Neonicotinoid Insecticide-Degrading Bacteria and Their Application Potential in Contaminated Agricultural Soil Remediation
Abstract
:1. Introduction
2. Current Status and Risks of Neonicotinoid Insecticide Pollution in Agricultural Soils
3. Neonicotinoid Insecticide–Degrading Bacteria
4. Neonicotinoid Insecticide-Degrading Bacterial Consortia
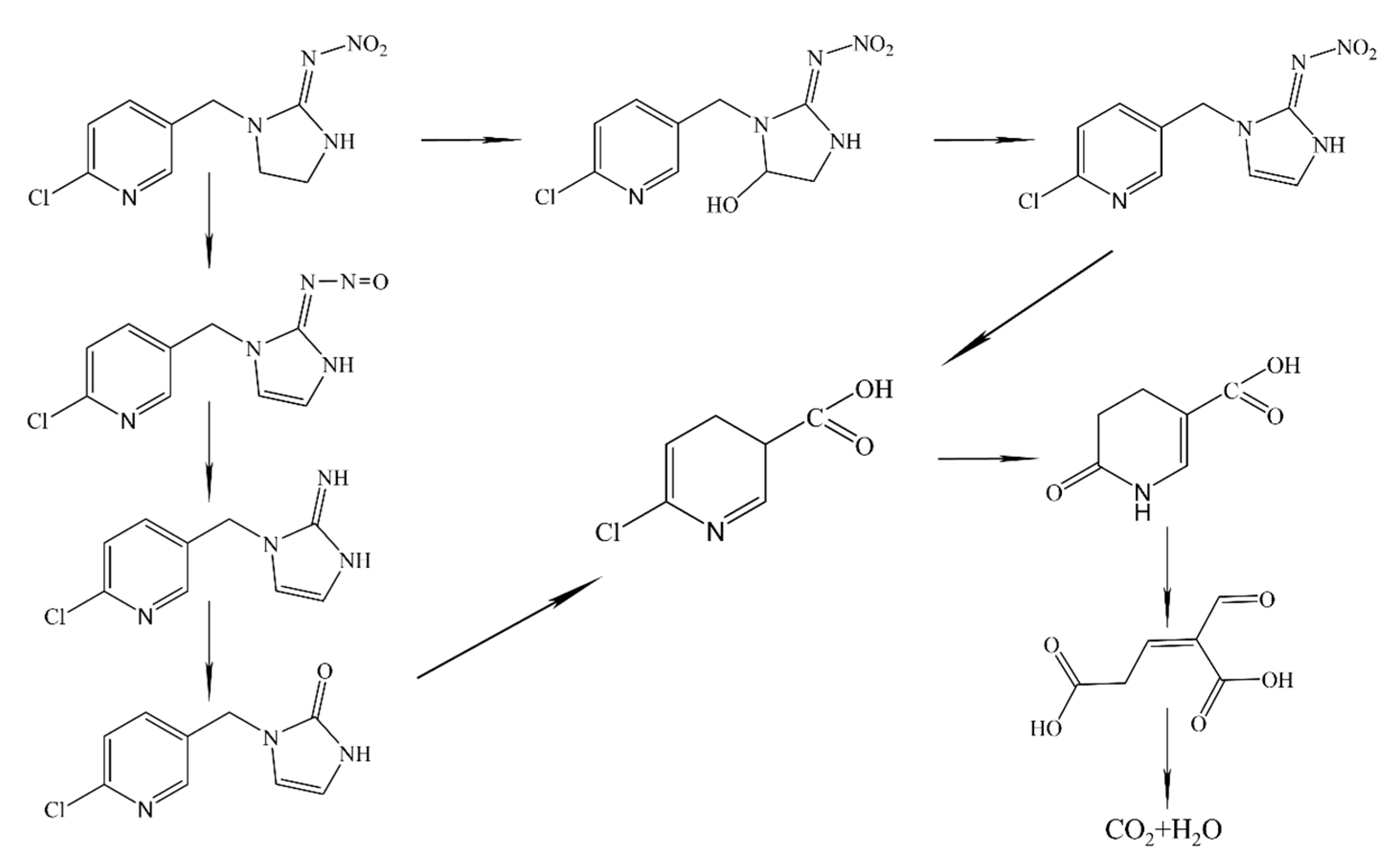
5. Neonicotinoid Insecticide Degradation Pathway
6. Application of NNI-Degrading Bacteria in the Remediation of Polluted Soil
7. Summary and Outlook
- (1)
- At present, most research studies on bacterial degradation of NNIs have been conducted in the laboratory setting, rather than on the remediation of real polluted agricultural soil. Since the environment in agricultural soil is very complex, the use of NNI-degrading bacteria for the remediation of real farm soil needs more testing.
- (2)
- Composite consortia are a research hotspot for the degradation of NNIs using bacteria. In the future, modern molecular biology methods, such as high-flow sequencing, stable isotope tracing, macro-genomics, and macro-transcriptomics, can be used to clarify the mechanisms underlying the synergistic interaction between different strains of bacteria in bacterial groups and to filter such strains for the construction of bacterial consortia with targeted and efficient NNI degradation for application in actual agricultural soil remediation.
- (3)
- In the future, NNI-degrading functional bacteria or consortia can be prepared as immobilized bacterial agents to improve the survival rate of degrading bacteria under in situ conditions and harsh climatic conditions, promote the biodegradation of NNIs, and achieve efficient and safe remediation of NNI-contaminated soil.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Marchand, P.A. Regulatory evolution of neonicotinoid insecticides as plant protection active substances in Europe. Agrochemicals 2023, 2, 446–457. [Google Scholar] [CrossRef]
- Luo, D.; Xia, F.; He, M.; Wu, S.; Zhao, X.; Liao, X. Sublethal effects of the cis-nitromethylene neonicotinoid insecticide cycloxaprid on the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Crop Prot. 2023, 166, 106172. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Brüggmann, D.; Groneberg, D.A. Neonicotinoids: A critical assessment of the global research landscape of the most extensively used insecticide. Environ. Res. 2022, 213, 113727. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Ma, S.Z. Current development and utilization of neonicotinoid insecticides. South. Hortic. 2023, 34, 69–73. (In Chinese) [Google Scholar]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Zhang, Z.; An, L.; Hough, R.; Hu, P.; Cui, S. A review of spatiotemporal patterns of neonicotinoid insecticides in water, sediment, and soil across China. Environ. Sci. Pollut. Res. 2022, 29, 55336–55347. [Google Scholar] [CrossRef]
- Addy-Orduna, L.M.; Brodeur, J.C.; Mateo, R. Oral acute toxicity of imidacloprid, thiamethoxam and clothianidin in eared doves: A contribution for the risk assessment of neonicotinoids in birds. Sci. Total Environ. 2019, 650, 1216–1223. [Google Scholar] [CrossRef]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Cui, X.; Wang, H.; Liu, Z.; Yang, Y.; Xu, B. Review on effects of some insecticides on honey bee health. Pestic. Biochem. Physiol. 2022, 188, 105219. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of selected neonicotinoid insecticides in soil–water systems: Current state of the art and knowledge gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef]
- Anjos, C.S.; Lima, R.N.; Porto, A.L. An overview of neonicotinoids: Biotransformation and biodegradation by microbiological processes. Environ. Sci. Pollut. Res. 2021, 28, 37082–37109. [Google Scholar] [CrossRef]
- Gautam, P.; Dubey, S.K. Biodegradation of Neonicotinoids: Current Trends and Future Prospects. Curr. Pollut. Rep. 2023, 9, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Bi, G.; Ward, T.J.; Li, L. Adsorption and degradation of neonicotinoid insecticides in agricultural soils. Environ. Sci. Pollut. Res. 2023, 30, 47516–47526. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.F.; Wang, S.; Liu, L.Y.; Zeng, E.Y. The human and ecological risks of neonicotinoid insecticides in soils of an agricultural zone within the Pearl River Delta, South China. Environ. Pollut. 2021, 284, 117358. [Google Scholar] [CrossRef]
- Limay-Rios, V.; Forero, L.G.; Xue, Y.; Smith, J.; Baute, T.; Schaafsma, A. Neonicotinoid insecticide residues in soil dust and associated parent soil in fields with a history of seed treatment use on crops in southwestern Ontario. Environ. Toxicol. Chem. 2016, 35, 303–310. [Google Scholar] [CrossRef]
- Xu, T.; Dyer, D.G.; McConnell, L.L.; Bondarenko, S.; Allen, R.; Heinemann, O. Clothianidin in agricultural soils and uptake into corn pollen and canola nectar after multiyear seed treatment applications. Environ. Toxicol. Chem. 2016, 35, 311–321. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; Field, R.W. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environmental Science: Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Chang, C.H.; Lou, J.L.; Zhao, M.R.; Lu, C. Potential human exposures to neonicotinoid insecticides: A review. Environ. Pollut. 2018, 236, 71–81. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. Human exposure to neonicotinoids and the associated health risks: A review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef]
- Stewart, S.D.; Lorenz, G.M.; Catchot, A.L.; Gore, J.; Cook, D.; Skinner, J.; Barber, J. Potential exposure of pollinators to neonicotinoid insecticides from the use of insecticide seed treatments in the mid-southern United States. Environ. Sci. Technol. 2014, 48, 9762–9769. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef]
- Heimbach, F.; Russ, A.; Schimmer, M.; Born, K. Large-scale monitoring of effects of clothianidin dressed oilseed rape seeds on pollinating insects in Northern Germany: Implementation of the monitoring project and its representativeness. Ecotoxicology 2016, 25, 1630–1647. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Chang, C.H.; Yu, C.; Wang, X.; Lu, C. Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Lu, C.; Chang, C.H.; Palmer, C.; Zhao, M.; Zhang, Q. Neonicotinoid residues in fruits and vegetables: An integrated dietary exposure assessment approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Tapparo, A. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Shang, N.; Xiao, Y.; Xiao, Y.; Liu, Y.; Xia, X. Identification and coexposure of Neonicotinoid insecticides and their transformation products in Retail Cowpea (Vigna unguiculata). Environ. Sci. Technol. 2023, 57, 20182–20193. [Google Scholar] [CrossRef]
- Ueyama, J.; Nomura, H.; Kondo, T.; Saito, I.; Ito, Y.; Osaka, A.; Kamijima, M. Biological monitoring method for urinary neonicotinoid insecticides using LC-MS/MS and its application to Japanese adults. J. Occup. Health 2014, 56, 461–468. [Google Scholar] [CrossRef]
- Marfo, J.T.; Fujioka, K.; Ikenaka, Y.; Nakayama, S.M.; Mizukawa, H.; Aoyama, Y.; Taira, K. Relationship between urinary N-desmethyl-acetamiprid and typical symptoms including neurological findings: A prevalence case-control study. PLoS ONE 2015, 10, e0142172. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, X.; Zhang, T.; Song, S.; Zhu, H.; Lu, S.; Sun, H. Neonicotinoid insecticides and their metabolites can pass through the human placenta unimpeded. Environ. Sci. Technol. 2022, 56, 17143–17152. [Google Scholar] [CrossRef]
- Shao, X.; Liu, Z.; Xu, X.; Li, Z.; Qian, X. Overall status of neonicotinoid insecticides in China: Production, application and innovation. J. Pestic. Sci. 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Tu, C.; Long, T.; Bu, Y.; Wang, H.; Deng, S. Remediation Technologies for Neonicotinoids in Contaminated Environments: Current State and Future Prospects. Environ. Int. 2023, 178, 108044. [Google Scholar] [CrossRef]
- Elango, D.; Siddharthan, N.; Alaqeel, S.I.; Subash, V.; Manikandan, V.; Almansour, A.I.; Jayanthi, P. Biodegradation of neonicotinoid insecticide acetamiprid by earthworm gut bacteria Brucella intermedium PDB13 and its ecotoxicity. Microbiol. Res. 2023, 268, 127278. [Google Scholar] [CrossRef]
- Zhou, G.C.; Wang, Y.; Zhai, S.; Ge, F.; Liu, Z.H.; Dai, Y.J.; Hou, J.Y. Biodegradation of the neonicotinoid insecticide thiamethoxam by the nitrogen-fixing and plant-growth-promoting rhizobacterium Ensifer adhaerens strain TMX-23. Appl. Microbiol. Biotechnol. 2013, 97, 4065–4074. [Google Scholar] [CrossRef]
- Phugare, S.S.; Kalyani, D.C.; Gaikwad, Y.B.; Jadhav, J.P. Microbial degradation of imidacloprid and toxicological analysis of its biodegradation metabolites in silkworm (Bombyx mori). Chem. Eng. J. 2013, 230, 27–35. [Google Scholar] [CrossRef]
- Gupta, M.; Mathur, S.; Sharma, T.K.; Rana, M.; Gairola, A.; Navani, N.K.; Pathania, R. A study on metabolic prowess of Pseudomonas sp. RPT 52 to degrade imidacloprid, endosulfan and coragen. J. Hazard. Mater. 2016, 301, 250–258. [Google Scholar] [CrossRef]
- Ma, Y.; Zhai, S.; Mao, S.Y.; Sun, S.L.; Wang, Y.; Liu, Z.H.; Yuan, S. Co-metabolic transformation of the neonicotinoid insecticide imidacloprid by the new soil isolate Pseudoxanthomonas indica CGMCC 6648. J. Environ. Sci. Health Part B 2014, 49, 661–670. [Google Scholar] [CrossRef]
- Akoijam, R.; Singh, B. Biodegradation of imidacloprid in sandy loam soil by Bacillus aerophilus. Int. J. Environ. Anal. Chem. 2015, 95, 730–743. [Google Scholar] [CrossRef]
- Pandey, G.; Dorrian, S.J.; Russell, R.J.; Oakeshott, J.G. Biotransformation of the neonicotinoid insecticides imidacloprid and thiamethoxam by Pseudomonas sp. 1G. Biochem. Biophys. Res. Commun. 2009, 380, 710–714. [Google Scholar] [CrossRef]
- Sabourmoghaddam, N.; Zakaria, M.P.; Omar, D. Evidence for the microbial degradation of imidacloprid in soils of Cameron Highlands. J. Saudi Soc. Agric. Sci. 2015, 14, 182–188. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Gupta, V.K. Assessment of imidacloprid degradation by soil-isolated Bacillus alkalinitrilicus. Environ. Monit. Assess. 2014, 186, 7183–7193. [Google Scholar] [CrossRef]
- Kandil, M.M.; Trigo, C.; Koskinen, W.C.; Sadowsky, M.J. Isolation and characterization of a novel imidacloprid-degrading Mycobacterium sp. strain MK6 from an Egyptian soil. J. Agric. Food Chem. 2015, 63, 4721–4727. [Google Scholar] [CrossRef]
- Tiwari, S.; Tripathi, P.; Mohan, D.; Singh, R.S. Imidacloprid biodegradation using novel bacteria Tepidibacillus decaturensis strain ST1 in batch and in situ microcosm study. Environ. Sci. Pollut. Res. 2023, 30, 61562–61572. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, Y.; Liu, B.; Song, F.; You, M. Isolation of an indigenous imidacloprid-degrading bacterium and imidacloprid bioremediation under simulated in situ and ex situ conditions. J. Microbiol. Biotechnol. 2013, 23, 1617–1626. [Google Scholar] [CrossRef]
- Shi, Z.; Dong, W.; Xion, F.; Liu, J.; Zhou, X.; Xu, F.; Jiang, M. Characteristics and metabolic pathway of acetamiprid biodegradation by Fusarium sp. strain CS-3 isolated from soil. Biodegradation 2018, 29, 593–603. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhang, L.J.; Sun, S.L.; Ge, F.; Mao, S.Y.; Ma, Y.; Yuan, S. Degradation of the neonicotinoid insecticide acetamiprid via the N-carbamoylimine derivate (IM-1-2) mediated by the nitrile hydratase of the nitrogen-fixing bacterium Ensifer meliloti CGMCC 7333. J. Agric. Food Chem. 2014, 62, 9957–9964. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Gao, H.; Yue, W.; Xiong, M.; Li, F.; Ge, W. Co-metabolic biodegradation of acetamiprid by Pseudoxanthomonas sp. AAP-7 isolated from a long-term acetamiprid-polluted soil. Bioresour. Technol. 2013, 150, 259–265. [Google Scholar] [CrossRef]
- Sun, S.; Fan, Z.; Zhao, Y.; Guo, L.; Dai, Y. A novel nutrient deprivation-induced neonicotinoid insecticide acetamiprid degradation by Ensifer adhaerens CGMCC 6315. J. Agric. Food Chem. 2018, 67, 63–71. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Yue, W.; Zhang, H.; Li, F.; Xiong, M. Microbial degradation of acetamiprid by Ochrobactrum sp. D-12 isolated from contaminated soil. PLoS ONE 2013, 8, e82603. [Google Scholar] [CrossRef]
- Phugare, S.S.; Jadhav, J.P. Biodegradation of acetamiprid by isolated bacterial strain Rhodococcus sp. BCH2 and toxicological analysis of its metabolites in silkworm (Bombax mori). Clean–Soil Air Water 2015, 43, 296–304. [Google Scholar] [CrossRef]
- Ji, W.; Chen, T.; Sang, Q.; Ge, F.; Yuan, S. Metabolism of chloronicotinyl neonicotinoid insecticides by fungi Penicillium oxalicum IM-3. J. Ecol. Rural. Environ. 2010, 26, 246–250. (In Chinese) [Google Scholar]
- Guo, L.; Fang, W.W.; Guo, L.L.; Yao, C.F.; Zhao, Y.X.; Ge, F.; Dai, Y.J. Biodegradation of the neonicotinoid insecticide acetamiprid by actinomycetes Streptomyces canus CGMCC 13662 and characterization of the novel nitrile hydratase involved. J. Agric. Food Chem. 2019, 67, 5922–5931. [Google Scholar] [CrossRef]
- Yao, X.H.; Min, H. Isolation, characterization and phylogenetic analysis of a bacterial strain capable of degrading acetamiprid. J. Environ. Sci. 2006, 18, 141–146. [Google Scholar]
- Dai, Y.; Zhao, Y.; Zhang, W.; Yu, C.; Ji, W.; Xu, W.; Yuan, S. Biotransformation of thianicotinyl neonicotinoid insecticides: Diverse molecular substituents response to metabolism by bacterium Stenotrophomonas maltophilia CGMCC 1. 1788. Bioresour. Technol. 2010, 101, 3838–3843. [Google Scholar] [CrossRef]
- Parte, S.G.; Kharat, A.S. Aerobic degradation of clothianidin to 2-chloro-methyl thiazole and methyl 3-(thiazole-yl) methyl guanidine produced by Pseudomonas stutzeri smk. J. Environ. Public Health 2019, 2019, 4807913. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhou, Q.W.; Zhou, G.C.; Cao, Y.M.; Dai, Y.J.; Ji, W.W.; Yuan, S. Biotransformation of the neonicotinoid insecticide thiacloprid by the bacterium Variovorax boronicumulans strain J1 and mediation of the major metabolic pathway by nitrile hydratase. J. Agric. Food Chem. 2012, 60, 153–159. [Google Scholar] [CrossRef]
- Ge, F.; Zhou, L.Y.; Wang, Y.; Ma, Y.; Zhai, S.; Liu, Z.H.; Yuan, S. Hydrolysis of the neonicotinoid insecticide thiacloprid by the N2-fixing bacterium Ensifer meliloti CGMCC 7333. Int. Biodeterior. Biodegrad. 2014, 93, 10–17. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Jiang, H.Y.; Cheng, X.; Zhu, Y.X.; Fan, Z.X.; Dai, Z.L.; Dai, Y.J. Neonicotinoid thiacloprid transformation by the N2-fixing bacterium Microvirga flocculans CGMCC 1.16731 and toxicity of the amide metabolite. Int. Biodeterior. Biodegrad. 2019, 145, 104806. [Google Scholar] [CrossRef]
- Dai, Y.J.; Ji, W.W.; Chen, T.; Zhang, W.J.; Liu, Z.H.; Ge, F.; Yuan, S. Metabolism of the Neonicotinoid Insecticides Acetamiprid and Thiacloprid by the Yeast Rhodotorula mucilaginosa Strain IM-2. J. Agric. Food Chem. 2010, 58, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Jindal, V.; Mandal, K.; Kaur, G.; Gupta, V.K. Thiamethoxam degradation by Pseudomonas and Bacillus strains isolated from agricultural soils. Environ. Monit. Assess. 2015, 187, 300. [Google Scholar] [CrossRef] [PubMed]
- Hegde, D.R.; Manoharan, T.; Sridar, R. Identification and characterization of bacterial isolates and their role in the degradation of neonicotinoid insecticide thiamethoxam. J. Pure Appl. Microbiol. 2017, 11, 393–400. [Google Scholar] [CrossRef]
- Dai, Z.L.; Yang, W.L.; Fan, Z.X.; Guo, L.; Liu, Z.H.; Dai, Y.J. Actinomycetes Rhodococcus ruber CGMCC 17550 degrades neonicotinoid insecticide nitenpyram via a novel hydroxylation pathway and remediates nitenpyram in surface water. Chemosphere 2021, 270, 128670. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Bhatt, K.; Sharma, A.; Zhang, W.; Mishra, S.; Chen, S. Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit. Rev. Biotechnol. 2021, 41, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castillo, G.; Molina-Rodríguez, M.; Cambronero-Heinrichs, J.C.; Quirós-Fournier, J.P.; Lizano-Fallas, V.; Jiménez-Rojas, C.; Rodríguez-Rodríguez, C.E. Simultaneous removal of neonicotinoid insecticides by a microbial degrading consortium: Detoxification at reactor scale. Chemosphere 2019, 235, 1097–1106. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, B.; Gupta, V.K. Biodegradation of imidacloprid by consortium of two soil isolated Bacillus sp. Bull. Environ. Contam. Toxicol. 2014, 93, 637–642. [Google Scholar] [CrossRef]
- Wang, X.; Xue, L.; Chang, S.; He, X.; Fan, T.; Wu, J.; Emaneghemi, B. Bioremediation and metabolism of clothianidin by mixed bacterial consortia enriched from contaminated soils in Chinese greenhouse. Process Biochem. 2019, 78, 114–122. [Google Scholar] [CrossRef]
- Xu, B.; Xue, R.; Zhou, J.; Wen, X.; Shi, Z.; Chen, M.; Jiang, M. Characterization of acetamiprid biodegradation by the microbial consortium ACE-3 enriched from contaminated soil. Front. Microbiol. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Perruchon, C.; Pantoleon, A.; Veroutis, D.; Gallego-Blanco, S.; Martin-Laurent, F.; Liadaki, K.; Karpouzas, D.G. Characterization of the biodegradation, bioremediation and detoxification capacity of a bacterial consortium able to degrade the fungicide thiabendazole. Biodegradation 2017, 28, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Rene, E.R.; Huang, Y.; Wu, X.; Zhou, Z.; Li, J.; Chen, S. Indigenous bacterial consortium-mediated cypermethrin degradation in the presence of organic amendments and Zea mays plants. Environ. Res. 2022, 212, 113137. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the microbial degradation and biochemical mechanisms of neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fang, S.; Wan, S.; Zhou, W.; Sun, C.; Li, J. Microorganisms capable of degrading neonicotinoids and their metabolic pathways: A review. Chin. J. Biotechnol. 2022, 38, 4462–4497. (In Chinese) [Google Scholar]
- Wang, J.; Ohno, H.; Ide, Y.; Ichinose, H.; Mori, T.; Kawagishi, H.; Hirai, H. Identification of the cytochrome P450 involved in the degradation of neonicotinoid insecticide acetamiprid in Phanerochaete chrysosporium. J. Hazard. Mater. 2019, 371, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Anhalt, J.C.; Moorman, T.B.; Koskinen, W.C. Biodegradation of imidacloprid by an isolated soil microorganism. J. Environ. Sci. Health Part B 2007, 42, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.Q.; Mao, S.Y.; Sun, S.L.; Yang, W.L.; Ge, F.; Dai, Y.J. Regulation of hydroxylation and nitroreduction pathways during metabolism of the neonicotinoid insecticide imidacloprid by Pseudomonas putida. J. Agric. Food Chem. 2016, 64, 4866–4875. [Google Scholar] [CrossRef]
- Yang, H.; Hu, S.; Wang, X.; Chuang, S.; Jia, W.; Jiang, J. Pigmentiphaga sp. strain D-2 uses a novel amidase to initiate the catabolism of the neonicotinoid insecticide acetamiprid. Appl. Environ. Microbiol. 2020, 86, e02425-19. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Zheng, J.; Wang, G.; Hong, Q.; Li, S.; Jiang, J. Biodegradation of acetamiprid by Pigmentiphaga sp. D-2 and the degradation pathway. Int. Biodeterior. Biodegrad. 2013, 85, 95–102. [Google Scholar] [CrossRef]
- Brunet, J.L.; Badiou, A.; Belzunces, L.P. In vivo metabolic fate of [14C]-acetamiprid in six biological compartments of the honeybee, Apis mellifera L. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 742–748. [Google Scholar] [CrossRef]
- Elumalai, P.; Yi, X.; Chen, Z.; Rajasekar, A.; de Paiva, T.C.B.; Hassaan, M.A.; Huang, M. Detection of Neonicotinoids in agriculture soil and degradation of thiacloprid through photo degradation, biodegradation and photo-biodegradation. Environ. Pollut. 2022, 306, 119452. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shan, L.; Chen, Z.; He, Z.; Li, J.; Yang, Y.; Zhong, W. Differential effects of homologous transcriptional regulators NicR2A, NicR2B1, and NicR2B2 and endogenous ectopic strong promoters on nicotine metabolism in Pseudomonas sp. Strain JY-Q. Appl. Environ. Microbiol. 2021, 87, e02457-20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yi, F.; Chen, G.; Pan, F.; Yang, Y.; Shu, M.; Zhong, W. Function enhancement of a metabolic module via endogenous promoter replacement for Pseudomonas sp. JY-Q to degrade nicotine in tobacco waste treatment. Appl. Biochem. Biotechnol. 2021, 193, 2793–2805. [Google Scholar] [CrossRef]


| Name | CAS | Abbreviation | CW | WS | VP | DT50(d) | log Koc | log Kow |
|---|---|---|---|---|---|---|---|---|
| Imidacloprid | 138261-41-3 | IMI | 255.70 | 610 | 4 × 10−7 | 104–228 | 2.19–2.9 | 0.57 |
| Thiamethoxam | 153719-23-4 | THIA | 291.72 | 4100 | 6.6 × 10−6 | 7–72 | 1.75 | −0.13 |
| Dinotefuran | 165252-70-0 | DIN | 202.21 | 39,830 | 1.7 × 10−3 | 50–100 | 1.41 | −0.64 |
| Acetamiprid | 135410-20-7 | ACE | 222.67 | 2950 | 1.73 × 10−4 | 2–20 | 2.3 | 0.80 |
| Thiacloprid | 111988-49-9 | THI | 252.72 | 184 | 3.0 × 10−7 | 9–27 | 3.67 | 1.26 |
| Clothianidin | 210880-92-5 | CLO | 249.68 | 340 | 2.8 × 10−8 | 13–1386 | 2.08 | 0.70 |
| Nitenpyram | 150824-47-8 | NIT | 270.71 | 590,000 | 1.1 × 10−3 | 1–15 | 1.78 | −0.66 |
| Imidaclothiz | 105843-36-5 | IMID | 261.69 | 500 | NA | 3.1 | NA | NA |
| Microorganism | Source | Reaction Condition | Degradation Rate | References |
|---|---|---|---|---|
| Imidacloprid | ||||
| Klebsiella pneumoniae BCH1 | Agricultural soil, India | 30 °C, pH 7, 7 d | 50 mg L−1, 78% | [38] |
| Pseudomonas sp. RPT52 | Agricultural soil, India | 37 °C, 200 r min−1, 24 h | 128 mg L−1, 46.5% | [39] |
| Pseudoxanthomonas indica CGMCC 6648 | Rhizosphere soil, Chian | 28 °C, pH 7, 6 d | 311 mg L−1, 70.1% | [40] |
| Bacillus aerophilus | Sugarcane field soils, India | Sandy loam soil, 60 d | 150 mg kg −1, 96.1% | [41] |
| Pseudomonas sp. 1G | Soil, Australia | 28 °C, microaerophilic | 50 mg L−1, about 70% | [42] |
| Rhizobium sp. | Oil field soil, Malaysia | 28 °C, 120 r min−1, 25 d | 25 mg L−1, 45.48% | [43] |
| Bacillus alkalinitrilicu | Sugarcane field soils, India | 28 °C, 56 d | 50 mg/kg, 98.02% | [44] |
| Mycobacterium sp. MK6 | Soil, Egypt | 28 °C, 14 d | 150 mg L−1, 99.7% | [45] |
| epidibacillus decaturensis. ST1 | Agricultural field, India | 30 °C, 120 rpm, 20 d | 200 mg L−1, 90% | [46] |
| Ochrobactrum sp. BCL-1 | Rhizosphere soil, China | 30 °C, pH 8, 48 h | 50 mg L−1, 67.67% | [47] |
| Acetamiprid | ||||
| Fusarium sp. CS-3 | Soil, China | 25–30 °C, pH 5–7, 96 h | 50 mg L−1, 98% | [48] |
| Ensifer meliloti CGMCC 7333 | Rhizosphere soil, China | 30 °C, pH 7.5, 220 r min−1 | 500 mg L−1, 65.1% | [49] |
| Pigmentiphaga sp. AAP-1 | Industrial soil, China | 30 °C, pH 7, 2.5 h | 100 mg L−1, 100% | [50] |
| Pseudoxanthomonas sp. AAP-7 | Industrial soil, China | 30 °C, pH 7, 60 h | 200 mg L−1, 95% 300 mg L−1, 93% 400 mg L−1, 87% 600 mg L−1, 73% | |
| Ensifer adhaerens CGMCC 6315 | Soil, China | 30 °C, 12 h | 200 mg L−1, 94.4% | [51] |
| Ochrobactrum sp. D-12 | Agricultural soil, China | 30 °C, pH 7, 14 h °C | 3000 mg L−1, 39.27% | [52] |
| Rhodococcus sp. BCH-2 | Contaminated soil, India | 35 °C, pH 7, 8 d | 50 mg L−1, 84.65% | [53] |
| Penicillium oxalicum IM-3 | Soil, China | 30 °C, 14 d | 500 mg L−1, 41.6% | [54] |
| Streptomyces canus CGMCC 13662 | Soil, China | 30 °C, pH 7, 4 d | 200 mg L−1, 87.6% | [55] |
| Pseudomonos sp. FH2 | Agriculture field soil, China | 30 °C, pH 7.0, 14 d | 800 mg L−1, 96.7% | [56] |
| Imidaclothiz | ||||
| Stenotrophomonas maltophilia CGMCC 1.1788 | Soil, China | 30 °C, 84 d | 500 mg L−1, 36.2% | [57] |
| Clothianidin | ||||
| Pseudomonas stutzeri smk | Agricultural Soil, China | 30 °C, pH 7, 14 d | 10 mg L−1, 62.0% | [58] |
| Thiacloprid | ||||
| Variovorax boronicumulans J1 | Agricultural soil, China | 30 °C, pH 7.2, 60 h | 200 mg L−1, 62.5% | [59] |
| Ensifer meliloti CGMCC 7333 | Rhizosphere soil, China | 30 °C, 60 h | 200 mg L−1, 86.8% | [60] |
| Microvirga flocculans CGMCC 1.16731 | Soil, China | 30 h | 159 mg L−1, 90.5% | [61] |
| Rhodotorula mucilaginosa IM-2 | Soil, China | 30 °C, 20 d | 200 mg L−1, 59.9% | [62] |
| Thiamethoxam | ||||
| Ensifer adhaerens TMX-23 | Rhizosphere soil, China | 30 °C, 10 d | 200 mg L−1, 21.6% | [63] |
| Bacillus aeromonas IMBL 4.1 | Soil, India | 37 °C, pH 6.0 °C–6.5, 15 d | 50 mg L−1, 45.28% | [64] |
| Pseudomonas putida IMBL 5.2 | Soil, India | 37 °C, pH 6.0 °C–6.5, 15 d | 50 mg L−1, 38.23% | |
| Acinetobacter sp. Enterobacter sp. Bacillus sp. | Agricultural soil, India | 15 d | 50 mg L−1, 94.72% 50 mg L−1, 90.78% 50 mg L−1, 82.06% | [65] |
| Name | Source | Reaction Condition | Degradation Rate | References |
|---|---|---|---|---|
| N1/2 | Contaminated soil, Costa Ric | Imidacloprid, thiamethoxam, 160 rpm, 25 °C, 5 d | 50 mg L−1, 60.1% (imidacloprid), 33.4% (thiamethoxam) | [67] |
| / | Sugarcane growing soils, India | Imidacloprid, 25 ± 2 °C, 56 d | 50 mg kg−1 soil, 93.6% 100 mg kg−1 soil, 94.2% 150 mg kg−1 soil, 93% | [68] |
| SCAH | Contaminated soil, China | Clothianidin, 150 rpm, 30 °C, 15 d | 500 mg L−1, 79.3% | [69] |
| ACE-3 | Acetamiprid-contaminated soil, China | Acetamiprid, pH 6.0–8.0, 20–42 °C, 144 h | 50 mg L−1, 100% | [70] |
| / | Wastewater disposal site, Greece | Thiabendazole, 28 d | 5 mg kg−1 soil, 100% 50 mg kg−1 soil, 100% 100 mg kg−1 soil, 100% | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Sun, S.; Li, P.; Zhou, X.; Wang, J. Neonicotinoid Insecticide-Degrading Bacteria and Their Application Potential in Contaminated Agricultural Soil Remediation. Agrochemicals 2024, 3, 29-41. https://doi.org/10.3390/agrochemicals3010004
Zeng Y, Sun S, Li P, Zhou X, Wang J. Neonicotinoid Insecticide-Degrading Bacteria and Their Application Potential in Contaminated Agricultural Soil Remediation. Agrochemicals. 2024; 3(1):29-41. https://doi.org/10.3390/agrochemicals3010004
Chicago/Turabian StyleZeng, Yuechun, Shaolin Sun, Pengfei Li, Xian Zhou, and Jian Wang. 2024. "Neonicotinoid Insecticide-Degrading Bacteria and Their Application Potential in Contaminated Agricultural Soil Remediation" Agrochemicals 3, no. 1: 29-41. https://doi.org/10.3390/agrochemicals3010004
APA StyleZeng, Y., Sun, S., Li, P., Zhou, X., & Wang, J. (2024). Neonicotinoid Insecticide-Degrading Bacteria and Their Application Potential in Contaminated Agricultural Soil Remediation. Agrochemicals, 3(1), 29-41. https://doi.org/10.3390/agrochemicals3010004






