Expression of Tribbles Pseudokinase 3 in Prostate Cancers and Its Roles in Cell Cycle Regulation
Abstract
1. Introduction
2. Results
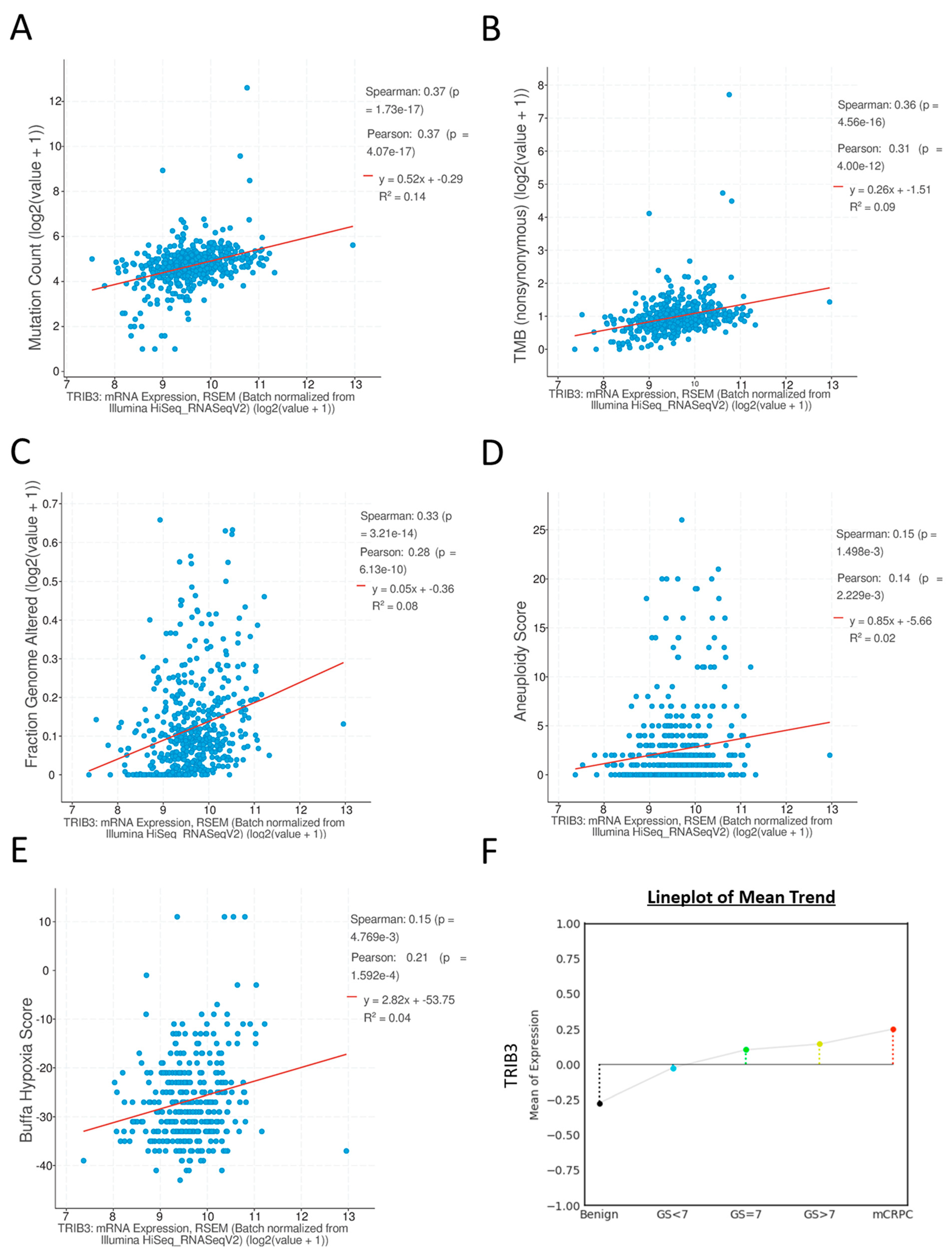
2.1. Expression of TRIB3 at mRNA Level in Prostate Cancers and Its Association with Genomic Mutations and Other Clinical Attributes
2.2. High mRNA Expression of TRIB3 Portends Poor Progression-Free or Poor Disease-Free Survival of Prostate Cancer Patients
2.3. Increased Expression of TRIB3 Leads to Cell Cycle Arrest and Reduced Tumor Cell Proliferation
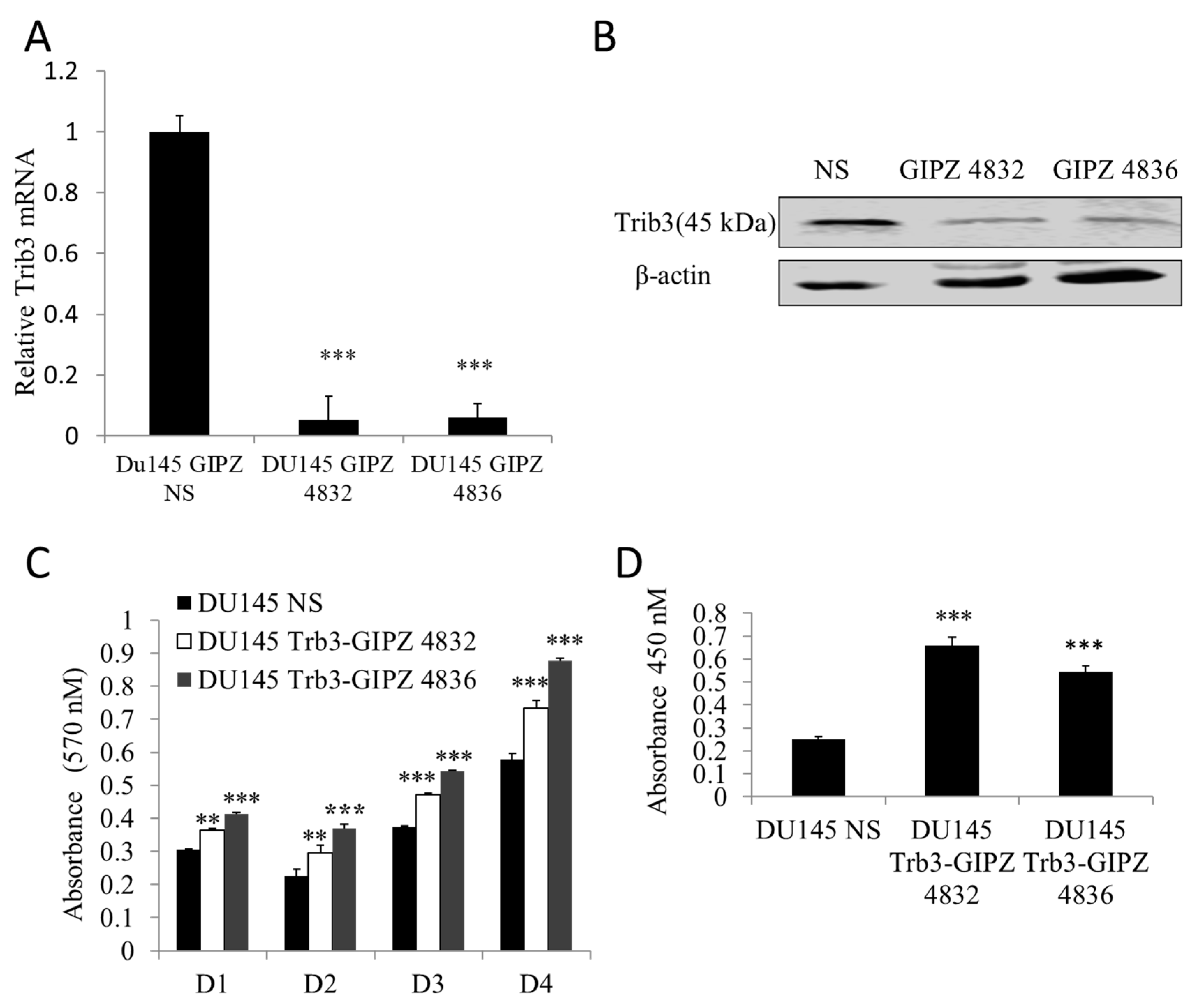
2.4. Depletion of TRIB3 in Prostate Cancer Cells Promotes Cellular Proliferation
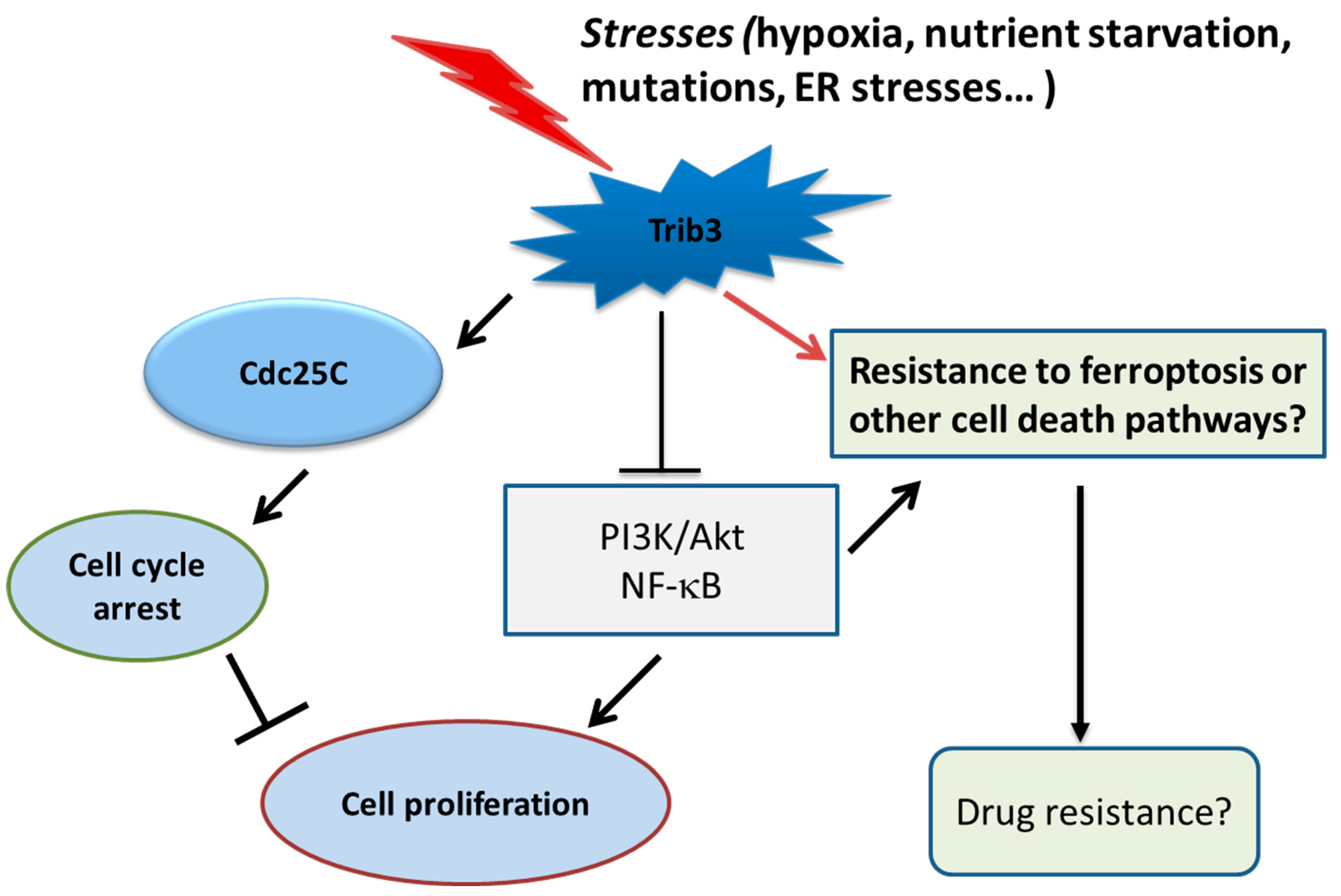
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hegedus, Z.; Czibula, A.; Kiss-Toth, E. Tribbles: Novel regulators of cell function; evolutionary aspects. Cell. Mol. Life Sci. 2006, 63, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, F.; Savonet, V.; Radulescu, A.; Petermans, J.; Dumont, J.E.; Maenhaut, C. Identification and characterization of novel genes modulated in the thyroid of dogs treated with methimazole and propylthiouracil. J. Biol. Chem. 1996, 271, 28451–28457. [Google Scholar] [CrossRef]
- Wilkin, F.; Suarez-Huerta, N.; Robaye, B.; Peetermans, J.; Libert, F.; Dumont, J.E.; Maenhaut, C. Characterization of a phosphoprotein whose mRNA is regulated by the mitogenic pathways in dog thyroid cells. Eur. J. Biochem. 1997, 248, 660–668. [Google Scholar] [CrossRef] [PubMed]
- de Lorenzo, C.; Greco, A.; Fiorentino, T.V.; Mannino, G.C.; Hribal, M.L. Variants of insulin-signaling inhibitor genes in type 2 diabetes and related metabolic abnormalities. Int. J. Genom. 2013, 2013, 376454. [Google Scholar] [CrossRef] [PubMed]
- Prudente, S.; Sesti, G.; Pandolfi, A.; Andreozzi, F.; Consoli, A.; Trischitta, V. The mammalian tribbles homolog TRIB3, glucose homeostasis, and cardiovascular diseases. Endocr. Rev. 2012, 33, 526–546. [Google Scholar] [CrossRef]
- Beguinot, F. Tribbles homologue 3 (TRIB3) and the insulin-resistance genes in type 2 diabetes. Diabetologia 2010, 53, 1831–1834. [Google Scholar] [CrossRef]
- Ord, T.; Ord, D.; Adler, P.; Vilo, J.; Ord, T. TRIB3 enhances cell viability during glucose deprivation in HEK293-derived cells by upregulating IGFBP2, a novel nutrient deficiency survival factor. Biochim. Biophys. Acta 2015, 1853, 2492–2505. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Chuang, G.C.; Hill, H.S.; Tian, L.; Fu, Y.; Moellering, D.R.; Garvey, W.T. Role of TRIB3 in regulation of insulin sensitivity and nutrient metabolism during short-term fasting and nutrient excess. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E908–E916. [Google Scholar] [CrossRef]
- Du, K.; Herzig, S.; Kulkarni, R.N.; Montminy, M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003, 300, 1574–1577. [Google Scholar] [CrossRef]
- Samper-Ternent, R.; Al Snih, S. Obesity in Older Adults: Epidemiology and Implications for Disability and Disease. Rev. Clin. Gerontol. 2012, 22, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Lorente, M.; Garcia-Taboada, E.; Perez Gomez, E.; Davila, D.; Zuniga-Garcia, P.; Maria Flores, J.; Rodriguez, A.; Hegedus, Z.; Mosen-Ansorena, D.; et al. Loss of Tribbles pseudokinase-3 promotes Akt-driven tumorigenesis via FOXO inactivation. Cell Death Differ. 2015, 22, 131–144. [Google Scholar] [CrossRef]
- Salazar, M.; Lorente, M.; Garcia-Taboada, E.; Gomez, E.P.; Davila, D.; Zuniga-Garcia, P.; Flores, J.M.; Rodriguez, A.; Hegedus, Z.; Mosen-Ansorena, D.; et al. TRIB3 suppresses tumorigenesis by controlling mTORC2/AKT/FOXO signaling. Mol. Cell. Oncol. 2015, 2, e980134. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xia, J.; Wang, H.; Sun, L.; Wu, Z.; Bin, J.; Liao, Y.; Li, N.; Liao, W. Overexpression of TRIB3 promotes angiogenesis in human gastric cancer. Oncol. Rep. 2016, 36, 2339–2348. [Google Scholar] [CrossRef]
- Wennemers, M.; Bussink, J.; Scheijen, B.; Nagtegaal, I.D.; van Laarhoven, H.W.; Raleigh, J.A.; Varia, M.A.; Heuvel, J.J.; Rouschop, K.M.; Sweep, F.C.; et al. Tribbles homolog 3 denotes a poor prognosis in breast cancer and is involved in hypoxia response. Breast Cancer Res. 2011, 13, R82. [Google Scholar] [CrossRef]
- Yu, J.M.; Sun, W.; Wang, Z.H.; Liang, X.; Hua, F.; Li, K.; Lv, X.X.; Zhang, X.W.; Liu, Y.Y.; Yu, J.J.; et al. TRIB3 supports breast cancer stemness by suppressing FOXO1 degradation and enhancing SOX2 transcription. Nat. Commun. 2019, 10, 5720. [Google Scholar] [CrossRef]
- Salazar, M.; Lorente, M.; Orea-Soufi, A.; Davila, D.; Erazo, T.; Lizcano, J.; Carracedo, A.; Kiss-Toth, E.; Velasco, G. Oncosuppressive functions of tribbles pseudokinase 3. Biochem. Soc. Trans. 2015, 43, 1122–1126. [Google Scholar] [CrossRef]
- Schwarzer, R.; Dames, S.; Tondera, D.; Klippel, A.; Kaufmann, J. TRB3 is a PI 3-kinase dependent indicator for nutrient starvation. Cell Signal. 2006, 18, 899–909. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Yang, Y.; Ren, H.; Ren, T.; Huang, Y.; Zhang, S.; Sun, Q.; Huang, H. TRIB3 inhibition by palbociclib sensitizes prostate cancer to ferroptosis via downregulating SOX2/SLC7A11 expression. Cell Death Discov. 2024, 10, 425. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, S.; Qin, Y.; Shu, F.; Xu, Y.; Chen, J.; Xu, B.E.; Sun, X.; Wu, J. TRB3 interacts with CtIP and is overexpressed in certain cancers. Biochim. Biophys. Acta 2007, 1770, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, H.J.; Guo, Z.M.; Zeng, M.S.; Li, M.Z.; Jiang, Y.E.; He, X.G.; Sun, C.Z. TRB3 overexpression due to endoplasmic reticulum stress inhibits AKT kinase activation of tongue squamous cell carcinoma. Oral Oncol. 2011, 47, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304 e296. [Google Scholar] [CrossRef]
- Ellrott, K.; Bailey, M.H.; Saksena, G.; Covington, K.R.; Kandoth, C.; Stewart, C.; Hess, J.; Ma, S.; Chiotti, K.E.; McLellan, M.; et al. Scalable Open Science Approach for Mutation Calling of Tumor Exomes Using Multiple Genomic Pipelines. Cell Syst. 2018, 6, 271–281 e277. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689 e673. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416 e411. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337 e310. [Google Scholar] [CrossRef]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Knudsen, B.S.; Erho, N.; Alshalalfa, M.; Takhar, M.; Al-Deen Ashab, H.; Davicioni, E.; Karnes, R.J.; Klein, E.A.; Den, R.B.; et al. Integrated Classification of Prostate Cancer Reveals a Novel Luminal Subtype with Poor Outcome. Cancer Res. 2016, 76, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. Embo J. 2005, 24, 1243–1255. [Google Scholar] [CrossRef]
- Carraro, V.; Maurin, A.C.; Lambert-Langlais, S.; Averous, J.; Chaveroux, C.; Parry, L.; Jousse, C.; Ord, D.; Ord, T.; Fafournoux, P.; et al. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2alpha/ATF4 pathway. PLoS ONE 2010, 5, e15716. [Google Scholar] [CrossRef]
- Abdullahi, A.; Stanojcic, M.; Parousis, A.; Patsouris, D.; Jeschke, M.G. Modeling Acute ER Stress in Vivo and in Vitro. Shock 2017, 47, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Chetram, M.A.; Odero-Marah, V.; Hinton, C.V. Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells. Mol. Cancer Res. 2011, 9, 90–102. [Google Scholar] [CrossRef]
- Hu, C.; Li, Q.; Xiang, L.; Luo, Y.; Li, S.; An, J.; Yu, X.; Zhang, G.; Chen, Y.; Wang, Y.; et al. Comprehensive pan-cancer analysis unveils the significant prognostic value and potential role in immune microenvironment modulation of TRIB3. Comput. Struct. Biotechnol. J. 2024, 23, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, Z.; Czibula, A.; Kiss-Toth, E. Tribbles: A family of kinase-like proteins with potent signalling regulatory function. Cell Signal. 2007, 19, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Rorth, P.; Szabo, K.; Texido, G. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol. Cell 2000, 6, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Seher, T.C.; Leptin, M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr. Biol. 2000, 10, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adom, D.; Wang, J.; Wang, M.-T.; Nie, D. Expression of Tribbles Pseudokinase 3 in Prostate Cancers and Its Roles in Cell Cycle Regulation. Kinases Phosphatases 2025, 3, 2. https://doi.org/10.3390/kinasesphosphatases3010002
Adom D, Wang J, Wang M-T, Nie D. Expression of Tribbles Pseudokinase 3 in Prostate Cancers and Its Roles in Cell Cycle Regulation. Kinases and Phosphatases. 2025; 3(1):2. https://doi.org/10.3390/kinasesphosphatases3010002
Chicago/Turabian StyleAdom, Djamilatou, Jiuhui Wang, Man-Tzu Wang, and Daotai Nie. 2025. "Expression of Tribbles Pseudokinase 3 in Prostate Cancers and Its Roles in Cell Cycle Regulation" Kinases and Phosphatases 3, no. 1: 2. https://doi.org/10.3390/kinasesphosphatases3010002
APA StyleAdom, D., Wang, J., Wang, M.-T., & Nie, D. (2025). Expression of Tribbles Pseudokinase 3 in Prostate Cancers and Its Roles in Cell Cycle Regulation. Kinases and Phosphatases, 3(1), 2. https://doi.org/10.3390/kinasesphosphatases3010002






