Sustainable Warm-Climate Forage Legumes: Versatile Products and Services
Abstract
1. Introduction
2. Pulses for Food and Pharmaceuticals
2.1. Primary Pulses
2.2. Nutritional Properties
2.3. Food Uses
2.4. Milk Substitutes
2.5. Leafy Vegetables
2.6. Pharmaceuticals
3. Forages
3.1. Monocultures
3.2. Mixtures with Grasses
3.3. Fodder Banks
3.4. Silvopasture
4. Bioenergy
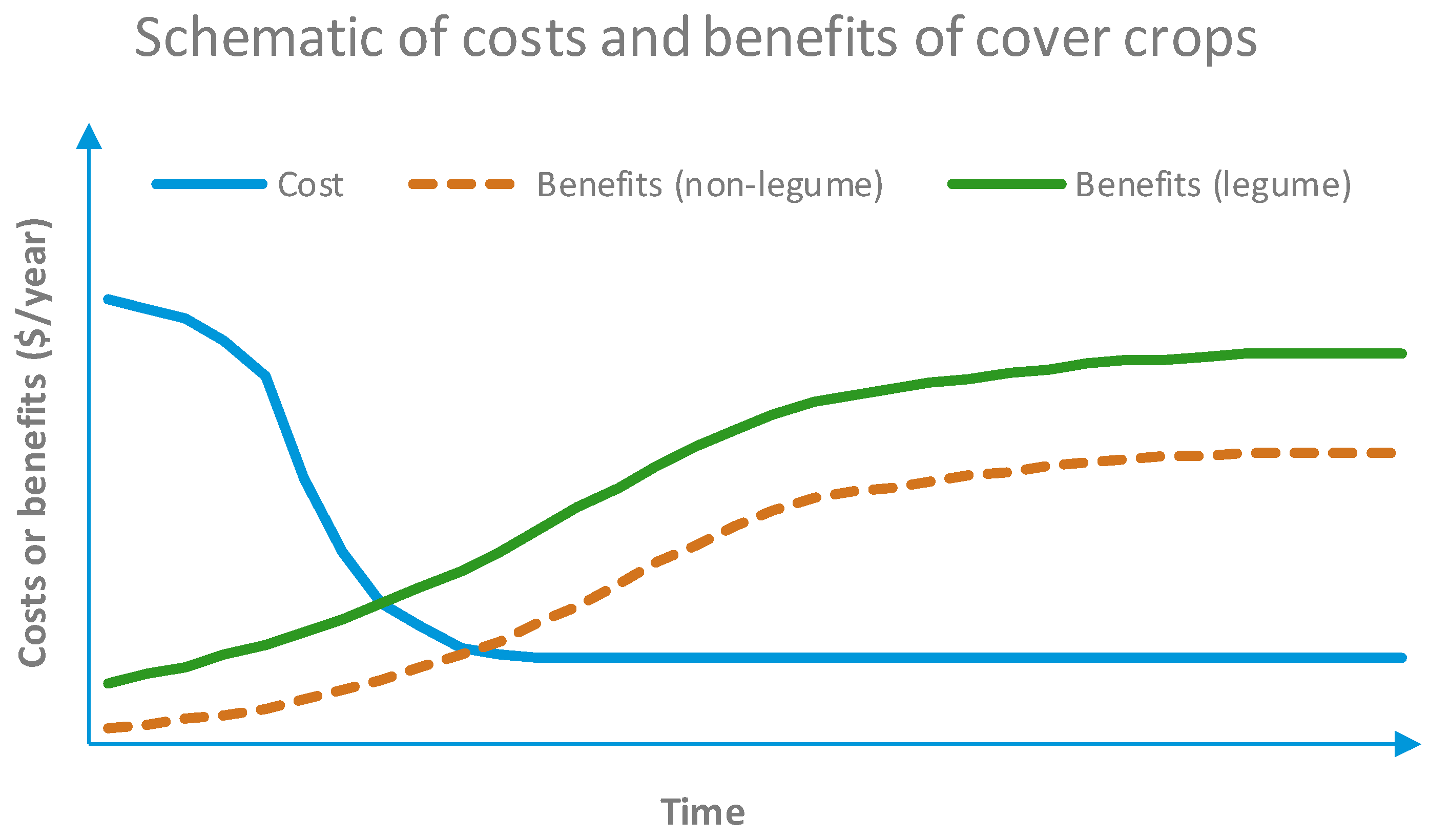
5. Cover Crops
6. Soil Health and Fertility
7. Ecosystem Services
8. Economics
8.1. Agroecosystem Profitability
8.2. Economic Flexibility and Risk Mitigation
8.3. Specific Contributions
8.4. Policy Challenges and Barriers to Adoption
9. Genomics
10. Conclusions: Versatility into the Future
Author Contributions
Funding
Conflicts of Interest
References
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for science and breeding. Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Bouton, J.H. An overview of the role of lucerne (Medicago sativa L.) in pastoral agriculture. Crop Pasture Sci. 2012, 63, 734–738. [Google Scholar] [CrossRef]
- Dubeux, J.C.B., Jr.; Muir, J.P.; Apolinário, V.X.O.; Ramachandran Nair, P.K.; Lira, M.A.; Sollenberger, L.E. Tree legumes: An underexploited resource in warm-climate silvopastures. Rev. Bras. Zootec. 2017, 46, 689–703. [Google Scholar] [CrossRef]
- Tomic, D.D.; Stevovic, V.L.; Durovic, D.; Marjanovic, M.; Madic, M.R.; Pavlovic, N.; Lazarevic, D.; Petrovic, M.; Radovanovic, M. Perennial forage legumes as an element of sustainable systems. Not. Bot. Horti Agrobot. 2023, 51, 13240. [Google Scholar] [CrossRef]
- Ehlers, J.D.; Hall, A.E. Cowpea (Vigna unguiculata L. Walp.). Field Crops Res. 1997, 53, 187–204. [Google Scholar] [CrossRef]
- Dubeux, J.C.B., Jr.; Sollenberger, L.E.; Muir, J.P.; Tedeschi, L.O.; Santos, M.V.F.; Cunha, M.V.; Mello, A.C.L.; DiLorenzo, N. Sustainable intensification of livestock production on pastures. Arch. Latinoam. Prod. Anim. 2017, 25, 97–111. [Google Scholar]
- Depablos, L.; Homem, B.G.C.; Ferreira, I.M.; Bernardes, T.F.; Boddey, R.M.; Lara, M.A.S.; Casagrande, D.R. Nitrogen cycling in tropical grass-legume pastures managed under canopy light interception. Nutr. Cycl. Agroecosyst. 2021, 121, 51–67. [Google Scholar] [CrossRef]
- Dubeux, J.C.B., Jr.; Sollenberger, L.E.; Mathews, B.W.; Scholberg, J.M.; Santos, H.Q. Nutrient cycling in warm-climate grasslands. Crop Sci. 2007, 47, 915–928. [Google Scholar] [CrossRef]
- Jaramillo, D.M.; Dubeux, J.C.B., Jr.; Sollenberger, L.; Mackowiak, C.; Vendramini, J.M.B.; DiLorenzo, N.; Queiroz, L.M.D.; Santos, E.R.S.; Garcia, L.; Ruiz-Moreno, M.; et al. Litter mass, deposition rate, and decomposition in nitrogen-fertilized or grass–legume grazing systems. Crop Sci. 2021, 61, 2176–2189. [Google Scholar] [CrossRef]
- Santos, E.R.S.; Dubeux, J.C.B., Jr.; Jaramillo, D.M.; Garcia, L.; Mackowiak, C.L.; Blount, A.R.S.; Pereira-Neto, J.D.; Queiroz, L.M.D.; Ruiz-Moreno, M. Herbage responses and nitrogen agronomic efficiency of bahiagrass–legume mixtures. Agron. J. 2020, 112, 4057–4068. [Google Scholar] [CrossRef]
- Cantarutti, R.B.; Tarré, R.; Macedo, R.; Cadisch, G.; Rezende, C.P.; Pereira, J.M.; Braga, J.M.; Gomide, J.A.; Ferreira, E.; Alves, B.J.R.; et al. The effect of grazing intensity and the presence of a forage legume on nitrogen dynamics in Brachiaria pastures in the Atlantic Forest region of the South of Bahia, Brazil. Nutr. Cycl. Agroecosyst. 2002, 64, 257–271. [Google Scholar] [CrossRef]
- dos Santos Souza, W.; de Paula Rezende, C.; Marques Pereira, J.; Cassador Monteiro, R.; dos Santos, C.A.; de Oliveira Macedo, R.; Barbosa Alecrim, F.; Machado Pinheiro, E.F.; de Campos, D.V.B.; Urquiaga, S.; et al. Can N2 fixation by forage legumes build soil organic matter to rival fertilizer N in a tropical forest biome? Geoderma Reg. 2023, 33, e00646. [Google Scholar] [CrossRef]
- Silva, L.S.; Sollenberger, L.E.; Kimberly Mullenix, M.; Kohmann, M.M.; Dubeux, J.C.B., Jr.; Silveira, M.L. Soil carbon and nitrogen stocks in nitrogen-fertilized grass and legume-grass forage systems. Nutr. Cycl. Agroecosyst. 2022, 122, 105–117. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Frossard, E.; Luscher, A. Effects of legumes and fertiliser on nitrogen balance and nitrate leaching from intact leys and after tilling for subsequent crop. Agric. Ecosyst. Environ. 2024, 360, 108776. [Google Scholar] [CrossRef]
- Garcia, L.; Dubeux, J.C.B., Jr.; Sollenberger, L.E.; Vendramini, J.M.B.; DiLorenzo, N.; Santos, E.R.S.; Jaramillo, D.M.; Ruiz-Moreno, M. Nutrient excretion from cattle grazing nitrogen-fertilized grass or grass–legume pastures. Agron. J. 2021, 113, 3110–3123. [Google Scholar] [CrossRef]
- Garzon, J.; Vendramini, J.M.B.; Silveira, M.L.; Dubeux, J.C.B., Jr.; Liao, H.-L.; Sollenberger, L.E.; da Silva, H.M.S.; Gomes, V.C.; de Oliveira, H.M.R. Overseeding aeschynomene and N fertilization effects on forage characteristics, N fixation, and N2O-N emissions of bahiagrass pastures. Crop Sci. 2023, 63, 2594–2607. [Google Scholar] [CrossRef]
- Neely, C.B.; Rouquette, F.M., Jr.; Morgan, C.L.S.; Hons, F.M.; Rooney, W.L.; Smith, G.R. Impact on soil organic C and total soil N from cool- and warm-season legumes used in a green manure-forage cropping system. Agric. Sci. 2024, 15, 333–357. [Google Scholar] [CrossRef]
- Carvalho, M.; Bebeli, P.J.; Pereira, G.; Castro, I.; Egea-Gilabert, C.; Matos, M.; Lazaridi, E.; Duarte, I.; Lino-Neto, T.; Ntatsi, G.; et al. European cowpea landraces for a more sustainable agriculture system and novel foods. J. Sci. Food Agric. 2017, 97, 4399–4407. [Google Scholar] [CrossRef]
- Martos-Fuentes, M.; Fernández, J.A.; Ochoa, J.; Carvalho, M.; Carnide, V.; Rosa, V.; Pereira, G.; Barcelos, C.; Bebeli, J.P.; Egea-Gilabert, C. Genotype by environment interactions in cowpea (Vigna unguiculata L. Walp.) grown in the Iberian Peninsula. Crop Pasture Sci. 2017, 68, 924–931. [Google Scholar] [CrossRef]
- Alharbi, N.H.; Adhikari, K.N. Factors of yield determination in faba bean (Vicia faba). Crop Pasture Sci. 2020, 71, 305–321. [Google Scholar] [CrossRef]
- Bunce, J.A. Contrasting responses of seed yield to elevated carbon dioxide under field conditions within Phaseolus vulgaris. Agric. Ecosyst. Environ. 2008, 128, 219–224. [Google Scholar] [CrossRef]
- Chaturvedi, G.S.; Aggarwal, P.K.; Sinha, S.K. Growth and yield of determinate and indeterminate cowpeas in dryland agriculture. J. Agric. Sci. 2018, 94, 137–144. [Google Scholar] [CrossRef]
- Asante, B.O.; Villano, B.A.; Patrick, I.W.; Battese, G.E. Impacts of exposure and access to seed on the adoption of dual-purpose cowpea and groundnut varieties in Ghana. J. Dev. Areas 2017, 51, 173–194. [Google Scholar] [CrossRef]
- Butkute, B.; Taugenis, L.; Norkeviciene, E. Small-seeded legumes as a novel food source. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds, sprouted seeds and microgreens. Molecules 2018, 24, 133. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A.A. Exploring the nutritional potential of wild and underutilized legumes. Compr. Rev. Food Sci. Food Saf. 2009, 8, 305–331. [Google Scholar] [CrossRef]
- de Lima Coêlho, D.; Dubeux, J.C.B.; dos Santos, M.V.F.; de Mello, A.C.L.; da Cunha, M.V.; de Freitas, E.V.; de Almeida, B.G.; de Oliveira Apolinário, V.X.; Ferraz, A.P.F.; Simili, F.F. Can silvopasture with arboreal legumes increase root mass at deeper soil layers and improve soil aggregation? Soil Sci. Soc. Am. J. 2024, 88, 2211–2226. [Google Scholar] [CrossRef]
- Dubeux Junior, J.C.B.; Trumpp, K.R.; Queiroz, L.D.; Brêtas, I.L.; Bernardini, M.; Portuguez, J.; Garcia, L.; Odour, K.; Simili, F.F.; Bizutti, B.; et al. Forage Legumes: A Potential Way to Reduce Fertilizer Inputs; The Florida Cattleman and Livestock Journal; Florida Cattleman Association: Kissimmee, FL, USA, 2024; pp. 42–44. [Google Scholar]
- Muller, M.-H.; Poncet, C.; Prosperi, J.M.; Santoni, S.; Ronfort, J. Domestication history in the Medicago sativa species complex: Inferences from nuclear sequence polymorphism. Mol. Ecol. 2006, 15, 1589–1602. [Google Scholar] [CrossRef]
- Desmae, H.; Janila, P.; Okori, P.; Pandey, M.K.; Motagi, B.N.; Monyo, E.; Mponda, O.; Okello, D.; Sako, D.; Echeckwu, C.; et al. Genetics, genomics and breeding of groundnut (Arachis hypogaea, L.). Plant Breed. 2019, 138, 425–444. [Google Scholar] [CrossRef]
- Vinithashri, G.; Manivannan, N.; Viswanathan, P.L.; Selvakumar, T. Genetic variability, heritability and genetic advance of yield and related traits in F3 generation of groundnut (Arachis hypogaea L.). Electron. J. Plant Breed. 2019, 10, 1292–1297. [Google Scholar] [CrossRef]
- Fuller, D.Q.; Harvey, E.L. The archaeobotany of Indian pulses: Identification, processing and evidence for cultivation. Environ. Arch. 2006, 11, 219–246. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agricultural Organization of the United Nations; FAOSTAT: Rome, Italy, 2020; Available online: http://www.fao.org/faostat/en/#search/chick%20pea (accessed on 1 March 2024).
- Campos-Vega, R.; Reynoso-Camacho, R.; Pedraza-Aboytes, G.; Acosta, J.A.; Guzman-Maldonado, S.H.; Paredes-Lopez, O.; Ooman, B.D.; Loarca-Pina, G. Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.). J. Food Sci. 2009, 74, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Geil, P.B.; Anderson, J.W. Nutrition and health implications of dry beans: A review. J. Am. Coll. Nutr. 1994, 13, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, S437–S442. [Google Scholar] [CrossRef] [PubMed]
- Sozer, N.; Holopainen-Mantila, U.; Poutanen, K. Traditional and new food uses of pulses. Cereal Chem. 2017, 94, 66–73. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Re-defining efficiency of feed use by livestock. Animal 2011, 5, 1014–1022. [Google Scholar] [CrossRef]
- Vallath, A.; Shanmugam, A.; Rawson, A. Prospects of future pulse milk variants from other healthier pulses—As an alternative to soy milk. Trends Food Sci. Technol. 2022, 124, 51–62. [Google Scholar] [CrossRef]
- Muir, J.P.; Tedeschi, L.O.; Dubeux, J.C.B., Jr.; Peters, M.; Burkart, S. Enhancing food security in Latin America with forage legumes. Arq. Lat. Am. De Produção Anim. 2017, 25, 113–131. Available online: https://ojs.alpa.uy/index.php/ojs_files/article/view/2577 (accessed on 1 March 2024).
- Ibrikci, H.; Knewtson, J.B.S.; Grusak, M.A. Chickpea leaves as a vegetable green for humans: Evaluation of mineral composition. J. Sci. Food Agric. 2003, 83, 945–950. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Ohler, T.A.; Mitchell, C.A. Cowpea leaves for human consumption: Production, utilization and nutrient composition. In Advances in Cowpea Research; Singh, B.B., Raj, D.R.M., Dashiell, K., Jackai, L.E.N., Eds.; Co-publication of International Institute of Tropical Agriculture (IITA) and Japan International Center for Agricultural Sciences (JIRCAS); IITA: Ibadan, Nigeria, 1997; pp. 326–332. Available online: https://pdfs.semanticscholar.org/e580/e3171955abe1e25ceb7bb86f62d78629d447.pdf (accessed on 1 March 2024).
- Singh, B.B. Cowpea: The Food Legume of the 21st Century; Crop Science Society of America: Madison, WI, USA, 2014. [Google Scholar] [CrossRef]
- Enyiukwu, D.N.; Amadioha, A.C.; and Ononuju, C.C. Nutritional significance of cowpea leaves for human consumption. Greener Trends Food Sci. Nutr. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Bittenbender, H.C. Handling and storage of cowpea (Vigna unguiculata L. Walp) as leaf vegetable. Trop. Agric. 1992, 69, 197–199. [Google Scholar]
- Emily, M.T.; Padhi, D.; Ramdath, D. A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. J. Funct. Foods 2017, 38, 635–643. [Google Scholar] [CrossRef]
- Magalhães, S.C.Q.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.M.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insigth into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Cho, S.J.; Juillerat, M.A.; Lee, C.H. Cholesterol lowering mechanism of soybean protein hydrolysate. J. Agric. Food Chem. 2007, 55, 10599–10604. [Google Scholar] [CrossRef]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38(Pt. B), 602–611. [Google Scholar] [CrossRef]
- Stephens, F.O. Phytoestrogens and prostate cancer: Possible preventive role. Med. J. Aust. 1997, 167, 138–140. [Google Scholar] [CrossRef]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef]
- Muir, J.P.; Santos, M.V.F.; Cunha, M.V.; Dubeux, J.C.B., Jr.; Lira, M.A., Jr.; Souza, R.T.A.; Souza, T.C. Value of endemic legumes for livestock production on caatinga rangelands. Rev. Bras. Ciências Agrárias 2019, 14, e5648. [Google Scholar] [CrossRef]
- Shelton, H.M.; Franzel, S.; Peters, M. Adoption of tropical legume technology around the world: Analysis of success. Trop. Grassl-Forrajes Trop. 2005, 39, 198–209. Available online: https://brill.com/edcollchap/book/9789086865512/BP000012.xml (accessed on 1 March 2024).
- Teixeira, V.I.; Dubeux, J.C.B., Jr.; Santos, M.V.F.; Lira, M.A., Jr.; Lira, M.A.; Silva, H.M.S. Agronomic and bromatologic aspects of forage legumes from Brazilian NE. Arch. Zootec. 2010, 59, 245–254. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0004-05922010000200010 (accessed on 1 March 2024). [CrossRef]
- Lira Junior, M.A.; Fracetto, F.J.C.; Ferreira, J.d.S.; Silva, M.B.; Fracetto, G.G.M. Legume silvopastoral systems enhance soil organic matter quality in a subhumid tropical environment. Soil Sci. Soc. Am. J. 2020, 84, 1209–1218. [Google Scholar] [CrossRef]
- Lira Junior, M.A.; Fracetto, F.J.C.; Ferreira, J.S.; Silva, M.B.; Fracetto, G.G.M. Legume-based silvopastoral systems drive C and N soil stocks in a subhumid tropical environment. Catena 2020, 189, 104508. [Google Scholar] [CrossRef]
- Putnam, D.H.; Summers, C.G.; Orloff, S.B. Alfalfa production systems in California. In Irrigated Alfalfa Management for Mediterranean and Desert Zones; Chapter 1; Summers, C.G., Putnam, D.H., Eds.; University of California Agriculture and Natural Resources publication: Oakland, CA, USA, 2007; Publication 8287; Available online: http://alfalfa.ucdavis.edu/IrrigatedAlfalfa (accessed on 7 June 2020).
- Garay, A.H.; Sollenberger, L.E.; Staples, C.R.; Pedreira, C.G.S. ‘Florigraze’ and ‘Arbrook’ rhizoma peanut as pasture for growing Holstein heifers. Crop Sci. 2004, 44, 1355–1360. [Google Scholar] [CrossRef]
- Jaramillo, D.M.; Dubeux, J.C.B., Jr.; Mackowiak, C.; Sollenberger, L.E.; DiLorenzo, N.; Rowland, D.L.; Blount, A.R.S.; Santos, E.R.S.; Garcia, L.; Ruiz-Moreno, M. Annual and perennial peanut mixed with ‘Pensacola’ bahiagrass in North Florida. Crop Sci. 2018, 58, 982–992. [Google Scholar] [CrossRef]
- Jaramillo, D.M.; Dubeux, J.C.B., Jr.; Mackowiak, C.; Sollenberger, L.E.; Santos, E.R.S.; Garcia, L.; Ruiz-Moreno, M.; Silva, C.S.; DiLorenzo, N. Annual and perennial peanut species as alternatives to nitrogen fertilizer in bermudagrass hay production systems. Agron. J. 2018, 110, 2390–2399. [Google Scholar] [CrossRef]
- Tamele, O.H.; Sá, O.A.A.L.; Bernardes, T.F.; Lara, M.A.S.; Casagrande, D.R. Optimal defoliation management of brachiaria grass—Forage peanut for balanced pasture establishment. Grass Forage Sci. 2018, 73, 522–531. [Google Scholar] [CrossRef]
- Ribeiro, O.L.; Cecato, U.; Iwamoto, B.S.; Pinheiro, A.; Jobim, C.C.; Damasceno, J.C. Performance of beef cattle grazing Tanzania grass fertilized with nitrogen or intercropped with Stylo. Rev. Bras. Saúde Produção Anim. 2011, 12, 275–285. Available online: https://www.cabdirect.org/cabdirect/abstract/20113132135 (accessed on 1 March 2024).
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Luscher, A. Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Casanova-Lugo, F.; Petit-Aldana, J.; Solorio-Sánchez, F.; Ramírez-Avilés, L.; Ward, S.E.; Villanueva-López, G.; Aryal, D.R. Carbon stocks in biomass and soils of woody species fodder banks in the dry topics of Mexico. Soil. Use Manag. 2018, 34, 500–509. [Google Scholar] [CrossRef]
- Smolikowski, B.; Puig, H.; Roose, E. Influence of soil protection techniques on runoff, erosion and plant production on semi-arid hillsides of Cabo Verde. Agric. Ecosyst. Environ. 2001, 87, 67–80. [Google Scholar] [CrossRef]
- Muir, J.P.; Pitman, W.D.; Foster, J.L.; Dubeux, J.C.B., Jr. Sustainable intensification of cultivated pastures using multiple herbivore species. Afr. J. Range Sci. 2015, 32, 97–112. [Google Scholar] [CrossRef]
- da Silva, I.A.G.; Dubeux, J.C.B., Jr.; Souza, C.G.; Moreno, M.R.; Dos Santos, M.V.F.; de Oliveira Apolinario, V.X.; de Mello, A.C.L.; da Cunha, M.V.; Muir, J.P.; Lira Junior, M.A. Nutritive value and condensed tannins of tree legumes in silvopasture systems. Sci. Rep. 2024, 14, 18080. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, V.X.O.; Dubeux, J.C.B., Jr.; Lira, M.A.; Ferreira, R.L.C.; Mello, A.C.L.; Santos, M.V.F.; Sampaio, E.V.S.B.; Muir, J.P. Tree legumes provide marketable wood and add nitrogen in warm-climate silvopasture systems. Agron. J. 2015, 107, 1915–1921. [Google Scholar] [CrossRef]
- Costa, S.B.M.; Mello, A.C.L.; Dubeux, J.C.B., Jr.; Santos, M.V.F.; Lira, M.A.; Oliveira, J.T.C.; Apolinário, V.X.O. Livestock performance in warm-climate silvopastures using tree legumes. Agron. J. 2016, 108, 2026–2035. [Google Scholar] [CrossRef]
- Santos, A.M.G.; Dubeux, J.C.B., Jr.; Santos, M.V.F.; Lira, M.A.; Apolinário, V.X.O.; Costa, S.B.M.; Coêlho, D.L.; Peixôto, T.V.F.R.; Santos, E.R.S. Animal performance in grass monoculture or silvopastures using tree legumes. Agrofor. Syst. 2019, 94, 615–626. [Google Scholar] [CrossRef]
- Apolinário, V.X.O.; Dubeux, J.V.B., Jr.; Lira, M.A.; Ferreira, R.L.C.; Mello, A.C.L.; Coelho, D.L.; Muir, J.P.; Sampaio, E.V.S.B. Decomposition of arboreal legume fractions in a silvopastoral system. Crop Sci. 2016, 56, 1356–1363. [Google Scholar] [CrossRef]
- Yang, L.; Takase, M.; Zhang, M.; Zhao, T.; Wu, X. Potential non-edible oil feedstock for biodiesel production in Africa: A survey. Renew. Sust. Energy Rev. 2014, 38, 461–477. [Google Scholar] [CrossRef]
- Babu, S.C.; Debnath, D. Bioenergy economy, food security, and development. In Biofuels, Bioenergy and Food Security; Debnath, D., Babu, S.C., Eds.; Academic Press: London, UK, 2019; pp. 3–22. [Google Scholar] [CrossRef]
- IEA. Data and Statistics. Available online: https://www.iea.org/data-and-statistics (accessed on 13 February 2019).
- Chel, A.; Kaushik, G. Renewable energy for sustainable agriculture. Agron. Sustain. Dev. 2011, 31, 91–118. [Google Scholar] [CrossRef]
- FAO. Conservation Agriculture. Food and Agriculture Organization of the United Nations. 2017. Available online: http://www.fao.org/3/a-i7480e.pdf (accessed on 1 March 2024).
- Seifert, T.; Ackerman, P.; Chirwa, P.W.; von Doderer, C.; du Toit, B.; Görgens, J.; Ham, C.; Kunneke, A.; Meincken, M. Biomass from wood in the tropics. In Bioenergy from Wood—Sustainable Production in the Tropics; Seifert, T., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–10. [Google Scholar]
- Heya, M.N.; Pournavab, R.F.; Parra, A.C.; Maiti, R.; Salas Cruz, L.R. Timber-yielding plants of the Tamaulipan Thorn Scrub: Forest, fodder, and bioenergy potential. In Biology, Productivity and Bioenergy of Timber-yielding Plants; SpringerBriefs in Plant Science; Springer: Cham, Switzerland, 2017; pp. 1–119. [Google Scholar] [CrossRef]
- Bessou, C.; Ferchaud, F.; Gabrielle, B.; Mary, B. Biofuels, greenhouse gases and climate change. In Sustainable Agriculture; Lichtfouse, E., Hamelin, M., Navarrete, M., Debaeke, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 2. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B.J.R.; Morrison, M.J. Legumes for mitigation of climate change and provision of feedstocks for biofuels and biorefineries. Rev. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Costa, K.C.P.; Lima, R.M.B.; Ferreira, M.J. Biomass and energy yield of leguminous trees cultivated in Amazonas. Floresta 2014, 45, 705–712. Available online: https://www.alice.cnptia.embrapa.br/handle/doc/1037660 (accessed on 1 March 2024). [CrossRef]
- Foroughbakhch, R.; Háuad, L.A.; Cespedes, A.E.; Ponce, E.E.; González, N. Evaluation of 15 indigenous and introduced species for reforestation and agroforestry in northeastern Mexico. Agrofor. Syst. 2001, 51, 213–221. [Google Scholar] [CrossRef]
- Kazakoff, S.H.; Gresshoff, P.M.; Scott, P.T. Pongamia pinnata, a sustainable feedstock for biodiesel production. In Energy Crops; Halford, N.G., Karp, A., Eds.; Energy and Environment Series No. 3; Royal Society of Chemistry: London, UK, 2010; pp. 233–254. Available online: https://books.google.com/books?hl=en&lr=&id=YHIoDwAAQBAJ&oi=fnd&pg=PA233&dq=Kazakoff+SH,+Gresshoff+PM,+Scott+PT+(2010)+Pongamia+pinnata,+a+sustainable+feedstock+for+biodiesel+production.+In:+Halford+N,+Karp+A+(eds)+Energy+crops.+Royal+Society+of+Chemistry,+London,+pp.+233%E2%80%93254.&ots=_UcHHCsrja&sig=0e9l-LYstcVtLIMPqj8MyEV28IM#v=onepage&q&f=false (accessed on 1 March 2024).
- Singh, Y.P.; Singh, G.; Sharma, D.K. Biomass and bio-energy production of ten multipurpose tree species planted in sodic soils of indo-gangetic plains. J. Res. 2010, 21, 19–24. [Google Scholar] [CrossRef]
- Biswas, B.; Scott, P.T.; Gresshoff, P.M. Tree legumes as feedstock for sustainable biofuel production: Opportunities and challenges. J. Plant Physiol. 2011, 168, 1877–1884. [Google Scholar] [CrossRef]
- Chen, W.; Thanapal, S.S.; Annamalai, K.; Ansley, R.J. Liquid yield from juniper and mesquite bio-fuel gasification. Int. J. Energy Res. 2015, 39, 621–633. [Google Scholar] [CrossRef]
- Biswas, B.; Kazakoff, S.H.; Jiang, Q.; Samuel, S.; Gresshoff, P.M.; Scott, P.T. Genetic and genomic analysis of the tree legume Pongamia pinnata as a feedstock for biofuel. Plant Genome 2013, 6, plantgenome2013-05. [Google Scholar] [CrossRef]
- Borchard, N.; Bulusu, M.; Hartwig, A.-M.; Ulrich, M.; Lee, S.M.; Baral, H. Screening potential bioenergy production of tree species in degraded and marginal land in the Tropics. Forests 2018, 9, 594. [Google Scholar] [CrossRef]
- Gresshoff, P.M. Modern biology analysis of the legume tree Pongamia pinnata as a sustainable biofuel source. Legume Perspect. 2015, 6, 25–28. [Google Scholar]
- Gresshoff, P.M.; Rangan, L.; Indrasumunar, A.; Scott, P.T. A new bioenergy crop based on oil-rich seeds from the legume tree Pongamia pinnata. Energy Emiss. Control Technol. 2017, 5, 19–26. [Google Scholar] [CrossRef]
- Kazakoff, S.H.; Imelfort, M.; Edwards, D.; Koehorst, J.; Biswas, B.; Batley, J.; Scott, P.T.; Gresshoff, P.M. Capturing the biofuel wellhead and powerhouse: The chloroplast and mitochondrial genomes of the leguminous feedstock tree Pongamia pinnata. PLoS ONE 2012, 7, e51687. [Google Scholar] [CrossRef]
- Murphy, H.T.; O’Connell, D.A.; Seaton, G.; Raison, R.J.; Rodriguez, L.C.; Braid, A.L.; Kriticos, D.J.; Jovanovic, T.; Abadi, A.; Betar, M. A common view of the opportunities, challenges and research actions for Pongamia in Australia. Bioenerg. Res. 2012, 5, 778–799. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium. Available online: https://www.cabi.org/isc/research (accessed on 11 June 2020).
- Global Biodiversity Information Facility—GBIF. Get Data. Occurrences. 2020. Available online: https://www.gbif.org/occurrence/search (accessed on 11 June 2020).
- JSTOR. Global Plants. Available online: https://plants.jstor.org (accessed on 11 June 2020).
- National Research Council. Firewood Crops: Shrub and Tree Species for Energy Production; The National Academies Press: Washington, DC, USA, 1980. [CrossRef]
- Useful Tropical Plants. Useful Tropical Plants Database. 2020. Available online: http://tropical.theferns.info (accessed on 11 June 2020).
- World Flora Online—WFO. An Online Flora of All Known Plants. 2020. Available online: http://www.worldfloraonline.org (accessed on 10 June 2020).
- Sollenberger, L.E.; Kohmann, M.M.; Dubeux, J.C.; Silveira, M.L. Grassland management affects delivery of regulating and supporting ecosystem services. Crop Sci. 2019, 59, 441–459. [Google Scholar] [CrossRef]
- Dubache, M.; Russelle, M.P. Forage legume roots and nodules and their role in nitrogen transfer. Agron. J. 1994, 86, 259–266. [Google Scholar] [CrossRef]
- Karlen, D.L.; Lemunyon, J.L.; Singer, J.W. Forages for conservation and improved soil quality. In Forages: The Science of Grassland Agriculture, 6th ed.; Barnes, R.F., Nelson, C.J., Moore, K.J., Collins, M., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2007. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=163970 (accessed on 1 March 2024).
- Katsvairo, T.W.; Rich, J.R.; Dunn, R.A. Perennial grass rotation: An effective and challenging tactic for nematode management with many other positive effects. Pest Manag. Sci. 2006, 62, 793–796. [Google Scholar] [CrossRef]
- Mahama, G.Y.; Vara Prasad, P.V.; Roozeboom, K.L.; Nippert, J.B.; Rice, C.W. Cover crops, fertilizer nitrogen rates, and economic return of grain sorghum. Agron. J. 2016, 108, 1–16. [Google Scholar] [CrossRef]
- Olson, R.A.; Brewer, M.J. Small mammal populations occurring in a diversified winter wheat cropping system. Agric. Ecosyst. Environ. 2003, 95, 311–319. [Google Scholar] [CrossRef]
- Barros, V.D.C.; Lira Junior, M.A.; Fracetto, F.J.C.; Fracetto, G.G.M.; Ferreira, J.S.; de Barros, D.J.; da Silva Júnior, A.F. Effects of different legume green manures on tropical soil microbiology after corn harvest. Bragantia 2020, 79, 505–515. [Google Scholar] [CrossRef]
- Unger, P.W.; Vigil, M.F. Cover crop effects on soil water relationships. J. Soil Water Conserv. 1998, 53, 200–207. Available online: https://www.jswconline.org/content/53/3/200 (accessed on 3 June 2020).
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality-A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; SSSA: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Quantitative indicators of soil quality: A minimum data set. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 25–37. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Delgado, A.; Gomez, J.A. The Soil. Physical, Chemical and Biological Properties. In Principles of Agronomy for Sustainable Agriculture; Villalobos, F., Fereres, E., Eds.; Springer: Cham, Switzerland, 2017; pp. 15–26. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of Permanganate-Oxidizable Carbon and Mineralizable Carbon for Assessment of Organic Matter Stabilization and Mineralization. Soil Water Manag. Conserv. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265. [Google Scholar] [CrossRef]
- Sainju, U.M.; Whitehead, W.F.; Singh, B.P. Biculture legume-cereal cover crops for enhanced biomass yield and carbon and nitrogen. Agron. J. 2005, 97, 1403–1412. [Google Scholar] [CrossRef]
- Allison, F.E. The fate of nitrogen applied to soils. Adv. Agron. 1966, 18, 219–258. [Google Scholar] [CrossRef]
- Russelle, M.P.; Allan, D.L.; Gourley, C.J.P. Direct assessment of symbiotically fixed nitrogen in the rhizosphere of alfalfa. Plant Soil 1994, 159, 233–243. [Google Scholar] [CrossRef]
- Smith, M.S.; Frye, W.W.; Varco, J.J. Legume winter cover crops. In Advances in Soil Science 7; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1987; pp. 95–139. [Google Scholar] [CrossRef]
- Mathenge, C.; Thuita, M.; Masso, C.; Gweyi-Onyango, J.; Vanlauwe, B. Variability of soybean response to rhizobia inoculant, vermicompost, and a legume-specific fertilizer blend in Siaya County of Kenya. Soil Tillage Res. 2019, 194, 104290. [Google Scholar] [CrossRef]
- Mortinho, E.S.; Jalal, A.; Oliveira, C.E.S.; Fernandes, G.C.; Pereira, N.C.M.; Rosa, P.A.L.; Do Nascimento, V.; de Sá, M.E.; Teixeira Filho, M.C.M. Co-Inoculations with Plant Growth-Promoting Bacteria in the Common Bean to Increase Efficiency of NPK Fertilization. Agronomy 2022, 12, 1325. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshne, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Herder, G.D.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef]
- Mabrouk, Y.; Hemissi, I.; Ben Salem, I.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. In Symbiosis; Rigobelo, E., Ed.; IntechOpen: London, UK, 2018; pp. 107–119. [Google Scholar] [CrossRef]
- Azcón, R.; Barea, J.M. Mycorrhizosphere interactions for legume improvement. In Microbes for Legume Improvement; Khan, M.S., Musarrat, J., Zaidi, A., Eds.; Springer: Vienna, Austria, 2010. [Google Scholar] [CrossRef]
- Desai, S.; Kumar, G.P.; Amalraj, L.D.; Bagyaraj, D.J.; Ashwin, R. Exploiting PGPR and AMF Biodiversity for Plant Health Management. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Theodorou, C.; Reddell, P. In vitro synthesis of ectomycorrhizas on Casuarinaceae with a range of mycorrhizal fungi. New Phytol. 1991, 118, 279–288. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Collados, C.; Barea, J.M.; Azcón, R. Arbuscular mycorrhizal symbiosis can alleviate drought-induced nodule senescence in soybean plants. New Phytol. 2001, 151, 493–502. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Dominguez-Nuñez, J.A.; Benito, B.; Berrocal-Lobo, M.; Albanesi, A. Mycorrhizal fungi: Role in the solubilization of potassium. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Staudinger, C.; Mehmeti-Tershania, V.; Gil-Quintan, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]
- Hohmann, P.; Messmer, M.M. Breeding for mycorrhizal symbiosis: Focus on disease resistance. Euphytica 2017, 213, 113. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shine, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Iqbal Khan, M.S.; Shahid, N.; Aaliya, K. Bottlenecks in commercialization and future prospects of PGPR. Appl. Soil. Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Grönemeyer, J.L.; Reinhold-Hurek, B. Diversity of Bradyrhizobia in Sub-Saharan Africa: A rich resource. Front. Microbiol. 2018, 9, 2194. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Khan, M.Y.; Waqas, M.R.; Binyamin, R.; Akhtar, S.; Zahir, Z.A. Arbuscular mycorrhizas: An overview. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia-the roots of a success story. Curr. Opin. Plant Biol. 2017, 44, 7–15. [Google Scholar] [CrossRef]
- Molina, R.; Horton, T.R. Mycorrhiza Specificity: Its Role in the Development and Function of Common Mycelial Networks. In Mycorrhizal Networks; Springer: Dordrecht, The Netherlands, 2015; Volume 224, pp. 1–39. [Google Scholar] [CrossRef]
- Lendenmann, M.; Thonar, C.; Barnard, R.L.; Salmon, Y.; Werner, R.A.; Frossard, E.; Jansa, J. Symbiont identity matters: Carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 689–702. [Google Scholar] [CrossRef]
- Denison, R.F.; Kiers, E.T. Life Histories of Symbiotic Rhizobia and Mycorrhizal Fungi. Curr. Biol. 2011, 21, R775–R785. [Google Scholar] [CrossRef]
- Kokkoris, V.; Li, Y.; Hamel, C.; Hanson, K.; Hart, M. Site specificity in establishment of a commercial arbuscular mycorrhizal fungal inoculant. Sci. Total Environ. 2019, 660, 1135–1143. [Google Scholar] [CrossRef]
- Backer, R.J.; Rokem, S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Lobo, C.B.; Juarez Tomas, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Mozafar, A.; Anken, T. Diversity and structure of, A.M.F communities as affected by tillage in a temperate soil. Mycorrhiza 2002, 12, 225–234. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, M.; Varma, A. Role of PGPR in soil fertility and plant health. In Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants; Egamberdieva, D., Shrivastava, S., Varma, A., Eds.; Soil Biology; Springer: Cham, Switzerland, 2015; Volume 42. [Google Scholar] [CrossRef]
- Djuuna, I.A.F. Arbuscular mycorrhizal colonization and agricultural Lland use history. In Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Solaiman, Z., Abbott, L., Varma, A., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 41. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, T.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Xu, Z.; Wang, K. Responses of soil diazotrophs to legume species and density in a karst grassland, southwest China. Agric. Ecosyst. Environ. 2020, 288, 106707. [Google Scholar] [CrossRef]
- Paterson, J.; Jahanshah, G.; Li, Y.; Wang, Q.; Mehnaz, A.; Gross, H. The contribution of genome mining strategies to the understanding of active principles of PGPR strains. FEMS Microbiol. Ecol. 2017, 93, fiw249. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R. Superior polymeric formulations and emerging innovative products of bacterial inoculants for sustainable agriculture and the environment. In Agriculturally Important Microorganisms; Singh, H., Sarma, B., Keswani, C., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Chiquito Contreras, C.J.; Murillo Amador, B.; Vidal Hernández, L.; Quiñones Aguilar, E.E.; Chiquito Contreras, R.G. Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J. Soil. Sci. Plant Nutr. 2017, 17, 1003–1012. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Nagahashia, G.; Hepperly, P.R. On-farm production of inoculum of indigenous arbuscular mycorrhizal fungi and assessment of diluents of compost for inoculum production. Bioresour. Technol. 2010, 101, 2326–2330. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S.; Vio, L.; Bonaria, E.; Giovannetti, M. Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol. Biochem. 2011, 43, 367–376. [Google Scholar] [CrossRef]
- Lehman, M.R.; Taheri, W.I.; Osborne, S.L.; Buyer, J.S.; Douds, D.D., Jr. Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil. Ecol. 2012, 61, 300–304. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Mehboob, I.; Naveed, M.; Zahir, Z.A.; Sessitsch, A. Potential of Rhizosphere Bacteria for Improving Rhizobium-Legume Symbiosis. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N., Ed.; Springer: New Delhi, India, 2013; pp. 305–349. [Google Scholar] [CrossRef]
- MEA—Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; Available online: https://www.researchgate.net/publication/297563785_Millennium_Ecosystem_Assessment_Ecosystems_and_human_well-being_synthesis (accessed on 1 March 2024).
- Sollenberger, L.E.; Batista Dubeux, J.C., Jr. Warm-climate, legume-grass forage mixtures versus grass-only swards: An ecosystem services comparison. Rev. Bras. Zootec. 2022, 24, e20210198. [Google Scholar] [CrossRef]
- Giovannetti, M.A.; Lema, V.; Bartoli, C.G.; Capparelli, A. Starch grain characterization of Prosopis chilensis (Mol.) Stuntz and P. flexuosa DC and the analysis of their archaeological remains in Andean South America. J. Archaeol. Sci. 2008, 35, 2973–2985. [Google Scholar] [CrossRef]
- García-Mateos, R.; Soto-Hernández, M.; Vibrans, H. Erythrina americana Miller (“Colorín”; Fabaceae), a versatile resource from Mexico: A review. Econ. Bot. 2001, 55, 391–400. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Naumann, H.; Sepela, R.; Rezaire, A.; Masih, S.; Zeller, W.; Reinhardt, L.; Robe, J.; Sullivan, M.; Hagerman, A. Relationships between structures of condensed tannins from texas legumes and methane production during in vitro rumen digestion. Molecules 2018, 23, 2123. [Google Scholar] [CrossRef]
- Norris, A.B.; Tedeschi, L.; Muir, J.P.; Foster, J.L. The effect of quebracho (Schinopsis balansae) condensed tannin extract fed to steers on seasonal fecal gas flux. J. Environ. Qual. 2020, 140, 155–163. [Google Scholar] [CrossRef]
- Acero, A.; Muir, J.P.; Wolfe, R.M. Nutritional composition and condensed tannin concentration changes as leaves become litter in nine trees and vines. J. Sci. Food Agric. 2010, 90, 2582–2585. [Google Scholar] [CrossRef]
- Nichols, J.D.; Rosemeyer, M.E.; Carpenter, F.L.; Kettler, J. Intercropping legume trees with native timber trees rapidly restores cover to eroded tropical pasture without fertilization. For. Ecol. Manag. 2001, 152, 195–209. [Google Scholar] [CrossRef]
- Sun, H.; Tang, Y.; Xie, J.S. Research and application of hedgerow intercropping in China. J. Soil Water Conserv. 2004, 18, 114–117. [Google Scholar]
- Potts, S.G.; Woodcock, B.A.; Roberts, S.P.M.; Tscheulin, T.; Pilgrim, E.S.; Brown, V.K.; Tallowin, J.R. Enhancing pollinator biodiversity in intensive grasslands. J. Appl. Ecol. 2009, 46, 369–379. [Google Scholar] [CrossRef]
- Peoples, M.B.; Hauggaard-Nielsen, H.; Huguenin-Elie, O.; Jensen, E.S.; Justes, E.; Williams, M. The contributions of legumes to reducing the environmental risk of agricultural production. In Agroecosystem Diversity: Reconciling Contemporary Agriculture and Environmental Quality; Lemaire, G., Carvalho, P.C.F., Kronberg, S., Recous, S., Eds.; Academic Press: Cambridge, UK, 2019; pp. 123–143. [Google Scholar] [CrossRef]
- Muir, J.P.; Bow, J.R.; Rodriguez, W.; Petterson, J.M. Defoliation of panicled tick-clover, Tweedy’s tick-clover, and tall bush-clover: II. Herbage nutritive value and condensed tannin concentrations. Agron. J. 2008, 100, 1635–1639. [Google Scholar] [CrossRef]
- Delate, K.; Duffy, M.; Chase, C.; Holste, A. An economic comparison of organic and conventional grain crops in a long-term agroecological research (LTAR) site in lowa. Am. J. Altern. Agric. 2003, 18, 59–69. [Google Scholar] [CrossRef]
- Goldsmith, P.D. Economics of soybean production, marketing, and utilization. In Soybeans: Chemistry, Production, Processing, and Utilization; Johnson, L.A., White, P.J., Galloway, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Angus, J.F.; Kirkegaard, J.A.; Hunt, J.R.; Ryan, M.H.; Ohlander, L.; Peoples, M.B. Break crops and rotations for wheat. Crop Pasture Sci. 2015, 66, 523–552. [Google Scholar] [CrossRef]
- Duffy, M. Continuous corn versus corn/soybeans: Do the relative prices change the profit comparison? Ag Decis. Mak. Newsl. 2015, 16, 1. Available online: https://lib.dr.iastate.edu/agdm/vol16/iss2/1 (accessed on 19 February 2019).
- Preissel, S.; Reckling, M.; Schläfke, M.; Zander, P. Magnitude and farm-economic value of grain legume pre-crop benefits in Europe: A review. Field Crop Res. 2015, 175, 64–79. [Google Scholar] [CrossRef]
- Schnitkey, G. Expected Corn Versus Soybean Returns in 2019. Farmdoc Daily (9): 29, Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign. 2019. Available online: https://farmdocdaily.illinois.edu/2019/02/expected-corn-versus-soybean-returns-in-2019.html (accessed on 19 February 2019).
- Yigezu, Y.A.; El-Shater, T.; Boughlala, M.; Bishaw, Z.; Niane, A.A.; Maalouf, F.; Degu, W.T.; Wery, J.; Boutfiras, M.; Aw-Hassan, A. Legume-based rotations have clear economic advantages over cereal monocropping in dry areas. Agron. Sustain. Dev. 2019, 39, 58. [Google Scholar] [CrossRef]
- Reckling, M.; Bergkvist, G.; Watson, C.A.; Stoddard, F.L.; Zander, P.M.; Walker, R.L.; Pristeri, A.; Toncea, I.; Bachinger, J. Trade-offs between economic and environmental impacts of introducing legumes into cropping systems. Front. Plant Sci. 2016, 7, 669. [Google Scholar] [CrossRef]
- Langemeier, M. Relative Profitability of Corn and Soybean Production in Indiana; Center for Commercial Agriculture, College of Agriculture, Purdue University: West Lafayette, IN, USA, 2018; Available online: https://ag.purdue.edu/commercialag/home/wp-content/uploads/2018/05/201805_-Langemeier_RelativeProfitabilityCornSoybeanIndiana.pdf (accessed on 12 March 2020).
- Dobbins, C.; Langemeier, M. Crop Cost and Return Guide; Center for Commercial Agriculture, College of Agriculture, Purdue University: West Lafayette, IN, USA, 2018; Available online: https://ag.purdue.edu/commercialag/home/resource/2018/03/2018-crop-cost-and-return-guide/ (accessed on 12 March 2020).
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Muir, J.P.; Bow, J.R. Forage, nitrogen, and phosphorous yield dynamics of cool-season annual forages overseeded onto Tifton 85 Bermudagrass. Agron. J. 2011, 103, 1019–1025. [Google Scholar] [CrossRef]
- Butler, T.J.; Muir, J.P.; Huo, C.; Gurestsky, J.A. Switchgrass biomass production with over-seeded cool-season legumes. Bioenergy Res. 2013, 6, 44–52. [Google Scholar] [CrossRef]
- Komarek, A.M.; De Pinto, A.; Smith, V.H. A review of types of risks in agriculture: What we know and what we need to know. Agric. Syst. 2019, 178, 102738. [Google Scholar] [CrossRef]
- Babcock, B.A. The effects of uncertainty on optimal nitrogen applications. Appl. Econ. Perspect. Policy 1992, 14, 271–280. [Google Scholar] [CrossRef]
- AGDaily. African Swine Fever Abroad Creates Waves for U.S. Agriculture. AGDaily. 31 May 2019. Available online: https://www.agdaily.com/news/african-swine-fever-us-agriculture/ (accessed on 17 March 2020).
- Mitchell, P. Meta-Economics of Cover Crops. Science of Cover Crops, Madison, Wisconsin. 14 March 2014. Available online: http://mccc.msu.edu/wp-content/uploads/2016/11/MCCC2016_6-Meta-Economics-of-Cover-Crops-2.pdf (accessed on 13 March 2020).
- Hansen, L.; Ribaudo, M. Economic Measures of Soil Conservation Benefits; United States Department of Agriculture: Washington, DC, USA, 2008. Available online: https://www.ers.usda.gov/publications/pub-details?pubid=47550 (accessed on 12 March 2020).
- DTN. DTN Retail Fertilizer Trends, 4 March 2020. Progressive Farmer, DTN. 2020. Available online: https://www.dtnpf.com/agriculture/web/ag/crops/article/2020/03/04/prices-fertilizers-end-february (accessed on 13 March 2020).
- Gabriel, J.L.; Garrido, A.; Quemada, M. Cover crops effect on farm benefits and nitrate leaching: Linking economic and environmental analysis. Agric. Syst. 2013, 121, 23–32. [Google Scholar] [CrossRef]
- Ruark, M.D.; Stute, J.K. Cover Crops and Nitrogen Credits. Proceedings of the 2009 Wisconsin Crop Management Conference, Volume 48. University of Wisconsin Extension. 2009. Available online: https://extension.soils.wisc.edu/wcmc/cover-crops-and-nitrogen-credits/ (accessed on 12 March 2020).
- USDA-ARS; U.S. Department of Agriculture, Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies 2011–2012; Food Surveys Research Group: Beltsville Wash, DC, USA, 2014. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/ (accessed on 1 March 2024).
- EIA. Energy Information Administration. Monthly Biodiesel Production Report. Available online: https://www.eia.gov/biofuels/biodiesel/production/ (accessed on 12 March 2020).
- Hay, F.J. Soybeans for Biodiesel Production. Available online: https://farm-energy.extension.org/soybeans-for-biodiesel-production/ (accessed on 12 March 2020).
- USDA-NASS. United States Department of Agriculture National Agricultural Statistics Service. QuickStats. 2020. Available online: https://quickstats.nass.usda.gov/ (accessed on 12 March 2020).
- Ciaian, P.; Kancs, A. Interdependencies in the energy–bioenergy–food price systems: A cointegration analysis. Resour. Energy Econ. 2011, 33, 326–348. [Google Scholar] [CrossRef]
- Womach, J. The 1990 Farm Bill: Issues Likely to Shape the Policy Debate; CRS Report for Congress, 88-700ENR; Food and Agriculture Section, Environment and Natural Resources Policy Division, Congressional Research Service, The Library of Congress: Washington, DC, USA, 1988.
- Young, D.L. Policy Barriers to Sustainable Agriculture. Am. J. Altern. Agric. 1989, 4, 135–143. Available online: http://www.jstor.org/stable/44507056 (accessed on 1 March 2024). [CrossRef]
- Olmstead, J.; Brummer, E.C. Benefits and barriers to perennial forage crops in Iowa corn and soybean rotations. Renew. Agric. Food Syst. 2008, 23, 97–107. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Smith, A.M.; Ellis, T.H.N.; Hedley, C.; Martin, C. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell 1990, 60, 115–122. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar]
- The Arabidopsis Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Sato, S.; Nakamura, Y.; Kaneko, T.; Asamizu, E.; Kato, T.; Nakao, M.; Sasamoto, S.; Watanabe, A.; Ono, A.; Kawashima, K.; et al. Genome structure of the legume, Lotus japonicus. DNA Res. 2008, 15, 227–239. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Miyahara, A.; Sato, S.; Kato, T.; Yoshikawa, M.; Taketa, M.; Hayashi, M.; Pedrosa, A.; Onda, R.; Imaizumi-Anraku, H. Construction of a genetic linkage map of the model legume Lotus japonicus using an intraspecific F2 population. DNA Res. 2001, 8, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kaneko, T.; Nakamura, Y.; Asamizu, E.; Kato, T.; Tabata, S. Structural analysis of a Lotus japonicus genome. I. Sequence features and mapping of fifty-six TAC clones which cover the 5.4 Mb regions of the genome. DNA Res. 2001, 8, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.A.; Etter, P.D.; Atwood, T.S.; Currey, M.C.; Shiver, A.L.; Lewis, Z.A.; Selker, E.U.; Cresko, W.A.; Johnson, E.A. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 2008, 3, e3376. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Miller, M.R.; Dunham, J.P.; Amores, A.; Cresko, W.A.; Johnson, E.A. Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Res. 2007, 17, 240–248. [Google Scholar] [CrossRef]
- Toonen, R.J.; Puritz, J.B.; Forsman, Z.H.; Whitney, J.L.; Fernandez-Silva, I.; Andrews, K.R.; Bird, C.E. ezRAD: A simplified method for genomic genotyping in non-model organisms. PeerJ 2013, 1, e203. [Google Scholar] [CrossRef]
- Grillo, M.A.; De Mita, S.; Burke, P.V.; Solórzano-Lowell, K.L.; Heath, K.D. Intrapopulation genomics in a model mutualist: Population structure and candidate symbiosis genes under selection in Medicago truncatula. Evolution 2016, 70, 2704–2717. [Google Scholar] [CrossRef]
- Pan, L.; Wang, N.; Wu, Z.; Guo, R.; Yu, X.; Zheng, Y.; Xia, Q.; Gui, S.; Chen, C. A high-density genetic map derived from RAD sequencing and its application in QTL analysis of yield-related traits in Vigna unguiculata. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Yates, S.A.; Swain, M.T.; Hegarty, M.J.; Chernukin, I.; Lowe, M.; Allison, G.G.; Ruttink, T.; Abberton, M.T.; Jenkins, G.; Skøt, L. De novo assembly of red clover transcriptome based on RNA-Seq data provides insight into drought response, gene discovery and marker identification. BMC Genom. 2014, 15, 453. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Li, L.; Wang, S. De novo assembly of the common bean transcriptome using short reads for the discovery of drought-responsive genes. PLoS ONE 2014, 9, e109262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chang, S.; Hartman, G.L.; Domier, L.L. Assembly and annotation of a draft genome sequence for Glycine latifolia, a perennial wild relative of soybean. Plant J. 2018, 95, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.C. The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Krishnakumar, V.; Bidwell, S.; Rosen, B.; Chan, A.; Zhou, S.; Gentzbittel, L.; Childs, K.L.; Yandell, M.; Gundlach, H.; et al. An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genom. 2014, 15, 312. [Google Scholar] [CrossRef]
- Zhuang, X.; Tang, R.; Garg, V.; Wang, X.; Tang, H.; Chow, C.-N.; Wang, J.; Deng, Y.; Wang, D.; Khan, A.W.; et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 2019, 51, 865–876. [Google Scholar] [CrossRef]
- Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Current strategies of polyploid plant genome sequence assembly. Front. Plant Sci. 2018, 9, 1660. [Google Scholar] [CrossRef]
- Saballos, A.; Williams, M.M. Genome-wide Association Study Identifies Candidate Loci with Major Contributions to the Genetic Control of Pod Morphological Traits in Snap Bean. J. Am. Soc. Hort. Sci. 2024, 149, 1–14. [Google Scholar] [CrossRef]
- Vleugels, T.; Ruttink, T.; Ariza-Suarez, D.; Dubey, R.; Saleem, A.; Roldán-Ruiz, I.; Muylle, H. GWAS for Drought Resilience Traits in Red Clover (Trifolium pratense L.). Genes 2024, 15, 1347. [Google Scholar] [CrossRef]
- Afzal, M.; Alghamdi, S.S.; Migdadi, H.H.; Khan, M.A.; Mirza, S.B.; El-Harty, E. Legume genomics and transcriptomics: From classic breeding to modern technologies. Saudi J. Biol. Sci. 2020, 27, 543–555. [Google Scholar] [CrossRef]
- Jain, M.; Garg, R. (Eds.) Legume Genomics: Methods and Protocols; Humana: New York, NY, USA, 2020. [Google Scholar]
- Pandey, M.K.; Roorkiwal, M.; Singh, V.K.; Ramalingam, A.; Kudapa, H.; Thudi, M.; Chitikineni, A.; Rathore, A.; Varshney, R.K. Emerging genomic tools for legume breeding: Current status and future prospects. Front. Plant Sci. 2016, 7, 455. [Google Scholar] [CrossRef]
- Bekele-Alemu, A.; Girma-Tola, D.; Ligaba-Osena, A. The Potential of CRISPR/Cas9 to Circumvent the Risk Factor Neurotoxin β-N-oxalyl-L-α, β-diaminopropionic acid Limiting Wide Acceptance of the Underutilized Grass Pea (Lathyrus sativus L.). Curr. Issues Mol. Biol. 2024, 46, 10570–10589. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Ma, C.; Chen, Y.; Liu, C.; Wang, Y.; Wang, S.; Chen, X. Editing the nuclear localization signals of E1 and E1Lb enables the production of tropical soybean in temperate growing regions. Plant Biotechnol. J. 2024, 22, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Neenu, S.; Rashmi, I.; Meena, B.P.; Jatav, R.C.; Lakaria, B.L.; Biswas, A.K.; Singh, M.; Patra, A.K. Ameliorating effects of leucaena biochar on soil acidity and exchangeable ions. Communs. Soil Sci. Plant Anal. 2016, 47, 1252. [Google Scholar] [CrossRef]
- Rengsirikul, K.; Kanjanakuha, R.; Ishii, Y.; Kangvansaichol, K.; Sripichitt, P.; Punsuvon, V.; Vaithanomsat, P.; Nakamanee, G.; Tudsri, S. Potential forage and biomass production of newly introduced varieties of leucaena (Leucaena leucocephala (Lam.) de Wit.) in Thailand. Grassl. Sci. 2011, 57, 94–100. [Google Scholar] [CrossRef]
- Sethi, P.; Kulkarni, P.R. Functional properties of protein isolate from Leucaena leucocephala seeds. Intern. J. Food Sci. Nutr. 2009, 45, 35–39. [Google Scholar] [CrossRef]
- Zarin, M.A.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2018, 5, 65–75. [Google Scholar] [CrossRef]
| Legume | System | Climate | Duration | N Contributed | Citation |
|---|---|---|---|---|---|
| Arachis pintoi | Forest biome | Tropical | 8 years | 72.5 kg N ha−1 yr−1 in ecosystem | [13] |
| Aeschynomene americana | Savannah | Subtropical | 3 years | 9 kg N ha−1 yr−1in legume | [16] |
| Pisum sativum | Winter annual grass mix | Subtropical | 2 years | 213 kg N ha−1 yr−1 in legume | [17] |
| Vigna radiata | Summer annual grass mix | Subtropical | 2 years | 167 kg N ha−1 yr−1 in legume | [17] |
| Trifolium spp. | Grasslands | Temperate | 4 years | 27 kg soil mineral N ha−1 0 to 60 cm | [14] |
| Calopogonium muconoides | Brachiaria brizantha + C. muconoides mixed pasture | Tropical | 2 years | 73 to 98 kg N ha−1 yr−1 from biological nitrogen fixation | [7] |
| Trait | Range | Biotic Factors | Abiotic Factors | Examples |
|---|---|---|---|---|
| Architecture | Dense to open | Plant competition | Wind | Rangeland |
| Canopy | Small to large | Plant competition | Light interception | |
| Habit | Decumbent to upright | Grazing | Soil moisture (added this based on the general trend of shorter stature plants in arid environments) | Rangeland |
| Root depth | Shallow to deep | Herb vs. tree | Soil texture Water table Rainfall distribution | Rangeland |
| Height | Decumbent to upright | Seedling populations | Soil texture Water table Rainfall distribution | Pulses |
| Climbing | Viney to upright | Plant competition Grazing (exposed meristem) | Light interception | Cowpeas |
| Flowering | Early to late | Seedling populations | Rainfall distribution | Soybean days to flowering |
| Seed set | In/determinant | Herbivory/harvest schedule | Soil moisture | Cowpea |
| Seed quantity | Low to large | Herbivory/haying | Climate/soil | Annuals vs. perennials |
| Seed size | Small to large | Ecological succession | Climate | Annuals vs. perennials |
| Rainfall requirements | 200 to 2000 mm | Annual vs. perennial | Climate | Days to flowering |
| Heat tolerance | Up to 40 °C | Leaf abscission | Altitude/latitude | Forage legumes |
| Cold tolerance | Freeze vs. tropical | Life cycle | Altitude/latitude | Forage legumes |
| Propagation | Seed vs. vegetative | Herbivory | Rainfall | Perennial peanut |
| Species | Habit | Origin |
|---|---|---|
| Acaciella angustissima (Mill.) Britton & Rose | Tree | North, Central and South America |
| Acacia auriculiformis A.Cunn. ex Benth. | Tree | Asia and Oceania |
| Acacia leptocarpa A.Cunn. ex Benth. | Tree | Oceania |
| Acacia mangium Wild. | Asia and Oceania | |
| Acacia mearnsii Wild. | Tree | Oceania |
| Acacia nilótica (L.) Delile | Tree | Africa and Asia |
| Senegalia polyacantha (Willd) Seigler & Ebinger | Tree | Africa |
| Senegalia senegal (L.) Willd. | Shrub | Africa and Asia |
| Aeschynomene histrix Poir. | Shrub | Central and South America |
| Albizia lebbeck (L.) Benth. | Tree | Africa and Asia |
| Brachystegia boehmii Taub. | Tree | Africa |
| Brachystegia spiciformis Benth. | Tree | Africa |
| Cajanus cajan (L.) Huth. | Shrub | Asia |
| Calliandra calothyrsus Meissner. | Tree | Central America |
| Dichrostachys cinerea (L.) Wight & Arn. | Shrub | Africa and Asia |
| Erythrina excelsa Baker. | Tree | Africa |
| Gliricidia sepium (Jacq.) Walp. | Shrub | Central America |
| Inga edulis Mart. | Tree | Central and South America |
| Leucaena collinsii Britton & Rose | Tree | Central America |
| Leucaena leucocephala (Lam.) De Wit. | Tree | Central America |
| Mimosa scabrella Benth. | Tree | South America |
| Mimosa caesalpiniifolia Benth. | Tree | South America |
| Pithecellobium dulce (Roxb.) Benth. | Tree | Central and South America |
| Pongamia pinnata (L.) Pierre. | Shrub | Asia |
| Prosopis alba Griseb. | Tree | South America |
| Prosopis juliflora (Sw.) DC. | Tree | Central and South America |
| Senna siamea (Lam.) H.S. Irwin & Barneby | Shrub | Asia |
| Senna spectabilis (DC.) H.S. Irwin & Barneby | Tree | South America |
| Sesbania bispinosa (Jacq.) W.Wight | Shrub | Africa and Asia |
| Sesbania sesban (L.) Merr. | Shrub | Africa, Asia and Oceania |
| Tamarindus indica L. | Tree | Africa |
| Tephrosia candida (Roxb.) DC. | Shrub | Asia |
| Tephrosia vogelii Hook.f. | Shrub | Africa |
| N Fixation | Enhance Nodules | Enhance AMF | P Solubilization | >K Uptake | Salinity and Drought Stress | Disease Suppression | Plant and Root Growth | |
|---|---|---|---|---|---|---|---|---|
| Agrobacterium | X | X | X | |||||
| Arthrobacter | X | |||||||
| Aspergillus | X | |||||||
| Azoarcus | X | |||||||
| Azotobacter | X | X | X | X | ||||
| Azospirillum | X | X | X | |||||
| Bacillus | X | X | x | X | X | X | x | X |
| Burkholderia | X | X | x | |||||
| Diazotrophicus | X | |||||||
| Enterobacter | x | X | X | X | ||||
| Herbaspirillum | X | |||||||
| Klebsiella | X | X | X | |||||
| Pseudomonas | x | x | X | X | X | x | X | |
| Rhizobium | X | X | X | x | X | |||
| Rhizopus | X | |||||||
| Serratia | X | x | X | |||||
| Trichoderma | x | X |
| Cover Crop | <15 cm Growth | 15 to 30 cm Growth |
|---|---|---|
| Alfalfa | USD 35 | USD 53–USD 88 |
| Red clover | USD 35 | USD 44–USD 70 |
| Sweet clover | USD 35 | USD 70–USD 106 |
| Vetch | USD 35 | USD 35–USD 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muir, J.P.; Batista Dubeux Junior, J.C.; Santos, M.V.F.d.; Foster, J.L.; Caraciolo Ferreira, R.L.; Lira, M.d.A., Jr.; Bellows, B.; Osei, E.; Singh, B.B.; Brady, J.A. Sustainable Warm-Climate Forage Legumes: Versatile Products and Services. Grasses 2025, 4, 16. https://doi.org/10.3390/grasses4020016
Muir JP, Batista Dubeux Junior JC, Santos MVFd, Foster JL, Caraciolo Ferreira RL, Lira MdA Jr., Bellows B, Osei E, Singh BB, Brady JA. Sustainable Warm-Climate Forage Legumes: Versatile Products and Services. Grasses. 2025; 4(2):16. https://doi.org/10.3390/grasses4020016
Chicago/Turabian StyleMuir, James P., José C. Batista Dubeux Junior, Mércia V. Ferreira dos Santos, Jamie L. Foster, Rinaldo L. Caraciolo Ferreira, Mário de Andrade Lira, Jr., Barbara Bellows, Edward Osei, Bir B. Singh, and Jeff A. Brady. 2025. "Sustainable Warm-Climate Forage Legumes: Versatile Products and Services" Grasses 4, no. 2: 16. https://doi.org/10.3390/grasses4020016
APA StyleMuir, J. P., Batista Dubeux Junior, J. C., Santos, M. V. F. d., Foster, J. L., Caraciolo Ferreira, R. L., Lira, M. d. A., Jr., Bellows, B., Osei, E., Singh, B. B., & Brady, J. A. (2025). Sustainable Warm-Climate Forage Legumes: Versatile Products and Services. Grasses, 4(2), 16. https://doi.org/10.3390/grasses4020016







