Performance Evaluation of Fiber Near-Infrared (NIR) Optic Probes for Quality Control of Curd Hardness in Cheese Produced by Spray-Dried Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk Preparation and Coagulation
2.2. Experimental Design
2.3. Temperature Control
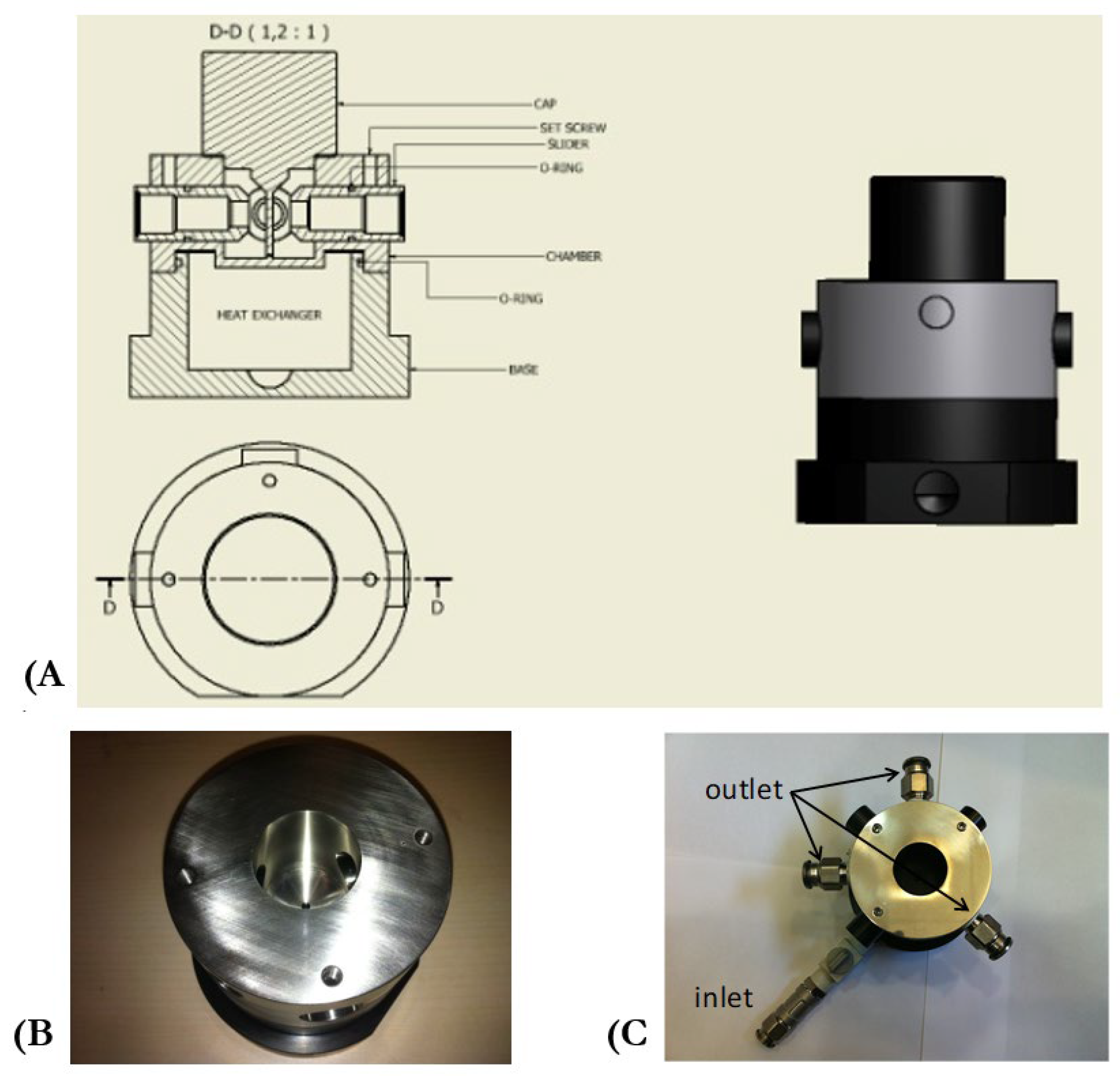
2.4. Monitoring Light Backscatter Profiles
2.5. Statistical Analysis
3. Results and Discussion
3.1. NIR Light Scattering Ratio through the Cell
3.2. Determination of Integration Time
3.3. Visual Cutting Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rangel-Ortega, S.d.C.; Campos-Múzquiz, L.G.; Charles-Rodriguez, A.V.; Chávez-Gonzaléz, M.L.; Palomo-Ligas, L.; Contreras-Esquivel, J.C.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Biological control of pathogens in artisanal cheeses. Int. Dairy J. 2023, 140, 105612. [Google Scholar] [CrossRef]
- Castillo, M.; Payne, F.A.; Hicks, C.L.; Lopez, M.B. Predicting cutting and clotting time of coagulating goat’s milk using diffuse reflectance: Effect of pH, temperature and enzyme concentration. Int. Dairy J. 2000, 10, 551–562. [Google Scholar] [CrossRef]
- Fox, P.F.; Cogan, T.M.; Guinee, T.P. Factors That Affect the Quality of Cheese. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 617–641. [Google Scholar]
- Bintsis, T.; Papademas, P. The Evolution of Fermented Milks, from Artisanal to Industrial Products: A Critical Review. Fermentation 2022, 8, 679. [Google Scholar] [CrossRef]
- Johnson, M.E. A 100-Year Review: Cheese production and quality. J. Dairy Sci. 2017, 100, 9952–9965. [Google Scholar] [CrossRef] [PubMed]
- Roupas, P. Predictive modelling of dairy manufacturing processes. Int. Dairy J. 2008, 18, 741–753. [Google Scholar] [CrossRef]
- Lei, T.; Sun, D.W. Developments of nondestructive techniques for evaluating quality attributes of cheeses: A review. Trends Food Sci. Technol. 2019, 88, 527–542. [Google Scholar] [CrossRef]
- Panikuttira, B.; Payne, F.A.; O’Shea, N.; Tobin, J.T.; O’Callaghan, D.J.; O’Donnell, C.P. Investigation of an in-line prototype fluorescence and infrared backscatter sensor to monitor rennet-induced coagulation of skim milk at different protein concentrations. Int. J. Food Sci. Technol. 2020, 55, 175–182. [Google Scholar] [CrossRef]
- Fagan, C.C. Infrared Spectroscopy. In Process Analytical Technology for the Food Industry; O’Donnell, C.P., Fagan, C., Cullen, P.J., Eds.; Springer: New York, NY, USA, 2014; pp. 73–101. [Google Scholar]
- Castillo, M.; Arango, O. A Method and a System for Determining Gel Firmness Values from Inline Optical. Measurements. Patent EP3036527 B1, 8 January 2015. [Google Scholar]
- Galli, B.D.; Hamed, A.M.; Sheehan, J.J.; King, N.; Abdel-Hamid, M.; Romeih, E. Technological solutions and adaptive processing tools to mitigate the impact of seasonal variations in milk composition on Cheddar cheese production—A review. Int. J. Dairy Technol. 2023, 76, 449–467. [Google Scholar] [CrossRef]
- Arango, O.; Trujillo, A.J.; Castillo, M. Monitoring the effect of inulin, protein, and calcium on milk coagulation phases using a fibre optic sensor. Int. Dairy J. 2018, 81, 80–86. [Google Scholar] [CrossRef]
- Salvador, D.; Arango, O.; Castillo, M. In-line estimation of the elastic module of milk gels with variation of temperature protein concentration. Int. J. Food Sci. Technol. 2019, 54, 354–360. [Google Scholar] [CrossRef]
- Pérez, B. Dispersión de luz NIR Durante la Coagulación de Leche Desnatada. Efecto de la Concentración de Proteína y Temperatura Sobre el Voltaje Inicial. Bachelor’s Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Horne, D.S.; Lucey, J.A. Rennet-Induced Coagulation of Milk. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Arango, O. Aplicación de Dispersión de Luz de Infrarrojo Próximo en la Producción de Derivados Lácteos Bajos en Grasa Con Inulina. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2015. [Google Scholar]
- Payne, F.A.; Crofcheck, C.L.; Nokes, S.E.; Kang, K.C. Light backscatter of milk products for transition sensing using optical fibers. Trans. Am. Soc. Agric. Eng. 1999, 42, 1771–1776. [Google Scholar] [CrossRef]
- Nicolau, N.; Buffa, M.; O’Callaghan, D.J.; Guamis, B.; Castillo, M. Estimation of clotting and cutting times in sheep cheese manufacture using NIR light backscatter. Dairy Sci. Technol. 2015, 95, 495–507. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC, 18th ed.; AOAC: Arlington, VA, USA, 2005. [Google Scholar]
- Strani, L.; Grassi, S.; De Juan, A. Effect of physicochemical factors and use of milk powder on milk rennet-coagulation: Process understanding by near infrared spectroscopy and chemometrics. Food Control 2021, 119, 107494. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci. Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]
- Cozzolino, D. The Ability of Near Infrared (NIR) Spectroscopy to Predict Functional Properties in Foods: Challenges and Opportunities. Molecules 2021, 26, 6981. [Google Scholar] [CrossRef]
- Zhang, M.; Li, G.; Wang, S.; Fu, Z.; Guan, Y.; Lin, L. The influence of different integration time on stoichiometric analysis in near infrared grating spectrometers. Infrared Phys. Technol. 2017, 86, 130–134. [Google Scholar] [CrossRef]
- Grassi, S.; Strani, L.; Alamprese, C.; Pricca, N.; Casiraghi, E.; Cabassi, G. A FT-NIR Process Analytical Technology Approach for Milk Renneting Control. Foods 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J. The measurement and significance of ionic calcium in milk—A review. Int. J. Dairy Technol. 2011, 64, 1–13. [Google Scholar] [CrossRef]
- Lin, M.J.; Lewis, M.J.; Grandison, A.S. Measurement of ionic calcium in milk. Int. J. Dairy Technol. 2006, 59, 192–199. [Google Scholar] [CrossRef]
- Villaquiran, Z.Y. Evaluación de Una Sonda Multifibra Para la Determinación Óptica en Línea Del Módulo Elástico del Gel Durante la Elaboración de Queso. Master’s Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2020. [Google Scholar]
- Fagan, C.C.; Castillo, M.; O’Donnell, C.P.; O’Callaghan, D.J. Online prediction of cheese making indices using backscatter of near infrared light. Int. Dairy J. 2008, 18, 120–128. [Google Scholar] [CrossRef]
- Mateo, M.J.; O’Callaghan, D.J.; Everard, C.D.; Fagan, C.C.; Castillo, M.; Payne, F.A. Influence of curd cutting programme and stirring speed on the prediction of syneresis indices in cheese making using NIR light backscatter. LWT Food Sci. Technol. 2009, 42, 950–955. [Google Scholar] [CrossRef]
- Taifi, N.; Bakkali, F.; Faiz, B.; Moudden, A.; Maze, G.D.D. Characterization of the syneresis and the firmness of the milk gel using an ultrasonic technique. Maeas. Sci. Technol. 2006, 17, 281–287. [Google Scholar] [CrossRef]
- Li, X.; Toyoda, K.; Ihara, I. Coagulation process of soymilk characterized by electrical impedance spectroscopy. J. Food Eng. 2010, 105, 563–568. [Google Scholar] [CrossRef]
- Lyndgaard, C.B.; Engelsen, S.B.; Van Der Berg, F.W.J. Real-time modeling of milk coagulation using in-line near infrared spectroscopy. J. Food Eng. 2012, 108, 345–352. [Google Scholar] [CrossRef]


| Chemical Composition | g/100 g |
|---|---|
| Fat | 1.25 ± 0.13 |
| Protein | 32.50 ± 1.55 |
| Lactose | 52.91 ± 2.72 |
| Minerals | 7.75 ± 0.83 |
| Moisture | 5.58 ± 0.77 |
| T.I. (ms) | Without Light | With Light | Wavelength (nm) |
|---|---|---|---|
| 100 | 11.73 ± 1.68 eA | 13.04 ± 2.76 fA | 780 |
| 15.40 ± 1.73 dA | 16.22 ± 3.12 eA | 880 | |
| 11.73 ± 1.70 eA | 13.04 ± 2.78 fA | 980 | |
| 500 | 29.00 ± 3.01 cA | 31.74 ± 3.19 cA | 780 |
| 33.01 ± 3.25 bA | 34.53 ± 3.48 bA | 880 | |
| 24.32 ± 3.21 cA | 25.77 ± 2.97 dA | 980 | |
| 1000 | 28.03 ± 2.41 cA | 33.28 ± 2.80 cA | 780 |
| 39.12 ± 2.18 aA | 41.44 ± 2.95 aA | 880 | |
| 27.44 ± 2.28 cA | 29.03 ± 3.02 cA | 980 |
| Medium | Without Light | With Light | Wavelength (nm) |
|---|---|---|---|
| Water | 29.12 ± 1.68 bA | 15,621.36 ± 2.76 aB | 780 |
| 32.86 ± 1.73 aA | 15,625.67 ± 3.12 aB | 880 | |
| 11.73 ± 1.70 dA | 15,620.81 ± 2.78 aB | 980 | |
| Air | 29.71 ± 3.01 bA | 15,633.97 ± 3.19 aB | 780 |
| 33.62± 3.25 aA | 15,635.77 ± 3.48 aB | 880 | |
| 25.00 ± 3.21 cA | 15,630.55 ± 2.97 aB | 980 |
| Time of Integration (ms) | Counts |
|---|---|
| 100 | 3914 ± 10.34 d |
| 150 | 5908 ± 13.45 c |
| 200 | 7849 ± 15.74 b |
| 500 | 16,023 ± 20.45 a |
| Optic Parameters | Protein Content (%) | ||
|---|---|---|---|
| 3% | 3.5% | 4% | |
| I0 (bits) | 434 ± 5.98 a | 472 ± 7.96 b | 506 ± 8.67 c |
| tmax (min) | 4.81 ± 0.54 a | 4.20 ± 0.32 a | 4.47 ± 0.27 a |
| Rmax (dimensionless) | 1.04 ± 0.02 a | 1.04 ± 0.01 a | 1.06 ± 0.02 b |
| R1max (dimensionless) | 0.0278 ± 0.001 a | 0.0331 ± 0.001 b | 0.0391 ± 0.001 c |
| R2min (dimensionless) | −0.0064 ± 0.001 a | −0.0105 ± 0.001 b | −0.0107 ± 0.001 b |
| t2min (min) | 13.83 ± 2.34 a | 6.09 ± 2.07 b | 5.65 ± 1.75 b |
| Optic Parameters | Wavelength (nm) | ||
|---|---|---|---|
| 870 nm | 880 nm | 890 nm | |
| I0 (bits) | 531 ± 5.87 a | 468 ± 6.76 b | 413 ± 4.77 c |
| tmax (min) | 4.34 ± 0.39 a | 4.68 ± 0.34 a | 4.46 ± 0.21 a |
| Rmax (dimensionless) | 1.04 ± 0.04 a | 1.05 ± 0.03 a | 1.05 ± 0.02 a |
| R1max (dimensionless) | 0.034 ± 0.001 a | 0.032 ± 0.001 a | 0.033 ± 0.001 a |
| R2min (dimensionless) | −0.00890 ± 0.001 a | −0.00906 ± 0.001 a | −0.00968 ± 0.001 a |
| t2min (min) | 6.09 ± 1.89 a | 8.70 ± 2.61 ab | 10.79 ± 3.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza, L.; Aleman, R.S.; Marcia, J.; Yadav, A.; Castillo, M. Performance Evaluation of Fiber Near-Infrared (NIR) Optic Probes for Quality Control of Curd Hardness in Cheese Produced by Spray-Dried Milk. Spectrosc. J. 2023, 1, 152-162. https://doi.org/10.3390/spectroscj1030013
Meza L, Aleman RS, Marcia J, Yadav A, Castillo M. Performance Evaluation of Fiber Near-Infrared (NIR) Optic Probes for Quality Control of Curd Hardness in Cheese Produced by Spray-Dried Milk. Spectroscopy Journal. 2023; 1(3):152-162. https://doi.org/10.3390/spectroscj1030013
Chicago/Turabian StyleMeza, Lesther, Ricardo S. Aleman, Jhunior Marcia, Ajitesh Yadav, and Manuel Castillo. 2023. "Performance Evaluation of Fiber Near-Infrared (NIR) Optic Probes for Quality Control of Curd Hardness in Cheese Produced by Spray-Dried Milk" Spectroscopy Journal 1, no. 3: 152-162. https://doi.org/10.3390/spectroscj1030013
APA StyleMeza, L., Aleman, R. S., Marcia, J., Yadav, A., & Castillo, M. (2023). Performance Evaluation of Fiber Near-Infrared (NIR) Optic Probes for Quality Control of Curd Hardness in Cheese Produced by Spray-Dried Milk. Spectroscopy Journal, 1(3), 152-162. https://doi.org/10.3390/spectroscj1030013







