Lethal and Sub-Lethal Effects of Spirotetramat on Red Spider Mite, Tetranychus macfarlanei Baker and Pritchard (Acari: Tetranychidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Spider Mites
2.2. Toxicity to Adult Females
2.3. Immature Development Duration of F1 Generation
2.4. Reproduction and Longevity of Females of F1 Generation
2.5. Life Table Parameters
2.6. Statistical Analyses
3. Results
3.1. Toxicity of Spirotetramat to T. macfarlanei Females of Parent Generation
3.2. The Development of Immatures in F1 Generation
3.3. Adult Reproduction and Longevity of F1 Generation
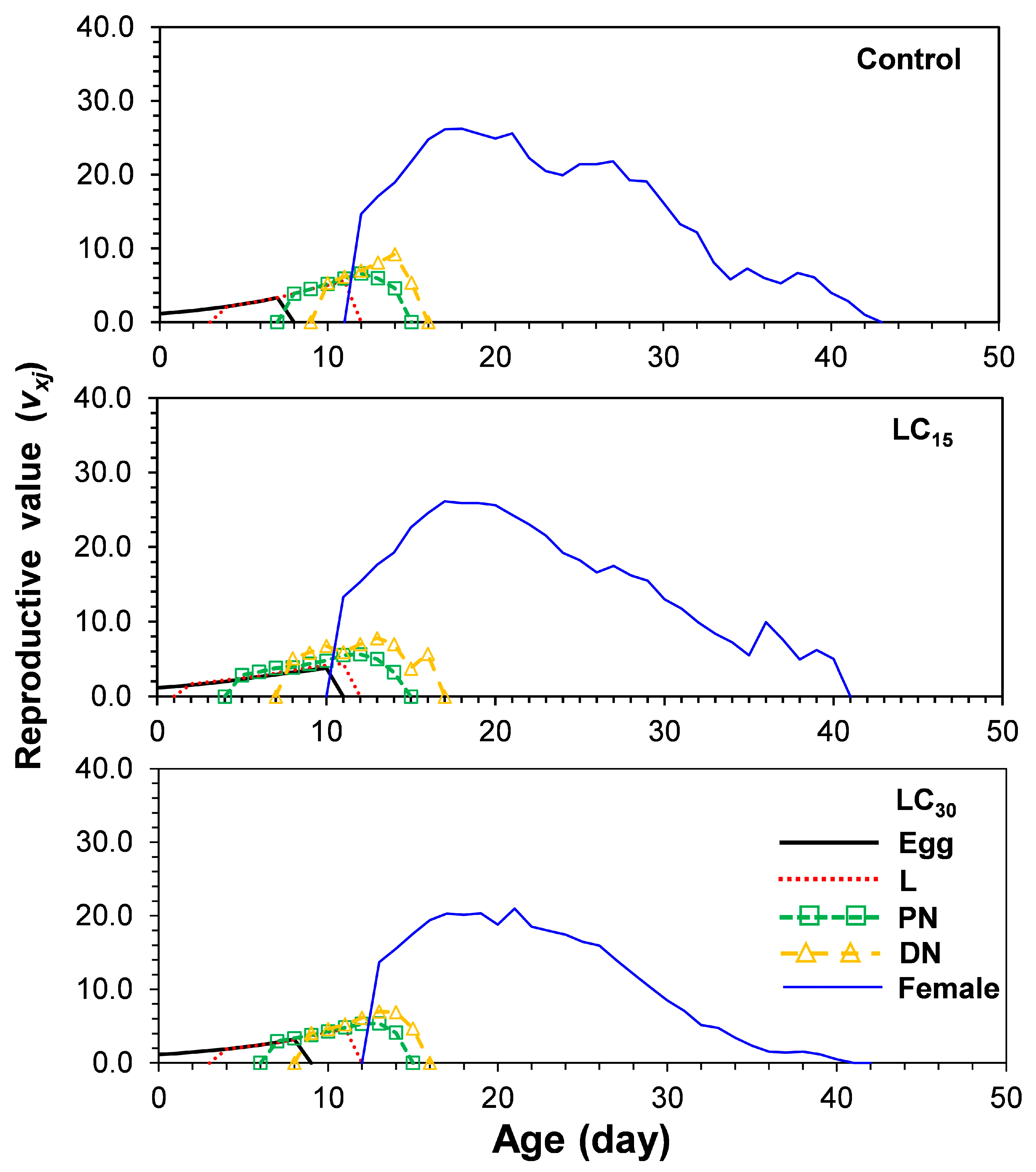
3.4. Life Table Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975; p. 614. [Google Scholar]
- Bolland, H.R.; Gutierrez, J.; Flechtmann, C.H. World Catalogue of the Spider Mite Family (Acari: Tetranychidae); Brill Academic Publishers: Leiden, The Netherlands, 1998; p. 392. [Google Scholar]
- Ullah, M.S.; Gotoh, T. Laboratory-based toxicity of some acaricides to Tetranychus macfarlanei and Tetranychus truncatus (Acari: Tetranychidae). Int. J. Acarol. 2013, 39, 244–251. [Google Scholar] [CrossRef]
- Ullah, M.S.; Haque, M.A.; Nachman, G.; Gotoh, T. Temperature dependent development and reproductive traits of Tetranychus macfarlanei (Acari: Tetranychidae). Exp. Appl. Acarol. 2012, 56, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.S.; Hossain, M.A.; Jahan, M.; Sarker, M.A.; Hamim, I. A Field Guide of Major Insect Pests and Diseases of Different Crops in Bangladesh; Bangladesh Agricultural University Extension Centre (BAUEC): Mymensingh, Bangladesh, 2021; p. 500. [Google Scholar]
- Migeon, A.; Dorkeld, F. Spider Mites Web: A Comprehensive Database for the Tetranychidae. 2024. Available online: https://www1.montpellier.inra.fr/CBGP/spmweb/ (accessed on 10 June 2024).
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Tirry, L. Mechanisms of acaricide resistance in the two-spotted spider mite Tetranychus urticae. In Biorational Control of Arthropod Pests; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 347–393. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. Sublethal effects of insecticide on longevity, fecundity, and behaviour of insect pests: A review. Biosci. J. 2000, 11, 107–112. [Google Scholar]
- Singh, J.P.; Marwaha, K.K. Effects of sublethal concentrations of some insecticides on growth and development of maize stalk borer, Chilo partellus (Swinhoe) larvae. Shashpa 2000, 7, 181–186. [Google Scholar]
- Babcock, J.M.; Gerwick, C.B.; Huang, J.X.; Loso, M.R.; Nakamura, G.; Nolting, S.P.; Rogers, R.B.; Sparks, T.C.; Thomas, J.; Watson, G.B.; et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest. Manag. Sci. 2011, 67, 328–334. [Google Scholar] [CrossRef]
- Brück, E.; Elbert, A.; Fischer, R.; Krueger, S.; Kühnhold, J.; Klueken, A.M.; Nauen, R.; Niebes, J.F.; Reckmann, U.; Schnorbach, H.J. Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: Biological profile and field performance. Crop Prot. 2009, 28, 838–844. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Sun, H.; Zhu, J. Evaluation of the demographic potential of Aphelinus albipodus (Hymenoptera: Aphelinidae) with Aphis glycines (Homoptera: Aphididae) as alternate host at various temperatures. Appl. Entomol. Zool. 2016, 51, 247–255. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Aldana-Madrid, M.L.; Silveira-Gramont, M.I.; Aguiar, J.L. Spirotetramat—An Alternative for the Control of Parasitic Sucking Insects and Its Fate in the Environment; IntechOpen: London, UK, 2016; ISBN 978-953-51-2258-6. [Google Scholar]
- Gong, Y.; Shi, X.; Desneux, N.; Gao, X. Effects of spirotetramat treatments on fecundity and carboxylesterase expression of Aphis gossypii Glover. Ecotoxicology 2016, 25, 655–663. [Google Scholar] [CrossRef]
- Arrese, E.L.; Canavoso, L.E.; Jouni, Z.E.; Pennington, J.E.; Tsuchida, K.; Wells, M.A. Lipid storage and mobilization in insects: Current status and future directions. Insect Biochem. Mol. Biol. 2001, 31, 7–17. [Google Scholar] [CrossRef]
- Ouyang, Y.; Montez, G.H.; Liu, L.; Grafton-Cardwell, E.E. Spirodiclofen and spirotetramat bioassays for monitoring resistance in citrus red mite, Panonychus citri (Acari: Tetranychidae). Pest Manag. Sci. 2012, 68, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Döker, İ.; Kazak, C.; Ay, R. Resistance status and detoxification enzyme activity in ten populations of Panonychus citri (Acari: Tetranychidae) from Turkey. Crop Prot. 2021, 141, 105488–105497. [Google Scholar] [CrossRef]
- Vermeer, R.; Baur, P. Movento® product development: Custom-made formulations for an exceptional active ingredient. Bayer CropSci. J. 2008, 61, 141–157. [Google Scholar]
- Sverdrup, L.E.; Bjørge, C.; Eklo, O.M.; Grung, M.; Källqvist, T.; Klingen, I.; Låg, M.; Ropstad, E.; Øvrebø, S. Risk assessment of the insecticide Movento 100 SC with the active substance spirotetramat. Eur. J. Nutr. Food Saf. 2022, 14, 9–11. [Google Scholar] [CrossRef]
- Lashkari, M.R.; Sahragard, A.; Ghadamyari, M. Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Sci. 2007, 14, 207–212. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Mahmoodi, L.; Mehrkhou, F.; Guz, N.; Forouzan, M.; Atlihan, R. Sublethal effects of three insecticides on fitness parameters and population projection of Brevicoryne brassicae (Hemiptera: Aphididae). J. Econ. Entomol. 2020, 113, 2713–2722. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef]
- Jie, M.; Gao, Y.; Kuang, D.; Shi, Y.; Wang, H.; Jing, W. Relationship between imidacloprid residues and control effect on cotton aphids in arid region. Environ. Geochem. Health 2021, 43, 1941–1952. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Yang, X.-M.; Sun, L.-J.; Zhao, C.-D.; Chi, H.; Zheng, C.-Y. Sublethal effect of spirotetramat on the life table and population growth of Frankliniella occidentalis (Thysanoptera: Thripidae). Entomol. Gen. 2021, 41, 219–231. [Google Scholar] [CrossRef]
- Iftikhar, A.; Hafeez, F.; Aziz, M.A.; Hashim, M.; Naeem, A.; Yousaf, H.K.; Saleem, M.J.; Hussain, S.; Hafeez, M.; Ali, Q.; et al. Assessment of sublethal and transgenerational effects of spirotetramat, on population growth of cabbage aphid, Brevicoryne brassicae L. (Hemiptera: Aphididae). Front. Physiol. 2022, 13, 1014190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, H.K.; Kim, G.H. Sublethal effects of spirotetramat, cyantraniliprole, and pymetrozine on Aphis gossypii (Hemiptera: Aphididae). Insects 2024, 15, 247. [Google Scholar] [CrossRef] [PubMed]
- Marcic, D.; Petronijevic, S.; Drobnjakovic, T.; Prijovic, M.; Peric, P.; Milenkovic, S. The effects of spirotetramat on life history traits and population growth of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2012, 56, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Beers, E.H.; Schmidt, R.A. Impacts of orchard pesticides on Galendromus occidentalis: Lethal and sublethal effects. Crop Prot. 2014, 56, 16–24. [Google Scholar] [CrossRef]
- Kim, M.; Sim, C.; Shin, D.; Suh, E.; Cho, K. Residual and sublethal effects of fenpyroximate and pyridaben on the instantaneous rate of increase of Tetranychus urticae. Crop Prot. 2006, 25, 542–548. [Google Scholar] [CrossRef]
- Li, Y.Y.; Fan, X.; Zhang, G.H.; Liu, Y.Q.; Chen, H.Q.; Liu, H.; Wang, J.J. Sublethal effects of Bifenazate on life history and population parameters of Tetranychus urticae (Acari: Tetranychidae). Syst. Appl. Acarol. 2017, 22, 148–158. [Google Scholar] [CrossRef]
- Rimy, S.J.; Das, G.; Gotoh, T.; Ullah, M.S. Lethal and sublethal effects of bifenazate on the biological parameters of Tetranychus truncatus Ehara (Acari: Tetranychidae). Syst. Appl. Acarol. 2021, 26, 2118–2132. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C.; et al. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell: Oxford, UK, 2000; p. 592. [Google Scholar]
- Iftikhar, A.; Hafeez, F.; Hafeez, M.; Farooq, M.; Asif Aziz, M.; Sohaib, M.; Naeem, A.; Lu, Y. Sublethal effects of a juvenile hormone analog, Pyriproxyfen on demographic parameters of non-target predator, Hippodamia convergens Guerin-Meneville (Coleoptera: Coccinellidae). Ecotoxicology 2020, 29, 1017–1028. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Zheng, Y.; Weng, Q.; Biondi, A.; Desneux, N.; Wu, K. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int. J. Biol. Sci. 2013, 9, 246–255. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.; Yousaf, H.K.; Khan, M.M.; Imran, M.; Zhang, Z.; Shah, S.; Wang, L. Sublethal effects of bistrifluron on key biological traits, macronutrients contents and vitellogenin (SeVg) expression in Spodoptera exigua (Hübner). Pestic. Biochem. Physiol. 2021, 174, 104802. [Google Scholar] [CrossRef]
- Hoy, M.A. Agricultural Acarology: Introduction to Integrated Mite Management; CRC Press: Boca Raton, FL, USA, 2011; p. 430. [Google Scholar]
- Nishi, A.N.; Chowdhury, S.; Mondal, P.; Akram, M.W.; Ullah, M.S. Efficacy of entomopathogen Cordyceps tenuipes (Peck) Kepler, B. Shrestha et Spatafora against spider mite Tetranychus piercei McGregor (Acari: Tetranychidae). Int. J. Acarol. 2023, 49, 239–246. [Google Scholar] [CrossRef]
- Ullah, M.S.; Kobayashi, Y.; Gotoh, T. Development and reproductive capacity of the miyake spider mite Eotetranychus kankitus (Acari: Tetranychidae) at different temperatures. Insects 2022, 13, 910. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age, Two Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2024. [Google Scholar]
- Mou, D.F.; Lee, C.C.; Smith, C.L.; Chi, H. Using viable eggs to accurately determine the demo graphic and predation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J. Appl. Entomol. 2015, 139, 579–591. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Tuan, S.J.; Yeh, C.C.; Atlihan, R.; Chi, H. Linking life table and predation rate for biological control: A comparative study of Eocanthecona furcellata (Hemiptera: Pentatomidae) fed on Spodoptera litura (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2016, 109, 13–24. [Google Scholar] [CrossRef]
- Chi, H. Life table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chen, G.M.; Chi, H.; Wang, R.C.; Wang, Y.P.; Xu, Y.Y.; Li, X.D.; Yin, P.; Zheng, F.Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef]
- Robertson, J.L.; Savin, N.E.; Savin, N.E.; Preisler, H.K. Bioassays with Arthropods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 224. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993; p. 456. [Google Scholar]
- Daniels, R.E.; Allan, J.D. Life table evaluation of chronic exposure to a pesticide. Can. J. Fish. Aquat. Sci. 1981, 38, 485–494. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide mode of action: Return of the ryanodine receptor. Pest Manag. Sci. 2006, 62, 690–692. [Google Scholar] [CrossRef]
- Moens, J.; De Clercq, P.; Tirry, L. Side effects of pesticides on the larvae of the hoverfly Episyrphus balteatus in the laboratory. Phytoparasitica 2011, 39, 1–9. [Google Scholar] [CrossRef]
- Mollaloo, M.G.; Kheradmand, K.; Sadeghi, R.; Talebi, A.A. Demographic analysis of sublethal effects of spiromesifen on Neoseiulus californicus (Acari: Phytoseiidae). Acarologia 2017, 57, 571–580. [Google Scholar] [CrossRef]
- Liang, P.Z.; Ma, K.S.; Chen, X.W.; Tang, C.Y.; Xia, J.; Chi, H.; Gao, X.W. Toxicity and sublethal effects of flupyradifurone, a novel butenolide insecticide, on the development and fecundity of Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2019, 112, 852–858. [Google Scholar] [CrossRef]
- Tang, Q.; Xiang, M.; Hu, H.; An, C.; Gao, X. Evaluation of sublethal effects of sulfoxaflor on the green peach aphid (Hemiptera: Aphididae) using life table parameters. J. Econ. Entomol. 2015, 108, 2720–2728. [Google Scholar] [CrossRef]



| Parameters | Values |
|---|---|
| Slope | 1.060 ± 0.103 |
| n | 480 |
| χ2 | 1.116 |
| Df | 6 |
| LC15 (95% CL) | 2.160 (1.226–3.265) |
| LC30 (95% CL) | 6.572 (4.546–8.866) |
| LC50 (95% CL) | 20.541 (15.596–27.504) |
| LC90 (95% CL) | 332.811 (193.320–718.028) |
| Treatment | Sex | N a | Egg | Larva | Protonymph | Deutonymph | Egg to Adult | Survival (%) |
|---|---|---|---|---|---|---|---|---|
| Control | ♀ | 40 | 6.35 ± 0.11 b | 2.73 ± 0.09 b | 2.25 ± 0.08 a | 2.92 ± 0.08 a | 14.25 ± 0.12 bc | 98.8 |
| ♂ | 39 | 6.44 ± 0.14 ab | 3.03 ± 0.13 ab | 2.36 ± 0.11 a | 2.62 ± 0.10 bc | 14.44 ± 0.13 ab | ||
| LC15 | ♀ | 34 | 6.24 ± 0.19 b | 2.74 ± 0.11 b | 2.18 ± 0.09 b | 2.85 ± 0.09 ab | 14.00 ± 0.21 c | 80.0 |
| ♂ | 30 | 6.93 ± 0.24 a | 3.10 ± 0.18 ab | 2.47 ± 0.11 a | 2.37 ± 0.18 c | 14.87 ± 0.20 a | ||
| LC30 | ♀ | 35 | 6.37 ± 0.10 b | 2.80 ±0.11 b | 2.31 ± 0.08 a | 2.91 ± 0.10 a | 14.40 ± 0.13 bc | 82.5 |
| ♂ | 31 | 6.65 ± 0.19 ab | 3.26 ± 0.16 a | 2.39 ± 0.10 a | 2.45 ± 0.12 c | 14.74 ± 0.20 ab |
| Treatment | N a | APOP | TPOP | Oviposition Days | Female Longevity | Eggs Per Female |
|---|---|---|---|---|---|---|
| Control | 40 | 1.82 ± 0.13 a | 16.07 ± 0.15 a | 11.47 ± 1.00 a | 28.80 ± 1.05 a | 59.20 ± 6.17 a |
| LC15 | 54 | 1.79 ± 0.13 a | 15.79 ± 0.24 a | 11.44 ± 1.04 a | 28.56 ± 1.09 a | 56.12 ± 6.60 ab |
| LC30 | 52 | 2.09 ± 0.25 a | 16.49 ± 0.29 a | 10.34 ± 1.10 a | 29.26 ± 1.27 a | 41.46 ± 4.96 b |
| Treatment | R0 | r | t | λ | GRR |
|---|---|---|---|---|---|
| Control | 29.60 ± 4.52 a | 0.1500 ± 0.0063 a | 22.59 ± 0.43 a | 1.1618 ± 0.0073 a | 55.60 ± 9.47 a |
| LC15 | 23.85 ± 4.15 ab | 0.1412 ± 0.0075 ab | 22.46 ± 0.44 a | 1.1516 ± 0.0086 ab | 56.24 ± 10.98 a |
| LC30 | 18.14 ± 3.143 b | 0.1276 ± 0.0076 b | 22.72 ± 0.38 a | 1.1361 ± 0.0086 b | 44.88 ± 5.99 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swarna, F.A.; Hayder, T.; Barsa, S.M.; Mondal, P.; Gotoh, T.; Ullah, M.S. Lethal and Sub-Lethal Effects of Spirotetramat on Red Spider Mite, Tetranychus macfarlanei Baker and Pritchard (Acari: Tetranychidae). Arthropoda 2024, 2, 212-225. https://doi.org/10.3390/arthropoda2030016
Swarna FA, Hayder T, Barsa SM, Mondal P, Gotoh T, Ullah MS. Lethal and Sub-Lethal Effects of Spirotetramat on Red Spider Mite, Tetranychus macfarlanei Baker and Pritchard (Acari: Tetranychidae). Arthropoda. 2024; 2(3):212-225. https://doi.org/10.3390/arthropoda2030016
Chicago/Turabian StyleSwarna, Farhana Afrose, Tasfia Hayder, Shreema Mandal Barsa, Powlomee Mondal, Tetsuo Gotoh, and Mohammad Shaef Ullah. 2024. "Lethal and Sub-Lethal Effects of Spirotetramat on Red Spider Mite, Tetranychus macfarlanei Baker and Pritchard (Acari: Tetranychidae)" Arthropoda 2, no. 3: 212-225. https://doi.org/10.3390/arthropoda2030016







