Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects
Abstract
:1. Introduction
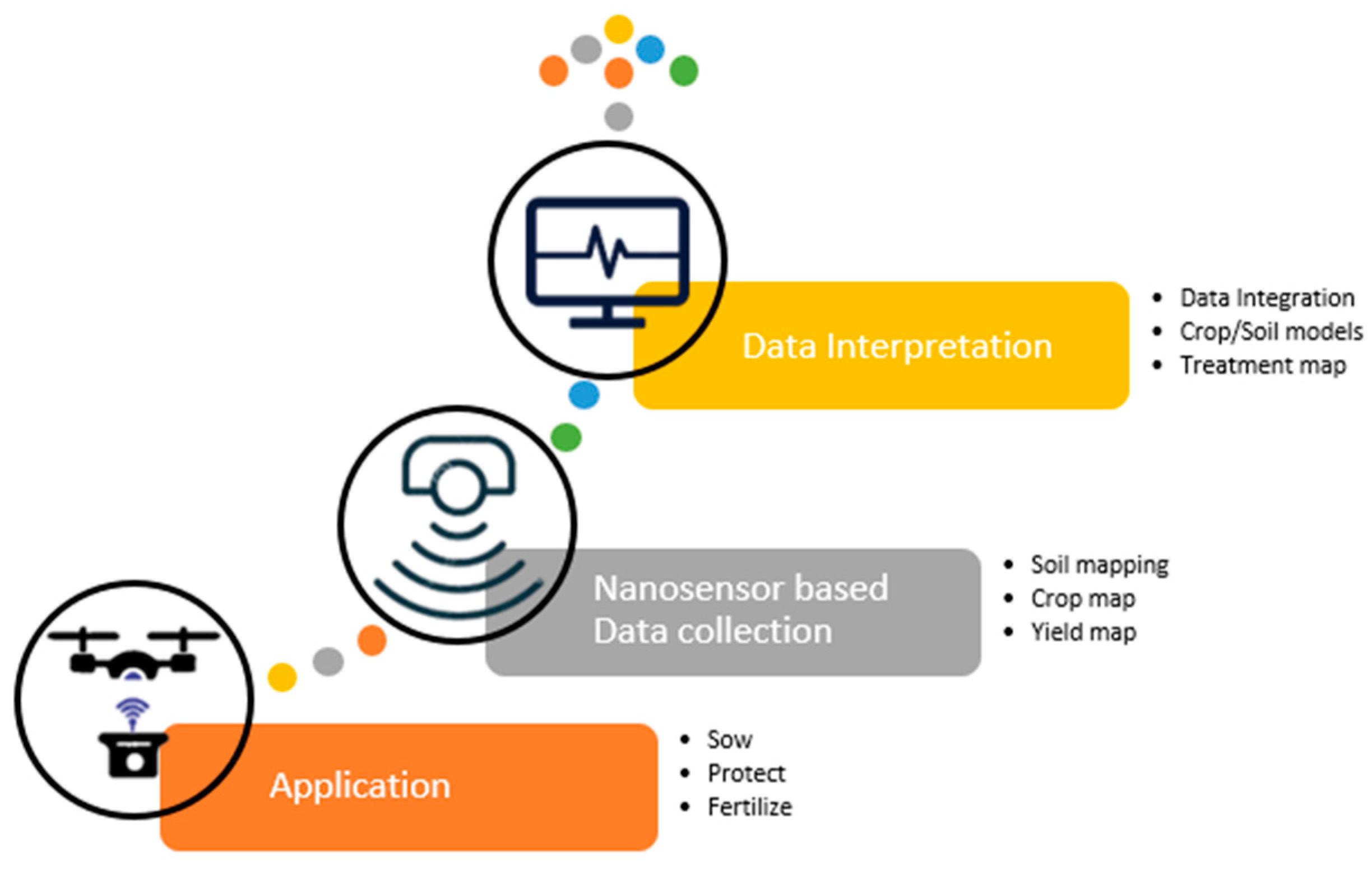
2. Synergies of Precision Agriculture and Nanotechnology for Sustainable Crop Growth
2.1. Improved Nutrient Utilization
2.2. Enhanced Pest Control
2.3. Advanced Environmental Monitoring
2.4. Variable Rate Technology (VRT)
2.5. Automated Machinery
2.6. Data Analytics
2.7. Nanomaterials Use in Plant Growth
2.8. Summary of Synergies between Precision Agriculture and Nanotechnology
3. Advantages of Nanotechnology in the Agriculture Systems
3.1. Improved Seed Germination and Plant Growth
3.2. Improved Micronutrient Supply
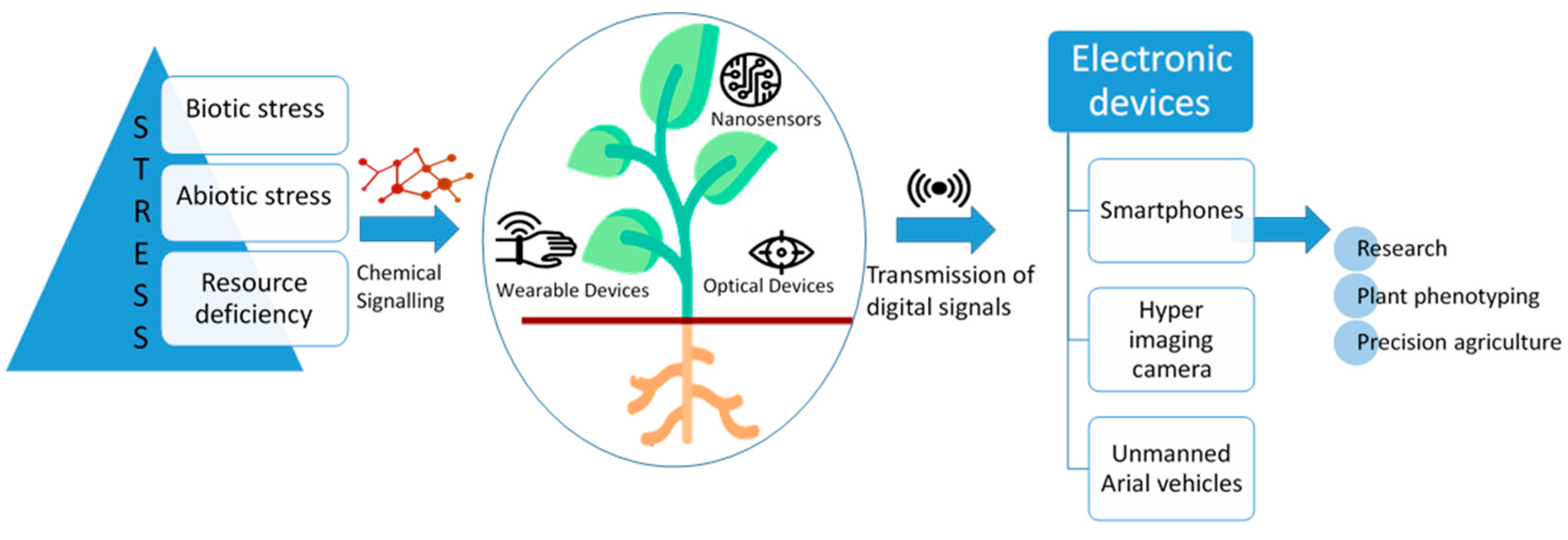
3.3. Biotic and Abiotic Plant Stress Alleviation
3.4. Improved Plant Fertilization in Lower Dosage
3.4.1. Delivery of Biofertilizers
3.4.2. Delivery of Chemical Fertilizers
3.5. Lowering the Dosage of Pesticides
3.5.1. Use as Nanoinsecticides
3.5.2. Use as Nanofungicides
3.5.3. Use as Nanoherbicides
3.6. Summary of Advantages of Nanotechnology in the Agriculture Systems
4. Disadvantages of Nanotechnology in Agriculture Systems
Summary of Disadvantages of Nanotechnology in Agriculture
5. Types of Nanotechnology Based Nanodiagnostic Systems
5.1. Metal Nanoparticle-Based Systems
5.2. Functional Quantum Dots
5.3. Nanofabrication Imaging
5.4. Nanopore System
5.5. Nanobarcodes
5.6. Kit-Based Systems
5.7. Summary of Nanotechnology Based Nanodiagnostic Systems
6. Nanobiosensors in Diagnostics and Precision Agriculture
6.1. Monitoring of Soil Quality Parameters
6.2. Monitoring Soil Pesticides/Herbicides
6.3. Monitoring Soil Nutrients
6.4. Monitoring Soil Humidity
6.5. Monitoring Plant Disease and Stress
6.6. Monitoring Irrigation
6.7. Summary of Biosensors in Precision Agriculture
7. Nanotechnological Applications to Reduce Agro-Waste and for Synthesizing High-Value Products
8. Tagging, Monitoring, and Tracking the Agroproducts Using Nanotechnology Methods and Devices
9. Smartphone-Based Biosensors in Precision Agriculture
10. Precision Agriculture and Cloud Computing
11. Nanotechnology and Agribusiness
12. Economic, Legal, Social, and Risk Implications of Nanotechnology
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Panhwar, Q.A.; Ali, A.; Naher, U.A.; Memon, M.Y. Chapter 2—Fertilizer Management Strategies for Enhancing Nutrient Use Efficiency and Sustainable Wheat Production, in Organic Farming; Chandran, S., Unni, M.R., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 17–39. [Google Scholar]
- Meyer, W.B.; Turner, B.L. Human population growth and global land-use/cover change. Annu. Rev. Ecol. Syst. 1992, 23, 39–61. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Nanosensors: Frontiers in precision agriculture. Nanotechnol. Agric. Paradig. 2017, 279–291. [Google Scholar]
- Chen, Y.; Zhang, D.; Wang, D.; Lu, L.; Wang, X.; Guo, G. A carbon-supported BiSn nanoparticles based novel sensor for sensitive electrochemical determination of Cd (II) ions. Talanta 2019, 202, 27–33. [Google Scholar] [CrossRef]
- Kiaee, G.; Dimitrakakis, N.; Sharifzadeh, S.; Kim, H.; Avery, R.K.; Moghaddam, K.M.; Haghniaz, R.; Yalcintas, E.P.; de Barros, N.R.; Karamikamkar, S.; et al. Laponite-Based Nanomaterials for Drug Delivery. Adv. Healthc. Mater. 2022, 11, 2102054. [Google Scholar] [CrossRef] [PubMed]
- Finch, H.J.S.; Samuel, A.M.; Lane, G.P.F. 10—Precision Farming, in Lockhart & Wiseman’s Crop Husbandry Including Grassland, 9th ed.; Finch, S., Samuel, A.M., Lane, G.P.F., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 235–244. [Google Scholar]
- Dhanaraju, M.; Chenniappan, P.; Ramalingam, K.; Pazhanivelan, S.; Kaliaperumal, R. Smart Farming: Internet of Things (IoT)—Based Sustainable Agriculture. Agriculture 2022, 12, 1745. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Cicek, S.; Nadaroglu, H. The use of nanotechnology in the agriculture. Adv. Nano Res. 2015, 3, 207. [Google Scholar] [CrossRef]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Muhammad Aamir, I. Nano-fertilizers for sustainable crop production under changing climate: A global perspective. In Sustainable Crop Production; Mirza, H., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 18. [Google Scholar]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299. [Google Scholar] [CrossRef] [PubMed]
- Senapaty, M.K.; Ray, A.; Padhy, N. IoT-Enabled Soil Nutrient Analysis and Crop Recommendation Model for Precision Agriculture. Computers 2023, 12, 61. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Z.; Ullah, S.; Melagraki, G.; Afantitis, A.; Lynch, I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat. Plants 2021, 7, 864–876. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Mailapalli, D. Nanopesticides for Pest Control; Springer: Cham, Switzerland, 2020; Volume 40, pp. 43–74. [Google Scholar]
- Adeyinka, O.S.; Riaz, S.; Toufiq, N.; Yousaf, I.; Bhatti, M.U.; Batcho, A.A.; Olajide, A.A.; Nasir, I.A.; Tabassum, B. Advances in exogenous RNA delivery techniques for RNAi-mediated pest control. Mol. Biol. Rep. 2020, 47, 6309–6319. [Google Scholar] [CrossRef]
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in targeted pesticides with environmentally responsive controlled release by nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. Enhancing smart farming through the applications of Agriculture 4.0 technologies. Int. J. Intell. Netw. 2022, 3, 150–164. [Google Scholar] [CrossRef]
- Khandelwal, N.; Barbole, R.S.; Banerjee, S.S.; Chate, G.P.; Biradar, A.V.; Khandare, J.J.; Giri, A.P. Budding trends in integrated pest management using advanced micro-and nanomaterials: Challenges and perspectives. J. Environ. Manag. 2016, 184, 157–169. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Marelli, B.; Zeng, X.; Mason, A.J.; Cao, C. Soil sensors and plant wearables for smart and precision agriculture. Adv. Mater. 2021, 33, 2007764. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M. Nanosensors and nanobiosensors for monitoring the environmental pollutants. In Waste Recycling Technologies for Nanomaterials Manufacturing; Springer: Cham, Switzerland, 2021; pp. 229–246. [Google Scholar]
- Tantalaki, N.; Souravlas, S.; Roumeliotis, M. Data-driven decision making in precision agriculture: The rise of big data in agricultural systems. J. Agric. Food Inf. 2019, 20, 344–380. [Google Scholar] [CrossRef]
- Dar, F.A.; Qazi, G.; Pirzadah, T.B. Nano-biosensors: NextGen diagnostic tools in agriculture. In Nanobiotechnology in Agriculture: Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 129–144. [Google Scholar]
- Šarauskis, E.; Kazlauskas, M.; Naujokienė, V.; Bručienė, I.; Steponavičius, D.; Romaneckas, K.; Jasinskas, A. Variable rate seeding in precision agriculture: Recent advances and future perspectives. Agriculture 2022, 12, 305. [Google Scholar] [CrossRef]
- Si, Y.; Liu, J.; Chen, Y.; Miao, X.; Ye, F.; Liu, Z.; Li, J. rGO/AuNPs/tetraphenylporphyrin nanoconjugate-based electrochemical sensor for highly sensitive detection of cadmium ions. Anal. Methods 2018, 10, 3631–3636. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Emam, T.M.; Elsherbiny, E.A. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater. Sci. Eng. C 2020, 109, 110617. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Jia, P.; Liu, J.; Liu, Y.; Sun, X.; Zhang, M.; Tian, Y.; Zhang, D.; Wang, J.; Wang, L. Diversely positive-charged gold nanoparticles based biosensor: A label-free and sensitive tool for foodborne pathogen detection. Food Chem. X 2019, 3, 100052. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Ren, W.; Chang, H.; Li, L.; Teng, Y. Effect of graphene oxide on growth of wheat seedlings: Insights from oxidative stress and physiological flux. Bull. Environ. Contam. Toxicol. 2020, 105, 139–145. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Nagarajan, K.; Ramanujam, N.; Sanjay, M.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Luan, Q.; Tang, H.; Huang, F.; Xiang, X.; Yang, C.; Bao, Y. Cellulose Anionic Hydrogels Based on Cellulose Nanofibers as Natural Stimulants for Seed Germination and Seedling Growth. J. Agric. Food Chem. 2017, 65, 3785–3791. [Google Scholar] [CrossRef]
- Saito, H.; Yamashita, Y.; Sakata, N.; Ishiga, T.; Shiraishi, N.; Usuki, G.; Nguyn, V.T.; Yamamura, E.; Ishiga, Y. Covering Soybean Leaves with Cellulose Nanofiber Changes Leaf Surface Hydrophobicity and Confers Resistance against Phakopsora pachyrhizi. Front. Plant Sci. 2021, 12, 726565. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Piotto, F.A.; Schmidt, D.; Peters, L.P.; Monteiro, C.C.; Azevedo, R.A. Seed priming with hormones does not alleviate induced oxidative stress in maize seedlings subjected to salt stress. Sci. Agric. 2011, 68, 598–602. [Google Scholar] [CrossRef]
- Batty, A.; Dixon, K.; Brundrett, M.; Sivasithamparam, K. Constraints to symbiotic germination of terrestrial orchid seed in a mediterranean bushland. New Phytol. 2001, 152, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.A.; Laware, S.L. Seed Priming: A Critical Review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Ko, K.-S.; Kong, I.C. Toxic effects of nanoparticles on bioluminescence activity, seed germination, and gene mutation. Appl. Microbiol. Biotechnol. 2014, 98, 3295–3303. [Google Scholar] [CrossRef] [PubMed]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 282. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Chinnappan, S.; Ramalingam, C.; Kumar, A. A novel approach to evaluate titanium dioxide nanoparticle–protein interaction through docking: An insight into mechanism of action. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 937–943. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Srivastava, P.; Ramalingam, C. A spectroscopic study on interaction between bovine serum albumin and titanium dioxide nanoparticle synthesized from microwave-assisted hybrid chemical approach. J. Photochem. Photobiol. B Biol. 2016, 161, 472–481. [Google Scholar] [CrossRef]
- Vemula, A. Chitosan Bionanocomposite: A Potential Approach for Sustainable Agriculture. Med. Agric. Environ. Sci. 2022, 2, 41–46. [Google Scholar]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Priya, B.; Srinivasarao, M.; Mukherjee, S. Screening of Phosphorus Nanoparticle Concentration Based on their Effects at Germination & Seedling Level in Mung, Urd and Cowpea. Vegetos Int. J. Plant Res 2015, 28, 169. [Google Scholar]
- Akhtar, N.; Ilyas, N. Role of nanosilicab to boost the activities of metabolites in Triticum aestivum facing drought stress. Plant Soil 2022, 477, 99–115. [Google Scholar] [CrossRef]
- Johns, D.A. Effect of Silicon Dioxide Nanoparticles on Seed Germination and Growth of Four Different Plant Species. Ph.D. Thesis, Deakin University, Geelong, Australia, 2018. [Google Scholar]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russ. Agric. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Kapoor, N.; Kaisar, A.; Dixit, R.; Singh, N.P.; Singh, J. A study to evaluate the effect of silver nanoparticles synthesized by Sonchus asper on fenugreek plant. J. Pharmacogn. Phytochem. 2018, 7, 1144–1149. [Google Scholar]
- Manesh, R.R.; Grassi, G.; Bergami, E.; Marques-Santos, L.; Faleri, C.; Liberatori, G.; Corsi, I. Co-exposure to titanium dioxide nanoparticles does not affect cadmium toxicity in radish seeds (Raphanus sativus). Ecotoxicol. Environ. Saf. 2018, 148, 359–366. [Google Scholar] [CrossRef]
- Zahra, Z.; Ali, M.A.; Parveen, A.; Kim, E.; Khokhar, M.F.; Baig, S.; Hina, K.; Choi, H.-K.; Arshad, M. Exposure–response of wheat cultivars to TiO2 nanoparticles in contrasted soils. Soil Sediment Contam. Int. J. 2019, 28, 184–199. [Google Scholar] [CrossRef]
- Goodarzi, G.R.; Noor, V.P.; Ahmadloo, F. Effects of nanoparticle treatments on propagation of Prunus mahaleb L. by seed. J. For. Sci. 2017, 63, 408–416. [Google Scholar] [CrossRef]
- Srinivasan, R.; Maity, A.; Singh, K.K.; Ghosh, P.K.; Kumar, S.; Srivastava, M.K.; Radhakrishna, A.; Srivastava, R.; Kumari, B. Influence of copper oxide and zinc oxide nano-particles on growth of fodder cowpea and soil microbiological properties. Range Manag. Agrofor. 2017, 38, 208–214. [Google Scholar]
- Alhammad, B.A.; Ahmad, A.; Seleiman, M.F.; Tola, E. Seed Priming with Nanoparticles and 24-Epibrassinolide Improved Seed Germination and Enzymatic Performance of Zea mays L. in Salt-Stressed Soil. Plants 2023, 12, 690. [Google Scholar]
- Bayat, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R.; Shkurkin, S.I. Ameliorating seed germination and seedling growth of nano-primed wheat and flax seeds using seven biogenic metal-based nanoparticles. Agronomy 2022, 12, 811. [Google Scholar] [CrossRef]
- Swaminathan, S.; Edward, B.; Kurpad, A. Micronutrient deficiency and cognitive and physical performance in Indian children. Eur. J. Clin. Nutr. 2013, 67, 467. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lee, S.-L.; Taylor, C.; Li, J.; Chan, Y.-M.; Agarwal, R.; Temple, R.; Throckmorton, D.; Tyner, K. Scientific and regulatory approach to botanical drug development: A US FDA perspective. J. Nat Prod. 2020, 83, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Monreal, C.; DeRosa, M.; Mallubhotla, S.; Bindraban, P.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Subramanian, K.; Paulraj, C.; Natarajan, S. Nanotechnological approaches in nutrient management. In Nanotechnology Applications in Agriculture; TNAU Technical Bulletin; Tamil Nadu Agricultural University: Coimbatore, India, 2008; pp. 37–42. [Google Scholar]
- Dey, J.K.; Das, S.; Mawlong, L.G. Nanotechnology and its Importance in Micronutrient Fertilization. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2306–2325. [Google Scholar]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Mashwani, Z.-U.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth. 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2016, 15, 15–22. [Google Scholar] [CrossRef]
- Chhipa, H.; Joshi, P. Nanofertilisers, nanopesticides and nanosensors in agriculture. In Nanoscience in Food and Agriculture 1. Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2016; pp. 247–282. [Google Scholar]
- Jampílek, J.; Kráľová, K. Chapter 3—Nanopesticides: Preparation, targeting, and controlled release. In New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 10, pp. 81–127. [Google Scholar]
- Khan, M.; Khan, A.U.; Hasan, M.A.; Yadav, K.K.; Pinto, M.; Malik, N.; Yadav, V.K.; Khan, A.H.; Islam, S.; Sharma, G.K. Agro-Nanotechnology as an Emerging Field: A Novel Sustainable Approach for Improving Plant Growth by Reducing Biotic Stress. Appl. Sci. 2021, 11, 2282. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, I.K.; Mishra, R.; Singh, A.; Ramawat, N.; Singh, A. The Role of Zinc Oxide Nanoparticles in Plants: A Critical Appraisal. In Nanomaterial Biointeractions at the Cellular, Organismal and System Levels; Springer: Cham, Switzerland, 2021; pp. 249–267. [Google Scholar]
- Abdel Latef, A.A.H.; Srivastava, A.K.; El-Sadek, M.S.A.; Kordrostami, M.; Tran, L.S.P. Titanium Dioxide Nanoparticles Improve Growth and Enhance Tolerance of Broad Bean Plants under Saline Soil Conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2019, 27, 4958–4968. [Google Scholar]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc Oxide Nanoparticles Improve Salt Tolerance in Rice Seedlings by Improving Physiological and Biochemical Indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) Nanoparticles Reduce Heavy Metals Uptake and Mitigate Their Toxicity in Wheat Seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.E.; Abdelhameed, R.M. Superiority of modified graphene oxide for enhancing the growth, yield, and antioxidant potential of pearl millet (Pennisetum glaucum L.) under salt stress. Plant Stress 2021, 2, 100025. [Google Scholar]
- Yuan, Z.; Zhang, Z.; Wang, X.; Li, L.; Cai, K.; Han, H. Novel impacts of functionalized multi-walled carbon nanotubes in plants: Promotion of nodulation and ni-trogenase activity in the rhizobium-legume system. Nanoscale 2017, 9, 9921–9937. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Gómez, C.; Zubair, M.; Ilyas, N.; Khan, N.; Shahid, M.A. Silicon Alleviate Hypoxia Stress by Improving Enzymatic and Non-enzymatic Antioxidants and Regulating Nutrient Uptake in Muscadine Grape (Muscadinia rotundifolia Michx.). Front. Plant Sci. 2021, 11, 618873. [Google Scholar] [CrossRef]
- AlKubaisi, N.A.; Aref, N.M.A. Dispersed gold nanoparticles potentially ruin gold barley yellow dwarf virus and eliminate virus infectivity hazards. Appl. Nanosci. 2016, 7, 31–40. [Google Scholar] [CrossRef]
- Jaskulski, D.; Jaskulska, I.; Majewska, J.; Radziemska, M.; Bilgin, A.; Brtnicky, M. Silver Nanoparticles (AgNPs) in Urea Solution in Laboratory Tests and Field Experiments with Crops and Vegetables. Materials 2022, 15, 870. [Google Scholar] [CrossRef]
- Ponmurugan, P.; Manjukarunambika, K.; Elango, V.; Gnanamangai, B.M. Antifungal activity of biosynthesised copper na-noparticles evaluated against red root-rot disease in tea plants. J. Exp. Nanosci. 2016, 11, 1019–1031. [Google Scholar]
- Anusuya, S.; Sathiyabama, M. Effect of Chitosan on Rhizome Rot Disease of Turmeric Caused by Pythium aphanidermatum. ISRN Biotechnol. 2014, 2014, 305349. [Google Scholar]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Silicon is an inducible and effective herbivore defence against Helicoverpa punctigera (Lepidoptera: Noctuidae) in soybean. Bull. Entomol. Res. 2019, 110, 417–422. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.; Zadeh, S.M.; Turral, H.; Burke, J. Water Pollution from Agriculture: A Global Review; Executive Summary; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Meena, V.; Rao, A.S. Utilization of nano rock phosphate by maize (Zea mays L.) crop in a vertisol of Central India. J. Agric. Sci. Technol. A 2014, 4, 5A. [Google Scholar]
- Yuvaraj, M.; Subramanian, K.S. Development of slow release Zn fertilizer using nano-zeolite as carrier. J. Plant Nutr. 2018, 41, 311–320. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y. Effects of graphene on seed germination and seedling growth. J. Nanoparticle Res. 2015, 17, 78. [Google Scholar] [CrossRef]
- Dehkourdi, E.H.; Mosavi, M. Effect of Anatase Nanoparticles (TiO2) on Parsley Seed Germination (Petroselinum crispum) In Vitro. Biol. Trace Elem. Res. 2013, 155, 283–286. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Effects of Nano-Materials on Seed Germination and Seedling Growth: Striking the Slight Balance between the Concepts and Controversies. Mater. Focus 2016, 5, 195–201. [Google Scholar] [CrossRef]
- Nadi, E.; Aynehband, A.; Mojaddam, M. Effect of nano-iron chelate fertilizer on grain yield, protein percent and chlorophyll content of Faba bean (Vicia faba L.). Int. J. Biosci. 2013, 3, 267–272. [Google Scholar]
- Shaymurat, T.; Gu, J.; Xu, C.; Yang, Z.; Zhao, Q.; Liu, Y.; Liu, Y. Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): A morphological study. Nanotoxicology 2012, 6, 241–248. [Google Scholar] [CrossRef]
- Thomas, L.; Singh, I. Microbial biofertilizers: Types and applications. In Biofertilizers for Sustainable Agriculture and Environment; Springer Nature International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Krishnaprabu, S. Liquid microbial consortium: A potential tool for sustainable soil health. J. Pharmacogn. Phytochem. 2020, 9, 2191–2199. [Google Scholar]
- Vandergheynst, J.; Scher, H.; Guo, H.-Y.; Schultz, D. Water-in-oil emulsions that improve the storage and delivery of the bio-larvacide Lagenidium giganteum. BioControl 2007, 52, 207–229. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kumar, R.; Mishra, R.K.; Pandey, A.; Pathak, A.; Zaidi, M.; Srivastava, S.K.; Dikshit, A. Prediction and validation of gold nanoparticles (GNPs) on plant growth promoting rhizobacteria (PGPR): A step toward development of nano-biofertilizers. Nanotechnol. Rev. 2015, 4, 439–448. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association: Paris, France, 1997; Volume 11. [Google Scholar]
- Elsayed, A.A.; Ahmed, E.-G.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Haider, G.; Zia, M.; Shah, G.A.; Iqbal, Z.; Hayat, A. Development and testing of zinc sulfate and zinc oxide nanoparticle-coated urea fertilizer to improve N and Zn use efficiency. Front. Plant Sci. 2022, 13, 1058219. [Google Scholar] [CrossRef]
- Amin, S.; Aziz, T.; Zia-ur-Rehman, M.; Saleem, I.; Rizwan, M.; Ashar, A.; Mussawar, H.A.; Maqsood, M.A. Zinc oxide nanoparticles coated urea enhances nitrogen efficiency and zinc bioavailability in wheat in alkaline calcareous soils. Environ. Sci. Pollut. Res. Int. 2023. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Main, A.R.; Webb, E.B.; Goyne, K.W.; Mengel, D. Neonicotinoid insecticides negatively affect performance measures of non-target terrestrial arthropods: A meta-analysis. Ecol. Appl. 2018, 28, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, K.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. Res. 2020, 60, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Scrinis, G.; Lyons, K. The Emerging Nano-Corporate Paradigm: Nanotechnology and the Transformation of Nature, Food and Agri-Food Systems. Int. J. Sociol. Agric. Food 2007, 15, 22–44. [Google Scholar]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in agricultural pest management and their environmental risks: A review. Int. J. Environ. Sci. Technol. 2023, 15, 1–26. [Google Scholar] [CrossRef]
- Allen, R. Agriculture during the industrial revolution. In The Economic History of Britain since 1700; Roderick, F., McCloskey, D., Eds.; Cambridge University Press: Cambridge, UK, 1994; Volume 1. [Google Scholar]
- Hao, Y.; Cao, X.; Ma, C.; Zhang, Z.; Zhao, N.; Ali, A.; Hou, T.; Xiang, Z.; Zhuang, J.; Wu, S.; et al. Potential Applications and Antifungal Activities of Engineered Nanomaterials against Gray Mold Disease Agent Botrytis cinerea on Rose Petals. Front. Plant Sci. 2017, 8, 1332. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and In Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Sushma; Kumar, S.; Dutta, P. Role of chitosan and chitosan-based nanoparticles in pesticide delivery: Avenues and applications. In Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences; Academic Press: Cambridge, MA, USA, 2022; pp. 401–434. [Google Scholar]
- Kumar, R.; Duhan, J.S.; Manuja, A.; Kaur, P.; Kumar, B.; Sadh, P.K. Toxicity assessment and control of early blight and stem rot of Solanum tuberosum L. by mancozeb-loaded chitosan–gum acacia nanocomposites. J. Xenobiot. 2022, 12, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yi, R.; Yang, H.; Xu, Q.; Huang, R.; Tang, J.; Li, X.; Liu, X.; Wu, L.; Yu, J.; et al. Antifungal effect of chitosan/nano-TiO2 composite coatings against Colletotrichum gloeosporioides, Cladosporium oxysporum and Penicillium steckii. Molecules 2021, 26, 4401. [Google Scholar] [CrossRef] [PubMed]
- Shende, S.; Gaikwad, N.; Bansod, S. Synthesis and evaluation of antimicrobial potential of copper nanoparticle against agri-culturally important phytopathogens. Synthesis 2016, 1, 41–47. [Google Scholar]
- Rajkuberan, C.; Rajiv, P.; Mostafa, M.; Abd-Elsalam, K.A. Multifunctional Copper-Based Nanocomposites in Agroecosystem Applications, in Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 595–613. [Google Scholar]
- Siddiqui, Z.A.; Parveen, A.; Ahmad, L.; Hashem, A. Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Sci. Hortic. 2019, 249, 374–382. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Khan, A.A.; Parveen, A.; Ansari, S.; Alam, M. Effect of Phyto-Assisted Synthesis of Magnesium Oxide Nanoparticles (MgO-NPs) on Bacteria and the Root-Knot Nematode. Bioinorg. Chem. Appl. 2022, 2022, 3973841. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Jayabaskaran, C.; Manikandan, A.; Anusuya, S. Synthesis of Nickel-Chitosan Nanoparticles for Controlling Blast Diseases in Asian Rice. Appl. Biochem. Biotechnol. 2022, 195, 2134–2148. [Google Scholar] [CrossRef]
- Mala, R.; Arunachalam, P.; Sivasankari, M. Synergistic bactericidal activity of silver nanoparticles and ciprofloxacin against phytopathogens. J. Cell Tissue Res. 2012, 12, 3249. [Google Scholar]
- Khan, M.R.; Siddiqui, Z.A.; Fang, X. Potential of metal and metal oxide nanoparticles in plant disease diagnostics and man-agement: Recent advances and challenges. Chemosphere 2022, 297, 134114. [Google Scholar] [CrossRef]
- Vizitiu, D.E.; Sardarescu, D.I.; Fierascu, I.; Fierascu, R.C.; Soare, L.C.; Ungureanu, C.; Buciumeanu, E.C.; Guta, I.C.; Pandelea, L.M. Grapevine Plants Management Using Natural Extracts and Phytosynthesized Silver Nanoparticles. Materials 2022, 15, 8188. [Google Scholar] [CrossRef]
- Wang, L.; Pan, T.; Gao, X.; An, J.; Ning, C.; Li, S.; Cai, K. Silica nanoparticles activate defense responses by reducing reactive oxygen species under Ralstonia solanacearum infection in tomato plants. NanoImpact 2022, 28, 100418. [Google Scholar] [CrossRef]
- Hamza, A.; Derbalah, A.; Mohamed, A. Recent Trends for Biocontrolling the Tomato Late Blight Disease under Field Condi-tions. Egypt. J. Biol. Pest Control. 2015, 25, 145–151. [Google Scholar]
- Li, Y.; Zhang, P.; Li, M.; Shakoor, N.; Adeel, M.; Zhou, P.; Guo, M.; Jiang, Y.; Zhao, W.; Lou, B.; et al. Application and mechanisms of metal-based nanoparticles in the control of bacterial and fungal crop diseases. Pest Manag. Sci. 2022, 79, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Nanohybrid Antifungals for Control of Plant Diseases: Current Status and Future Perspectives. J. Fungi 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- González-Merino, A.M.; Hernández-Juárez, A.; Betancourt-Galindo, R.; Ochoa-Fuentes, Y.M.; Valdez-Aguilar, L.A.; Limón-Corona, M.L. Antifungal activity of zinc oxide nanoparticles in Fusarium oxysporum-Solanum lycopersicum pathosystem under controlled conditions. J. Phytopathol. 2021, 169, 533–544. [Google Scholar] [CrossRef]
- Rouhani, M.; Samih, M.; Kalantari, S. Insecticidal effect of silica and silver nanoparticles on the cowpea seed beetle, Callo-sobruchus maculatus F. (Col.: Bruchidae). J. Entomol. Res. 2013, 4, 297–305. [Google Scholar]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for pro-grammed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; do Espirito Santo Pereira, A.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: New Delhi, India, 2013. [Google Scholar]
- Rouhani, M.; Samih, M.A.; Kalantari, S. Insecticide effect of silver and zinc nanoparticles against Aphis nerii Boyer De Fonscolombe (Hemiptera: Aphididae). Chil. J. Agric. Res. 2012, 72, 590. [Google Scholar] [CrossRef]
- Ulrichs, C.; Mewis, I.; Goswami, A. Crop diversification aiming nutritional security in West Bengal: Biotechnology of stinging capsules in nature’s water-blooms. Ann. Tech. Issue State Agric. Technol. Serv. Assoc. 2005, 8, 1–18. [Google Scholar]
- Kamel, A.; Abd-Elsalam, K.A.; Alghuthaymi, M.A. Nanobiofungicides: Is it the Next-Generation of Fungicides? J. Nanotechnol. Mater. Sci. 2015, 2, 38–40. [Google Scholar]
- Pimentel, D. Pesticides and Pest Control, in Integrated Pest Management: Innovation-Development Process; Springer: Berlin/Heidelberg, Germany, 2009; pp. 83–87. [Google Scholar]
- Park, H.-J.; Kim, S.-H.; Kim, H.-J.; Choi, S.-H. A New Composition of Nanosized Silica-Silver for Control of Various Plant Diseases. Plant Pathol. J. 2006, 22, 295–302. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, R.; Shit, S.; Gupata, S. Nanotechnological application on diagnosis of a plant disease. Int. Conf. Adv. Biol. Med. Sci. 2012. [Google Scholar]
- Paul, A.; Roychoudhury, A. Go green to protect plants: Repurposing the antimicrobial activity of biosynthesized silver nano-particles to combat phytopathogens. Nanotechnol. Environ. Eng. 2021, 6, 10. [Google Scholar] [CrossRef]
- Agrawal, S.; Rathore, P. Nanotechnology pros and cons to agriculture: A review. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 43–55. [Google Scholar]
- Schnoor, B.; Elhendawy, A.; Joseph, S.; Putman, M.; Chacón-Cerdas, R.; Flores-Mora, D.; Bravo-Moraga, F.; Bravo-Moraga, F.; Salvador-Morales, C. Engineering atrazine loaded poly (lactic-co-glycolic acid) nanoparticles to ameliorate environmental chal-lenges. J. Agric. Food Chem. 2018, 66, 7889–7898. [Google Scholar] [CrossRef]
- Sookhtanlou, M.; Allahyari, M.S.; Surujlal, J. Health risk of potato farmers exposed to overuse of chemical pesticides in Iran. Saf. Health Work 2022, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.F.M.; Gomes, D.G.; Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F.; Stolf-Moreira, R.; Oliveira, H.C. Post-Emergence Herbicidal Activity of Nanoatrazine against Susceptible Weeds. Front. Environ. Sci. 2018, 6, 12. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.; Fraceto, L.F.; De Lima, R. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N. Biswas, Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Ananda, S.; Shobha, G.; Shashidhara, K.S.; Mahadimane, V. Nano-cuprous oxide enhances seed germination and seedling growth in Lycopersicum esculentum plants. J. Drug Deliv. Ther. 2019, 9, 296–302. [Google Scholar]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Yasmeen, F.; Raja, N.I.; Razzaq, A.; Komatsu, S. Gel-free/label-free proteomic analysis of wheat shoot in stress tolerant varieties under iron nanoparticles exposure. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1586–1598. [Google Scholar] [CrossRef]
- Shang, H.; Ma, C.; Li, C.; Zhao, J.; Elmer, W.; White, J.C.; Xing, B. Copper oxide nanoparticle-embedded hydrogels enhance nutrient supply and growth of lettuce (Lactuca sativa) infected with Fusarium oxysporum f. sp. lactucae. Environ. Sci. Technol. 2021, 55, 13432–13442. [Google Scholar] [CrossRef]
- Kottegoda, N.; Madusanka, N.; Sandaruwan, C. Two new plant nutrient nanocomposites based on urea coated hydroxyapatite: Efficacy and plant uptake. Indian J. Agric. Sci. 2016, 86, 494–499. [Google Scholar]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2020, 269, 116134. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Ardebili, Z.O.; Ebadi, M. Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE 2020, 15, e0244207. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.C.H.T.; Carvalho, L.B.; Pereira, A.E.S.; Montanha, G.S.; Corrêa, C.G.; Carvalho, H.W.P.; Ganin, A.Y.; Fraceto, L.F.; Yiu, H.H.P. Localization of coated iron oxide (Fe3O4) nanoparticles on tomato seeds and their effects on growth. ACS Appl. Bio Mater. 2020, 3, 4109–4117. [Google Scholar] [CrossRef]
- Namjoyan, S.; Sorooshzadeh, A.; Rajabi, A.; Aghaalikhani, M. Nano-silicon protects sugar beet plants against water deficit stress by improving the antioxidant systems and compatible solutes. Acta Physiol. Plant. 2020, 42, 157. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Kavitha, K.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotechnol. 2014, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yuan, W.; Xu, C.; Li, F.; Cao, L.; Huang, Q. Enhancement of Spirotetramat Transfer in Cucumber Plant Using Mesoporous Silica Nanoparticles as Carriers. J. Agric. Food Chem. 2018, 66, 11592–11600. [Google Scholar] [CrossRef] [PubMed]
- Bapat, G.; Zinjarde, S.; Tamhane, V. Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Colloids Surf. B Biointerfaces 2020, 193, 111079. [Google Scholar] [CrossRef]
- Tabatabaee, S.; Iranbakhsh, A.; Shamili, M.; Oraghi Ardebili, Z. Copper nanoparticles mediated physiological changes and tran-scriptional variations in microRNA159 (miR159) and mevalonate kinase (MVK) in pepper; potential benefits and phytotoxicity assessment. J. Environ. Chem. Eng. 2021, 9, 106151. [Google Scholar] [CrossRef]
- Namburi, K.R.; Kora, A.J.; Chetukuri, A.; Kota, V.S.M.K. Biogenic silver nanoparticles as an antibacterial agent against bacterial leaf blight causing rice phytopathogen Xanthomonas oryzae pv. oryzae. Bioprocess Biosyst. Eng. 2021, 44, 1975–1988. [Google Scholar] [CrossRef]
- Chen, J.-N.; Wu, L.-T.; Kun, S.; Zhu, Y.-S.; Wei, D. Nonphytotoxic copper oxide nanoparticles are powerful “nanoweapons” that trigger resistance in tobacco against the soil-borne fungal pathogen Phytophthora nicotianae. J. Integr. Agric. 2022, 21, 3245–3262. [Google Scholar] [CrossRef]
- Shabbir, A.; Khan, M.; Ahmad, B.; Sadiq, Y.; Jaleel, H.; Uddin, M. Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash. Photosynth. 2019, 57, 599–606. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Ke, W.-J.; Hsieh, C.-T.; Lin, K.-S.; Tzou, D.-Y.; Chiang, C.-L. ZnO nanoparticles affect Bacillus subtilis cell growth and biofilm formation. PLoS ONE 2015, 10, e0128457. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, D.; Luo, Y.; He, D.; Zhang, H.; El-Naggar, A.; Palansooriya, K.N.; Chen, K.; Yan, Y.; Lu, X. Nanoplastics induce molecular toxicity in earthworm: Integrated multi-omics, morphological, and intestinal microorganism analyses. J. Hazard. Mater. 2023, 442, 130034. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. A 2009, 1216, 503–509. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1513. [Google Scholar] [CrossRef] [PubMed]
- Axelos, M.A.; Van de Voorde, M. Nanotechnology in Agriculture and Food Science; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.-A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; Von Mandach, U. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Akin, H.; Yeo, S.K.; Wirz, C.D.; Scheufele, D.A.; Brossard, D.; Xenos, M.A.; Corley, E.A. Are attitudes toward labeling nano products linked to attitudes toward GMO? Exploring a potential ‘spillover’effect for attitudes toward controversial technologies. J. Responsible Innov. 2019, 6, 50–74. [Google Scholar] [CrossRef]
- Pandey, G. Challenges and future prospects of agri-nanotechnology for sustainable agriculture in India. Environ. Technol. Innov. 2018, 11, 299–307. [Google Scholar] [CrossRef]
- Mitter, N.; Hussey, K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat. Nanotechnol. 2019, 14, 508–510. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO nanoparticles: A threat to soil organisms, plants, and human health. Environ. Geochem. Health 2019, 42, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.D.; White, J.C. Nanotechnology in agriculture: Next steps for understanding engineered nanoparticle exposure and risk. Nanoimpact 2016, 1, 9–12. [Google Scholar] [CrossRef]
- Smykov, I.T. Neophobia: Socio-ethical problems of innovative technologies of the food industry. Food Syst. 2023, 5, 308–318. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Sharma, P. Gold nanoparticles-based point-of-care colorimetric diagnostic for plant diseases. In Biosensors in Agriculture: Recent Trends and Future Perspectives; Springer: Cham, Switzerland, 2021; pp. 191–204. [Google Scholar]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K. Nanodiagnostics for plant pathogens. Environ. Chem. Lett. 2017, 15, 7–13. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Rai, P.; Sharma, S.; Chakdar, H.; Kumar, S.; Pandiyan, K.; Srivastava, A.K. Nanotechnology for the detection and diagnosis of plant pathogens. In Nanoscience in Food and Agriculture 2; Springer: Cham, Switzerland, 2016; pp. 253–276. [Google Scholar]
- Li, L.; Wang, C.; Nie, Y.; Yao, B.; Hu, H. Nanofabrication enabled lab-on-a-chip technology for the manipulation and detection of bacteria. TrAC Trends Anal. Chem. 2020, 127, 115905. [Google Scholar] [CrossRef]
- Rippa, M.; Castagna, R.; Brandi, S.; Fusco, G.; Monini, M.; Chen, D.; Zhou, J.; Zyss, J.; Petti, L. Octupolar plasmonic nanosensor based on ordered arrays of triangular Au nanopillars for selective rotavirus detection. ACS Appl. Nano Mater. 2020, 3, 4837–4844. [Google Scholar] [CrossRef]
- Mauriz, E. Recent progress in plasmonic biosensing schemes for virus detection. Sensors 2020, 20, 4745. [Google Scholar] [CrossRef]
- Liefting, L.W.; Waite, D.W.; Thompson, J.R. Application of Oxford Nanopore Technology to Plant Virus Detection. Viruses 2021, 13, 1424. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Ramalingam, C. Applications of nanotechnology in agriculture and water quality management. Environ. Chem. Lett. 2017, 15, 591–605. [Google Scholar] [CrossRef]
- Shivashakarappa, K.; Reddy, V.; Tupakula, V.K.; Farnian, A.; Vuppula, A.; Gunnaiah, R. Nanotechnology for the detection of plant pathogens. Plant Nano Biology 2022, 2, 100018. [Google Scholar] [CrossRef]
- Ghormade, V.; Rahi, S.; Rawal, K. Nanosensors for the detection of plant and human fungal pathogens. In Progress in Mycology: Biology and Biotechnological Applications; Satyanarayana, T., Deshmukh, S.K., Deshpande, M.V., Eds.; Springer Nature: Singapore, 2021; pp. 263–288. [Google Scholar]
- Khiyami, M.A.; Almoammar, H.; Awad, Y.M.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Plant pathogen nanodiagnostic techniques: Forthcoming changes? Biotechnol. Biotechnol. Equip. 2014, 28, 775–785. [Google Scholar] [CrossRef] [PubMed]
- John, S.A.; Chattree, A.; Ramteke, P.W.; Shanthy, P.; Nguyen, T.A.; Rajendran, S. Nanosensors for plant health monitoring. In Nanosensors for Smart Agriculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 449–461. [Google Scholar]
- Kumar, V.; Arora, K. Trends in nano-inspired biosensors for plants. Mater. Sci. Energy Technol. 2019, 3, 255–273. [Google Scholar] [CrossRef]
- Jang, H.; Kwak, C.H.; Kim, G.; Kim, S.M.; Huh, Y.S.; Jeon, T.-J. Identification of genetically modified DNA found in Roundup Ready soybean using gold nanoparticles. Microchim. Acta 2016, 183, 2649–2654. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Azinheiro, S.; Carvalho, J.; Abalde-Cela, S.; Carbo-Argibay, E.; Diéguez, L.; Piotrowski, M.; Kolen’Ko, Y.; Prado, M. Combination of Microfluidic Loop-Mediated Isothermal Amplification with Gold Nanoparticles for Rapid Detection of Salmonella spp. in Food Samples. Front. Microbiol. 2017, 8, 2159. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Tang, Y.; Wang, L. DNA Modified Fe3O4@Au Magnetic Nanoparticles as Selective Probes for Simultaneous Detection of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 3940–3947. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Hashim, A.F.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Nano-carbon: Plant growth promotion and protection. In Nanobiotechnology Applications in Plant Protection; Springer: Cham, Switzerland, 2018; pp. 155–188. [Google Scholar]
- Han, S.; Kim, W.; Lee, H.J.; Joyce, R.; Lee, J. Continuous and Real-Time Measurement of Plant Water Potential Using an AAO-Based Capacitive Humidity Sensor for Irrigation Control. ACS Appl. Electron. Mater. 2022, 4, 5922–5932. [Google Scholar] [CrossRef]
- Mukhopadhyay, S. Nanotechnology in agriculture: Prospects and constraints. Nanotechnol. Sci. Appl. 2014, 7, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.; Brooke, T.; Beckwith, R. Sensor and actuator networks—Vineyard computing: Sensor networks in agricultural production. IEEE Pervasive Comput. 2004, 3, 38–45. [Google Scholar] [CrossRef]
- Antonacci, A.; Arduini, F.; Moscone, D.; Palleschi, G.; Scognamiglio, V. Nanostructured (Bio)sensors for smart agriculture. TrAC Trends Anal. Chem. 2018, 98, 95–103. [Google Scholar] [CrossRef]
- Compagnone, D.; McNeil, C.; Athey, D.; Di Ilio, C.; Guilbault, G. An amperometric NADH biosensor based on NADH oxidase from Thermus aquaticus. Enzym. Microb. Technol. 1995, 17, 472–476. [Google Scholar] [CrossRef]
- Hossain, M.; Ghosh, S.; Boontongkong, Y.; Thanachayanont, C.; Dutta, J. Growth of Zinc Oxide Nanowires and Nanobelts for Gas Sensing Applications. J. Metastable Nanocrystalline Mater. 2005, 23, 27–30. [Google Scholar] [CrossRef]
- Huang, H.; Lee, Y.C.; Tan, O.K.; Zhou, W.; Peng, N.; Zhang, Q. High sensitivity SnO2 single-nanorod sensors for the detection of H2 gas at low temperature. Nanotechnology 2009, 20, 115501. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Jung, N.; Lee, M.; Yun, M.; Jeon, S. Electronic Nose Based on Multipatterns of ZnO Nanorods on a Quartz Resonator with Remote Electrodes. ACS Nano 2013, 7, 6685–6690. [Google Scholar] [CrossRef]
- Wegner, L.H. Using the Multifunctional Xylem Probe for in situ Studies of Plant Water and Ion Relations Under Saline Conditions. Methods Mol. Biol. 2012, 913, 35–66. [Google Scholar] [PubMed]
- Bandyopadhyay, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Advanced analytical techniques for the measurement of na-nomaterials in food and agricultural samples: A review. Environ. Eng. Sci. 2013, 30, 118–125. [Google Scholar] [CrossRef]
- Cursino, L.; Li, Y.; Zaini, P.A.; De La Fuente, L.; Hoch, H.C.; Burr, T.J. Twitching motility and biofilm formation are associated with tonB1 in Xylella fastidiosa. FEMS Microbiol. Lett. 2009, 299, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yada, R. Nanotechnologies in agriculture: New tools for sustainable development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Ditta, A. How helpful is nanotechnology in agriculture? Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 033002. [Google Scholar] [CrossRef]
- Omanović-Mikličanina, E.; Maksimović, M. Nanosensors applications in agriculture and food industry. Bull Chem. Technol. Bosnia. Herzegovina 2016, 47, 59–70. [Google Scholar]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Duraisamy, P.; Govindarajan, M.; Buhroo, A.A.; Prasad, R. Nano-biofungicides: Emerging trend in insect pest control. In Advances and Applications Through Fungal Nanobiotechnology; Springer: Cham, Switzerland, 2016; pp. 307–319. [Google Scholar]
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.L.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 2022, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Baer, K.N.; Marcel, B.J. Glyphosate. In Encyclopedia of Toxicology; Wexler, P., Ed.; Elsevier: San Diego, CA, USA, 2015; pp. 767–769. [Google Scholar]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent developments in nanotechnology transforming the agricultural sector: A transition replete with opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Inter. 2018, 10, 39124–39133. [Google Scholar] [CrossRef]
- Fiol, D.F.; Terrile, M.C.; Frik, J.; Mesas, F.A.; Álvarez, V.A.; Casalongué, C.A. Nanotechnology in plants: Recent advances and challenges. J. Chem. Technol. Biotechnol. 2021, 96, 2095–2108. [Google Scholar] [CrossRef]
- Wang, F.; Jian, J.; Geng, X.; Gou, G.; Cui, W.; Cui, J.; Qiao, Y.; Fu, J.; Yang, Y.; Ren, T.-L. A miniaturized integrated SAW sensing system for relative humidity based on graphene oxide film. IEEE Sens. J. 2020, 20, 9733–9739. [Google Scholar] [CrossRef]
- Mahdizadeh, M.; Najafi, N. Application of nano-sensors in the determination of soil moisture and temperature. Land Manag. J. 2019, 6, 169–178. [Google Scholar]
- Azzuhri, S.; Amiri, I.; Zulkhairi, A.; Salim, M.; Razak, M.; Khyasudeen, M.; Ahmad, H.; Zakaria, R.; Yupapin, P. Application of graphene oxide based Microfiber-Knot resonator for relative humidity sensing. Results Phys. 2018, 9, 1572–1577. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Humplík, J.F.; Lazár, D.; Husičková, A.; Spíchal, L. Automated phenotyping of plant shoots using imaging methods for analysis of plant stress responses—A review. Plant Methods 2015, 11, 29. [Google Scholar] [CrossRef]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H. Estimating stomatal conductance with thermal imagery. Plant Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, N.; Brien, C.; Oakey, H.; Berger, B.; Saade, S.; Ho, Y.S.; Schmöckel, S.M.; Tester, M.; Negrão, S. Salinity tolerance loci revealed in rice using high-throughput non-invasive phenotyping. Nat. Commun. 2016, 7, 13342. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Meron, M.; Saranga, Y.; Tsipris, J. Estimation of leaf water potential by thermal imagery and spatial analysis. J. Exp. Bot. 2005, 56, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.; Sirault, X.; Furbank, R.; Jones, H. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010, 61, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Pai, P.; Shetty, M.G.; Babitha, K.S. Gold nanoparticle based biosensors for rapid pathogen detection: A review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100756. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Aversa, P.; Antolini, F.; Cusano, A.; Consales, M.; Giordano, M.; Nicolais, L. Carbon nanotubes-coated multi-transducing sensors for VOCs detection. Sens. Actuators B Chem. 2005, 111–112, 171–180. [Google Scholar] [CrossRef]
- Hafaiedh, I.; Elleuch, W.; Clement, P.; Llobet, E.; Abdelghani, A. Multi-walled carbon nanotubes for volatile organic compound detection. Sens. Actuators B Chem. 2013, 182, 344–350. [Google Scholar] [CrossRef]
- Mehta, L.; Srivastava, S.; Adam, H.N.; Bose, S.; Ghosh, U.; Kumar, V.V. Climate change and uncertainty from ‘above’and ‘below’: Perspectives from India. Reg. Environ. Chang. 2019, 19, 1533–1547. [Google Scholar]
- Jasra, R.; Bajaj, H.; Mody, H. Clay as a versatile material for catalysts and adsorbents. Bull. Catal. Soc. India 1999, 9, 113–121. [Google Scholar]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Verma, N.; Lugani, Y.; Kumar, S.; Asadnia, M. Conventional and Advanced Techniques of Wastewater Monitoring and Treatment. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–48. [Google Scholar]
- John, A.T.; Murugappan, K.; Nisbet, D.R.; Tricoli, A. An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring. Sensors 2021, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- Virutkar, P.D.; Mahajan, A.P.; Meshram, B.H.; Kondawar, S.B. Conductive polymer nanocomposite enzyme immobilized biosensor for pesticide detection. J. Mater. NanoScience 2019, 6, 7–12. [Google Scholar]
- Akdag, A.; Işık, M.; Göktaş, H. Conducting polymer-based electrochemical biosensor for the detection of acetylthiocholine and pesticide via acetylcholinesterase. Biotechnol. Appl. Biochem. 2021, 68, 1113–1119. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Wang, X.; Yang, Q.; Wang, M.; Liu, G.; Yao, L. An All-Solid-State Nitrate Ion-Selective Electrode with Nanohybrids Composite Films for In-Situ Soil Nutrient Monitoring. Sensors 2020, 20, 2270. [Google Scholar] [CrossRef]
- Huang, S.-F.; Shih, W.-L.; Chen, Y.-Y.; Wu, Y.-M.; Chen, L.-C. Ion composition profiling and pattern recognition of vegetable sap using a solid-contact ion-selective electrode array. Biosens. Bioelectron. X 2021, 9, 100088. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Hashim, A.; Al-Khafaji, Y.; Hadi, A. Synthesis and Characterization of Flexible Resistive Humidity Sensors Based on PVA/PEO/CuO Nanocomposites. Trans. Electr. Electron. Mater. 2019, 20, 530–536. [Google Scholar]
- Fang, Y.; Ramasamy, R.P. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [PubMed]
- Khasim, S.; Pasha, A.; Dastager, S.G.; Panneerselvam, C.; Hamdalla, T.A.; Al-Ghamdi, S.; Alfadhli, S.; Makandar, M.B.; Albalawi, J.B.; Darwish, A. Design and development of multi-functional graphitic carbon nitride heterostructures embedded with copper and iron oxide nanoparticles as versatile sensing platforms for environmental and agricultural applications. Ceram. Int. 2023, 49, 20688–20698. [Google Scholar]
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Soil Moisture and Nutrient Monitoring. IEEE Access 2021, 9, 14095–14121. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Javourez, U.; O’donohue, M.; Hamelin, L. Waste-to-nutrition: A review of current and emerging conversion pathways. Biotechnol. Adv. 2021, 53, 107857. [Google Scholar] [CrossRef]
- Rai, S.; Solanki, M.K.; Anal, A.K.D.; Sagar, A.; Solanki, A.C.; Kashyap, B.K.; Pandey, A.K. Emerging frontiers of microbes as agro-waste recycler. In Waste to Energy: Prospects and Applications; Springer: Cham, Switzerland, 2020; pp. 3–27. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Liuzzi, S.; Rubino, C.; Stefanizzi, P.; Martellotta, F. The Agro-Waste Production in Selected EUSAIR Regions and Its Potential Use for Building Applications: A Review. Sustainability 2022, 14, 670. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Bayoumi, Y.; Shalaby, T.A.; El-Mahrouk, M.E.; Taha, N.; Elbasiouny, H.; Elbehiry, F.; Amer, M.; Abdalla, N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-Waste Nexus. Sustainability 2022, 14, 15717. [Google Scholar]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formu-lations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Anal, A.K.; Sadiq, M.B.; Singh, M. Emerging trends in traceability techniques in food systems. In Food Traceability and Authenticity, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 66–89. [Google Scholar]
- Mosadegh Sedghy, B. Evolution of radio frequency identification (RFID) in agricultural cold chain monitoring: A literature review. J. Agric. Sci. 2018, 11, 43–58. [Google Scholar] [CrossRef]
- Kuzma, J. Nanotechnology in animal production—Upstream assessment of applications. Livest. Sci. 2010, 130, 14–24. [Google Scholar] [CrossRef]
- Caon, T.; Martelli, S.M.; Fakhouri, F.M. New Trends in the Food Industry: Application of Nanosensors in Food Packaging. In Nanobiosensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 773–804. [Google Scholar]
- Tongrod, N.; Tuantranont, A.; Kerdcharoen, T. Adoption of precision agriculture in vineyard. In Proceedings of the 2009 6th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Chonburi, Thailand, 6–9 May 2009; IEEE: Piscataway, NJ, USA, 2009. [Google Scholar]
- Popp, J.; Griffin, T. Adoption trends of early adopters of precision farming in Arkansas. In Proceedings of the 5th International Conference on Precision Agriculture, Bloomington, MI, USA, 16–19 July 2000. [Google Scholar]
- Pivoto, D.; Waquil, P.D.; Talamini, E.; Finocchio, C.P.; Dalla Corte, V.F.; de Vargas Mores, G. Scientific development of smart farming technologies and their application in Brazil. Inf. Process. Agric. 2018, 5, 21–32. [Google Scholar] [CrossRef]
- Li, J. Nanotechnology Based Cell-All Phone-Sensors for Extended Network Chemical Sensing. In Proceedings of the Electrochemical Society Meeting Abstracts 225, Orlando, FL, USA, 11–16 May 2014; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2014; p. 456. [Google Scholar]
- Pongnumkul, S.; Chaovalit, P.; Surasvadi, N. Applications of Smartphone-Based Sensors in Agriculture: A Systematic Review of Research. J. Sens. 2015, 2015, 195308. [Google Scholar] [CrossRef]
- Mu, T.; Wang, S.; Li, T.; Wang, B.; Ma, X.; Huang, B.; Zhu, L.; Guo, J. Detection of Pesticide Residues Using Nano-SERS Chip and a Smartphone-Based Raman Sensor. IEEE J. Sel. Top. Quantum Electron. 2018, 25, 5200206. [Google Scholar] [CrossRef]
- Maksimović, M.; Omanović-Mikličanin, E. Green internet of things and green nanotechnology role in realizing smart and sustainable agriculture. In Proceedings of the VIII International Scientific Agriculture Symposium “AGROSYM 2017”, Jahorina, Bosnia and Herzegovina, 5–8 October 2017. [Google Scholar]
- Hooley, G.; Piercy, N.F.; Nicoulaud, B.M. Marketing Strategy and Competitive Positioning; Prentice Hall: Kent, OH, USA, 2012. [Google Scholar]
- Feeney, R.; Harmath, P.; Ramoni-Perazzi, J.; Mac Clay, P. Relationship between brand and dealer loyalty in the agricultural equipment market. Int. Food Agribus Manag. Rev. 2022, 25, 347–360. [Google Scholar] [CrossRef]
- Acharya, A.; Pal, P.K. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact 2020, 19, 100232. [Google Scholar] [CrossRef]
- Lu, J.; Bowles, M. How will nanotechnology affect agricultural supply chains? Int. Food Agribus Manag. Rev. 2013, 16, 21–42. [Google Scholar]
- Neme, K.; Nafady, A.; Uddin, S.; Tola, Y.B. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: Food security implication and challenges. Heliyon 2021, 7, e08539. [Google Scholar] [CrossRef]
- Khan, Z.; Ansari, M. Impact of Engineered Si Nanoparticles on Seed Germination, Vigour Index and Genotoxicity Assessment via DNA Damage of Root Tip Cells in Lens culinaris. J. Plant Biochem. Physiol. 2018, 6, 2. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K. Analysis, Fate, and Toxicity of Engineered Nanomaterials in Plants; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Lu, S.; Duffin, R.; Poland, C.; Daly, P.; Murphy, F.; Drost, E.; MacNee, W.; Stone, V.; Donaldson, K. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environ. Health Perspect. 2009, 117, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre. Toxicol. 2013, 10, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Bansalb, K.; Raib, R. Capturing thematic intervention of nanotechnology in agriculture sector: A scientometric approach. In Analysis, Fate, and Toxicity of Engineered Nanomaterials in Plants; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, p. 313. [Google Scholar]
- Santos, J.; Silva Calpa, L.d.R.; Gomes de Souza, F., Jr. Main Countries Contributing on Nanotechnology. Available online: https://www.qeios.com/read/4MZGZR (accessed on 1 January 2020).
- Bowman, D.M.; Hodge, G.A. ‘Governing’nanotechnology without government? Sci. Public Policy 2008, 35, 475–487. [Google Scholar] [CrossRef]

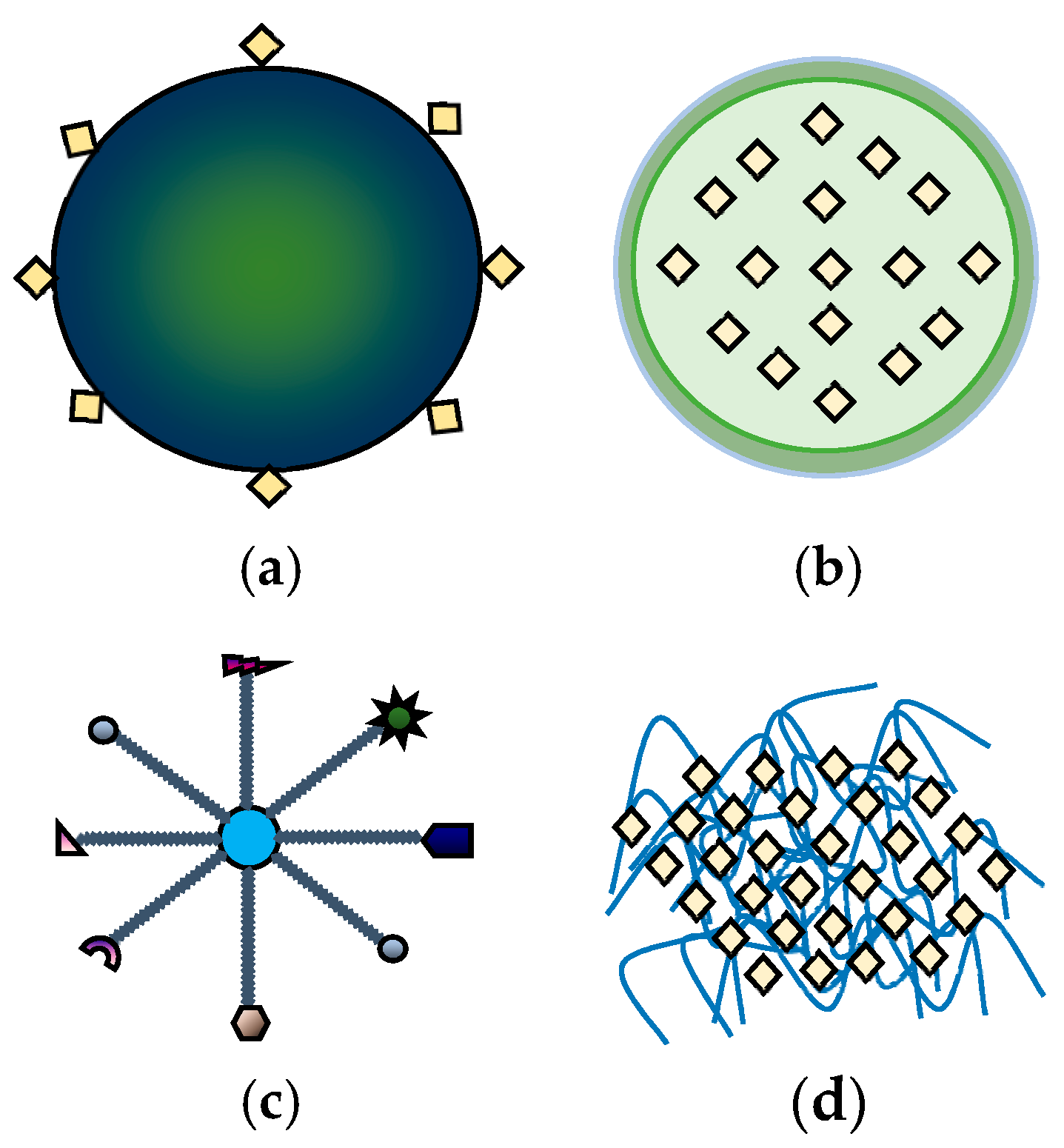


| Nanoparticle | Plant | Germination % Improvement | Reference |
|---|---|---|---|
| Chitosan and zinc oxide | rice | 20.00 | [46] |
| Ferric oxide | wheat | 41.60 | [47] |
| Nano phosphorus | mung, black gram and cowpea | 20.83, 38.1 and 20.83 | [48] |
| Silicon dioxide | wheat | 16.78 | [49] |
| Silicon dioxide | soybean, maize, wheat and lupine | 11.14, 4.65, 9.61 and 2.31 | [50] |
| Silver | wheat | 20.0 | [51] |
| Silver | fenugreek | 5.30 | [52] |
| Titanium dioxide | radish | 20.00 | [53] |
| Titanium dioxide | wheat | 16.30 | [54] |
| Titanium dioxide | perfumed cherry | 65.00 | [55] |
| Zinc oxide | cowpea | 3.18 | [56] |
| Zinc oxide | canola | 7.23 | [57] |
| Zinc oxide | wheat | 13.80 | [58] |
| Stress Type | Stressor (Biotic/Abiotic) | Nanoparticle | Plant | Effect on Plant | Reference |
|---|---|---|---|---|---|
| Abiotic | salinity | titanium dioxide | broad bean | protects photosynthetic machinery, enhances salinity tolerance | [72] |
| drought | silica | wheat | improves water retention and nutrient uptake | [73] | |
| salinity | zinc oxide | rice | enhances salt tolerance by maintaining ion balance | [74] | |
| heavy metal contamination | iron | wheat | chelates heavy metals, reducing toxicity | [75] | |
| UV radiation | cerium oxide | arabidopsis | protects chlorophyll from UV degradation | [76] | |
| cold stress | graphene oxide | pearl millet | protects the cellular structure, enhances cold tolerance | [77] | |
| nitrogen deficiency | carbon nanotubes | birdsfoot trefoil | facilitates nitrogen fixation | [78] | |
| phosphorus deficiency | hydroxyapatite | wheat | enhances phosphorus availability | [79] | |
| oxygen deficiency | silver | muscadine | combat hypoxia by boosting antioxidant activity | [80] | |
| Biotic | viral infections | gold | barley | antiviral properties reduce disease incidence | [81] |
| fungal infections | silver | barley, peas, oilseed rape, radish, cucumber, lettuce | antifungal properties reduce infection rates | [82] | |
| bacterial infections | copper | tea plant | antibacterial properties reduce disease occurrence | [83] | |
| pest infestation | chitosan | turmeric plant | insecticidal properties decrease pest damage | [84] | |
| herbivory | silica | soybean | reduces plant palatability to herbivores | [85] |
| Nanoparticle | In Vivo/In Vitro | Phytopathogen | Reference |
|---|---|---|---|
| Carbon nanotubes | In vivo | Gray mold disease agent Notrytis cinerea on rose petals | [116] |
| Chitosan | In vivo | Fusarium. oxysporum, P. capsici, Erwinia carotovora subsp. carotovora and f Xanthomonas campestris pv. vesicatoria on tomato plants | [117] |
| Chitosan and chitosan-based | In vivo | Pseudomonas syringae, Alternaria solani and F. oxysporum | [118] |
| Chitosan–Gum Acacia Nanocomposites | In vivo | F. oxysporum f. sp. lycopersici in potato plants | [119] |
| Chitosan/Nano-TiO2 Composite Coatings | In vitro | Colletotrichum gloeosporioides, Cladosporium oxysporum and Penicillium steckii | [120] |
| Copper oxide | In vivo | A. carthami, Aspergillus niger, F. oxysporum f.sp udum, Xanthomonas axonopodis pv. punicae | [121] |
| Copper oxide-graphene oxide nanocomposites | In vitro | F. graminearum and Rhizoctonia solani | [122] |
| Graphene oxide and zinc oxide | In vitro and In vivo | Pectobacterium carotovorum, Xanthomonas campestris pv. carotae, Meloidogyne javanica, A. dauci and F. solani on carrot | [123] |
| Iron oxide NPs | In vitro | P. expansum, A. niger, A. alternata, M. plumbeus, P. chrysogenum, T. roseum, and R. solani | [117] |
| Magnesium oxide | In vitro | Root-knot nematode (Meloidogyne incognita) and Ralstonia solanacearum | [124] |
| Magnesium oxide | In vitro | P. expansum, A. niger, A. alternata, M. plumbeus, P. chrysogenum, T. roseum, and R. solani | [117] |
| Magnesium oxide NPs-chitosan nanocomposites | In vivo | Fusarium wilt disease in tomato plants | [29] |
| Nickel-Chitosan | In vivo | Blast diseases in Asian rice (Pyricularia oryzae) | [125] |
| Silver | In vitro | X. campestris, Pseudomonas syringae, and F. oxysporum | [126] |
| Silicon dioxide, zinc oxide and titanium dioxide | In vivo | Fusarium wilt on Meloidogyne incognita | [127] |
| Silicon dioxide | In vivo | Powdery mildew in grapevine | [128] |
| Silica | In vivo | Control of bacterial wilt disease (Ralstonia solanacearum) in tomato plants | [129] |
| Titanium dioxide | In vivo | Tomato late blight | [130] |
| Zinc oxide | In vivo | Rice blast disease (Magnaporthe oryzae) in rice | [131] |
| Zinc oxide-chitosan nanocomposites | In vitro | Rhizoctonia solani and Sclerotinia sclerotiorum | [132] |
| Zinc oxide | In vivo | F. oxysporum on tomato plants | [133] |
| Effect on Plant | Nanoparticle | Plant | Reference |
|---|---|---|---|
| Growth enhancement | zinc oxide | tomato | [150] |
| Improved seed germination through soil water retention | copper oxide | tomato | [151] |
| silver | fenugreek | [51] | |
| silver | rice | [152] | |
| silicon dioxide | tomato | [91] | |
| hydrogels | wheat | [153] | |
| Improved micronutrient supply through slow release | copper oxide nanoparticle-embedded hydrogels | lettuce | [154] |
| nanocomposites of urea-coated hydroxyapatite and potassium encapsulated in nanoclay | tall fescue | [155] | |
| silicon dioxide | rice | [156] | |
| selenate and selenium | tomato | [157] | |
| iron oxide | tomato | [158] | |
| Abiotic and biotic stress alleviation | silicon dioxide | sugar beet and maize | [159,160] |
| Lowering the dosage of pesticides | silicon dioxide | cucumber | [161] |
| silicon dioxide | tomato | [162] | |
| copper oxide | pepper | [163] | |
| Reduces pests | silver | rice | [164] |
| copper oxide | tobacco | [165] | |
| Photosynthesis enhancement | titanium dioxide | khus | [166] |
| Disadvantage | Description | Reference |
|---|---|---|
| Ecological risks | Accumulate in soil, water, and air, disturbing soil microbes and lowering soil fertility and health. Accumulate in plants and animals, posing health risks. | [177] |
| Human health risks | Exposure can lead to health issues, especially for workers producing and applying nanomaterials. | [171] |
| High costs | Costly and could lead to an imbalance in the distribution of benefits, as small-scale farmers may not be able to afford it. | [178] |
| Ethical concerns | Raises concerns about food safety and security, with limited research on the long-term effects of consuming NPs and ethical concerns about GMOs. | [179] |
| Lack of regulation | Limited regulation and oversight raise concerns about potential risks and the need for robust regulations to protect human health and the environment. | [176] |
| Nanodiagnostic System | Application in Agriculture | Reference |
|---|---|---|
| Nanosensors | Soil nutrient monitoring, plant disease detection, pest detection | [191] |
| Quantum dots | Detection of plant viruses, monitoring of transgenic plants | [192] |
| Gold NPs | Identification of GM crops, pathogen detection | [193,194] |
| Magnetic NPs | Detection of heavy metals in soil, water monitoring | [195] |
| Nanobarcodes | Tracking and identification of plant species, traceability of agri-food products | [187] |
| Carbon nanotubes | Monitoring of plant growth, detection of pesticides | [196] |
| Nanofluidic devices | Control of irrigation, soil water content measurement | [197] |
| Type of Biosensors | Function | Material Type | Reference |
|---|---|---|---|
| Environmental biosensors, chemiresistor sensors | monitoring of soil quality parameters | polymers, metal oxides | [234] |
| Pesticide biosensors, electrochemical biosensors | monitoring soil pesticides/herbicides | enzymes, conducting polymers | [235,236] |
| Nutrient biosensors, potentiometric biosensors | monitoring soil nutrients | ion-selective electrodes, polymers | [237,238] |
| Moisture sensors, capacitive humidity sensors | monitoring soil humidity | ceramics, polymers | [239,240] |
| Plant disease biosensors, fluorescence-based biosensors | monitoring plant disease and stress | quantum dots, fluorescent proteins | [194,241] |
| Irrigation biosensors, soil moisture sensors | monitoring irrigation | ceramics, metal oxides | [242,243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects. Agrochemicals 2023, 2, 220-256. https://doi.org/10.3390/agrochemicals2020016
Yadav A, Yadav K, Ahmad R, Abd-Elsalam KA. Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects. Agrochemicals. 2023; 2(2):220-256. https://doi.org/10.3390/agrochemicals2020016
Chicago/Turabian StyleYadav, Anurag, Kusum Yadav, Rumana Ahmad, and Kamel A. Abd-Elsalam. 2023. "Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects" Agrochemicals 2, no. 2: 220-256. https://doi.org/10.3390/agrochemicals2020016
APA StyleYadav, A., Yadav, K., Ahmad, R., & Abd-Elsalam, K. A. (2023). Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects. Agrochemicals, 2(2), 220-256. https://doi.org/10.3390/agrochemicals2020016







