Nanotechnology-Based Delivery Systems for Enhanced Targeting of Tyrosine Kinase Inhibitors: Exploring Inorganic and Organic Nanoparticles as Targeted Carriers
Abstract
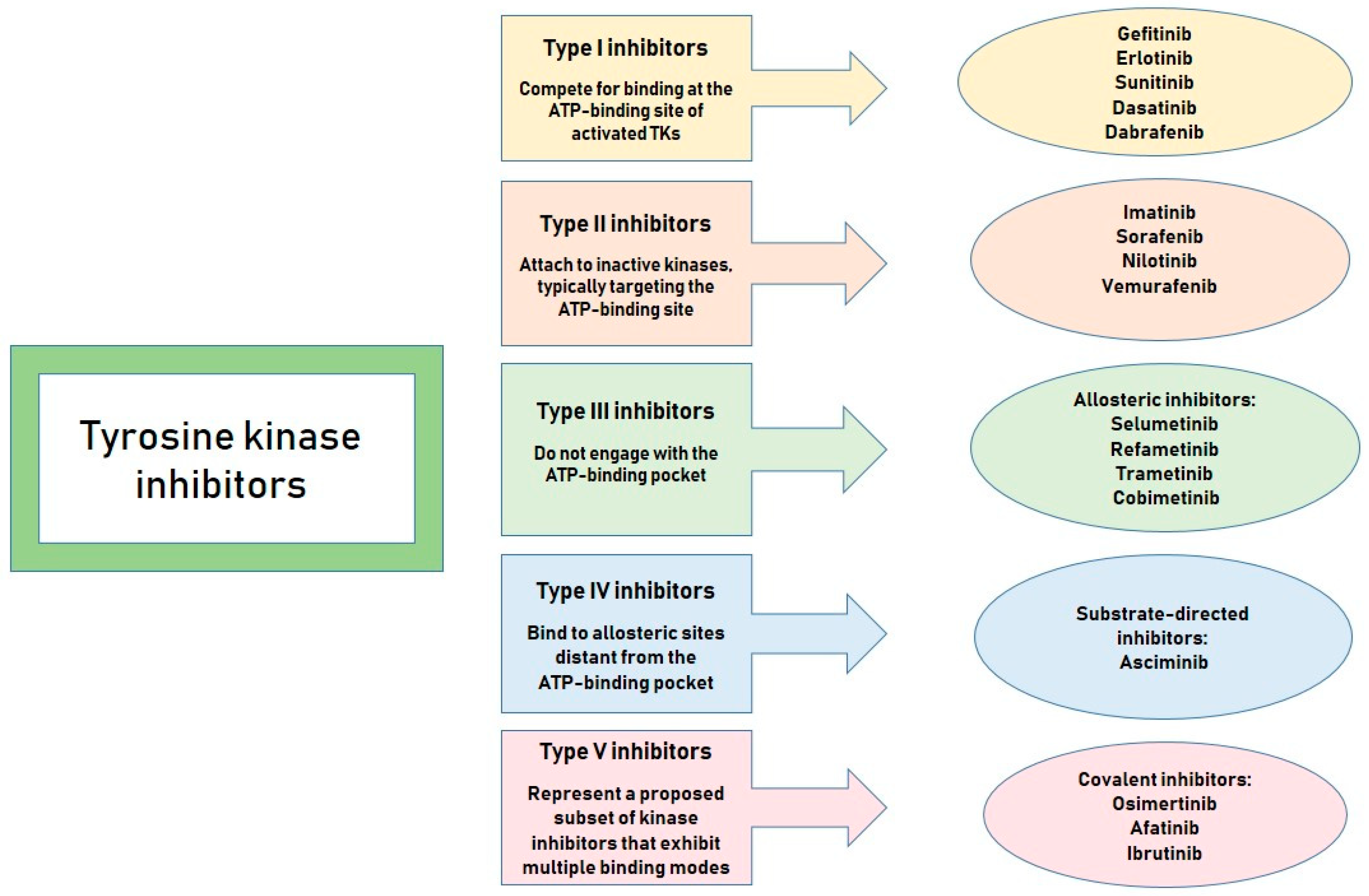
1. Introduction
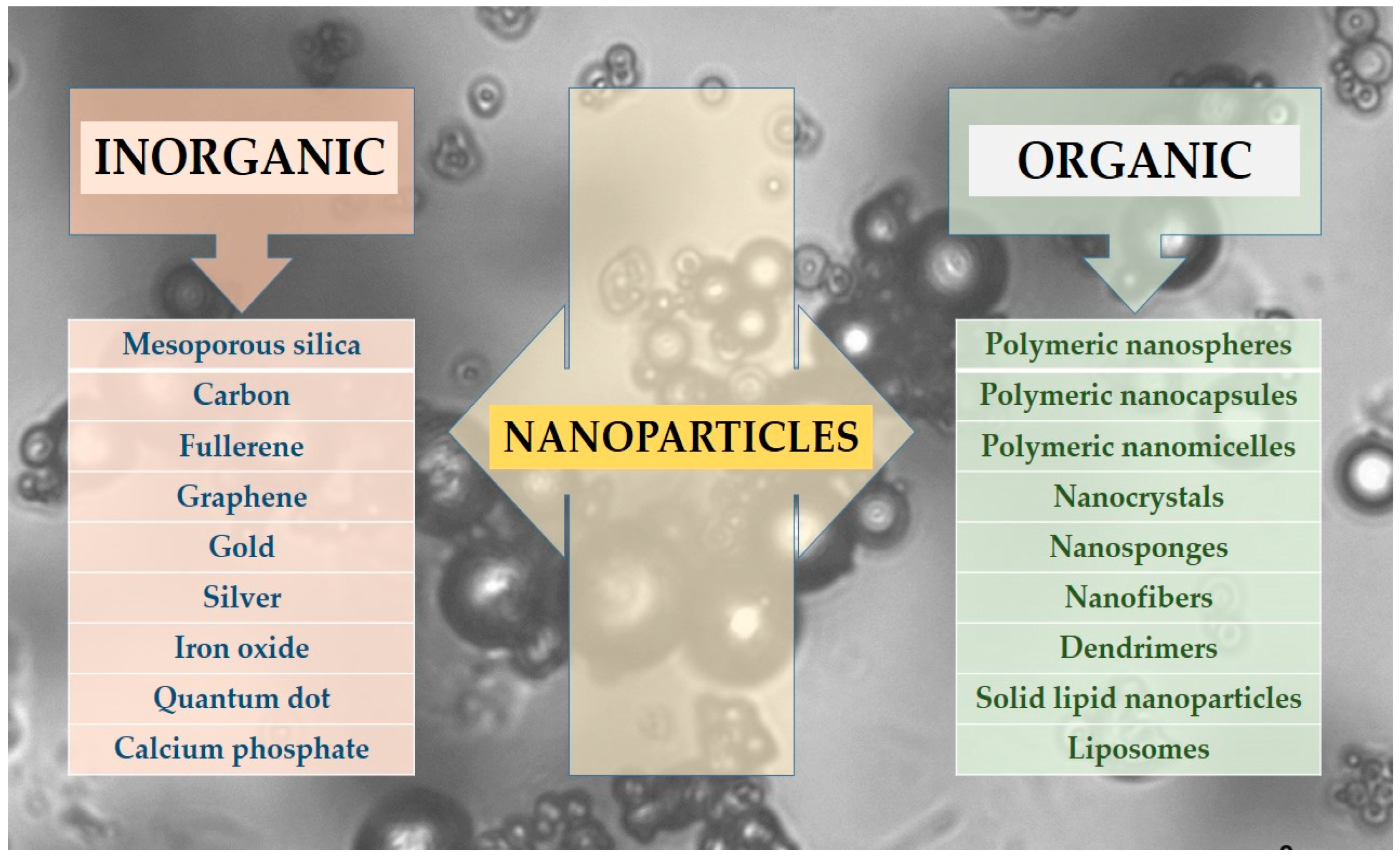
2. Nano-Scale Drug Delivery Systems
- Inorganic NPs, including silica, carbon-based, gold, and other metallic NPs.
- Organic NPs, which encompass polymeric NPs such as nanospheres and nanocapsules, protein-based carriers, micelles, liposomes, solid lipid nanoparticles (SLNs), dendrimers, nanotubes, nanofibers, nanocrystals, and others.
2.1. Inorganic/Metallic and Non-Metallic NPs
2.1.1. Gold NPs (AuNPs)
2.1.2. Silver NPs (AgNPs)
2.1.3. Mesoporous Silica NPs
2.1.4. Carbon Nanotubes
2.1.5. Magnetic NPs (MNPs)
2.2. Organic NPs
2.2.1. Polymeric NPs
2.2.2. Polymeric Nanocapsules/Nanospheres
2.2.3. Polymeric Micelles (PMs)
2.2.4. Solid Lipid NPs
2.2.5. Liposomes
2.2.6. Nanocrystals
2.2.7. Nanosponges (NSs)
2.2.8. Nanofibers
2.2.9. Dendrimers
3. Possibilities and Limitations of Nanotechnology-Based TKIs Carriers
4. Future Directions: Clinical Trials of Nano-Scaled TKI Carriers
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TKs | Tyrosine kinases |
| TKI | Tyrosine kinase inhibitor |
| TKIs | Tyrosine kinase inhibitors |
| ATP | Adenosine triphosphate |
| BCS | Biopharmaceutics Classification System |
| NPs | Nanoparticles |
| CNTs | Carbon nanotubes |
| AuNPs | Gold nanoparticles |
| AgNPs | Silver nanoparticles |
| CLL | Chronis lymphocytic leukemia |
| MSNPs | Mesoporous silica nanoparticles |
| HMSNPs | Hollow mesoporous silica nanoparticles |
| MNPs | Magnetic nanoparticles |
| MRI | Magnetic resonance imaging |
| PMs | Polymeric micelles |
| SN-38 | 7-ethyl-10-hydroxy-camptothecin |
| SLNs | Solid lipid nanoparticles |
| CML | Chronic myeloid leukemia |
| EPR | Enhanced permeability and retention |
| IMA | Imatinib |
| NSs | Nanosponges |
| ITB | Ibrutinib |
| SPNs | Supramolecular peptide nanofibers |
| Da | Dabrafenib |
| DOx | Doxorubicin |
| TZ | Trastuzumab |
| i.v. | Intravenous |
| PLA | Poly(lactic) acid |
| PLGA | Poly(lactic-co-glycolic) acid |
| PEG | Polyethylene glycol |
References
- Korucu Aktas, P.; Baysal, I.; Yabanoglu-Ciftci, S.; Lamprecht, A.; Arica, B. Recent Progress in Drug Delivery Systems for Tyrosine Kinase Inhibitors in the Treatment of Lung Cancer. Int. J. Pharm. 2024, 650, 123703. [Google Scholar] [CrossRef] [PubMed]
- Nishal, S.; Jhawat, V.; Gupta, S.; Phaugat, P. Utilization of Kinase Inhibitors as Novel Therapeutic Drug Targets: A Review. Oncol. Res. 2022, 30, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef]
- Foroughi-Nia, B.; Barar, J.; Memar, M.Y.; Aghanejad, A.; Davaran, S. Progresses in Polymeric Nanoparticles for Delivery of Tyrosine Kinase Inhibitors. Life Sci. 2021, 278, 119642. [Google Scholar] [CrossRef]
- Lu, Y.; Bian, D.; Zhang, X.; Zhang, H.; Zhu, Z. Inhibition of Bcl-2 and Bcl-xL Overcomes the Resistance to the Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer. Mol. Med. Rep. 2021, 23, 48. [Google Scholar] [CrossRef]
- Caponnetto, S.; Cantale, O.; Friedlaender, A.; Gomes, F.; Daryanani, S.; Gelibter, A.; Cortellini, A.; Giuffrida, D.; Addeo, A.; Banna, G.L. A Comparison Between First-, Second- and Third-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with Non-Small-Cell Lung Cancer and Brain Metastases. J. Mol. Pathol. 2021, 2, 1. [Google Scholar] [CrossRef]
- Yin, Y.; Yuan, X.; Gao, H.; Yang, Q. Nanoformulations of Small Molecule Protein Tyrosine Kinases Inhibitors Potentiate Targeted Cancer Therapy. Int. J. Pharm. 2020, 573, 118785. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, H.; Solanki, R.; Rawat, L.; Tabasum, S.; Tanwar, P.; Pal, S.; Sabarwal, A. Recent Developments in Receptor Tyrosine Kinase Inhibitors: A Promising Mainstay in Targeted Cancer Therapy. Med. Drug Discov. 2024, 23, 100195. [Google Scholar] [CrossRef]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Ojo, D.A.; Okeowo, O.T.; Omotuyi, I.O. Exploring Receptor Tyrosine Kinases-Inhibitors in Cancer Treatments. Egypt. J. Med. Hum. Genet. 2019, 20, 35. [Google Scholar] [CrossRef]
- Das, R.; Choithramani, A.; Shard, A. A Molecular Perspective for the Use of Type IV Tyrosine Kinase Inhibitors as Anticancer Therapeutics. Drug Discov. Today 2022, 27, 808–821. [Google Scholar] [CrossRef]
- Moradpour, Z.; Barghi, L. Novel Approaches for Efficient Delivery of Tyrosine Kinase Inhibitors. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2019, 22, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Herbrink, M.; Schellens, J.H.M.; Beijnen, J.H.; Nuijen, B. Inherent Formulation Issues of Kinase Inhibitors. J. Control Release Off. J. Control Release Soc. 2016, 239, 118–127. [Google Scholar] [CrossRef]
- Satapathy, S.; Patro, C.S. Solid Lipid Nanoparticles for Efficient Oral Delivery of Tyrosine Kinase Inhibitors: A Nano Targeted Cancer Drug Delivery. Adv. Pharm. Bull. 2021, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Devadasu, V.R.; Deb, P.K.; Maheshwari, R.; Sharma, P.; Tekade, R.K. Physicochemical, Pharmaceutical, and Biological Considerations in GIT Absorption of Drugs. In Dosage Form Design Considerations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 149–178. ISBN 978-0-12-814423-7. [Google Scholar]
- Blaesi, A.H.; Saka, N. Gastroretentive Fibrous Dosage Forms for Prolonged Delivery of Sparingly-Soluble Tyrosine Kinase Inhibitors. Part 1: Dosage Form Design, and Models of Expansion, Post-Expansion Mechanical Strength, and Drug Release. Int. J. Pharm. 2024, 2024, 124360. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology of Tyrosine Kinase Inhibitors in Cancer Therapy: A Perspective. Int. J. Mol. Sci. 2021, 22, 6538. [Google Scholar] [CrossRef] [PubMed]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse Effects of Tyrosine Kinase Inhibitors in Cancer Therapy: Pathophysiology, Mechanisms and Clinical Management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Rajendran, R.L.; Miruka, C.O.; Sivamani, P.; Maran, B.A.V.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.-C. A Review on Tyrosine Kinase Inhibitors for Targeted Breast Cancer Therapy. Pathol.-Res. Pract. 2024, 263, 155607. [Google Scholar] [CrossRef]
- Indermun, S.; Kumar, P.; Govender, M.; Choonara, Y.E. Can Nanomedicinal Approaches Provide an Edge to the Efficacy of Tyrosine Kinase Inhibitors? Curr. Med. Chem. 2023, 30, 1482–1501. [Google Scholar] [CrossRef]
- Kumar, A.; Vaiphei, K.K.; Gulbake, A. A Nanotechnology Driven Effectual Localized Lung Cancer Targeting Approaches Using Tyrosine Kinases Inhibitors: Recent Progress, Preclinical Assessment, Challenges, and Future Perspectives. Int. J. Pharm. 2024, 666, 124745. [Google Scholar] [CrossRef]
- Brusini, R.; Varna, M.; Couvreur, P. Advanced Nanomedicines for the Treatment of Inflammatory Diseases. Adv. Drug Deliv. Rev. 2020, 157, 161–178. [Google Scholar] [CrossRef]
- Yang, J.; Li, D.; Zhang, M.; Lin, G.; Hu, S.; Xu, H. From the Updated Landscape of the Emerging Biologics for IBDs Treatment to the New Delivery Systems. J. Control. Release 2023, 361, 568–591. [Google Scholar] [CrossRef] [PubMed]
- Dacoba, T.G.; Olivera, A.; Torres, D.; Crecente-Campo, J.; Alonso, M.J. Modulating the Immune System through Nanotechnology. Semin. Immunol. 2017, 34, 78–102. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Olayide, I.I.; Akalabu, M.C.; Ajiboye, B.O.; Ojo, A.B.; Oyinloye, B.E.; Ramalingam, M. Nanoparticles and Their Biomedical Applications. Biointerface Res. Appl. Chem. 2020, 11, 8431–8445. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, K.; Hao, Y.; Yang, C.; Zha, R.; Yi, C.; Qian, Z. Advances in Nanotechnology-Based Delivery Systems for EGFR Tyrosine Kinases Inhibitors in Cancer Therapy. Asian J. Pharm. Sci. 2020, 15, 26–41. [Google Scholar] [CrossRef]
- Varna, M.; Xuan, H.V.; Fort, E. Gold Nanoparticles in Cardiovascular Imaging. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1470. [Google Scholar] [CrossRef]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold Nanoparticle Surface Functionalization: A Necessary Requirement in the Development of Novel Nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef]
- Tarighatnia, A.; Foroughi-Nia, B.; Nader, N.D.; Aghanejad, A. Recent Trends and Advances in Nanosystems with Tyrosine Kinase Inhibitors for Image-Guided Cancer Treatments. J. Drug Deliv. Sci. Technol. 2023, 88, 104938. [Google Scholar] [CrossRef]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. [Google Scholar] [CrossRef]
- Elmetwalli, A.; Abdel-Monem, M.O.; El-Far, A.H.; Ghaith, G.S.; Albalawi, N.A.N.; Hassan, J.; Ismail, N.F.; El-Sewedy, T.; Alnamshan, M.M.; ALaqeel, N.K.; et al. Probiotic-Derived Silver Nanoparticles Target mTOR/MMP-9/BCL-2/Dependent AMPK Activation for Hepatic Cancer Treatment. Med. Oncol. 2024, 41, 106. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Zivic, F.; Grujovic, N.; Mitrovic, S.; Ahad, I.U.; Brabazon, D. Characteristics and Applications of Silver Nanoparticles. In Commercialization of Nanotechnologies—A Case Study Approach; Brabazon, D., Pellicer, E., Zivic, F., Sort, J., Dolors Baró, M., Grujovic, N., Choy, K.-L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 227–273. ISBN 978-3-319-56978-9. [Google Scholar]
- Adamo, F.M.; Silva Barcelos, E.C.; De Falco, F.; Dorillo, E.; Rompietti, C.; Sorcini, D.; Stella, A.; Del Papa, B.; Baldoni, S.; Esposito, A.; et al. Therapeutic Targeting Potential of Novel Silver Nanoparticles Coated with Anti-CD20 Antibody against Chronic Lymphocytic Leukemia. Cancers 2023, 15, 3618. [Google Scholar] [CrossRef]
- Torabi, M.; Aghanejad, A.; Savadi, P.; Barzegari, A.; Omidi, Y.; Barar, J. Fabrication of Mesoporous Silica Nanoparticles for Targeted Delivery of Sunitinib to Ovarian Cancer Cells. BioImpacts 2023, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Joshi, V.; Reddy, K.P.; Singh, N.; Das, P.; Datta, P. Biopharmaceutical and Pharmacokinetic Attributes to Drive Nanoformulations of Small Molecule Tyrosine Kinase Inhibitors. Asian J. Pharm. Sci. 2024, 19, 100980. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Bhattacharya, R.; Moin, A.; Al Hagbani, T.; Abdallah, M.H.; Danish Rizvi, S.M.; Khafagy, E.-S.; Hussain, T.; Gangadharappa, H.V. Dual Targeting Multiwalled Carbon Nanotubes for Improved Neratinib Delivery in Breast Cancer. RSC Adv. 2023, 13, 24309–24318. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Chang, J.; Sun, J.; Zhang, T.; Zhao, Y.; Li, Y.; Dong, H. Nanomedicine-Mediated Ferroptosis Targeting Strategies for Synergistic Cancer Therapy. J. Mater. Chem. B 2023, 11, 1171–1190. [Google Scholar] [CrossRef]
- Mohaghegh, S.; Tarighatnia, A.; Omidi, Y.; Barar, J.; Aghanejad, A.; Adibkia, K. Multifunctional Magnetic Nanoparticles for MRI-Guided Co-Delivery of Erlotinib and L-Asparaginase to Ovarian Cancer. J. Microencapsul. 2022, 39, 394–408. [Google Scholar] [CrossRef]
- Jafari, H.; Mahdavinia, G.R.; Kazemi, B.; Ehrlich, H.; Joseph, Y.; Rahimi-Nasrabadi, M. Highly Efficient Sunitinib Release from pH-Responsive mHPMC@Chitosan Core-Shell Nanoparticles. Carbohydr. Polym. 2021, 258, 117719. [Google Scholar] [CrossRef]
- Xu, W.; Ye, C.; Qing, X.; Liu, S.; Lv, X.; Wang, W.; Dong, X.; Zhang, Y. Multi-Target Tyrosine Kinase Inhibitor Nanoparticle Delivery Systems for Cancer Therapy. Mater. Today Bio 2022, 16, 100358. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (Lactic acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.-X.; Shu, N.; Zheng, S.-J.; Ma, M.; Zhao, Z.-B.; Cao, Z.-T.; Zhou, Q.; Du, J.-Z.; Wang, J. A Polymeric Nanoformulation Improves the Bioavailability and Efficacy of Sorafenib for Hepatocellular Carcinoma Therapy. Biomater. Sci. 2021, 9, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Fabrication and Characterization of Chitosan-Based Polymeric Nanoparticles of Imatinib for Colorectal Cancer Targeting Application. Int. J. Biol. Macromol. 2020, 151, 104–115. [Google Scholar] [CrossRef]
- Zhong, T.; Liu, X.; Li, H.; Zhang, J. Co-Delivery of Sorafenib and Crizotinib Encapsulated with Polymeric Nanoparticles for the Treatment of in Vivo Lung Cancer Animal Model. Drug Deliv. 2021, 28, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- El Asmar, A.; Morandi, G.; Burel, F. Synthesis of Dual Sensitive Lipid- b -Poly(Dimethylaminoethyl Methacrylate) Copolymers, Self-Assemblies and Modulation of Cloud Point Temperatures through Physical Blends with Lipid- b -Poly(2-Isopropyl-2-Oxazoline). Macromolecules 2019, 52, 9160–9167. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, J.; Liu, H.; Zheng, L.; Luo, H.; Liu, Z.; Chen, X.; Wang, F.; Li, D.; Pei, H.; et al. Biodegradable Interlayer-Crosslinked Polymer Micelles with Reduction Sensitivity for Non-Small Cell Lung Cancer Therapy. J. Biomed. Nanotechnol. 2018, 14, 1225–1238. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Peng, C.-L.; Chiang, P.-F.; Shieh, M.-J. Dual-Functional Polymeric Micelles Co-Loaded with Antineoplastic Drugs and Tyrosine Kinase Inhibitor for Combination Therapy in Colorectal Cancer. Pharmaceutics 2022, 14, 768. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A Brief Review on Solid Lipid Nanoparticles: Part and Parcel of Contemporary Drug Delivery Systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid Lipid Nanoparticles for Enhanced Oral Absorption: A Review. Colloids Surf. B Biointerfaces 2020, 196, 111305. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Aldawsari, M.F.; Alsaidan, Y.S.M.; Alfaiz, S.A.; Haque, A.; Az, A.; Alhazzani, K. Development and Characterization of Brigatinib Loaded Solid Lipid Nanoparticles: In-Vitro Cytotoxicity against Human Carcinoma A549 Lung Cell Lines. Chem. Phys. Lipids 2020, 233, 105003. [Google Scholar] [CrossRef]
- Moinuddin; Neekhra, S.; Ahmad, S.; Swarnkar, S.; Gupta, D.; Khunteta, A.; Jain, P.; Jain, S. Evaluation of Oral Bioavailability and In-Vivo Anti-Leukemic Potential of Dasatinib Loaded Solid Lipid Nanoparticles. Int. J. Pharm. Qual. Assur. 2023, 14, 587–591. [Google Scholar] [CrossRef]
- Salmaso, S.; Mastrotto, F.; Roverso, M.; Gandin, V.; De Martin, S.; Gabbia, D.; De Franco, M.; Vaccarin, C.; Verona, M.; Chilin, A.; et al. Tyrosine Kinase Inhibitor Prodrug-Loaded Liposomes for Controlled Release at Tumor Microenvironment. J. Control. Release 2021, 340, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR Effect and beyond: Strategies to Improve Tumor Targeting and Cancer Nanomedicine Treatment Efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.-W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef]
- Yueh, P.-F.; Chiang, C.-S.; Tsai, I.-J.; Tseng, Y.-L.; Chen, H.-R.; Lan, K.-L.; Hsu, F.-T. A Multifunctional PEGylated Liposomal-Encapsulated Sunitinib Enhancing Autophagy, Immunomodulation, and Safety in Renal Cell Carcinoma. J. Nanobiotechnol. 2024, 22, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Wang, S.; Ouyang, L.; Li, H.; Ding, J.; Deng, G.; Zhou, W. Imatinib Co-Loaded Targeted Realgar Nanocrystal for Synergistic Therapy of Chronic Myeloid Leukemia. J. Control. Release 2021, 338, 190–200. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Nanosponges for Drug Delivery and Cancer Therapy: Recent Advances. Nanomaterials 2022, 12, 2440. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in Vitro Evaluation of Topical Nanosponge-Based Gel Containing Butenafine for the Treatment of Fungal Skin Infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef]
- Fatima, F.; Anwer, M.K. Development and Characterization of Ibrutinib-Loaded Ethylcellulose-Based Nanosponges: Cytotoxicity Assay against MCF-7 Cell Lines. Appl. Sci. 2023, 13, 4984. [Google Scholar] [CrossRef]
- Pirdadeh-Beiranvand, M.; Afkhami, A.; Madrakian, T. Magnetic Molecularly Imprinted Electrospun Nanofibers for Selective Extraction of Nilotinib from Human Serum. Anal. Bioanal. Chem. 2020, 412, 1629–1637. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Erol, I.; Durgun, E.; Uyar, T. Fast-Dissolving Electrospun Gelatin Nanofibers Encapsulating Ciprofloxacin/Cyclodextrin Inclusion Complex. Colloids Surf. B Biointerfaces 2019, 178, 129–136. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Kotrotsiou, O.; Kiparissides, C. Water Treatment by Molecularly Imprinted Materials. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–230. ISBN 978-0-12-813926-4. [Google Scholar]
- Chen, P.; Cai, X.; Mu, G.; Duan, Y.; Jing, C.; Yang, Z.; Yang, C.; Wang, X. Supramolecular Nanofibers Co-Loaded with Dabrafenib and Doxorubicin for Targeted and Synergistic Therapy of Differentiated Thyroid Carcinoma. Theranostics 2023, 13, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Karpagavinayagam, P.; Vedhi, C. Green Synthesis of Iron Oxide Nanoparticles Using Avicennia Marina Flower Extract. Vacuum 2019, 160, 286–292. [Google Scholar] [CrossRef]
- Iacobazzi, R.M.; Porcelli, L.; Lopedota, A.A.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Altamura, E.; Di Fonte, R.; Azzariti, A.; Franco, M.; et al. Targeting Human Liver Cancer Cells with Lactobionic Acid-G(4)-PAMAM-FITC Sorafenib Loaded Dendrimers. Int. J. Pharm. 2017, 528, 485–497. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Alqahtani, F.Y.; Setó, S.; Khalil, N.; Aleshaiwi, L.; Alghamdi, M.; Alquadeib, B.; Alkahtani, H.; Aldarwesh, A.; Alqahtani, Q.H.; et al. Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. Int. J. Nanomed. 2020, 15, 5433–5443. [Google Scholar] [CrossRef]
- Holder, J.E.; Ferguson, C.; Oliveira, E.; Lodeiro, C.; Trim, C.M.; Byrne, L.J.; Bertolo, E.; Wilson, C.M. The Use of Nanoparticles for Targeted Drug Delivery in Non-Small Cell Lung Cancer. Front. Oncol. 2023, 13, 1154318. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Arora, R. Nanoformulations: A Novel Approach Against Hypoxia. In Management of High Altitude Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–256. ISBN 978-0-12-813999-8. [Google Scholar]
- Khan, S.; Hossain, M.K. Classification and Properties of Nanoparticles. In Nanoparticle-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–54. ISBN 978-0-12-824272-8. [Google Scholar]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Kasina, V.; Mownn, R.J.; Bahal, R.; Sartor, G.C. Nanoparticle Delivery Systems for Substance Use Disorder. Neuropsychopharmacology 2022, 47, 1431–1439. [Google Scholar] [CrossRef]
- Abdi Syahputra, R.; Dalimunthe, A.; Utari, Z.D.; Halim, P.; Sukarno, M.A.; Zainalabidin, S.; Salim, E.; Gunawan, M.; Nurkolis, F.; Park, M.N.; et al. Nanotechnology and Flavonoids: Current Research and Future Perspectives on Cardiovascular Health. J. Funct. Foods 2024, 120, 106355. [Google Scholar] [CrossRef]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive Insights into Mechanism of Nanotoxicity, Assessment Methods and Regulatory Challenges of Nanomedicines. Discov. Nano 2024, 19, 165. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Farag, H.A.M.; Salih, T.H.; Awlqadr, F.H.; Al-Manhel, A.J.A.; Vieira, I.R.S.; Conte-Junior, C.A. Application of Nanoparticles in Human Nutrition: A Review. Nutrients 2024, 16, 636. [Google Scholar] [CrossRef] [PubMed]
- Kole, E.; Jadhav, K.; Singh, R.; Mandpe, S.; Abhang, A.; Verma, R.K.; Naik, J. Recent Developments in Tyrosine Kinase Inhibitor-Based Nanotherapeutics for EGFR-Resistant Non-Small Cell Lung Cancer. Curr. Drug Deliv. 2025, 22, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Petrazzuolo, A.; Maiuri, M.C.; Zitvogel, L.; Kroemer, G.; Kepp, O. Trial Watch: Combination of Tyrosine Kinase Inhibitors (TKIs) and Immunotherapy. OncoImmunology 2022, 11, 2077898. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Illi, J.A.; Ko, A.H.; Korn, W.M.; Fong, L.; Chen, L.; Kashani-Sabet, M.; Ryan, C.J.; Rosenberg, J.E.; Dubey, S.; et al. A Phase I Study of a 2-Day Lapatinib Chemosensitization Pulse Preceding Nanoparticle Albumin-Bound Paclitaxel for Advanced Solid Malignancies. Clin. Cancer Res. 2009, 15, 5569–5575. [Google Scholar] [CrossRef]
| Nano-Scale Drug Delivery Systems | |
|---|---|
| Advantages | Disadvantages |
|
|
| Type of Nanoparticles | Advantages | Disadvantages | |
| Inorganic nanoparticles, including silica, carbon-based, gold, and other metallic nanoparticles | 1. Possess tunable electrical, magnetic, and optical characteristics 2. Offer flexibility in size, shape, and structural design 3. Well-suited for theranostic applications combining therapy and diagnostics 4. Easier tracking 5. Exhibit catalytic properties 6. Respond to various external stimuli 7. Support diverse and adaptable surface functionalization 8. Promote increased cellular uptake through ionic interactions with the blood–brain barrier | 1. Limited information on long-term toxicity and stability 2. Prone to aggregation and stability challenges 3. Involves intricate synthesis processes and high production costs 4. Raises environmental safety concerns 5. Exhibits poor solubility under certain conditions 6. Lacks biodegradability, leading to persistence in the body 7. Potential for accumulation in vital organs 8. Associated with various toxicity risks 9. Risk of toxicity due to the metal accumulation | |
| Organic nanoparticles | Polymeric nanoparticles | 1. Enhanced stability under various conditions 2. Biodegradable and biocompatible 3. Broad acceptance across biomedical applications 4. Easy surface modification 5. Capable of encapsulating both hydrophilic and hydrophobic drugs 6. High capacity for drug loading 7. Enables sustained and controlled drug release 8. Versatile design adaptability 9. Allows for precise control over particle size, shape, and surface characteristics | 1. Involves complex and expensive fabrication techniques 2. Susceptible to aggregation/agglomeration, necessitating surface functionalization 3. Faces significant challenges in large-scale production 4. Limited ability for real-time tracking and monitoring 5. Typically results in low production yield 6. Risk of pseudoallergic reactions 7. Uncontrolled or premature drug release into the bloodstream remains a major concern |
| Lipid-based nanoparticles | 1. Capable of interacting with cells and facilitating membrane transfer 2. Facilitates ligand attachment to enhance circulation time in the bloodstream 3. Supports encapsulation of both hydrophilic and hydrophobic drug molecules 4. Simple formulation process 5. Flexible payload capacity for diverse therapeutic agents 6. Biocompatible and biodegradable materials 7. Generally non-toxic 8. Allows for straightforward surface modification | 1. Possible cytotoxic effects resulting from non-specific cellular uptake 2. Pseudoallergy and inflammation 3. Limited encapsulation efficiency 4. Inconsistencies between production batches 5. Low efficiency in incorporating certain therapeutic agents 6. Slow drug release 7. Relatively short shelf life 8. Suboptimal physical and chemical stability | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdeva, Y. Nanotechnology-Based Delivery Systems for Enhanced Targeting of Tyrosine Kinase Inhibitors: Exploring Inorganic and Organic Nanoparticles as Targeted Carriers. Kinases Phosphatases 2025, 3, 9. https://doi.org/10.3390/kinasesphosphatases3020009
Gvozdeva Y. Nanotechnology-Based Delivery Systems for Enhanced Targeting of Tyrosine Kinase Inhibitors: Exploring Inorganic and Organic Nanoparticles as Targeted Carriers. Kinases and Phosphatases. 2025; 3(2):9. https://doi.org/10.3390/kinasesphosphatases3020009
Chicago/Turabian StyleGvozdeva, Yana. 2025. "Nanotechnology-Based Delivery Systems for Enhanced Targeting of Tyrosine Kinase Inhibitors: Exploring Inorganic and Organic Nanoparticles as Targeted Carriers" Kinases and Phosphatases 3, no. 2: 9. https://doi.org/10.3390/kinasesphosphatases3020009
APA StyleGvozdeva, Y. (2025). Nanotechnology-Based Delivery Systems for Enhanced Targeting of Tyrosine Kinase Inhibitors: Exploring Inorganic and Organic Nanoparticles as Targeted Carriers. Kinases and Phosphatases, 3(2), 9. https://doi.org/10.3390/kinasesphosphatases3020009






