow, M.; Bąk, A.; Heise, M.; Haus, O. A novel mutation in collagen transport protein, MIA3 gene, detected in a patient with clinical symptoms of Ehlers-Danlos hypermobile syndrome. Adv. Clin. Exp. Med. 2023, 32, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Guillemyn, B.; Nampoothiri, S.; Syx, D.; Malfait, F.; Symoens, S. Loss of TANGO1 Leads to Absence of Bone Mineralization. JBMR Plus 2021, 5, e10451. [Google Scholar] [CrossRef] [PubMed]
- Lekszas, C.; Foresti, O.; Raote, I.; Liedtke, D.; König, E.M.; Nanda, I.; Vona, B.; De Coster, P.; Cauwels, R.; Malhotra, V.; et al. Biallelic TANGO1 mutations cause a novel syndromal disease due to hampered cellular collagen secretion. eLife 2020, 9, e51319. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, Y.; Yu, L.; Liu, J.; Wang, T.; Sun, P.; Feng, Y.; Zhang, D.; Shi, L.; He, K.; et al. Mea6/cTAGE5 cooperates with TRAPPC12 to regulate PTN secretion and white matter development. iScience 2024, 27, 109180. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Wang, T.; Yao, L.; Lam, S.M.; Huang, X.; Fan, J.; Wang, Q.; Liu, L.; Jiang, Y.; et al. cTAGE5/MEA6 plays a critical role in neuronal cellular components trafficking and brain development. Proc. Natl. Acad. Sci. USA 2018, 115, E9449–E9458. [Google Scholar] [CrossRef]
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2020, 8, 963–974. [Google Scholar] [CrossRef]
- Fisher, M.; Nathan, S.D.; Hill, C.; Marshall, J.; Dejonckheere, F.; Thuresson, P.O.; Maher, T.M. Predicting Life Expectancy for Pirfenidone in Idiopathic Pulmonary Fibrosis. J. Manag. Care Spec. Pharm. 2017, 23, S17–S24. [Google Scholar] [CrossRef]
- McCaughey, J.; Stevenson, N.L.; Mantell, J.M.; Neal, C.R.; Paterson, A.; Heesom, K.; Stephens, D.J. A general role for TANGO1, encoded by MIA3, in secretory pathway organization and function. J. Cell Sci. 2021, 134, jcs259075. [Google Scholar] [CrossRef]
- Saito, K.; Yamashiro, K.; Ichikawa, Y.; Erlmann, P.; Kontani, K.; Malhotra, V.; Katada, T. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol. Biol. Cell 2011, 22, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Raote, I.; Ortega-Bellido, M.; Santos, A.J.; Foresti, O.; Zhang, C.; Garcia-Parajo, M.F.; Campelo, F.; Malhotra, V. TANGO1 builds a machine for collagen export by recruiting and spatially organizing COPII, tethers and membranes. eLife 2018, 7, e32723. [Google Scholar] [CrossRef] [PubMed]
- Raote, I.; Rosendahl, A.H.; Häkkinen, H.M.; Vibe, C.; Küçükaylak, I.; Sawant, M.; Keufgens, L.; Frommelt, P.; Halwas, K.; Broadbent, K.; et al. TANGO1 inhibitors reduce collagen secretion and limit tissue scarring. Nat. Commun. 2024, 15, 3302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artlett, C.M. The Road Well Traveled: From Inflammasomes to Collagen Export During Fibrosis. Sclerosis 2024, 2, 378-393. https://doi.org/10.3390/sclerosis2040025
Artlett CM. The Road Well Traveled: From Inflammasomes to Collagen Export During Fibrosis. Sclerosis. 2024; 2(4):378-393. https://doi.org/10.3390/sclerosis2040025
Chicago/Turabian StyleArtlett, Carol M. 2024. "The Road Well Traveled: From Inflammasomes to Collagen Export During Fibrosis" Sclerosis 2, no. 4: 378-393. https://doi.org/10.3390/sclerosis2040025
APA StyleArtlett, C. M. (2024). The Road Well Traveled: From Inflammasomes to Collagen Export During Fibrosis. Sclerosis, 2(4), 378-393. https://doi.org/10.3390/sclerosis2040025





