The Use of Augmented Reality on a Self-Paced Treadmill to Quantify Attention and Footfall Placement Variability in Middle-Aged to Older-Aged Adults with Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
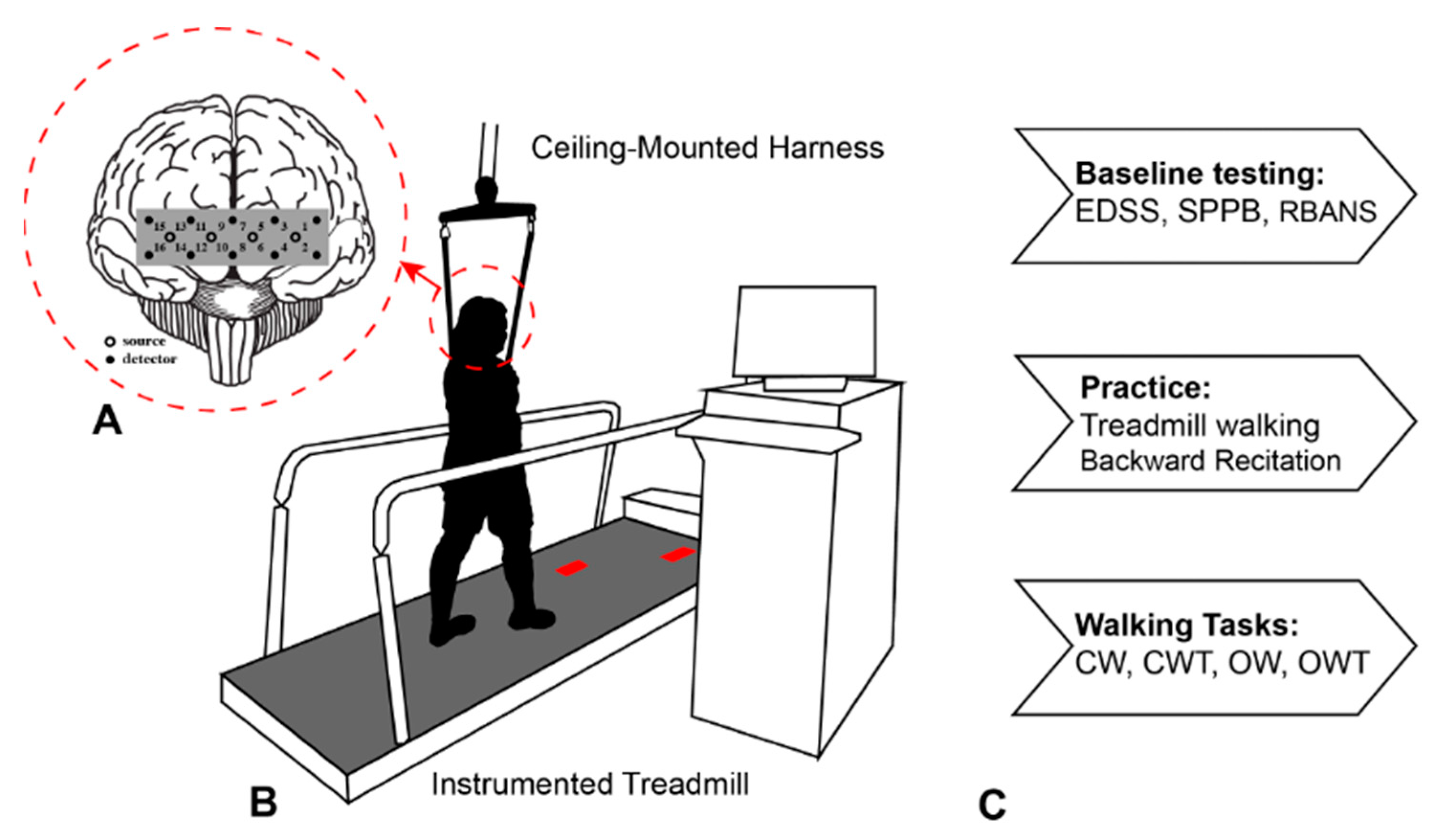
2.2. Procedure
2.3. Baseline Cognitive and Mobility Function Assessments
2.4. Data Collection
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
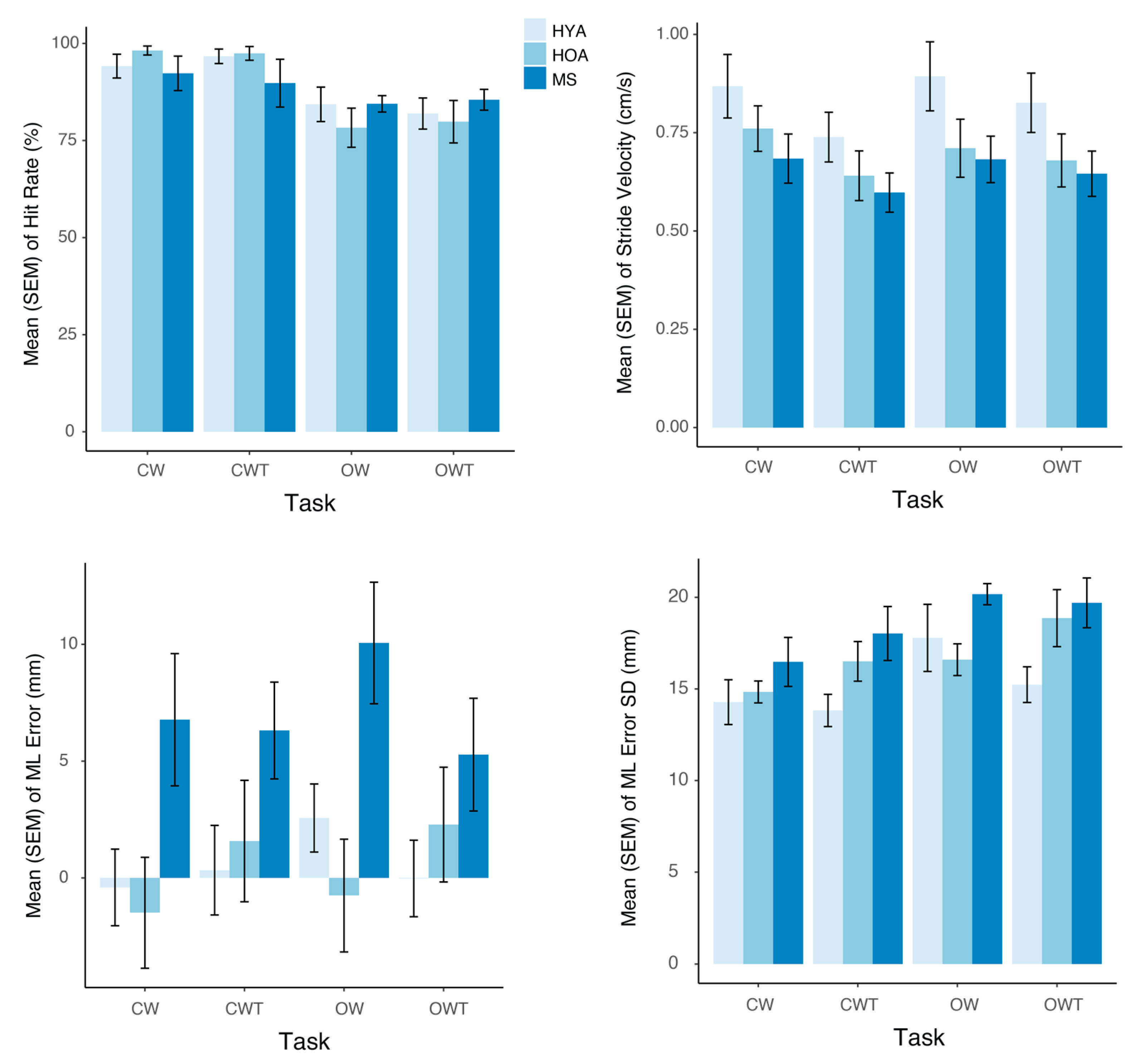
3.1. Behavioral Performance
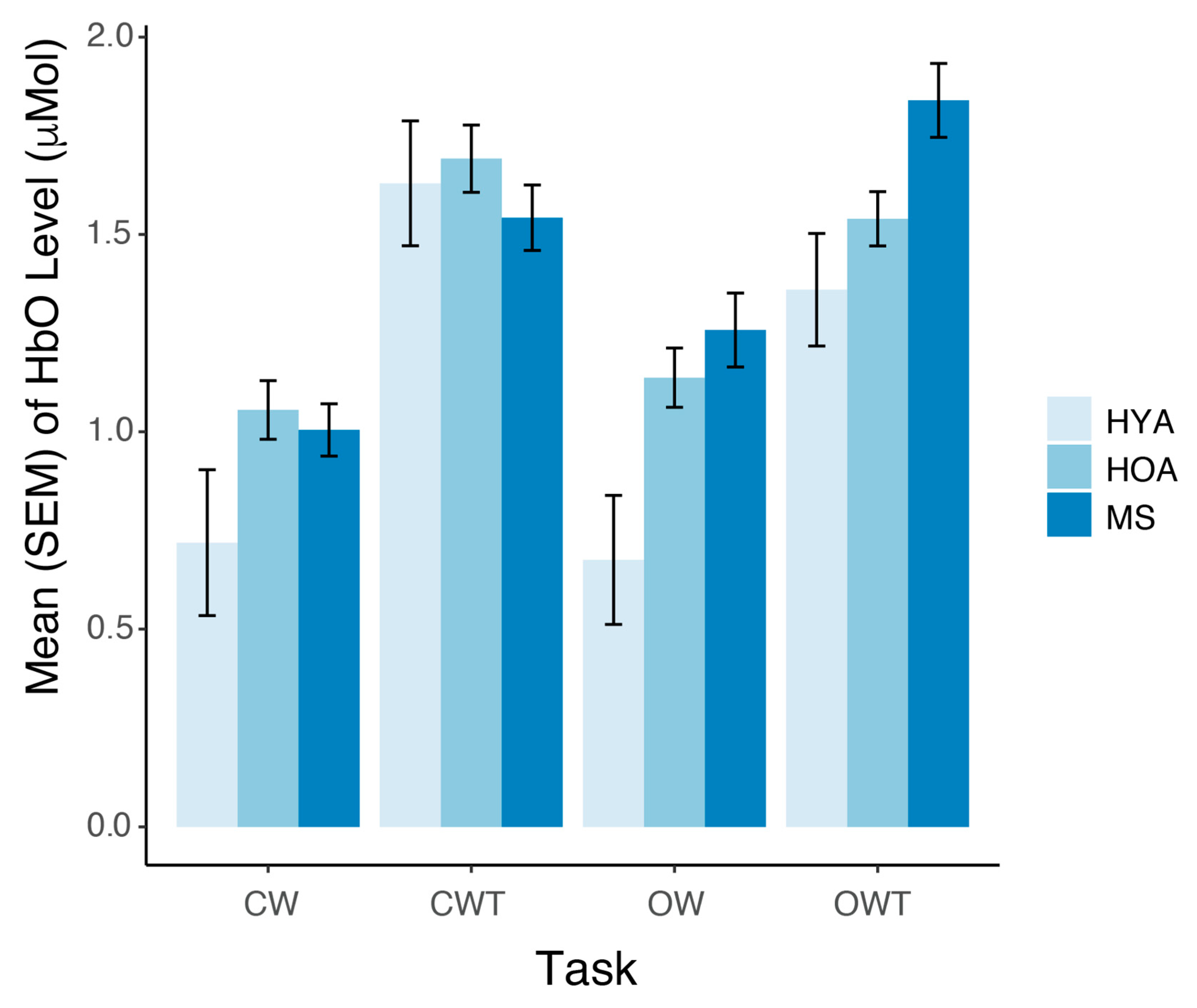
3.2. Prefrontal Cortex Oxygenated Hemoglobin Levels
3.3. Association Between Behavioral Performance and Disability and Cognitive Function in MS
4. Discussion
4.1. Addition of Obstacles Increases PFC Activity and Footfall Placement Variability
4.2. Differential Effect of Obstacles on PFC Activity in Older Adults and MS
4.3. Increases in PFC Activity and Decreases in Performance While Dual-Task Walking
4.4. Implications of Current Findings
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple Sclerosis. New Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.; Chelune, G.J.; Tulsky, D.S.; Lengenfelder, J.; Chiaravalloti, N.D. Is Speed of Processing or Working Memory the Primary Information Processing Deficit in Multiple Sclerosis? J. Clin. Exp. Neuropsychol. 2004, 26, 550–562. [Google Scholar] [CrossRef]
- Motl, R.W. Ambulation and Multiple Sclerosis. Phys. Med. Rehabil. Clin. 2013, 24, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Webber, S.C.; Porter, M.M.; Menec, V.H. Mobility in Older Adults: A Comprehensive Framework. Gerontologist 2010, 50, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Gopaul, K.; Montero Odasso, M.M. The Role of Cognitive Impairment in Fall Risk among Older Adults: A Systematic Review and Meta-Analysis. Age Ageing 2012, 41, 299–308. [Google Scholar] [CrossRef]
- Portaccio, E.; Magyari, M.; Havrdova, E.K.; Ruet, A.; Brochet, B.; Scalfari, A.; Di Filippo, M.; Tur, C.; Montalban, X.; Amato, M.P. Multiple Sclerosis: Emerging Epidemiological Trends and Redefining the Clinical Course. Lancet Reg. Health–Eur. 2024, 44, 100977. [Google Scholar] [CrossRef] [PubMed]
- Socie, M.J.; Sandroff, B.M.; Pula, J.H.; Hsiao-Wecksler, E.T.; Motl, R.W.; Sosnoff, J.J. Footfall Placement Variability and Falls in Multiple Sclerosis. Ann. Biomed. Eng. 2013, 41, 1740–1747. [Google Scholar] [CrossRef]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; McGinley, J.L.; Srikanth, V.K. Ageing and Gait Variability—A Population-Based Study of Older People. Age Ageing 2010, 39, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Blizzard, L.; Wood, A.G.; Srikanth, V.; Thomson, R.; Sanders, L.M.; Callisaya, M.L. Cognitive Function, Gait, and Gait Variability in Older People: A Population-Based Study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 726–732. [Google Scholar] [CrossRef]
- Brach, J.S.; Perera, S.; Studenski, S.; Katz, M.; Hall, C.; Verghese, J. Meaningful Change in Measures of Gait Variability in Older Adults. Gait Posture 2010, 31, 175–179. [Google Scholar] [CrossRef]
- Socie, M.J.; Motl, R.W.; Pula, J.H.; Sandroff, B.M.; Sosnoff, J.J. Gait Variability and Disability in Multiple Sclerosis. Gait Posture 2013, 38, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A. Association between Gait Variability, Falls and Mobility in People with Multiple Sclerosis: A Specific Observation on the EDSS 4.0–4.5 Level. NeuroRehabilitation 2017, 40, 579–585. [Google Scholar] [CrossRef]
- Hernandez, M.E.; O’Donnell, E.; Chaparro, G.; Holtzer, R.; Izzetoglu, M.; Sandroff, B.M.; Motl, R.W. Brain Activation Changes during Balance- And Attention-Demanding Tasks in Middle- And Older-Aged Adults with Multiple Sclerosis. Mot. Control 2019, 23, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Roerdink, M.; Bood, R.J.; Duysens, J.; Beek, P.J.; Peper, C.L.E. Attentional Costs of Visually Guided Walking: Effects of Age, Executive Function and Stepping-Task Demands. Gait Posture 2014, 40, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Peper, C.L.E.; Oorthuizen, J.K.; Roerdink, M. Attentional Demands of Cued Walking in Healthy Young and Elderly Adults. Gait Posture 2012, 36, 378–382. [Google Scholar] [CrossRef]
- Mazaheri, M.; Hoogkamer, W.; Potocanac, Z.; Verschueren, S.; Roerdink, M.; Beek, P.J.; Peper, C.E.; Duysens, J. Effects of Aging and Dual Tasking on Step Adjustments to Perturbations in Visually Cued Walking. Exp. Brain Res. 2015, 233, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Santinelli, F.B.; Sebastião, E.; Kuroda, M.H.; Moreno, V.C.; Pilon, J.; Vieira, L.H.P.; Barbieri, F.A. Cortical Activity and Gait Parameter Characteristics in People with Multiple Sclerosis during Unobstructed Gait and Obstacle Avoidance. Gait Posture 2021, 86, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.W.; Crum, J.; Pinti, P.; Aichelburg, C.; Oliver, D.; Lind, F.; Power, S.; Swingler, E.; Hakim, U.; Merla, A.; et al. Prefrontal Cortical Activation Associated with Prospective Memory While Walking around a Real-World Street Environment. Neuroimage 2022, 258, 119392. [Google Scholar] [CrossRef]
- Chatterjee, S.A.; Seidler, R.D.; Skinner, J.W.; Lysne, P.E.; Sumonthee, C.; Wu, S.S.; Cohen, R.A.; Rose, D.K.; Woods, A.J.; Clark, D.J. Obstacle Negotiation in Older Adults: Prefrontal Activation Interpreted through Conceptual Models of Brain Aging. Innov. Aging. 2020, 4, igaa034. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Shustack, S.; Giladi, N.; Hausdorff, J.M. Effects of Aging on Prefrontal Brain Activation during Challenging Walking Conditions. Brain Cogn. 2017, 115, 41–46. [Google Scholar] [CrossRef]
- Casuso-Holgado, M.J.; Martin-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of Virtual Reality Training for Balance and Gait Rehabilitation in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar] [CrossRef]
- Baram, Y.; Miller, A. Virtual Reality Cues for Improvement of Gait in Patients with Multiple Sclerosis. Neurology 2006, 66, 178–181. [Google Scholar] [CrossRef]
- Patil, V.; Narayan, J.; Sandhu, K.; Dwivedy, S.K. Integration of Virtual Reality and Augmented Reality in Physical Rehabilitation: A State-of-the-Art Review. In Revolutions in Product Design for Healthcare; Subburaj, K., Sandhu, K., Ćuković, S., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 177–205. [Google Scholar]
- Peruzzi, A.; Zarbo, I.R.; Cereatti, A.; Della Croce, U.; Mirelman, A. An Innovative Training Program Based on Virtual Reality and Treadmill: Effects on Gait of Persons with Multiple Sclerosis. Disabil. Rehabil. 2017, 39, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; Verghese, J.; Xue, X.; Lipton, R.B. Cognitive Processes Related to Gait Velocity: Results from the Einstein Aging Study. Neuropsychology 2006, 20, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, R.; Baert, I.; Kalron, A.; Romberg, A.; Tacchino, A.; Giffroy, X.; Coninx, K.; Feys, P. Associations between Clinical Characteristics and Dual Task Performance in Multiple Sclerosis Depend on the Cognitive and Motor Dual Tasks Used. Mult. Scler. Relat. Disord. 2021, 56, 103230. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.J.D.; Lord, S.R.; Schoene, D.; Pelicioni, P.H.S.; Sturnieks, D.L.; Menant, J.C. Age-Related Changes in Gait Adaptability in Response to Unpredictable Obstacles and Stepping Targets. Gait Posture 2016, 46, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Potocanac, Z.; Smulders, E.; Pijnappels, M.; Verschueren, S.; Duysens, J. Response Inhibition and Avoidance of Virtual Obstacles during Gait in Healthy Young and Older Adults. Hum. Mov. Sci. 2015, 39, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.; Wilkie, R.M.; Wann, J.P. Stepping over Obstacles: Attention Demands and Aging. Gait Posture 2009, 29, 428–432. [Google Scholar] [CrossRef]
- Holtzer, R.; Choi, J.; Motl, R.W.; Foley, F.W.; Wagshul, M.E.; Hernandez, M.E.; Izzetoglu, M. Brain Control of Dual-Task Walking Can Be Improved in Aging and Neurological Disease. Geroscience 2024, 46, 3169–3184. [Google Scholar] [CrossRef]
- Bishnoi, A.; Holtzer, R.; Hernandez, M.E. Brain Activation Changes While Walking in Adults with and without Neurological Disease: Systematic Review and Meta-Analysis of Functional near-Infrared Spectroscopy Studies. Brain Sci. 2021, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, G.; Balto, J.M.; Sandroff, B.M.; Holtzer, R.; Izzetoglu, M.; Motl, R.W.; Hernandez, M.E. Frontal Brain Activation Changes Due to Dual-Tasking under Partial Body Weight Support Conditions in Older Adults with Multiple Sclerosis. J. Neuroeng. Rehabil. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Holtzer, R.; Chaparro, G.; Jean, K.; Balto, J.M.; Sandroff, B.M.; Izzetoglu, M.; Motl, R.W. Brain Activation Changes during Locomotion in Middle-Aged to Older Adults with Multiple Sclerosis. J. Neurol. Sci. 2016, 370, 277–283. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Motl, R.W.; Foley, F.W.; Picone, M.A.; Izzetoglu, M.; Lipton, M.L.; Wagshul, M.; Holtzer, R. Disability Moderates Dual Task Walking Performance and Neural Efficiency in Older Adults With Multiple Sclerosis. Neurorehabil. Neural. Repair. 2024, 38, 795–807. [Google Scholar] [CrossRef]
- Motl, R.W.; Foley, F.W.; Picone, M.A.; Lipton, M.L.; Izzetoglu, M.; Hernandez, M.E.; Holtzer, R. Initial Validation of the University of Alabama Birmingham Study of Aging Life-Space Assessment in Older Adults with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 82, 105354. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; Motl, R.W.; Wagshul, M.E.; Picone, M.A.; Hernandez, M.E.; Izzetoglu, M.; Lipton, M.L.; Foley, F.W. Life Space Assessment and Falls in Older Adults with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 87, 105671. [Google Scholar] [CrossRef] [PubMed]
- Wagshul, M.E.; Foley, F.W.; Chaudhary, K.; Lipton, M.L.; Motl, R.W.; Izzetoglu, M.; Hernandez, M.E.; Picone, M.A.; Holtzer, R. Differential Associations of Mobility With Fronto-Striatal Integrity and Lesion Load in Older Adults With and Without Multiple Sclerosis. Neurorehabil. Neural. Repair. 2023, 37, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Audoin, B.; Ibarrola, D.; Ranjeva, J.-P.; Confort-Gouny, S.; Malikova, I.; Ali-Chérif, A.; Pelletier, J.; Cozzone, P. Compensatory Cortical Activation Observed by FMRI during a Cognitive Task at the Earliest Stage of Multiple Sclerosis. Hum. Brain Mapp. 2003, 20, 51–58. [Google Scholar] [CrossRef]
- Rocca, M.A.; De Meo, E.; Filippi, M. Functional MRI in Investigating Cognitive Impairment in Multiple Sclerosis. Acta Neurol. Scand. 2016, 134, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Riccitelli, G.; Rodegher, M.; Ceccarelli, A.; Falini, A.; Falautano, M.; Meani, A.; Comi, G.; Filippi, M. Functional MR Imaging Correlates of Neuropsychological Impairment in Primary-Progressive Multiple Sclerosis. Am. J. Neuroradiol. 2010, 31, 1240–1246. [Google Scholar] [CrossRef]
- Rypma, B.; Berger, J.S.; D’esposito, M. The Influence of Working-Memory Demand and Subject Performance on Prefrontal Cortical Activity. J. Cogn. Neurosci. 2002, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Spencer, M.; Folstein, M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1988, 1, 111–117. [Google Scholar]
- Lines, C.R.; McCarroll, K.A.; Lipton, R.B.; Block, G.A. Telephone Screening for Amnestic Mild Cognitive Impairment. Neurology 2003, 60, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Motl, R.W.; Chaparro, G.; Hernandez, M.E.; Balto, J.M.; Sandroff, B.M. Physical Function in Older Adults with Multiple Sclerosis: An Application of the Short Physical Performance Battery. J. Geriatr. Phys. Ther. 2018, 41, 155–160. [Google Scholar] [CrossRef]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Verghese, J. FNIRS Study of Walking and Walking While Talking in Young and Old Individuals. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2011, 66, 879–887. [Google Scholar] [CrossRef]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Wang, C.; England, S.; Verghese, J. Online Fronto-Cortical Control of Simple and Attention-Demanding Locomotion in Humans. Neuroimage 2015, 112, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Rynders, C.A.; Sosnoff, J.J. Deficits in Medio-Lateral Balance Control and the Implications for Falls in Individuals with Multiple Sclerosis. Gait Posture 2016, 49, 148–154. [Google Scholar] [CrossRef]
- Huisinga, J.M.; Mancini, M.; George, R.J.S.; Horak, F.B. Accelerometry Reveals Differences in Gait Variability between Patients with Multiple Sclerosis and Healthy Controls. Ann. Biomed. Eng. 2013, 41, 1670–1679. [Google Scholar] [CrossRef]
- Weed, L.; Little, C.; Kasser, S.L.; McGinnis, R.S. A Preliminary Investigation of the Effects of Obstacle Negotiation and Turning on Gait Variability in Adults with Multiple Sclerosis. Sensors 2021, 21, 5806. [Google Scholar] [CrossRef] [PubMed]
- Izzetoglu, M.; Holtzer, R. Effects of Processing Methods on FNIRS Signals Assessed during Active Walking Tasks in Older Adults. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 699–709. [Google Scholar] [CrossRef]
- Boas, D.A.; Franceschini, M.A.; Dunn, A.K.; Strangman, G. Noninvasive Imaging of Cerebral Activation with Diffuse Optical Tomography. In Vivo Optical Imaging of Brain Function, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 193–221. [Google Scholar]
- Harada, T.; Miyai, I.; Suzuki, M.; Kubota, K. Gait Capacity Affects Cortical Activation Patterns Related to Speed Control in the Elderly. Exp. Brain Res. 2009, 193, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Varas, R.; Costa; Iáñez, E.; Úbeda, A.; Hortal, E.; Azorín, J.M. Analyzing EEG Signals to Detect Unexpected Obstacles during Walking. J. Neuroeng. Rehabil. 2015, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Varas, R.; Costa, Á.; Úbeda, A.; Iáñez, E.; Azorin, J.M. Changes in Brain Activity Due to the Sudden Apparition of an Obstacle during Gait. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015; pp. 110–113. [Google Scholar]
- Haefeli, J.; Vögeli, S.; Michel, J.; Dietz, V. Preparation and Performance of Obstacle Steps: Interaction between Brain and Spinal Neuronal Activity. Eur. J. Neurosci. 2011, 33, 338–348. [Google Scholar] [CrossRef]
- Maidan, I.; Shustak, S.; Sharon, T.; Bernad-Elazari, H.; Geffen, N.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Prefrontal Cortex Activation during Obstacle Negotiation: What’s the Effect Size and Timing? Brain Cogn. 2018, 122, 45–51. [Google Scholar] [CrossRef]
- Lin, M.-I.B.; Lin, K.-H. Walking While Performing Working Memory Tasks Changes the Prefrontal Cortex Hemodynamic Activations and Gait Kinematics. Front. Behav. Neurosci. 2016, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.J. Automaticity of Walking: Functional Significance, Mechanisms, Measurement and Rehabilitation Strategies. Front. Hum. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive Motor Interference While Walking: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Postigo-Alonso, B.; Galvao-Carmona, A.; Benitez, I.; Conde-Gavilan, C.; Jover, A.; Molina, S.; Pena-Toledo, M.A.; Agüera, E. Cognitive-Motor Interference during Gait in Patients with Multiple Sclerosis: A Mixed Methods Systematic Review. Neurosci. Biobehav. Rev. 2018, 94, 126–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, C.; Zhang, H.; Xue, G.; Dong, Q.; Jin, Z.; Zhang, L.; Peng, C.; Zhao, H.; Guo, Y.; et al. Neural Substrates for Forward and Backward Recitation of Numbers and the Alphabet: A Close Examination of the Role of Intraparietal Sulcus and Perisylvian Areas. Brain Res. 2006, 1099, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Forebrain Control of Locomotor Behaviors. Brain Res. Rev. 2008, 57, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Neurophysiology of Gait: From the Spinal Cord to the Frontal Lobe. Mov. Disord. 2013, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Billington, J.; Field, D.T.; Wilkie, R.M.; Wann, J.P. An FMRI Study of Parietal Cortex Involvement in the Visual Guidance of Locomotion. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 1495. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor Control and Aging: Links to Age-Related Brain Structural, Functional, and Biochemical Effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
| Variable | HYA (n = 10) | HOA (n = 12) | MS (n = 10) | p Value | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 21.9 | 3.4 | 63.1 | 4.4 | 56.2 | 5.1 | <0.001 |
| Height (m) | 1.7 | 0.1 | 1.7 | 0.09 | 1.7 | 0.09 | 0.942 |
| Weight (kg) | 73.1 | 15.5 | 77.6 | 18 | 70.4 | 9.6 | 0.536 |
| RBANS | 98.6 | 19.1 | 105.6 | 11.3 | 98.1 | 13.4 | 0.411 |
| SPPB (0–12) | 11.5 | 0.5 | 11.6 | 0.7 | 10.2 | 1.8 | 0.012 |
| Number | Percent | Number | Percent | Number | Percent | ||
| Females | 5 | 50.0 | 9 | 75.0 | 8 | 80.0 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, M.E.; Holtzer, R.; Izzetoglu, M.; Motl, R.W. The Use of Augmented Reality on a Self-Paced Treadmill to Quantify Attention and Footfall Placement Variability in Middle-Aged to Older-Aged Adults with Multiple Sclerosis. Sclerosis 2025, 3, 3. https://doi.org/10.3390/sclerosis3010003
Hernandez ME, Holtzer R, Izzetoglu M, Motl RW. The Use of Augmented Reality on a Self-Paced Treadmill to Quantify Attention and Footfall Placement Variability in Middle-Aged to Older-Aged Adults with Multiple Sclerosis. Sclerosis. 2025; 3(1):3. https://doi.org/10.3390/sclerosis3010003
Chicago/Turabian StyleHernandez, Manuel E., Roee Holtzer, Meltem Izzetoglu, and Robert W. Motl. 2025. "The Use of Augmented Reality on a Self-Paced Treadmill to Quantify Attention and Footfall Placement Variability in Middle-Aged to Older-Aged Adults with Multiple Sclerosis" Sclerosis 3, no. 1: 3. https://doi.org/10.3390/sclerosis3010003
APA StyleHernandez, M. E., Holtzer, R., Izzetoglu, M., & Motl, R. W. (2025). The Use of Augmented Reality on a Self-Paced Treadmill to Quantify Attention and Footfall Placement Variability in Middle-Aged to Older-Aged Adults with Multiple Sclerosis. Sclerosis, 3(1), 3. https://doi.org/10.3390/sclerosis3010003








