Raman Spectra of Blood Serum as Holistic Biomarker for Differential Auxiliary Diagnoses of Attention Deficit and Hyperactivity Disorder (ADHD) in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Phase
2.1.1. Patient and Control Group Selection
2.1.2. Samples Preparation
2.2. Spectroscopic Phase
2.2.1. Sample Measurements
2.2.2. Data Pre-Processing
2.3. Statistical Phase
Classification Model Development and Testing
3. Results and Discussion
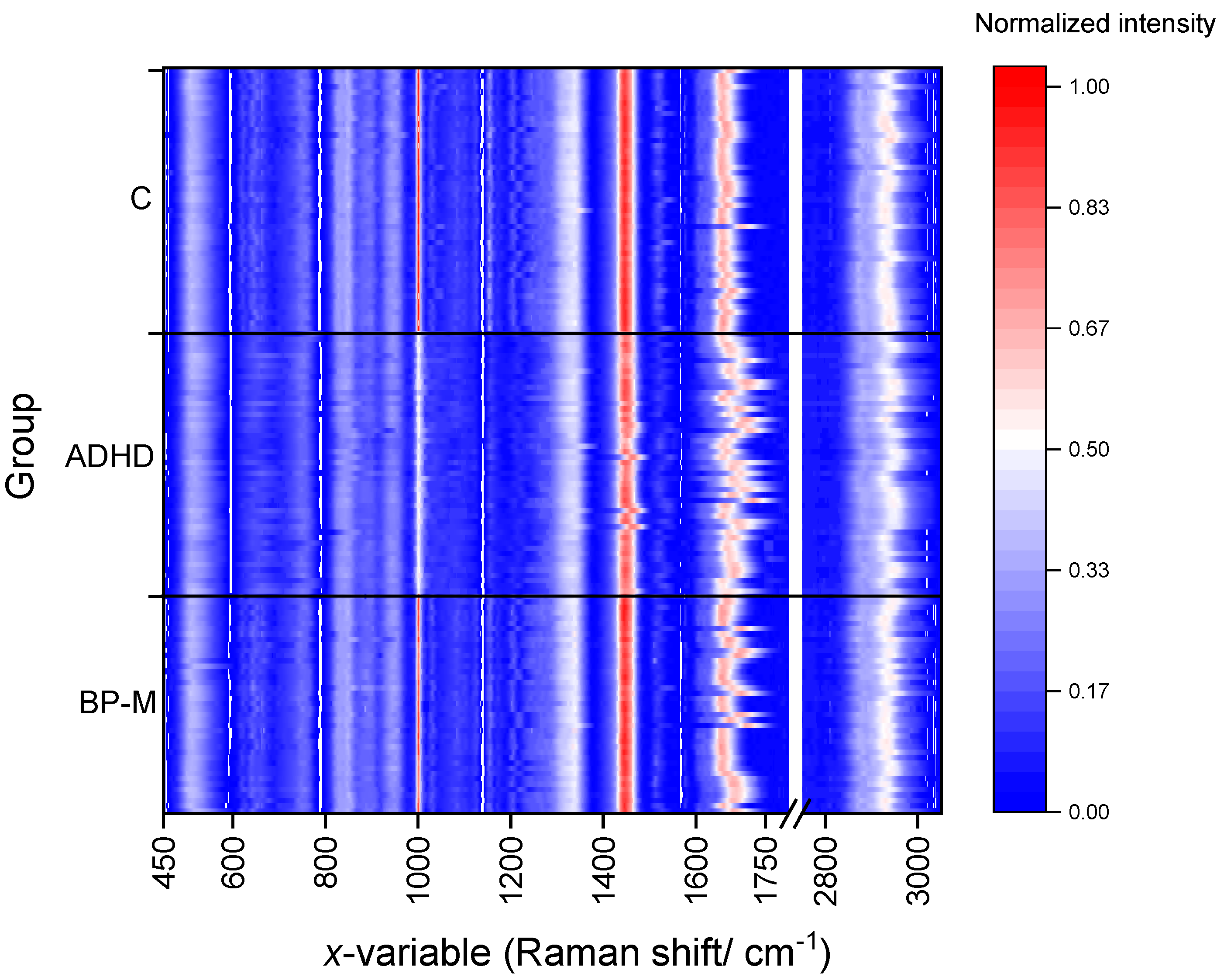
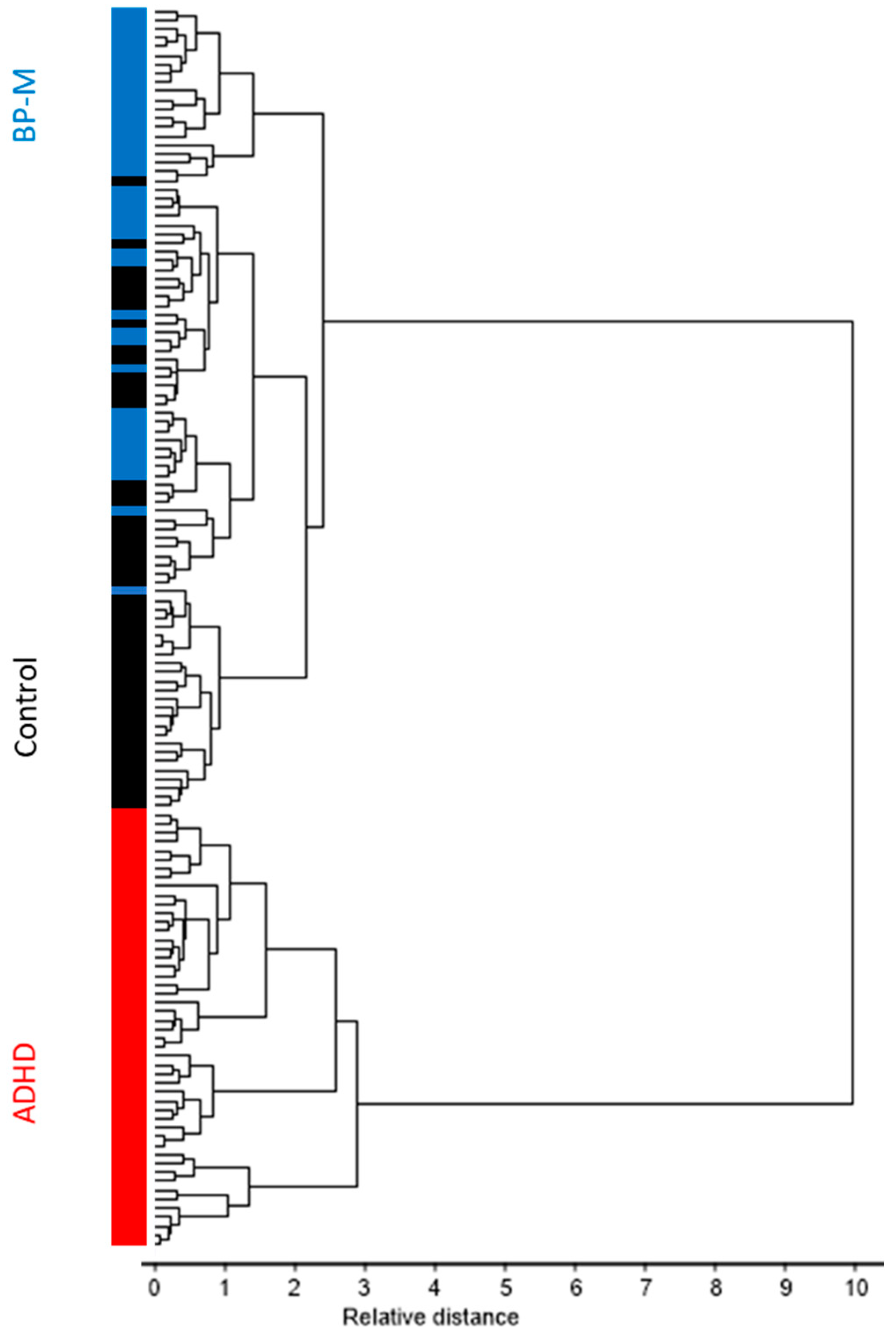
3.1. Preliminary Analysis of the Data
3.2. Classification Model Development
3.3. Predictions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Ramsay, J.R. Cognitive Behavioral Therapy for Adult ADHD; Routledge: Oxfordshire, UK, 2007; pp. 25–26. [Google Scholar]
- Steinau, S. Diagnostic Criteria in Attention Deficit Hyperactivity Disorder—Changes in DSM 5. Front. Psychiatry 2013, 4, 49. [Google Scholar] [CrossRef]
- Kooij, S.J.; Bejerot, S.; Blackwell, A.; Caci, H.; Casas-Brugué, M.; Carpentier, P.J.; Edvinsson, D.; Fayyad, J.; Foeken, K.; Fitzgerald, M.; et al. European Consensus Statement on Diagnosis and Treatment of Adult ADHD: The European Network Adult ADHD. BMC Psychiatry 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Bálint, S.; Czobor, P.; Mészáros, A.; Simon, V.; Bitter, I. Neuropsychological Impairments in Adult Attention Deficit Hyperactivity Disorder: A Literature Review. Psychiatr. Hung. 2008, 23, 324–335. [Google Scholar] [PubMed]
- Ginsberg, Y.; Quintero, J.; Anand, E.; Casillas, M.; Upadhyaya, H.P. Underdiagnosis of Attention-Deficit/Hyperactivity Disorder in Adult Patients: A Review of the Literature. Prim. Care Companion CNS Disord. 2014, 16, PCC.13r01600. [Google Scholar] [CrossRef] [PubMed]
- Cross River Therapy. ADHD Statistics: How Many People Have ADHD? Key ADHD Statistics and Facts. Available online: https://www.crossrivertherapy.com/adhd-statistics (accessed on 6 January 2024).
- Sroubek, A.; Kelly, M.; Li, X. Inattentiveness in Attention-Deficit/Hyperactivity Disorder. Neurosci. Bull. 2013, 29, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Rostain, A.L.; Blader, J.; Busch, B.; Childress, A.C.; Connor, D.F.; Newcorn, J.H. Practitioner Review: Emotional Dysregulation in Attention-Deficit/Hyperactivity Disorder—Implications for Clinical Recognition and Intervention. J. Child Psychol. Psychiatry Allied Discip. 2019, 60, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, R.B.; Musser, E.D.; Morris, S.; Ward, A.R.; Raiker, J.S.; Coles, E.K.; Pelham, W.E. Response Inhibition, Response Execution, and Emotion Regulation among Children with Attention-Deficit/Hyperactivity Disorder. J. Abnorm. Child Psychol. 2019, 47, 589–603. [Google Scholar] [CrossRef]
- Millichap, J.G. Chapter 2: Causative Factors. In Attention Deficit Hyperactivity Disorder Handbook: A Physician’s Guide to ADHD, 2nd ed.; Springer Science: New York, NY, USA, 2010. [Google Scholar]
- Thapar, A.; Cooper, M.; Eyre, O.; Langley, K. What have we learnt about the causes of ADHD? J. Child Psychol. Psychiatry Allied Discip. 2013, 54, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mourek, J.; Pokorny, J. ADHD—What is the Meaning of Sex-dependent Incidence Differences? Prague Med. Rep. 2022, 123, 215–224. [Google Scholar] [CrossRef]
- Momany, A.M.; Kamradt, J.M.; Ullsperger, J.M.; Elmore, A.L.; Nigg, J.T.; Nikolas, M.A. Sex Moderates the Impact of Birth Weight on Child Externalizing Psychopathology. J. Abnorm. Psychol. 2017, 126, 244–256. [Google Scholar] [CrossRef]
- Shaw, J.C.; Crombie, G.K.; Zakar, T.; Palliser, H.K.; Hirst, J.J. Perinatal Compromise Contributes to Programming of GABAergic and Glutamatergic Systems Leading to Long-term Effects on Offspring Behaviour. J. Neuroendocrinol. 2020, 32, e12814. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Attention Deficit Hyperactivity Disorder: Diagnosis and Management; National Guideline Centre: London, UK, 2019. [Google Scholar]
- Walitza, S.; Drechsler, R.; Ball, J. The School Child with ADHD. Ther. Umsch. 2012, 69, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Wilens, T.E.; Spencer, T.J. Understanding Attention-Deficit/Hyperactivity Disorder from Childhood to Adulthood. Postgrad. Med. 2010, 122, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Bonvicini, C.; Scassellati, C. Biomarkers in the Diagnosis of ADHD—Promising Directions. Curr. Psych. Rep. 2014, 16, 497. [Google Scholar] [CrossRef] [PubMed]
- Levent, L.; Tümkaya, S.; Ateşçi, F.; Tüysüzoğlu, H.; Varma, G.; Oğuzhanoğlu, N. A Neuropsychological Comparison of Bipolar Disorder and Adult Attention Deficit Hyperactivity Disorder. Turk Psikiyatri Derg. 2014, 23, 1–8. [Google Scholar]
- Marangoni, C.; De Chiara, L.; Faedda, G.L. Bipolar Disorder and ADHD: Comorbidity and Diagnostic Distinctions. Curr. Psych. Rep. 2015, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Brown, E.C.; Aydemir, O.; Brüne, M.; Lysaker, P.H. Metacognition in Psychosis: Comparison of Schizophrenia with Bipolar Disorder. Psychiatry Res. 2014, 219, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Tadin, D.; Glasser, D.; Hong, S.W.; Blake, R.; Park, S. Visual Context Processing in Bipolar Disorder: A Comparison with Schizophrenia. Front. Psychol. 2013, 4, 569. [Google Scholar] [CrossRef] [PubMed]
- Mueser, K.T.; Drake, R.E.; Wallach, M.A. Dual Diagnosis: A Review of Etiological Theories. Addict. Behav. 1998, 23, 717–734. [Google Scholar] [CrossRef]
- Thome, J.; Ehlis, A.C.; Fallgatter, A.J.; Krauel, K.; Lange, K.W.; Riederer, P.; Romanos, M.; Taurines, R.; Tucha, O.; Uzbekov, M.; et al. Biomarkers for attention-deficit/hyperactivity disorder (ADHD). A consensus report of the WFSBP task force on biological markers and the World Federation of ADHD. World J. Biol. Psychiatry 2012, 13, 379–400, This is a comprehensive review on clinical, imaging, genetic and proteomic markers and ADHD. The review concluded that, although some promising candidates such as olfactory sensitivity, substantial echogenicity, no biomarkers for ADHD are available. [Google Scholar] [CrossRef]
- Ogruc Ildiz, G.; Bayari, S.; Yorguner, N.; Fausto, R. Blood Serum Infrared Spectra Based Chemometric Models for Auxiliary Diagnosis of Autism Spectrum Disorder. In Autism Spectrum Disorder: Diagnosis and Treatment; El-Bazm, A.S., Mahmoud, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2021; Volume 1: Imaging and Signal Analysis, Chapter 10; pp. 185–213. [Google Scholar]
- Ogruc Ildiz, G.; Bayari, S.; Karadag, A.; Kaygisiz, E.; Fausto, R. Fourier Transform Infrared Spectroscopy Based Complementary Diagnosis Tool for Autism Spectrum Disorder in Children and Adolescents. Molecules 2020, 25, 2079. [Google Scholar] [CrossRef] [PubMed]
- Ogruc Ildiz, G.; Bayari, S.; Aksoy, U.M.; Yorguner, N.; Bulut, H.; Yilmaz, S.S.; Halimoglu, G.; Nur Kabuk, H.; Yavuz, G.; Fausto, R. Auxiliary Differential Diagnosis of Schizophrenia and Phases of Bipolar Disorder Based on the Blood Serum Raman Spectra. J. Raman Spectrosc. 2020, 51, 2233–2244. [Google Scholar] [CrossRef]
- Ogruc Ildiz, G.; Karadag, A.; Kaygisiz, E.; Fausto, R. PLS-DA Model for the Evaluation of Attention Deficit and Hyperactivity Disorder in Children and Adolescents through Blood Serum FTIR Spectra. Molecules 2021, 26, 3400. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.A.; Mantsch, H.H. Infrared Spectroscopy of Biological Fluids in Clinical and Diagnostic Analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 2, pp. 1–24. [Google Scholar]
- Ahmed, S.S.S.J.; Santosh, W.; Kumar, S.; Christlet, T.H.T. Neural Network Algorithm for the Early Detection of Parkinson’s Disease from Blood Plasma by FTIR Micro-Spectroscopy. Vib. Spectrosc. 2010, 53, 181–188. [Google Scholar] [CrossRef]
- Deleris, G.; Petibois, C. Applications of FT-IR Spectrometry to Plasma Contents Analysis and Monitoring. Vib. Spectrosc. 2003, 32, 129–136. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Ghasemi, K.; Garmarudi, A.B.; Ramin, M. Diagnostic Prediction of Renal Failure from Blood Serum Analysis by FTIR Spectrometry and Chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Mostaco-Guidolin, L.B.; Bachmann, L. Application of FTIR Spectroscopy for Identification of Blood and Leukemia Biomarkers: A Review over the Past 15 Years. Appl. Spectrosc. Rev. 2011, 46, 388–404. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Talyshinsky, M.; Souprun, Y.; Huleihel, M. FTIR Spectroscopy Examination of Leukemia Patients Plasma. Vibrat. Spectrosc. 2006, 40, 40–46. [Google Scholar] [CrossRef]
- Lewis, P.D.; Lewis, K.E.; Ghosal, R.; Bayliss, S.; Lloyd, A.J.; Wills, J.; Godfrey, R.; Kloer, P.; Mur, L.A.J. Evaluation of FTIR Spectroscopy as a Diagnostic Tool for Lung Cancer Using Sputum. BMC Cancer 2010, 10, 640. [Google Scholar] [CrossRef]
- Mordechai, S.; Shufan, E.; Katz Porat, B.S.; Salman, A. Early Diagnosis of Alzheimer’s Disease Using Infrared Spectroscopy of Isolated Blood Samples Followed by Multivariate Analyses. Analyst 2017, 142, 1276–1284. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational Spectroscopy and Conformation of Peptides, Polypeptides and Proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Liu, K.Z.; Shi, M.H.; Mantsch, H.H. Molecular and Chemical Characterization of Blood Cells by Infrared Spectroscopy: A New Optical Tool in Hematology. Blood Cells Mol. Dis. 2005, 35, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Ustun, B. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry 2018, 74, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, J.L.; Wieweg, B.W. The Hamilton Rating Scale for Depression: A Comprehensive Review. Operat. Psychiatry 1979, 10, 149–165. [Google Scholar]
- Menges, F. Spectragryph—Optical Spectroscopy Software (v. 1.2.16.1). 2021. Available online: www.effemm2.de/spectragryph/ (accessed on 1 January 2024).
- CAMO Software Inc. The UnscramblerTM Version 10.5; CAMO A/S: Trondheim, Norway, 2018. [Google Scholar]
- Pearson, K. On Lines and Planes of Closest Fit to Systems of Points in Space. Phil. Mag. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a Complex of Statistical Variables into Principal Components. J. Educat. Psychol. 1933, 24, 417–441, ibidim 498–520. [Google Scholar] [CrossRef]
- Hotelling, H. Relations Between Two Sets of Variates. Biometrika 1936, 28, 321–377. [Google Scholar] [CrossRef]
- Wold, H. Quantitative Sociology: International Perspectives on Mathematical and Statistical Model Building; Academic Press: New York, NY, USA, 1975; pp. 307–357. [Google Scholar]
- Ward, J.H.J. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Sys. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Wold, H. Estimation of Principal Components and Related Models by Iterative Least Squares. In Multivariate Analysis; Krishnaiah, P.R., Ed.; Academic Press: New York, NY, USA, 1966; pp. 391–420. [Google Scholar]
- Bair, E.; Hastie, T.; Paul, D.; Tibshirani, R. Prediction by Supervised Principal Components. J. Am. Stat. Assoc. 2006, 101, 119–137. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial Least Squares for Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Wilkinson, L.; Friendly, M. The History of the Cluster Heat Map. Am. Stat. 2009, 63, 179–184. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, A.; Rong, Z.; Wang, C.; Jia, X.; Zhang, K.; Xiao, R.; Wang, S. Fast and Non-Invasive Serum Detection Technology Based on Surface-Enhanced Raman Spectroscopy and Multivariate Statistical Analysis for Liver Disease. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 451–459. [Google Scholar] [CrossRef]
- Pérez, A.; Prada, Y.A.; Cabanzo, R.; González, C.I.; Mejía-Ospin, E. Diagnosis of Chagas Disease from Human Blood Serum Using Surface-Enhanced Raman Scattering (SERS) Spectroscopy and Chemometric Methods. Sens. Bio-Sens. Res. 2018, 21, 40–45. [Google Scholar] [CrossRef]
- Atkins, C.G.; Buckley, K.; Blades, M.W.; Turner, R.F.B. Raman Spectroscopy of Blood and Blood Components. Appl. Spectrosc. 2017, 71, 767–793. [Google Scholar] [CrossRef]
- Nargis, H.F.; Nawaz, H.; Ditta, A.; Mahmood, T.; Majeed, M.I.; Rashid, N.; Muddassar, M.; Bhatti, H.N.; Saleem, M.; Jilani, K.; et al. Raman Spectroscopy of Blood Plasma Samples from Breast Cancer Patients at Different Stages. Spectrochim. Acta Part A Mol. Biomolec. Spectrosc. 2019, 222, 117210. [Google Scholar] [CrossRef]
- Saade, J.; Silva, J.N.; Farias, P.M.A.; Lopes, D.F.; Santos, C.T.; Farias, B.A.; Rodrigues, K.C.; Martin, A.A. Glicemical Analysis of Human Blood Serum Using FT-Raman: A New Approach. Photomed. Laser Surg. 2012, 30, 388–392. [Google Scholar] [CrossRef]
- Faber, K.; Kowalski, K.F. Prediction Error in Least Squares Regression: Further Critique on the Deviation Used in the Unscrambler. Chemom. Intellig. Lab. Syst. 1996, 34, 283–292. [Google Scholar] [CrossRef]
- De Vries, S.; Ter Braak, C.J.F. Prediction Error in Partial Least Squares Regression: A Critique on the Deviation Used in the Unscrambler. Chemom. Intell. Lab. Syst. 1995, 30, 239–245. [Google Scholar] [CrossRef]
- Brereton, R.G. The Mahalanobis Distance and Its Relationship to Principal Component Scores. J. Chemom. 2015, 29, 143–145. [Google Scholar] [CrossRef]
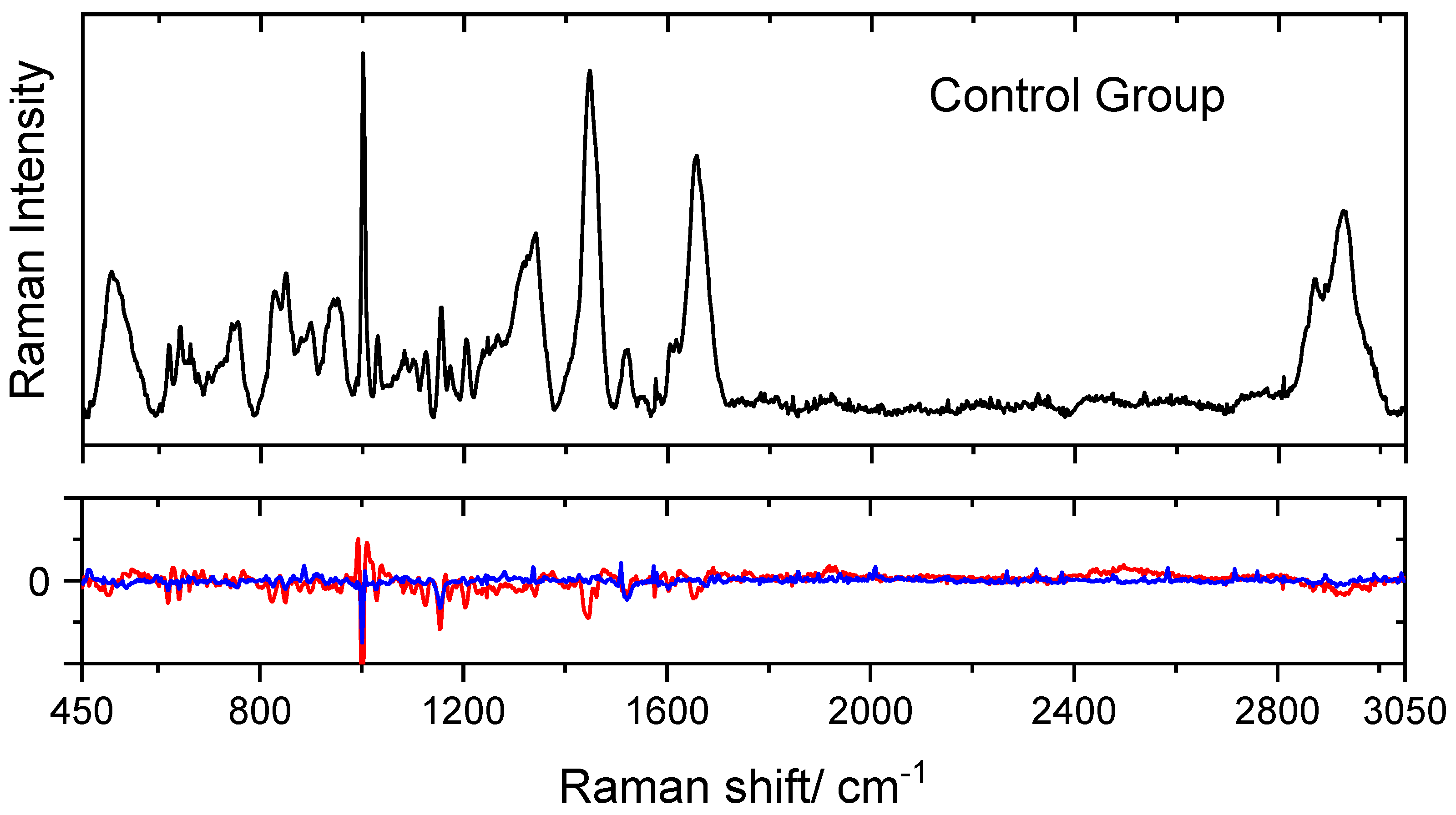




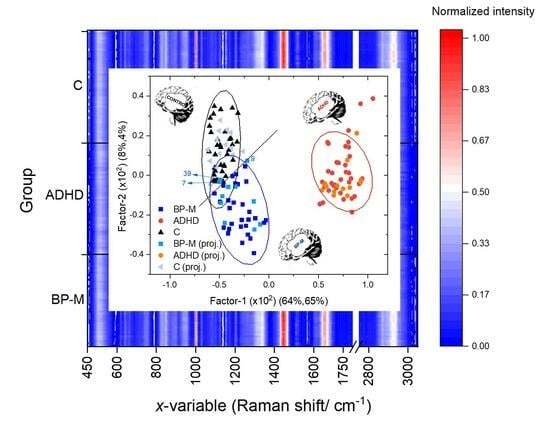
| Raman Shift | Assignment | Raman Shift | Assignment |
|---|---|---|---|
| 2929 | Lipids ν(CH) | 1205 | Amino acids ν(C=C) |
| 1655 | Protein (Amide I) ν(C=O) | 1173 | Cytosine, guanine |
| 1609 | Phenylalanine ν(C=C) | 1002 | Phenylalanine ν(C–H) |
| 1445 | Lipoproteins, phospholipids, | 945 | Phenylalanine ν(C–C) |
| δ(CH2), δ(CH3) | 850 | Tyrosine | |
| 1338 | Proteins (tryptophan) | 754 | Guanine, thymine |
| 1267 | Phospholipids δ(CH) | 654 | Amide IV (proteins) δ(NC=O) |
| 513 | Cystine ν(S–S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogan, G.Y.; Halimoglu, G.; Kaplanoglu, D.; Aksoy, U.M.; Kandeger, A.; Yavuz, E.; Kartal, S.; Fausto, R.; Ildiz, G.O. Raman Spectra of Blood Serum as Holistic Biomarker for Differential Auxiliary Diagnoses of Attention Deficit and Hyperactivity Disorder (ADHD) in Adults. Spectrosc. J. 2024, 2, 53-67. https://doi.org/10.3390/spectroscj2020004
Dogan GY, Halimoglu G, Kaplanoglu D, Aksoy UM, Kandeger A, Yavuz E, Kartal S, Fausto R, Ildiz GO. Raman Spectra of Blood Serum as Holistic Biomarker for Differential Auxiliary Diagnoses of Attention Deficit and Hyperactivity Disorder (ADHD) in Adults. Spectroscopy Journal. 2024; 2(2):53-67. https://doi.org/10.3390/spectroscj2020004
Chicago/Turabian StyleDogan, Gizem Yavuz, Gokhan Halimoglu, Dilara Kaplanoglu, Umut Mert Aksoy, Ali Kandeger, Elif Yavuz, Sehban Kartal, Rui Fausto, and Gulce Ogruc Ildiz. 2024. "Raman Spectra of Blood Serum as Holistic Biomarker for Differential Auxiliary Diagnoses of Attention Deficit and Hyperactivity Disorder (ADHD) in Adults" Spectroscopy Journal 2, no. 2: 53-67. https://doi.org/10.3390/spectroscj2020004