The Importance of Kinases in Retinal Degenerative Diseases
Abstract
:1. Introduction
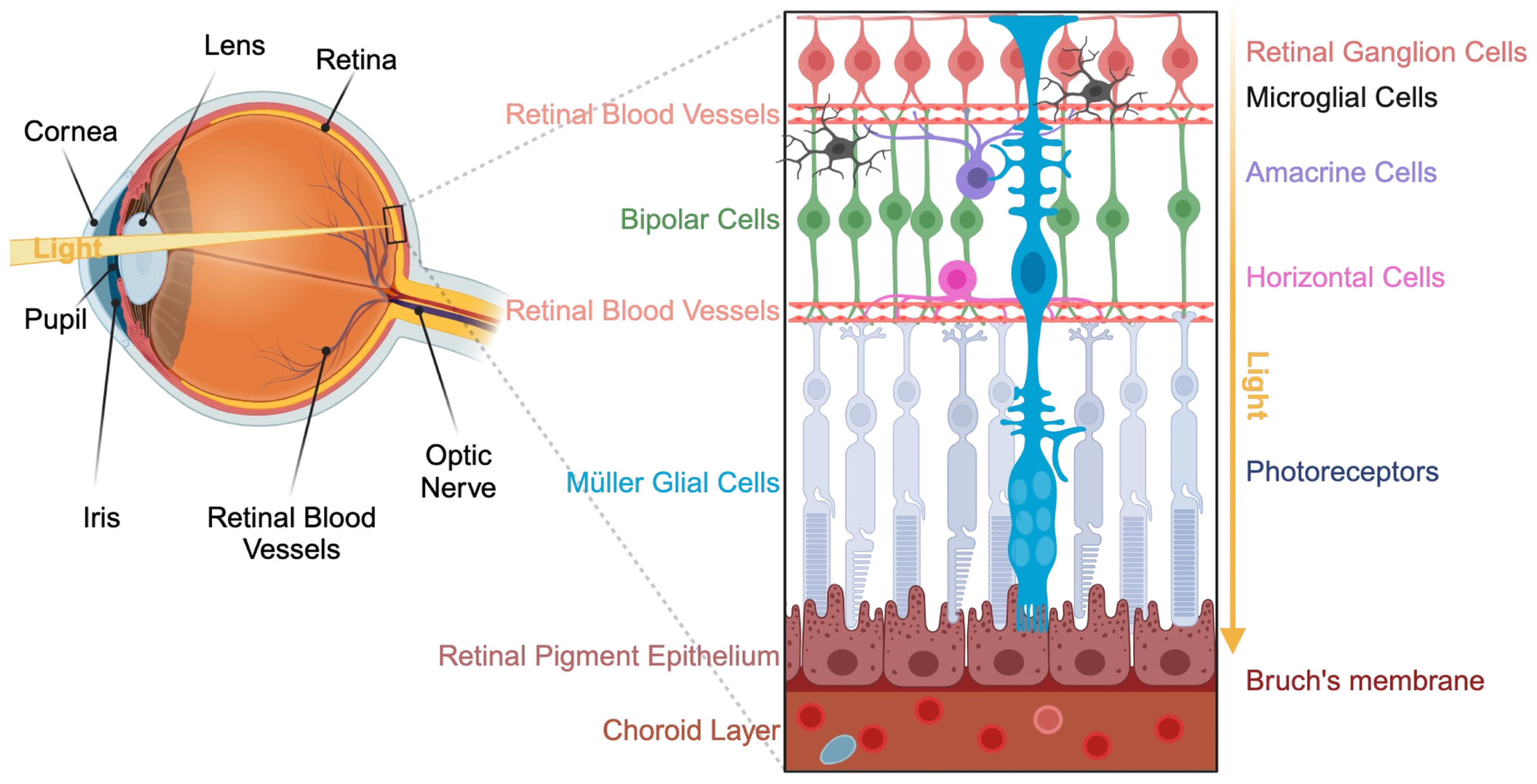
2. Presentation of the Visual System
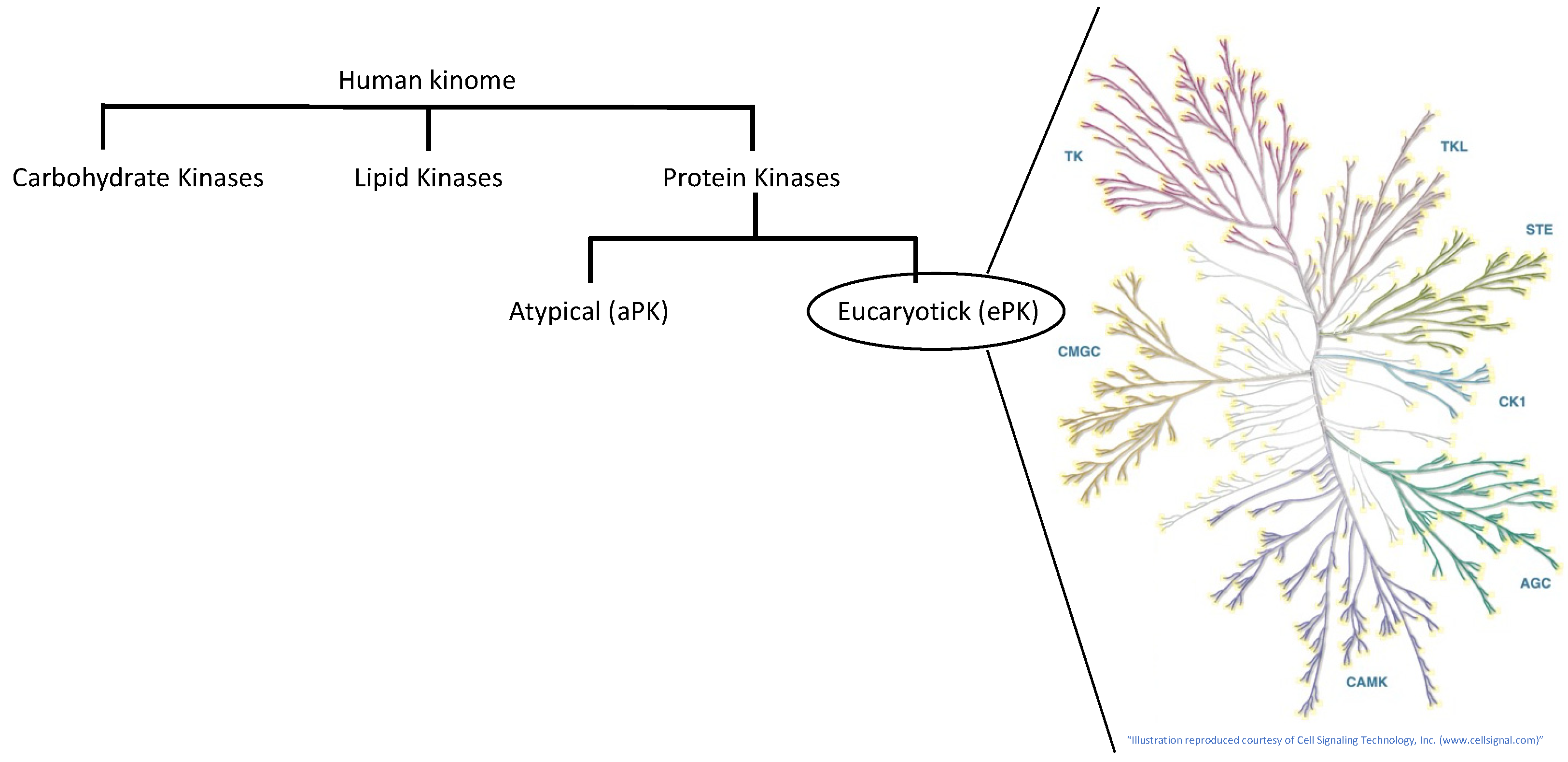
3. The Human Kinome
3.1. The Carbohydrate Kinases
3.2. The Lipid Kinases
3.2.1. The Sphingosine Kinases
3.2.2. The Diacylglycerol Kinase (DGK)
3.2.3. The Phosphoinositide Kinases (PIKs)
3.3. The Protein Kinases
3.3.1. The Tyrosine Kinases (TKs)
3.3.2. The Tyrosine Kinase-like (TKL) Kinases
3.3.3. The Sterile (STE) Protein Kinases Group
3.3.4. The Casein Kinase 1 (CK1) Family
3.3.5. The AGC Kinases
3.3.6. The Ca2+/Calmodulin-Dependent Protein Kinases (CAMKs)
3.3.7. The CMGC Group
3.3.8. The Receptor Guanylyl Cyclase (RGC) Group
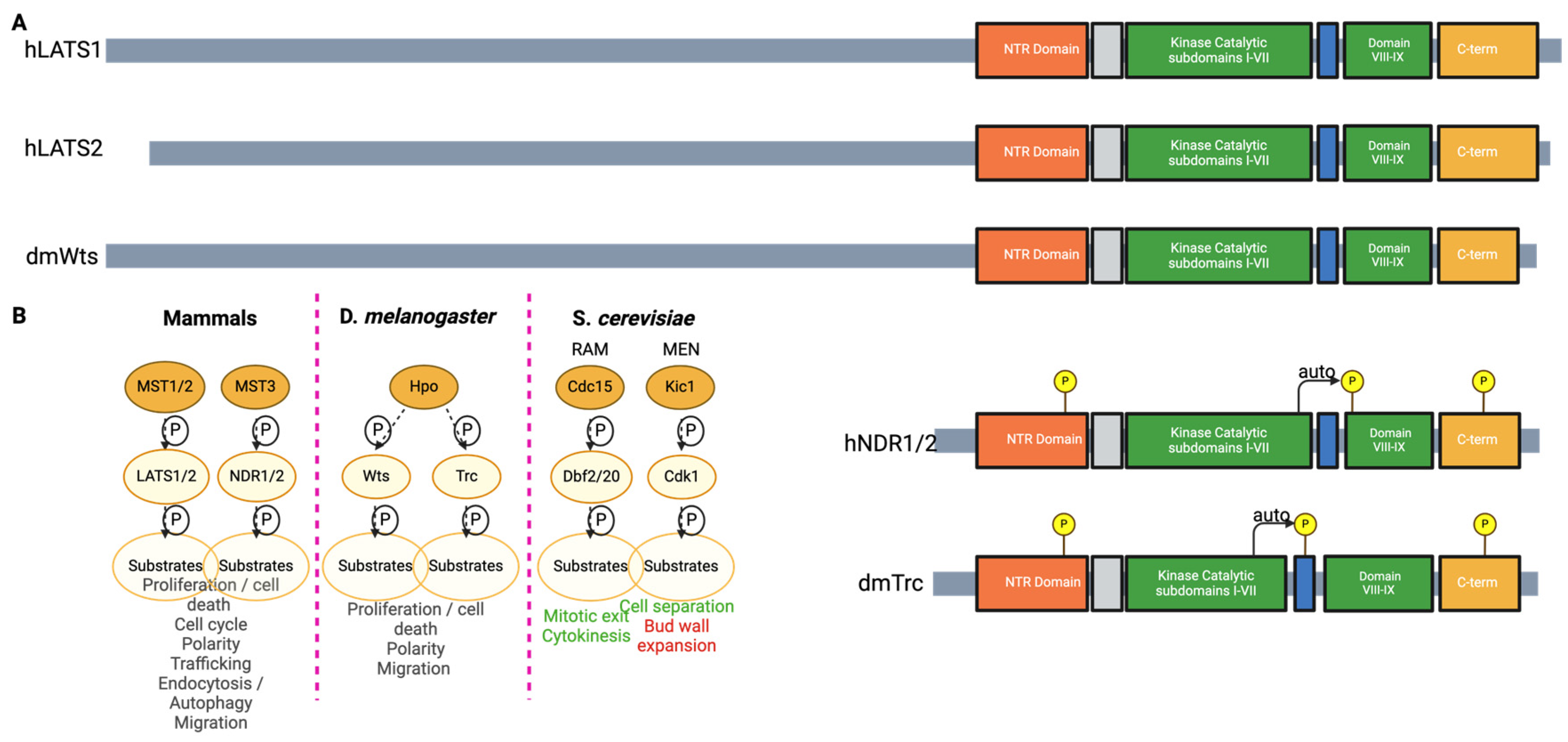
3.4. The Serine/Threonine (Ser/Thr) Kinases
The Nuclear Dbf2-Related Kinases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, A.; Chauhan, P.; Saha, B.; Kubatzky, K.F. Conceptual Evolution of Cell Signaling. Int. J. Mol. Sci. 2019, 20, 3292. [Google Scholar] [CrossRef]
- Wosilait, W.D.; Sutherland, E.W. The relationship of epinephrine and glucagon to liver phosphorylase. II. Enzymatic Inactivation of Liver Phosphorylase. J. Biol. Chem. 1956, 218, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Edelman, A.M.; Blumenthal, D.K.; Krebs, E.G. Protein serine/threonine kinases. Annu. Rev. Biochem. 1987, 56, 567–613. [Google Scholar] [CrossRef]
- Cohen, P.; Campbell, D.G.; Dent, P.; Gomez, N.; Lavoinne, A.; Nakielny, S.; Stokoe, D.; Sutherland, C.; Traverse, S. Dissection of the protein kinase cascades involved in insulin and nerve growth factor action. Biochem. Soc. Trans. 1992, 20, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The Yin and Yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Peterson, J.R. The Human Kinome and Kinase Inhibition. Curr. Protoc. Pharmacol. 2013, 60, 2.9.1–2.9.14. [Google Scholar] [CrossRef] [PubMed]
- Senthil, M.P.; Khadka, J.; Gilhotra, J.S.; Simon, S.; Pesudovs, K. Exploring the quality of life issues in people with retinal diseases: A qualitative study. J. Patient-Rep. Outcomes 2017, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Vyawahare, H.; Shinde, P. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 2022, 14, e29583. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lorenz, R.; Arold, S.T.; Reger, A.S.; Sankaran, B.; Casteel, D.E.; Herberg, F.W.; Kim, C. Crystal Structure of PKG I:cGMP Complex Reveals a cGMP-Mediated Dimeric Interface that Facilitates cGMP-Induced Activation. Structure 2016, 24, 710–720. [Google Scholar] [CrossRef]
- Li, S.; Ma, H.; Yang, F.; Ding, X. cGMP Signaling in Photoreceptor Degeneration. Int. J. Mol. Sci. 2023, 24, 11200. [Google Scholar] [CrossRef]
- Das, S.; Chen, Y.; Yan, J.; Christensen, G.; Belhadj, S.; Tolone, A.; Paquet-Durand, F. The role of cGMP-signalling and calcium-signalling in photoreceptor cell death: Perspectives for therapy development. Pflügers Arch. Eur. J. Physiol. 2021, 473, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Vega, M.V.; Harmer, N.J. Carbohydrate Kinases: A Conserved Mechanism Across Differing Folds. Catalysts 2019, 9, 29. [Google Scholar] [CrossRef]
- Hottin, C.; Perron, M.; Roger, J.E. GSK3 Is a Central Player in Retinal Degenerative Diseases but a Challenging Therapeutic Target. Cells 2022, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Rajala, R.V.S. Signaling roles of phosphoinositides in the retina. J. Lipid Res. 2021, 62, 100041. [Google Scholar] [CrossRef]
- Luján, L.M.L.; McCarty, M.F.; Di Nicolantonio, J.J.; Ruiz, J.C.G.; Rosas-Burgos, E.C.; Plascencia-Jatomea, M.; Assanga, S.B.I. Nutraceuticals/Drugs Promoting Mitophagy and Mitochondrial Biogenesis May Combat the Mitochondrial Dysfunction Driving Progression of Dry Age-Related Macular Degeneration. Nutrients 2022, 14, 1985. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Hexokinase-2-Linked Glycolytic Overload and Unscheduled Glycolysis—Driver of Insulin Resistance and Development of Vascular Complications of Diabetes. Int. J. Mol. Sci. 2022, 23, 2165. [Google Scholar] [CrossRef]
- Min, J.; Zeng, T.; Roux, M.; Lazar, D.; Chen, L.; Tudzarova, S. The Role of HIF1α-PFKFB3 Pathway in Diabetic Retinopathy. J. Clin. Endocrinol. Metab. 2021, 106, 2505–2519. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.L.; Stiles, M.A.; Gurley, J.M.; Grambergs, R.C.; Gu, X.; Elliott, M.H.; Proia, R.L.; Mandal, N.A. Sphingosine Kinase-1 Is Essential for Maintaining External/Outer Limiting Membrane and Associated Adherens Junctions in the Aging Retina. Mol. Neurobiol. 2019, 56, 7188–7207. [Google Scholar] [CrossRef]
- Porter, H.; Qi, H.; Prabhu, N.; Grambergs, R.; McRae, J.; Hopiavuori, B.; Mandal, N. Characterizing Sphingosine Kinases and Sphingosine 1-Phosphate Receptors in the Mammalian Eye and Retina. Int. J. Mol. Sci. 2018, 19, 3885. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.V.; Spalm, F.H.P.; Vera, M.S.; Rotstein, N.P. Sphingolipids as Emerging Mediators in Retina Degeneration. Front. Cell. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, Y.; Matsui, H.; Sakane, F.; Watanabe, M.; Goto, K. Distinct Expression and Localization of Diacylglycerol Kinase Isozymes in Rat Retina. J. Histochem. Cytochem. 2013, 61, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, Y.; Li, W.; Yang, B.; Yu, S.; Zhou, H.; Yang, C.; Xu, F.; Wang, J.; Gao, Y.; et al. Diacylglycerol Kinase (DGK) Inhibitor II (R59949) Could Suppress Retinal Neovascularization and Protect Retinal Astrocytes in an Oxygen-Induced Retinopathy Model. J. Mol. Neurosci. 2015, 56, 78–88. [Google Scholar] [CrossRef]
- Sasaki, T.; Takasuga, S.; Sasaki, J.; Kofuji, S.; Eguchi, S.; Yamazaki, M.; Suzuki, A. Mammalian phosphoinositide kinases and phosphatases. Prog. Lipid Res. 2009, 48, 307–343. [Google Scholar] [CrossRef] [PubMed]
- Rajala, R.V. Phosphoinositide 3-kinase signaling in the vertebrate retina. J. Lipid Res. 2010, 51, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, I.; Anderson, R.E.; Le, Y.Z.; Fliesler, S.J.; Sherry, D.M.; Rajala, R.V.S. Deletion of the p85α Regulatory Subunit of Phosphoinositide 3-Kinase in Cone Photoreceptor Cells Results in Cone Photoreceptor Degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 3775–3783. [Google Scholar] [CrossRef]
- Rajala, R.V.S. Aerobic Glycolysis in the Retina: Functional Roles of Pyruvate Kinase Isoforms. Front. Cell Dev. Biol. 2020, 8, 266. [Google Scholar] [CrossRef]
- Ivanovic, I.; Allen, D.T.; Dighe, R.; Le, Y.Z.; Anderson, R.E.; Rajala, R.V.S. Phosphoinositide 3-Kinase Signaling in Retinal Rod Photoreceptors. Investig. Opthalmol. Vis. Sci. 2011, 52, 6355–6362. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, X.; Tang, M.; Zhong, G.; Si, Y.; Li, H.; Zhu, F.; Liao, Q.; Li, L.; Zhao, J.; et al. A subcellular map of the human kinome. eLife 2021, 10, e64943. [Google Scholar] [CrossRef]
- Kanev, G.K.; de Graaf, C.; de Esch, I.J.; Leurs, R.; Würdinger, T.; Westerman, B.A.; Kooistra, A.J. The Landscape of Atypical and Eukaryotic Protein Kinases. Trends Pharmacol. Sci. 2019, 40, 818–832. [Google Scholar] [CrossRef]
- Rygiel, K.A.; Elkins, J.M. Recent advances in the structural biology of tyrosine kinases. Curr. Opin. Struct. Biol. 2023, 82, 102665. [Google Scholar] [CrossRef]
- Nagar, B. c-Abl Tyrosine Kinase and Inhibition by the Cancer Drug Imatinib (Gleevec/STI-571). J. Nutr. 2007, 137, 1518S–1523S. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Ricci, F.; Paris, L.P.; Korn, C.; Quezada-Ruiz, C.; Zarbin, M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: A review of preclinical data. Eye 2021, 35, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chaurasia, S.; Singh, R.P. A review of emerging tyrosine kinase inhibitors as durable treatment of neovascular age-related macular degeneration. Expert Opin. Emerg. Drugs 2023, 28, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, Q.; Zhang, H.; Li, X.; Jiang, Q.; Yao, J. A small molecular multi-targeting tyrosine kinase inhibitor, anlotinib, inhibits pathological ocular neovascularization. Biomed. Pharmacother. 2021, 138, 111493. [Google Scholar] [CrossRef] [PubMed]
- Sergeys, J.; Van Hove, I.; Hu, T.-T.; Temps, C.; Carragher, N.O.; Unciti-Broceta, A.; Feyen, J.H.; Moons, L.; Porcu, M. The retinal tyrosine kinome of diabetic Akimba mice highlights potential for specific Src family kinase inhibition in retinal vascular disease. Exp. Eye Res. 2020, 197, 108108. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.H.Y.; Francescut, L.; Lingor, P.; Bähr, M.; Nicotera, P. Identification of new kinase clusters required for neurite outgrowth and retraction by a loss-of-function RNA interference screen. Cell Death Differ. 2008, 15, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kuang, X.; Zou, Y.; Zeng, J.; Du, H.; Tang, H.; Long, C.; Mao, Y.; Yu, X.; Wen, C.; et al. MAP4K4 is involved in the neuronal development of retinal photoreceptors. Exp. Eye Res. 2023, 233, 109524. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Yang, Y.; Wang, Y.; Zhang, Y.; Zhuang, J.; Zhang, Y.; Shen, M.; Zhao, J.; Zhang, R.; et al. MAP4Ks inhibition promotes retinal neuron regeneration from Müller glia in adult mice. NPJ Regen. Med. 2023, 8, 36. [Google Scholar] [CrossRef]
- Lourenço, R.; Brandão, A.S.; Borbinha, J.; Gorgulho, R.; Jacinto, A. Yap Regulates Müller Glia Reprogramming in Damaged Zebrafish Retinas. Front. Cell Dev. Biol. 2021, 9, 667796. [Google Scholar] [CrossRef]
- Hoang, A.T.N.; Lee, H.; Lee, S.-J. Casein kinase I inhibitor D4476 influences autophagy and apoptosis in chloroquine-induced adult retinal pigment epithelial-19 cells. Exp. Eye Res. 2022, 218, 109004. [Google Scholar] [CrossRef]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef]
- Dreixler, J.C.; Hemmert, J.W.; Shenoy, S.K.; Shen, Y.; Lee, H.T.; Shaikh, A.R.; Rosenbaum, D.M.; Roth, S. The role of Akt/protein kinase B subtypes in retinal ischemic preconditioning. Exp. Eye Res. 2009, 88, 512–521. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Starr, C.; Mohns, E.J.; Li, Y.; Chen, E.P.; Yoon, Y.; Kellner, C.P.; Tanaka, K.; Wang, H.; et al. Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell 2021, 184, 4299–4314.e12. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Zhou, F.; Zhu, L. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca2+/CaMKII-dependent activation of AMPK. Arch. Pharmacal Res. 2018, 41, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Chaya, T.; Furukawa, T. Post-translational modification enzymes as key regulators of ciliary protein trafficking. J. Biochem. 2021, 169, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Dickman, M.B.; Yarden, O. Serine/Threonine Protein Kinases and Phosphatases in Filamentious Fungi. Fungal Genet. Biol. 1999, 26, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Yaron, T.M.; Huntsman, E.M.; Kerelsky, A.; Song, J.; Regev, A.; Lin, T.-Y.; Liberatore, K.; Cizin, D.M.; Cohen, B.M.; et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature 2023, 613, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Edward, A.M.I. Role of protein kinases in neurodegenerative disease: Cyclin-dependent kinases in Alzheimer’s disease. Front. Biosci. 2005, 10, 143–159. [Google Scholar] [CrossRef]
- Hergovich, A.; Stegert, M.R.; Schmitz, D.; Hemmings, B.A. NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 2006, 7, 253–264. [Google Scholar] [CrossRef]
- Santos, P.F.; Fazendeiro, B.; Luca, F.C.; Ambrósio, A.F.; Léger, H. The NDR/LATS protein kinases in neurobiology: Key regulators of cell proliferation, differentiation and migration in the ocular and central nervous system. Eur. J. Cell Biol. 2023, 102, 151333. [Google Scholar] [CrossRef]
- Bichsel, S.J.; Tamaskovic, R.; Stegert, M.R.; Hemmings, B.A. Mechanism of Activation of NDR (Nuclear Dbf2-related) Protein Kinase by the hMOB1 Protein. J. Biol. Chem. 2004, 279, 35228–35235. [Google Scholar] [CrossRef] [PubMed]
- Record, C.J.; Chaikuad, A.; Rellos, P.; Das, S.; Pike, A.C.W.; Fedorov, O.; Marsden, B.D.; Knapp, S.; Lee, W.H. Structural Comparison of Human Mammalian Ste20-Like Kinases. PLoS ONE 2010, 5, e11905. [Google Scholar] [CrossRef]
- Chen, S.; Fang, Y.; Xu, S.; Reis, C.; Zhang, J. Mammalian Sterile20-like Kinases: Signalings and Roles in Central Nervous System. Aging Dis. 2018, 9, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Meima, M.E.; Douglass, J.; Gunaratne, R.; Bradford, D.; Saeed, F.; Hoffert, J.D.; Steinbach, P.J.; Knepper, M.A.; Pisitkun, T. Predicting kinase-substrate interactions in the era of proteomics: Focus on “Identifying protein kinase target preferences using mass spectrometry”. Am. J. Physiol. Cell Physiol. 2012, 303, C711–C712. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Yu, Z.; Joy, S.; Mi, T.; Yazdanpanah, G.; Burgess, K.; de Paiva, C.S. New, potent, small molecule agonists of tyrosine kinase receptors attenuate dry eye disease. Front. Med. 2022, 9, 937142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Suk, K. Kinase-Based Taming of Brain Microglia Toward Disease-Modifying Therapy. Front. Cell. Neurosci. 2018, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Mahadevan, D. A comprehensive review of protein kinase inhibitors for cancer therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.A.; Mehta, S.; Lad, E.M.; Emerson, G.G.; Jumper, J.M.; Awh, C.C. Intravitreal Injection Therapy: Current Techniques and Supplemental Services. J. Vitreoretin. Dis. 2021, 5, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Campos, A.; Martins, J.; Ambrósio, A.F.; Campos, E.J. Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses. ACS Biomater. Sci. Eng. 2020, 6, 6587–6597. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Boia, R.; Dias, P.A.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Braga, M.E.; et al. Porous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control. Release 2019, 316, 331–348. [Google Scholar] [CrossRef]
- Samoilă, L.; Voștinaru, O.; Dinte, E.; Bodoki, A.E.; Iacob, B.-C.; Bodoki, E.; Samoilă, O. Topical Treatment for Retinal Degenerative Pathologies: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 8045. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.F.; Ambrósio, A.F.; Léger, H. The Importance of Kinases in Retinal Degenerative Diseases. Kinases Phosphatases 2024, 2, 93-109. https://doi.org/10.3390/kinasesphosphatases2010006
Santos PF, Ambrósio AF, Léger H. The Importance of Kinases in Retinal Degenerative Diseases. Kinases and Phosphatases. 2024; 2(1):93-109. https://doi.org/10.3390/kinasesphosphatases2010006
Chicago/Turabian StyleSantos, Paulo F., António Francisco Ambrósio, and Hélène Léger. 2024. "The Importance of Kinases in Retinal Degenerative Diseases" Kinases and Phosphatases 2, no. 1: 93-109. https://doi.org/10.3390/kinasesphosphatases2010006
APA StyleSantos, P. F., Ambrósio, A. F., & Léger, H. (2024). The Importance of Kinases in Retinal Degenerative Diseases. Kinases and Phosphatases, 2(1), 93-109. https://doi.org/10.3390/kinasesphosphatases2010006







