Single-Molecule Analysis of Alkaline Phosphatase
Abstract
1. Introduction
2. First Single-Enzyme Molecule Assay
3. Individual Molecules of a Given Enzyme Have Different Properties
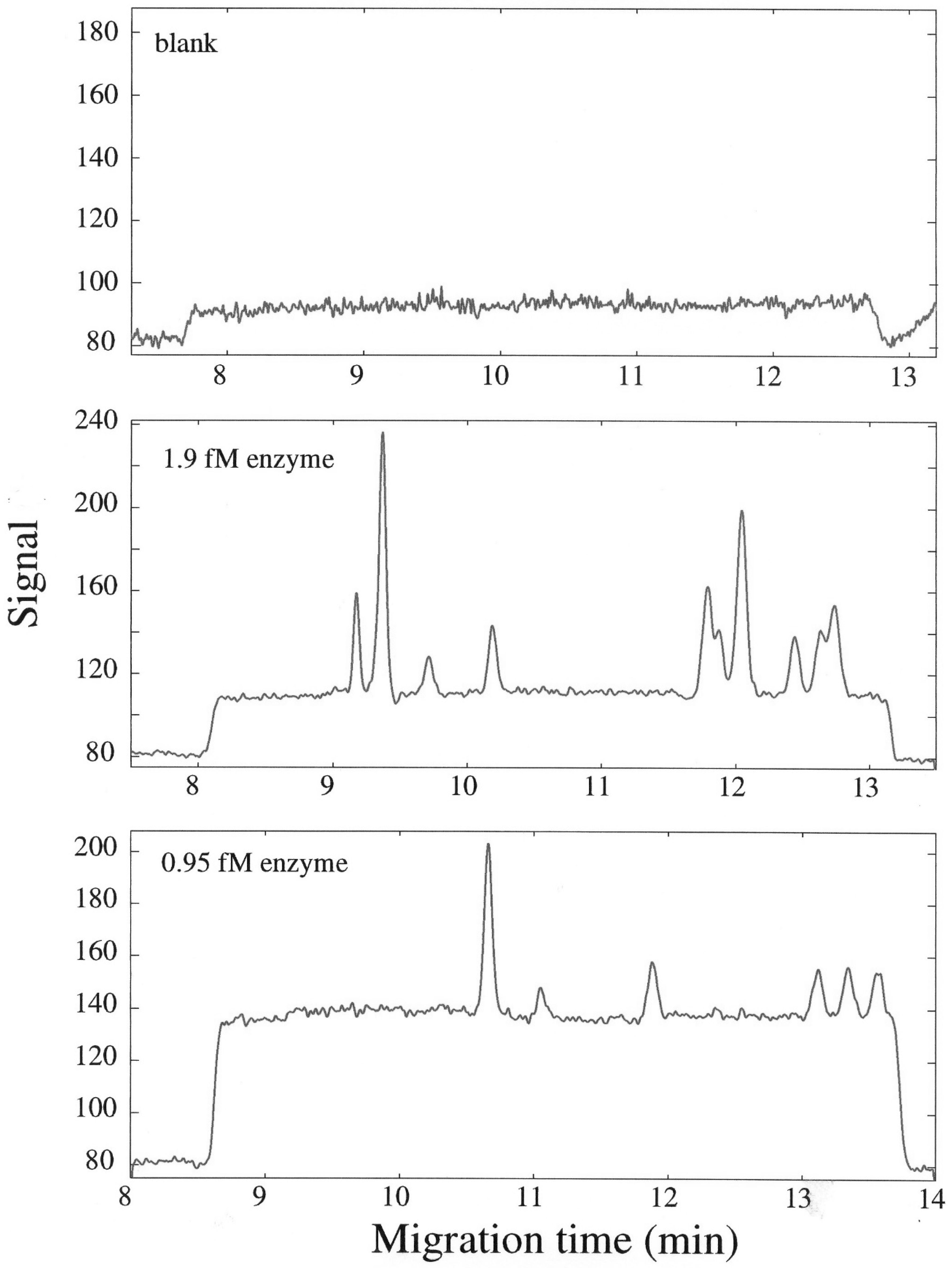
4. Single-Molecule Alkaline Phosphatase Assays Using Capillary Electrophoresis
5. Single-Molecule Alkaline Phosphatase Assays Using Microscopic Wells
6. In-Solution Single-Particle Tracking
7. Significance of Enzyme Heterogeneity
8. Summary
Funding
Conflicts of Interest
References
- Xue, Q.; Yeung, E.S. Differences in the Chemical Reactivity of Individual Molecules of an Enzyme. Nature 1995, 373, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Arriaga, E.A.; Wong, J.C.Y.; Lu, H.; Dovichi, N.J. Studies on Single Alkaline phosphatase Molecules: Reaction Rate and Activation Energy of a Reaction Catalyzed by a Single Molecule and the Effect of Thermal Denaturation—The Death of an Enzyme. J. Am. Chem. Soc. 1996, 118, 5245–5253. [Google Scholar] [CrossRef]
- Craig, D.B.; Arriaga, E.A.; Wong, J.C.Y.; Lu, H.; Dovichi, N.J. Single Alkaline Phosphatase Molecule Assay by Capillary Electrophoresis Laser-Induced Fluorescence Detection. Tech. Protein Chem. 1997, 8, 121–131. [Google Scholar]
- Craig, D.B.; Arriaga, E.A.; Wong, J.C.Y.; Lu, H.; Dovichi, N.J. Life and Death of a Single Enzyme Molecule. Anal. Chem. 1998, 70, 39A–43A. [Google Scholar] [CrossRef]
- Shoemaker, G.K.; Juers, D.H.; Coombs, J.M.L.; Matthews, B.W.; Craig, D.B. Crystallization of β-Galactosidase Does Not Reduce the Range of Activity of Individual Molecules. Biochemistry 2003, 42, 1707–1710. [Google Scholar] [CrossRef]
- Gorris, H.-H.; Walt, D.R. Mechanistic Aspects of Horseradish Peroxidase Elucidated Through Single-Molecule Studies. J. Am. Chem. Soc. 2009, 131, 6277–6282. [Google Scholar] [CrossRef]
- Liebherr, R.B.; Renner, M.; Gorris, H.-H. A Single molecule Perspective on the Functional Diversity of In Vitro Evolved β-Glucuronidase. J. Am. Chem. Soc. 2014, 136, 5949–5955. [Google Scholar] [CrossRef]
- Bai, C.; Wang, C.; Xie, X.S.; Wolynes, P.G. Single Molecule Physics and Chemistry. Proc. Natl. Acad. Sci. USA 1999, 96, 11075–11076. [Google Scholar] [CrossRef]
- Lu, H.P.; Xun, L.; Xie, X.S. Single-Molecule Enzymatic Dynamics. Science 1998, 282, 1877–1882. [Google Scholar] [CrossRef]
- Gorris, H.-H.; Rissin, D.M.; Walt, D.R. Stochastic Inhibitor Release and binding from Single-Enzyme Molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 17680–17685. [Google Scholar] [CrossRef]
- Chen, Z.; Shaw, A.; Wilson, H.; Woringer, M.; Darzacq, X.; Marqusee, S.; Wang, Q.; Bustamante, C. Single-Molecule Diffusometry Reveals No Catalysis-Induced Diffusion Enhancement of Alkaline Phosphatase as Proposed by FCS Experiments. Proc. Natl. Acad. Sci. USA 2020, 117, 21328–21335. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.R.; Shadabi, E.; Craig, D.B. Effect of Alteration of Translational Error Rate on Enzyme Microheterogeneity as Assessed by Variation in Single Molecule Electrophoretic Mobility and catalytic Activity. Biochem. Cell Biol. 2009, 87, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Haslam, A.M.; Coombs, J.M.L.; Nichols, E.R. Kinetic Studies on Unmodified Individual Escherichia coli β-Galactosidase Molecules in Free Solution. Biochem. Cell Biol. 2009, 88, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.J.; Hollett, J.W.; Craig, D.B. Determination of the Inhibitor Dissociation Constant of an Individual Unmodified Enzyme Molecule in Free Solution. Electrophoresis 2016, 37, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Hall, T.; Goltz, D.M. E. coli β-Galactosidase is Heterogeneous with Respect to a Requirement for Magnesium. BioMetals 2000, 13, 223–229. [Google Scholar] [CrossRef]
- Craig, D.B.; Nichols, E.R. Continuous Flow Assay for the Simultaneous Measurement of the electrophoretic Mobility, Catalytic Activity and its Variation Over time of Individual Molecules of Escherichia coli β-Galactosidase. Electrophoresis 2008, 29, 4296–4303. [Google Scholar] [CrossRef]
- Sakamoto, S.; Komatsu, T.; Watanabe, R.; Zhang, Y.; Inoue, T.; Kawaguchi, M.; Nakagawa, H.; Ueno, T.; Okusaka, T.; Honda, K.; et al. Multiplexed Single-Molecule Enzyme Activity Analysis for Counting Disease-Related proteins in Biological Samples. Sci. Adv. 2020, 6, eaay0888. [Google Scholar] [CrossRef]
- Ueno, H.; Kato, M.; Minagawa, Y.; Hirose, Y.; Noji, H. Elucidation and Control of low and High Active Populations of Alkaline Phosphatase Molecules for Quantitative Digital Bioassay. Protein Sci. 2021, 30, 1628–1639. [Google Scholar] [CrossRef]
- Rotman, B. Measurement of activity of single molecules of β-D-galactosidase. Proc. Natl. Acad. Sci. USA 1961, 47, 1981–1991. [Google Scholar] [CrossRef]
- Xue, Q.; Yeung, E.S. Variability of Intracellular Lactate Dehydrogenase Isoenzymes in Single Human Erythrocytes. Anal. Chem. 1994, 66, 1175–1178. [Google Scholar] [CrossRef]
- Craig, D.B.; King, S.D.; Reinfelds, G.; Henderson, A.R.P.; Wood, T.E.H. Electrophoretic Mobility, Catalytic Rate, and Activation Energy of Catalysis of Single molecules of the Enzyme β-Glucuronidase from Escherichia coli. Int. J. Biol. Macromol. 2017, 96, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, L. Studies on Calf-Intestinal Alkaline phosphatase 1. Chromatographic Purification, Microheterogeneity and Some Other Properties of the Purified Enzyme. Biochim. Biophys. Acta 1961, 52, 36–48. [Google Scholar] [CrossRef]
- Saini, P.K.; Done, J. The diversity of Alkaline Phosphatase from Rat Intestine: Isolation and Purification of the Enzyme(s). Biochim. Biophys. Acta 1972, 258, 147–153. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Cancedda, F.; Ericcson, I.H.; Neumann, P.A.; Piccoli, S.P.; Schlesinger, M.J.; Shriefer, K.; Walsh, K.A. Amino Acid sequence of Escherichia coli Alkaline Phosphatase. Proc. Natl. Acad. Sci. USA 1981, 78, 3473–3477. [Google Scholar] [CrossRef]
- Schlesinger, M.J.; Andersen, L. Multiple Molecular Forms of the Alkaline Phosphatase of Escherichia coli. Ann. N Y Acad. Sci. 1968, 151, 159–170. [Google Scholar] [CrossRef]
- Polakowski, R.; Craig, D.B.; Skelley, A.; Dovichi, N.J. Single molecules of Highly Purified Bacterial Alkaline Phosphatase Have Identical Activity. J. Am. Chem. Soc. 2000, 122, 4853–4855. [Google Scholar] [CrossRef]
- Crawford, J.J.; Itzkow, F.; MacLean, J.; Craig, D.B. Conformational Change in Individual Enzyme Molecules. Biochem. Cell. Biol. 2015, 93, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Winzor, D.J.; Jones, S.; Harding, S.E. Determination of Protein Charge by Capillary Zone Electrophoresis. Anal. Biochem. 2004, 333, 225–229. [Google Scholar] [CrossRef]
- Winzor, D.J. Determination of the Net Charge (Valence) of a Protein: A Fundamental but Elusive Parameter. Anal. Biochem. 2004, 325, 1–20. [Google Scholar] [CrossRef]
- Nichols, E.R.; Craig, D.B. Measurement of the Differences in Electrophoretic Mobility of Individual Molecules of E. coli β-Galactosidase Provides Insight Into Structural Differences Which Underlie Enzyme Microheterogeneity. Electrophoresis 2008, 29, 1–13. [Google Scholar] [CrossRef]
- Craig, D.B.; Malhi, S.; Ahmad, B.; Breckman, K.; Patal, A. Electrophoretic Mobility of Individual Molecules of Alkaline Phosphatase. Biochem. Cell Biol. 2022, 100, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Hoffstatter-Kuhn, S.; Paulus, A.; Gassmann, E.; Widmer, H.M. Influence of Borate Complexation on the Electrophoretic Behaviour of Carbohydrates in Capillary Electrophoresis. Anal. Chem. 1991, 63, 1541–1547. [Google Scholar] [CrossRef]
- Dyck, A.C.; Craig, D.B. Individual Molecules of Thermostable Alkaline Phosphatase Support Different Catalytic Rates at Room Temperature. Luminescence 2002, 17, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Hanlon-Dearman, F.; Beaudry, S.; Shek, K.; King, S.D. Comparison of the Single Molecule Activity Distribution of Recombinant and Non-Recombinant Bovine Intestinal Alkaline Phosphatase. Protein Expr. Purif. 2015, 114, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.R.; Craig, D.B. Single Molecule Assays Reveal Differences Between In Vitro and In Vivo Synthesized β-Galactosidase. Protein J. 2008, 27, 376–383. [Google Scholar] [CrossRef]
- Gilboa, T.; Ogata, A.F.; Walt, D.R. Single-Molecule Enzymology for Diagnostics: Profiling Alkaline Phosphatase Activity in Clinical Samples. ChemBioChem 2022, 23, e202100358. [Google Scholar] [CrossRef]
- Rissin, D.M.; Gorris, H.-H.; Walt, D.R. Distinct and Long-Lived Activity States of Single Enzyme Molecules. J. Am. Chem. Soc. 2008, 130, 5349–5353. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Minigawa, Y.; Noji, H.; Tabata, K.V. Multidimensional Digital Bioassay Platform Based on an Air-Sealed Femtoliter Reactor Array Device. Anal. Chem. 2021, 93, 5494–5502. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Walt, D.R. Single-Molecule Analysis Determines Isozymes of Human Alkaline Phosphatase in Serum. Agnew. Chem. Int. Ed. Engl. 2020, 59, 18010–18015. [Google Scholar] [CrossRef]
- Gilboa, T.; Ogata, A.F.; Reilly, C.B.; Walt, D.R. Single-Molecules Studies Reveal Method for Tuning the heterogeneous Activity of Alkaline Phosphatase. Biophys. J. 2022, 121, 2027–2034. [Google Scholar] [CrossRef]
- Obayashi, Y.; Iino, R.; Noji, H. A Single-Molecule Digital Enzyme assay Using Alkaline Phosphatase with a Cumarin-Based Fluorogenic Substrate. Analyst 2015, 140, 5065–5073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Craig, D.B.; Schwab, T.; Sterner, R. Random mutagenesis suggests that sequence errors are not a major cause of variation in the activity of individual molecules of β-galactosidase. Biochem. Cell. Biol. 2012, 90, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Hall, T. Newly induced β-galactosidase molecules have a higher activity than the basally expressed enzyme. J. Clin. Laser Med. Surg. 2000, 18, 209–214. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craig, D.B. Single-Molecule Analysis of Alkaline Phosphatase. Kinases Phosphatases 2024, 2, 306-314. https://doi.org/10.3390/kinasesphosphatases2040020
Craig DB. Single-Molecule Analysis of Alkaline Phosphatase. Kinases and Phosphatases. 2024; 2(4):306-314. https://doi.org/10.3390/kinasesphosphatases2040020
Chicago/Turabian StyleCraig, Douglas B. 2024. "Single-Molecule Analysis of Alkaline Phosphatase" Kinases and Phosphatases 2, no. 4: 306-314. https://doi.org/10.3390/kinasesphosphatases2040020
APA StyleCraig, D. B. (2024). Single-Molecule Analysis of Alkaline Phosphatase. Kinases and Phosphatases, 2(4), 306-314. https://doi.org/10.3390/kinasesphosphatases2040020




