The Prevalence of Egg Parasitoids of Two Cobweb Spiders in a Tropical Urban Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
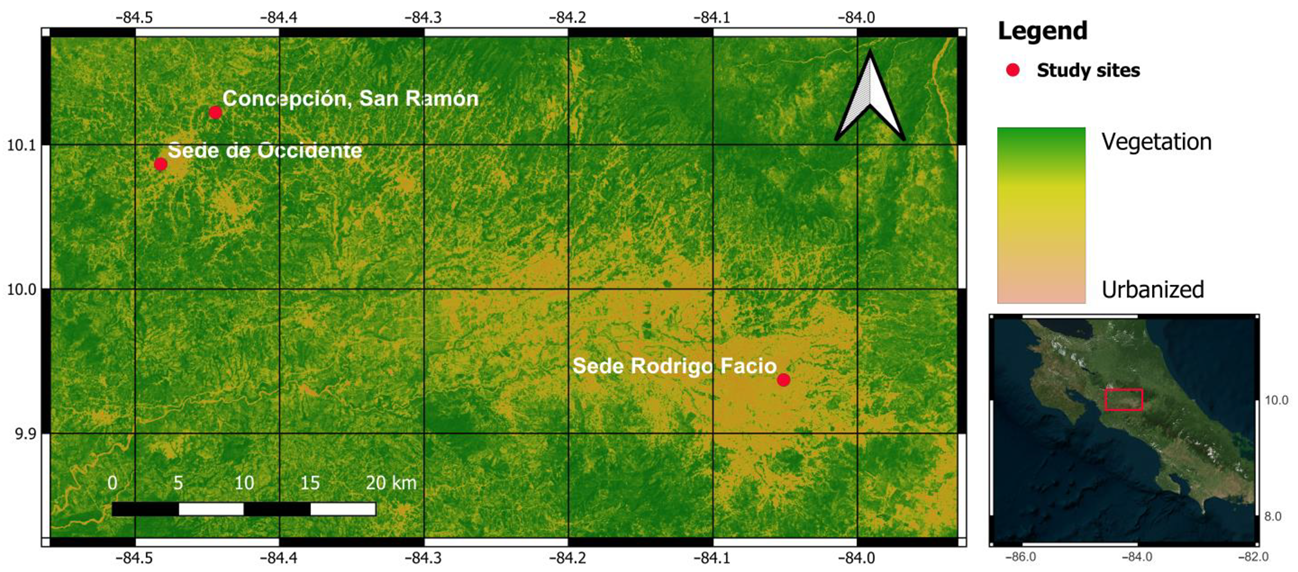
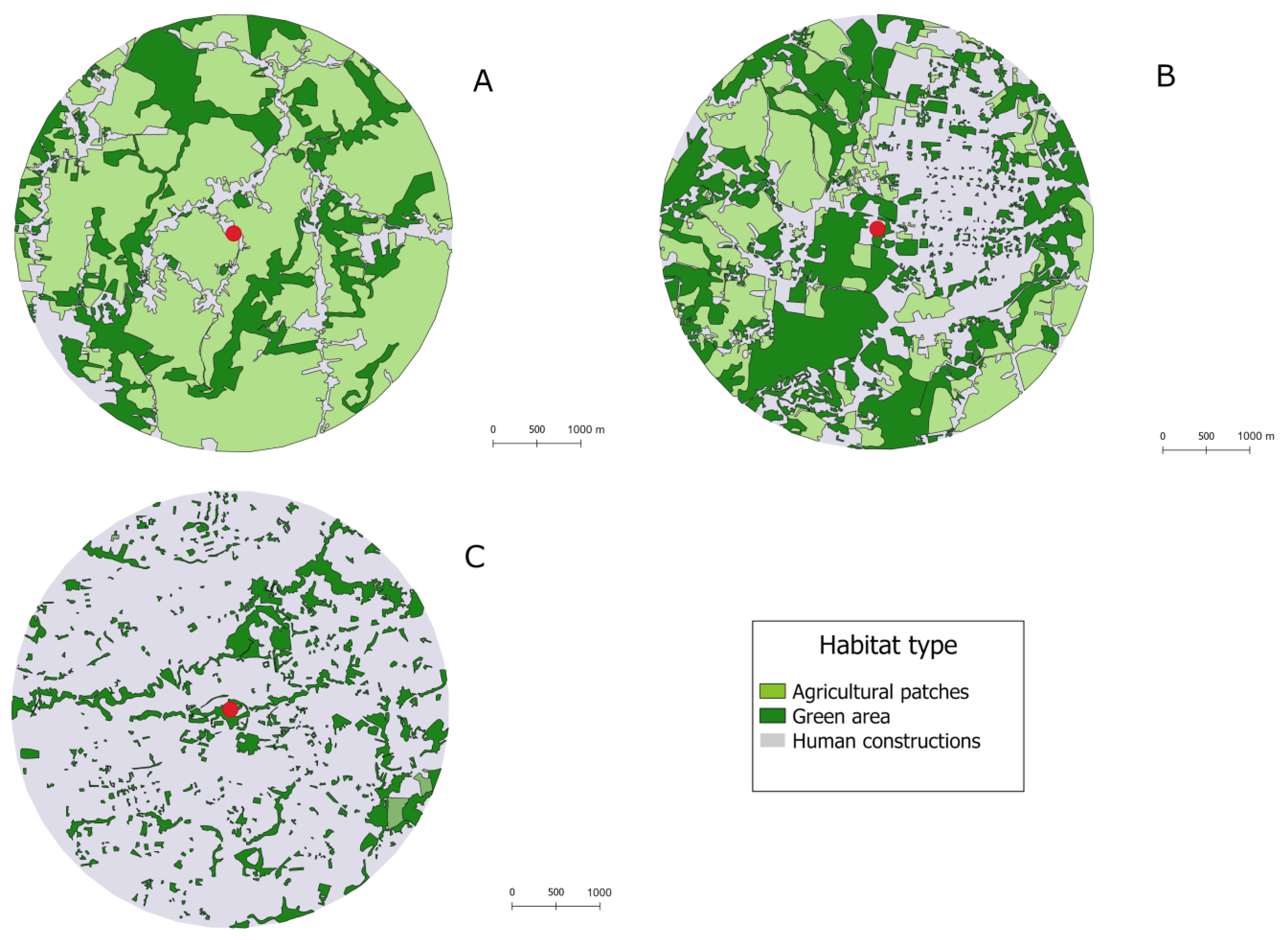
2.2. Study Site
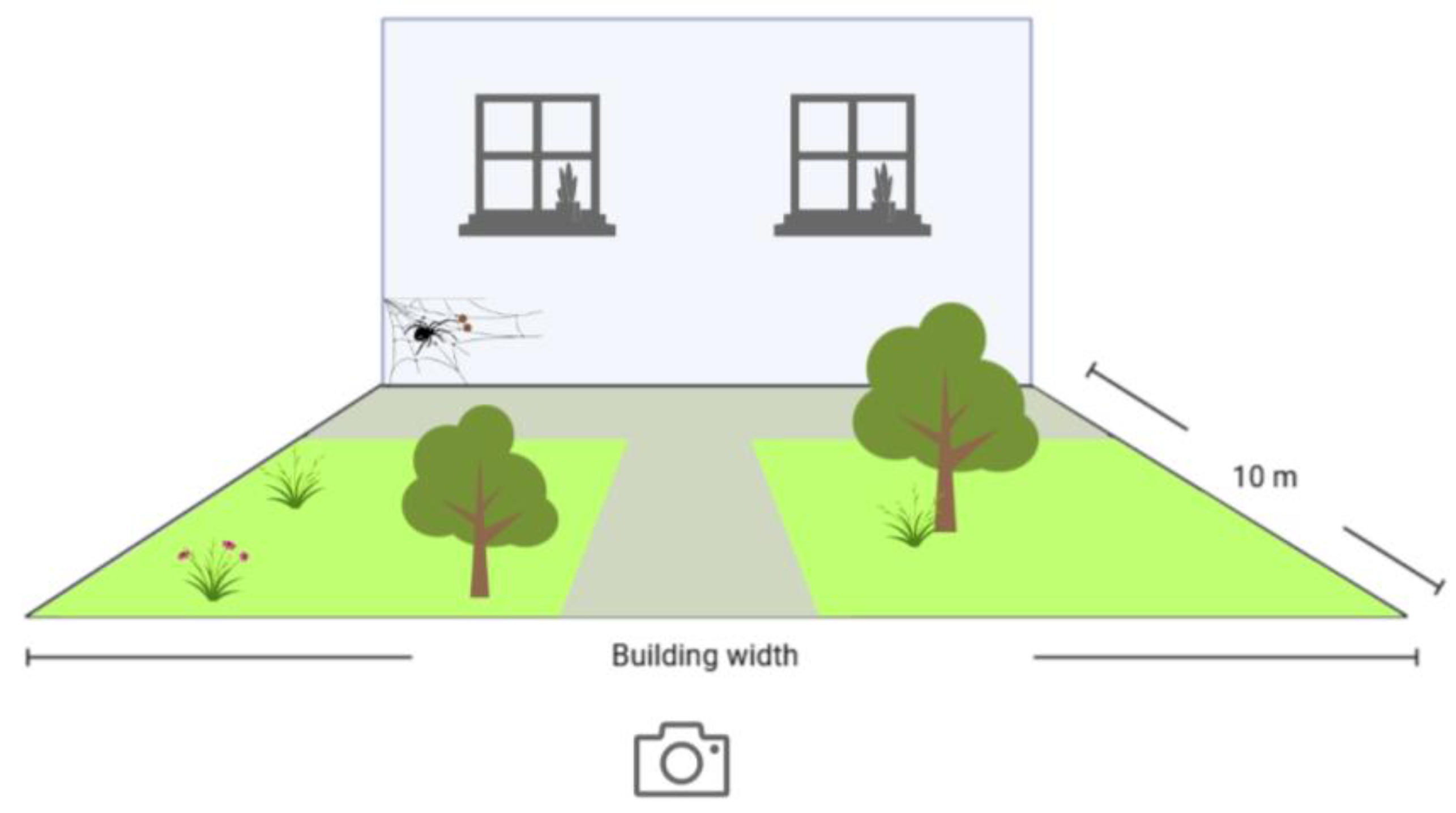
2.3. Data Collection
2.4. Data Analyses
3. Results
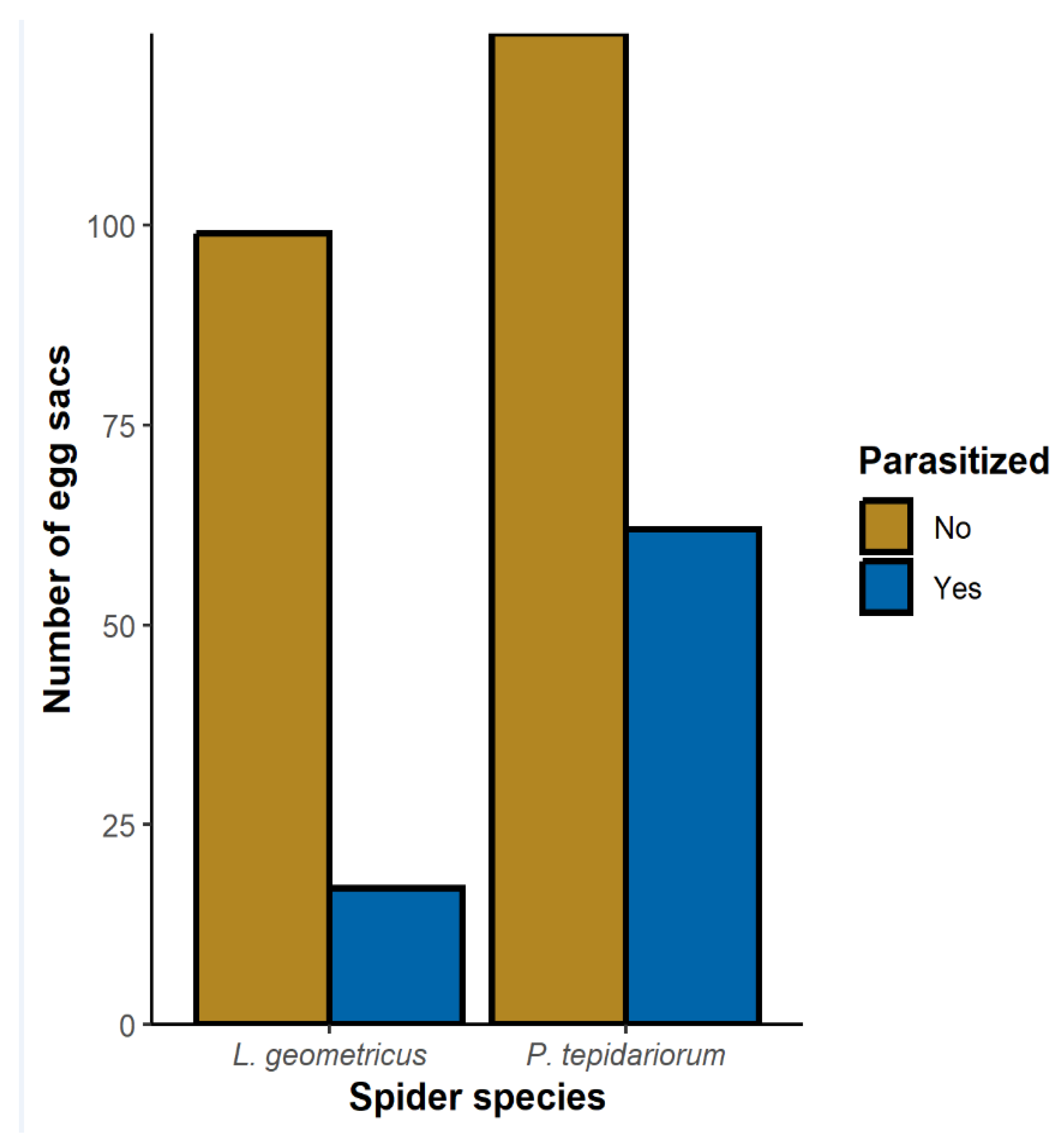
3.1. Egg Sacs Parasitized
3.2. Proportion of Parasitism
3.3. Parasitoids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durkin, E.S.; Cassidy, S.T.; Gilbert, R.; Richardson, E.A.; Roth, A.M.; Shablin, S.; Keiser, C.N. Parasites of spiders: Their impacts on host behavior and ecology. J. Arachnol. 2021, 49, 281–298. [Google Scholar] [CrossRef]
- Fei, M.; Gols, R.; Harvey, J.A. The bology and ecology of parasitoid wasps of predatory arthropods. Annu. Rev. Entomol. 2023, 68, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.A.; Arabesky, V.; Rozenberg, T.; Lubin, Y.; Segoli, M.; Mowery, M.A. Parasitoid development and superparasitism in invasive versus native widow spider host egg sacs. Biol. Invasions 2023, 25, 2519–2530. [Google Scholar] [CrossRef]
- Austin, A.D. The function of spider egg sacs in relation to parasitoids and predators, with special reference to the Australian fauna. J. Nat. Hist. 1985, 19, 359–376. [Google Scholar] [CrossRef]
- Matsumoto, R.; Konishi, K. Life histories of two ichneumonid parasitoids of Cyclosa octotuberculata (Araneae): Reclinervellus tuberculatus (Uchida) and its new sympatric congener (Hymenoptera: Ichneumonidae: Pimplinae). Entomol. Sci. 2007, 10, 267–278. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. 1986, 61, 395–434. [Google Scholar] [CrossRef]
- Horváth, R.; Magura, T.; Tóthmérész, B. Ignoring ecological demands masks the real effect of urbanization: A case study of ground-dwelling spiders along a rural-urban gradient in a lowland forest in Hungary. Ecol. Res. 2012, 27, 1069–1077. [Google Scholar] [CrossRef]
- Argañaraz, C.I.; Gleiser, R.M. Does urbanization have positive or negative effects on Crab spider (Araneae: Thomisidae) diversity? Zoologia 2017, 34, e19987. [Google Scholar] [CrossRef]
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, competition, and biodiversity loss in urban ecosystems. BioScience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Alberti, M. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 2015, 30, 114–126. [Google Scholar] [CrossRef]
- Egerer, M.; Fairbairn, M. Gated gardens: Effects of urbanization on community formation and commons management in community gardens. Geoforum 2018, 96, 61–69. [Google Scholar] [CrossRef]
- Rodríguez-Bardía, M.; Fuchs, E.J.; Barrantes, G.; Madrigal-Brenes, R.; Sandoval, L. Genetic structure in neotropical birds with different tolerance to urbanization. Sci. Rep. 2022, 12, 6054. [Google Scholar] [CrossRef]
- Alberti, M. The effects of urban patterns on ecosystem function. Int. Reg. Sci. Rev. 2005, 28, 168–192. [Google Scholar] [CrossRef]
- Ramalho, C.E.; Hobbs, R.J. Time for a change: Dynamic urban ecology. Trends Ecol. Evol. 2012, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Desneux, N.; Monticelli, L.; Fernandez, X.; Michel, T.; Lavoir, A.V. Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. Biomed Res. Int. 2015, 2015, 342982. [Google Scholar] [CrossRef]
- Bennett, A.B.; Gratton, C. Local and landscape scale variables impact parasitoid assemblages across an urbanization gradient. Landsc. Urban Plan. 2012, 104, 26–33. [Google Scholar] [CrossRef]
- Finch, O.D. The parasitoid complex and parasitoid-induced mortality of spiders (Araneae) in a Central European woodland. J. Nat. Hist. 2005, 39, 2339–2354. [Google Scholar]
- Magura, T.; Horváth, R.; Tóthmérész, B. Effects of urbanization on ground-dwelling spiders in forest patches, in Hungary. Landsc. Ecol. 2010, 25, 621–629. [Google Scholar] [CrossRef]
- Moura, R.R.; Neto, A.T.; Gonzaga, M.O. Don’t put all your eggs in small baskets: Ineffective guardians, incidence of parasitoids and clutch size of Latrodectus geometricus (Araneae, Theridiidae) along an urban gradient. Zool. Anz. 2010, 295, 120–125. [Google Scholar] [CrossRef]
- Vetter, R.S.; Vincent, L.S.; Itnyre, A.A.; Clarke, D.E.; Reinker, K.I.; Danielsen, W.R.D.; Robinson, L.J.; Kabashima, J.M.; Rust, M.K. Predators and parasitoids of egg sacs of the widow spiders, Latrodectus geometricus and Latrodectus hesperus (Araneae: Theridiidae) in southern California. J. Arachnol. 2012, 40, 209–214. [Google Scholar] [CrossRef]
- Marie, J.; Vetter, R.S. Establishment of the brown widow spider (Araneae: Theridiidae) and infestation of its egg sacs by a parasitoid, Philolema latrodecti (Hymenoptera: Eurytomidae), in French Polynesia and the Cook Islands. J. Med. Entomol. 2015, 52, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.S.; Vetter, R.S.; Wrenn, W.J.; Kempf, J.K.; Berrian, J.E. The brown widow spider Latrodectus geometricus CL Koch, 1841, in southern California. Pan-Pac. Entomol. 2009, 84, 344–349. [Google Scholar] [CrossRef]
- Valerio, C.E. Parasitismo en huevos de araña Achaearanea tepidariorum (Koch) (Aranea: Theridiidae) en Costa Rica. Rev. Biol. Trop. 1971, 18, 99–106. [Google Scholar]
- Valerio, C.E. Dimorfismo en avispas macho de la familia Scelionidae (Hymenoptera). Brenesia 1974, 1974, 1–9. [Google Scholar]
- Loiácono, M.S.; Margaría, C.B. Las especies del género Baeus (Hymenoptera: Scelionidae) endoparasitoides de ootecas de arañas en la región neotropical. Acta Zool. Mex. 2004, 20, 83–90. [Google Scholar] [CrossRef]
- Triana, E.; Barrantes, G.; Hanson, P.E. Incidence of parasitoids and predators on eggs of seven species of Theridiidae (Araneae). Arachnology 2012, 15, 293–298. [Google Scholar]
- Corcos, D.; Cerretti, P.; Caruso, V.; Mei, M.; Falco, M.; Marini, L. Impact of urbanization on predator and parasitoid insects at multiple spatial scales. PLoS ONE 2019, 14, e0214068. [Google Scholar] [CrossRef]
- Hanson, P.E.; Gauld, I.D. (Eds.) Hymenoptera de la Región Neotropical; Memoirs of the American Entomological Institute: Totnes, UK, 2006; Volume 77, pp. 1–997. [Google Scholar]
- Garb, J.E.; González, A.; Gillespie, R.G. The black widow spider genus Latrodectus (Araneae: Theridiidae): Phylogeny, biogeography, and invasion history. Mol. Phylogenetics Evol. 2004, 31, 1127–1142. [Google Scholar] [CrossRef]
- Taucare-Ríos, A. Primer registro de la viuda marrón, Latrodectus geometricus (Araneae: Theridiidae) en el norte de Chile. Rev. Chil. Entomol. 2011, 36, 39–42. [Google Scholar]
- Levi, H.W. Cosmopolitan and pantropical species of theridiid spiders (Araneae: Theridiidae). Pac. Insects 1967, 9, 175–186. [Google Scholar]
- Torchin, M.E.; Lafferty, K.D.; Dobson, A.P.; McKenzie, V.J.; Kuris, A.M. Introduced species and their missing parasites. Nature 2003, 421, 628–630. [Google Scholar] [CrossRef]
- Phillips, B.L.; Kelehear, C.; Pizzatto, L.; Brown, G.P.; Barton, D.; Shine, R. Parasites and pathogens lag behind their host during periods of host range advance. Ecology 2010, 91, 872–881. [Google Scholar] [CrossRef]
- Edwards, G.B. The Common House Spider, Parasteatoda tepidariorum (Koch) (Arachnida: Araneae: Theridiidae); University of Florida Institute of Food and Agricultural Sciences Extension: Gainesville, Florida, 2001; Volume 238, pp. 1–2. [Google Scholar]
- Mittmann, B.; Wolff, C. Embryonic development and staging of the cobweb spider Parasteatoda tepidariorum CL Koch, 1841 (syn.: Achaearanea tepidariorum; Araneomorphae; Theridiidae). Dev. Genes Evol. 2012, 222, 189–216. [Google Scholar] [CrossRef]
- McGregor, A.P.; Hilbrant, M.; Pechmann, M.; Schwager, E.E.; Prpic, N.M.; Damen, W.G. Cupiennius salei and Achaearanea tepidariorum: Spider models for investigating evolution and development. Bioessays 2008, 30, 487–498. [Google Scholar] [CrossRef]
- Taucare-Ríos, A.; Bizama, G.; Bustamante, R.O. Using global and regional species distribution models (SDM) to infer the invasive stage of Latrodectus geometricus (Araneae: Theridiidae) in the Americas. Environ. Entomol. 2016, 45, 1379–1385. [Google Scholar] [CrossRef]
- Barrantes, G.B. La viuda negra en Costa Rica: Información general. Biocenosis 2016, 30, 63–96. [Google Scholar]
- Vetter, R.S.; Penas, L.M.; Hoddle, M.S. Laboratory refugia preferences of the brown widow spider, Latrodectus geometricus (Araneae: Theridiidae). J. Arachnol. 2016, 44, 52–57. [Google Scholar] [CrossRef]
- Schoeninger, K.; de Pádua, D.G.; Salvatierra, L.; de Oliveira, M.L. First record of Pediobius pyrgo Walker (Hymenoptera: Eulophidae) in South America and its emergence from egg sacs of Latrodectus geometricus CL Koch (Araneae: Theridiidae). EntomoBrasilis 2015, 8, 79–81. [Google Scholar] [CrossRef]
- Taucare-Ríos, A.; Alvarenga, T.M.; Costa, V.A. Occurrence of Philolema sp. (Hymenoptera: Eurytomidae) in Latrodectus thoracicus (Nicolet, 1849) (Araneae: Theridiidae) egg sacs. Arachnology 2018, 17, 334–336. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2019. Available online: https://qgis.org/ (accessed on 10 January 2024).
- Iqbal, M.; Austin, A. Systematics of the wasp genus Ceratobaeus Ashmead (Hymenoptera: Scelionidae) from Australasia: Parasitoids of spider eggs. Rec. South Aust. Mus. Monogr. Ser. 2000, 6, 1–164. [Google Scholar]
- Rasband, W.S. ImageJ; US National Institutes of Health: Bethesda, MA, USA, 1997–2016. Available online: https://imagej.nih.gov/ij/ (accessed on 10 January 2024).
- Masner, L. Revisionary notes and keys to world genera of Scelionidae (Hymenoptera: Proctorupoidea). Mem. Entomol. Soc. Can. 1976, 108, 1–87. [Google Scholar] [CrossRef]
- Hansson, C. Eulophidae of Costa Rica, 1; Memoirs of the American Entomological Institute: Logan, UT, USA, 2002; Volume 67, pp. 1–292. [Google Scholar]
- Gibson, G.A.P.; Huber, J.T.; Woolley, J.B. Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); National Research Council Research Press: Ottawa, ON, Canada, 1997; pp. 1–794. [Google Scholar]
- Lotfalizadeh, H.; Delvare, G.; Rasplus, J.Y. Phylogenetic analysis of Eurytominae (Chalcidoidea: Eurytomidae) based on morphological characters. Zool. J. Linn. Soc. 2007, 151, 441–510. [Google Scholar] [CrossRef]
- Wheeler, T.A. Chloropidae. In Manual of Central American Diptera, 1st ed.; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbado, M.A., Eds.; National Research Council: Ottawa, ON, Canada, 2010; Volume 2, pp. 1137–1153. [Google Scholar]
- Helicon Focus (RRID: SCR_014462) Editing Photographs Program. Available online: https://www.heliconsoft.com/focus/help/spanish/HeliconFocus.html (accessed on 21 October 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: www.R-project.org (accessed on 10 January 2024).
- Odum, E.P. Fundamentals of Ecology; W. B. Saunders Co.: Philadelphia, PA, USA, 1953; pp. 1–384. [Google Scholar]
- Elton, C.S. Ecology of Invasions by Animals and Plants; Chapman and Hall: London, UK, 1958; pp. 7–261. [Google Scholar]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Landi, P.; Minoarivelo, H.O.; Brännström, Å.; Hui, C.; Dieckmann, U. Complexity and stability of ecological networks: A review of the theory. Popul. Ecol. 2018, 60, 319–345. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Johnson, J.C.; Urcuyo, J.; Moen, C.; Stevens, D.R. Urban heat island conditions experienced by the Western black widow spider (Latrodectus hesperus): Extreme heat slows development but results in behavioral accommodations. PLoS ONE 2019, 14, e0220153.m. [Google Scholar] [CrossRef]
- Damien, D.; Pierre, J.S.; van Baaren, J.; van Alphen, J.J.M. How temperature and habitat quality affect parasitoid lifetime reproductive success—A simulation study. Ecol. Modell. 2011, 222, 1604–1613. [Google Scholar]
- Burkman, C.E.; Gardiner, M.M. Urban greenspace composition and landscape context influence natural enemy community composition and function. Biol. Control 2014, 75, 58–67. [Google Scholar] [CrossRef]
- Rudd, H.; Vala, J.; Schaefer, V. Importance of backyard habitat in a comprehensive biodiversity conservation strategy: A connectivity analysis of urban green spaces. Restor. Ecol. 2002, 10, 368–375. [Google Scholar] [CrossRef]
- Rojas, M. Manejo sostenible de la broca del café (Hypothenemus hampei) mediante poda sistemática del cafeto en Costa Rica. Agron. Costarric. 2012, 36, 71–79. [Google Scholar] [CrossRef]
- Monge, P.; Partanen, T.; Wesseling, C.; Bravo, V.; Ruepert, C.; Burstyn, I. Assessment of pesticide exposure in the agricultural population of Costa Rica. Ann. Occup. Hyg. 2005, 49, 375–384. [Google Scholar] [PubMed]
- Blackman, A.; Naranjo, M.A. Does eco-certification have environmental benefits? Organic coffee in Costa Rica. Ecol. Econ. 2012, 83, 58–66. [Google Scholar] [CrossRef]
- Guido Cruz, F.; Castro Sánchez, S. Viviendo con la crisis cafetalera: Perspectivas futuras de pequeños y medianos productores de café en San Ramón de Alajuela, Costa Rica. InterSedes 2011, 7, 11–28. [Google Scholar]
- Pinheiro, L.A.; Dader, B.; Wanumen, A.C.; Pereira, J.A.; Santos, S.A.P.; Medina, P. Side Effects of pesticides on the Olive Fruit Fly parasitoid Psyttalia concolor (Szepligeti): A Review. Agronomy 2020, 10, 1755. [Google Scholar] [CrossRef]
- Mowery, M.A.; Arabesky, V.; Lubin, Y.; Segoli, M. Differential parasitism of native and invasive widow spider egg sacs. Behav. Ecol. 2022, 33, 565–572. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Vetter, R.S.; Howe, A.K. Egg toxicity in diverse spider taxa. J. Arachnol. 2017, 45, 209–212. [Google Scholar] [CrossRef]
- Austin, A.D. The fecundity, development and host relationships of Ceratobaeus spp. (Hymenoptera: Scelionidae), parasites of spider eggs. Ecol. Entomol. 1984, 9, 125–138. [Google Scholar] [CrossRef]
- Barrantes, G.; Eberhard, W.G.; Weng, J.L. Seasonal patterns of parasitism of the tropical spiders Theridion evexum (Araneae, Theridiidae) and Allocyclosa bifurca (Araneae, Araneidae) by the wasps Zatypota petronae and Polysphincta gutfreundi (Hymenoptera, Ichneumonidae). Rev. Biol. Trop. 2008, 56, 749–754. [Google Scholar] [CrossRef] [PubMed]



| Effect | Coefficients | SE | LCI | UCI |
|---|---|---|---|---|
| A Between species | ||||
| Intercept | −1.07 | 0.29 | −1.63 | −0.51 |
| L. geometricus | −1.23 | 0.34 | −1.90 | −0.55 |
| B Parasteatoda tepidariorum | ||||
| Intercept | −1.07 | 0.31 | 0.46 | 1.68 |
| Inter. Urbanization | 0.54 | 0.30 | −0.06 | 1.14 |
| Low urbanization | −0.66 | 0.91 | −2.44 | 1.11 |
| PC1 | 0.09 | 0.22 | −0.34 | 0.52 |
| C Latrodectus geometricus | ||||
| Intercept | −2.80 | 0.45 | −3.68 | −1.92 |
| Inter. Urbanization | 1.20 | 0.55 | 0.13 | 2.28 |
| PC1 | 0.07 | 0.15 | −0.22 | 0.36 |
| Effect | Coefficients | SE | LCI | UCI |
|---|---|---|---|---|
| A Between species | ||||
| Intercept | −2.15 | 0.55 | −3.22 | −1.08 |
| L. geometricus | −0.92 | 0.81 | −2.50 | 0.66 |
| B Parasteatoda tepidariorum | ||||
| Intercept | −1.42 | 0.46 | −2.31 | −0.52 |
| Inter. Urbanization | 0.22 | 0.49 | −0.74 | 1.18 |
| Low urbanization | −1.92 | 1.46 | −4.79 | 0.95 |
| PC1 | 0.12 | 0.32 | −0.51 | 0.75 |
| C Latrodectus geometricus | ||||
| Intercept | −5.25 | 1.91 | −9.00 | −1.50 |
| Inter. Urbanization | 2.81 | 1.85 | −0.81 | 6.44 |
| PC1 | 0.00 | 0.22 | −0.44 | 0.44 |
| Urbanism | Number of Egg Sacs Parasitized | Total Egg Sacs | Percentage of Egg Sacs Parasitized | Parasitoids | Predators |
|---|---|---|---|---|---|
| (A) Parasteatoda tepidariorum | |||||
| High | 21 | 67 | 31.3 | Baeus achaeraneus Idris sp. | No |
| Intermediate | 33 | 58 | 56.9 | ||
| Low | 8 | 36 | 22.2 | ||
| (B) Latrodectus geometricus | |||||
| High | 5 | 84 | 5.9 | Philolema sp. Pediobius pyrgo | Pseudogaurax sp. |
| Intermediate | 12 | 57 | 21.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Conejo, N.; Hanson, P.E.; Chacón-Madrigal, E.; Rojas-Malavasi, G. The Prevalence of Egg Parasitoids of Two Cobweb Spiders in a Tropical Urban Gradient. Arthropoda 2024, 2, 250-263. https://doi.org/10.3390/arthropoda2040018
Jiménez-Conejo N, Hanson PE, Chacón-Madrigal E, Rojas-Malavasi G. The Prevalence of Egg Parasitoids of Two Cobweb Spiders in a Tropical Urban Gradient. Arthropoda. 2024; 2(4):250-263. https://doi.org/10.3390/arthropoda2040018
Chicago/Turabian StyleJiménez-Conejo, Natalia, Paul E. Hanson, Eduardo Chacón-Madrigal, and Geovanna Rojas-Malavasi. 2024. "The Prevalence of Egg Parasitoids of Two Cobweb Spiders in a Tropical Urban Gradient" Arthropoda 2, no. 4: 250-263. https://doi.org/10.3390/arthropoda2040018
APA StyleJiménez-Conejo, N., Hanson, P. E., Chacón-Madrigal, E., & Rojas-Malavasi, G. (2024). The Prevalence of Egg Parasitoids of Two Cobweb Spiders in a Tropical Urban Gradient. Arthropoda, 2(4), 250-263. https://doi.org/10.3390/arthropoda2040018






