Benchtop 19F Nuclear Magnetic Resonance (NMR) Spectroscopy-Optimized Knorr Pyrazole Synthesis of Celecoxib and Mavacoxib, 3-(Trifluoromethyl) Pyrazolyl Benzenesulfonamides, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Synthesis Procedure (Micro-Scale)
2.2. Kinetic Parameters
3. Results and Discussion
3.1. Initial Rate
3.2. Final Conversion
3.3. Regioisomer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheikhi, N.; Bahraminejad, M.; Saeedi, M.; Mirfazli, S.S. A review: FDA-approved fluorine-containing small molecules from 2015 to 2022. Eur. J. Med. Chem. 2023, 260, 115758. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Shabir, G.; Saeed, A.; Zahid, W.; Naseer, F.; Riaz, Z.; Khalil, N.A.; Muneeba; Alberício, F. Chemistry and Pharmacology of Fluorinated Drugs Approved by the FDA (2016–2022). Pharmaceuticals 2023, 16, 1162. [Google Scholar] [CrossRef]
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef]
- O’Hagan, D.; Young, R.J. Future Challenges and Opportunities with Fluorine in Drugs? Med. Chem. Res. 2023, 32, 1231–1234. [Google Scholar] [CrossRef]
- Niu, Z.-X.; Hu, J.; Sun, J.-F.; Wang, Y.-T. Fluorine in the Pharmaceutical Industry: Synthetic Approaches and Application of Clinically Approved Fluorine-Enriched Anti-Infectious Medications. Eur. J. Med. Chem. 2024, 271, 116446. [Google Scholar] [CrossRef]
- Henary, E.; Casa, S.; Dost, T.L.; Sloop, J.C.; Henary, M. The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications. Pharmaceuticals 2024, 17, 281. [Google Scholar] [CrossRef]
- Barnes-Seeman, D.; Beck, J.; Springer, C. Fluorinated Compounds in Medicinal Chemistry: Recent Applications, Synthetic Advances and Matched-Pair Analyses. Curr. Top. Med. Chem. 2014, 14, 855–864. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.; Al-aizari, F.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2020, 121, 1670–1715. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The Therapeutic Voyage of Pyrazole and Its Analogs: A Review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. Review: Biologically Active Pyrazole Derivatives. New J. Chem. 2016, 41, 16–41. [Google Scholar] [CrossRef]
- Du, Y.; Li, G.; Cheng, Y.; Han, C.; Song, C.; Huang, N. Pyrazole-Containing Pharmaceuticals: Target, Pharmacological Activity, and Their SAR Studies. RSC Med. Chem. 2022, 13, 1300–1321. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547742/ (accessed on 30 September 2024).
- Dhondt, L.; Devreese, M.; Croubels, S.; De Baere, S.; Haesendonck, R.; Goessens, T.; Gehring, R.; De Backer, P.; Antonissen, G. Comparative Population Pharmacokinetics and Absolute Oral Bioavailability of COX-2 Selective Inhibitors Celecoxib, Mavacoxib and Meloxicam in Cockatiels (Nymphicus Hollandicus). Sci. Rep. 2017, 7, 12043. [Google Scholar] [CrossRef]
- Gong, L.; Thorn, C.F.; Bertagnolli, M.M.; Grosser, T.; Altman, R.B.; Klein, T.E. Celecoxib Pathways. Pharmacogenetics Genom. 2012, 22, 310–318. [Google Scholar] [CrossRef]
- Miller, S.B. Prostaglandins in Health and Disease: An Overview. Semin. Arthritis Rheum. 2006, 36, 37–49. [Google Scholar] [CrossRef]
- Qureshi, O.; Dua, A. COX Inhibitors; StatPearls: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549795/ (accessed on 30 September 2024).
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Levchenko, V.; Dmytriv, Y.V.; Tymtsunik, A.V.; Liubchak, K.; Rudnichenko, A.; Melnyk, A.V.; Veselovych, S.Y.; Borodulin, Y.V.; Otsalyuk, O.M.; Tolmachev, A.A.; et al. Preparation of 5-Fluoropyrazoles from Pyrazoles and N-Fluorobenzenesulfonimide (NFSI). J. Org. Chem. 2018, 83, 3265–3274. [Google Scholar] [CrossRef]
- Bonacorso, H.; Pittaluga, E.; Porte, L.; Libero, F.; Junges, A.; Zanatta, N.; Martins, M. Unexpected Metal-Free Fluorination and Oxidation at the C-4 Position of Pyrazoles Promoted by Selectfluor. Synlett 2015, 26, 2009–2013. [Google Scholar] [CrossRef]
- Breen, J.; Sandford, G.; Patel, B.; Fray, J. Synthesis of 4,4-Difluoro-1H-pyrazole Derivatives. Synlett 2014, 26, 51–54. [Google Scholar] [CrossRef]
- Fabra, F.; Vilarrasa, J.; Coll, J. Fluoroazoles. III. Synthesis and 1H and ¹⁹F NMR Spectra of 3-, 4-, and 5-Fluoro-1-Methylpyrazole. J. Heterocycl. Chem. 1978, 15, 1447–1449. [Google Scholar] [CrossRef]
- Joseph, C.S.; Cory, H.; Maged, H. Selective Incorporation of Fluorine in Pyrazoles. Eur. J. Org. Chem. 2015, 2015, 3405–3422. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Uraguchi, D.; Yamamoto, K.; Tokuhisa, K.; Yamakawa, T. Syntheses of 2-(Trifluoromethyl)-1,3-Dicarbonyl Compounds through Direct Trifluoromethylation with CF3I and Their Application to Fluorinated Pyrazoles Syntheses. Tetrahedron 2012, 68, 2636–2649. [Google Scholar] [CrossRef]
- Flood, D.T.; Hintzen, J.C.J.; Bird, M.J.; Cistrone, P.A.; Chen, J.S.; Dawson, P.E. Leveraging the Knorr Pyrazole Synthesis for the Facile Generation of Thioester Surrogates for Use in Native Chemical Ligation. Angew. Chem. Int. Ed. 2018, 57, 11634–11639. [Google Scholar] [CrossRef]
- Hassani, A.E.; Rouzi, K.; Assila, H.; Karrouchi, K.; Ansar, M.H. Recent Advances in the Synthesis of Pyrazole Derivatives: A Review. Reactions 2023, 4, 478–504. [Google Scholar] [CrossRef]
- Reddy, A.R.; Sampath, A.; Goverdhan, G.; Yakambaram, B.; Mukkanti, K.; Reddy, P.P. An Improved and Scalable Process for Celecoxib: A Selective Cyclooxygenase-2 Inhibitor. Org. Process Res. Dev. 2008, 13, 98–101. [Google Scholar] [CrossRef]
- Sthalam, V.K.; Singh, A.K.; Pabbaraja, S. An Integrated Continuous Flow Micro-Total Ultrafast Process System (μ-TUFPS) for the Synthesis of Celecoxib and Other Cyclooxygenase Inhibitors. Org. Process Res. Dev. 2019, 23, 1892–1899. [Google Scholar] [CrossRef]
- Scholtz, C.; Riley, D.L. Improved batch and flow syntheses of the nonsteroidal anti-inflammatory COX-2 inhibitor celecoxib. React. Chem. Eng. 2021, 6, 138–146. [Google Scholar] [CrossRef]
- Sloop, J.C.; Bumgardner, C.L.; Washington, G.; Loehle, W.D.; Sankar, S.S.; Lewis, A.B. Keto–Enol and Enol–Enol Tautomerism in Trifluoromethyl-β-Diketones. J. Fluor. Chem. 2006, 127, 780–786. [Google Scholar] [CrossRef]
- Medjahed, N.; Kibou, Z.; Berrichi, A.; Choukchou-Braham, N. Advances in Pyrazoles Rings’ Syntheses by Heterogeneous Catalysts, Ionic Liquids, and Multicomponent Reactions-A Review. Curr. Org. Chem. 2023, 27, 471–509. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Y.; Lin, Y.; Ren, Q. The Combination of Lewis Acid with N-Heterocyclic Carbene (NHC) Catalysis. Catalysts 2019, 9, 863. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Salimi, M.; Esmaeili, A.A. Cellulose Sulfuric Acid as a Bio-Supported and Efficient Solid Acid Catalyst for Synthesis of Pyrazoles in Aqueous Medium. RSC Adv. 2014, 4, 61193–61199. [Google Scholar] [CrossRef]
- Ponra, S.; Majumdar, K.C. Brønsted Acid-Promoted Synthesis of Common Heterocycles and Related Bio-Active and Functional Molecules. RSC Adv. 2016, 6, 37784–37922. [Google Scholar] [CrossRef]
- Portilla-Zúñiga, O.; Bautista-Aguilera, Ó.M.; Martínez, J.J.; Rojas, H.; Macías, M.A.; Iriepa, I.; Romanelli, G.P. Synthesis of N-Substituted Pyrroles Catalyzed by Low-Cost and Commercially Available Aluminas. Catalysts 2023, 13, 603. [Google Scholar] [CrossRef]
- Sanz, R.; Miguel, D.; Martínez, A.; Álvarez-Gutiérrez, J.M.; Rodríguez, F. Brønsted Acid-Catalyzed Benzylation of 1,3-Dicarbonyl Derivatives. Org. Lett. 2007, 9, 2027–2030. [Google Scholar] [CrossRef]
- Motokura, K.; Fujita, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Brønsted Acid Mediated Heterogeneous Addition Reaction of 1,3-Dicarbonyl Compounds to Alkenes and Alcohols. Angew. Chem. Int. Ed. 2006, 45, 2605–2609. [Google Scholar] [CrossRef]
- Danbo, X.; Dan, S.; Qiliang, C.; Jiaqi, Z.; Xiaofei, Z.; Guofu, Z. N-Heterocyclic Carbene/Lewis Acid Catalyzed Enantioselective Aerobic Annulation of α,β-Unsaturated Aldehydes with 1,3-Dicarbonyl Compounds. J. Org. Chem. 2016, 81, 6136–6141. [Google Scholar] [CrossRef]
- Wang, X.; Vu, J.; Luk, C.; Njoo, E. Benchtop 19F nuclear magnetic resonance spectroscopy enabled kinetic studies and optimization of the synthesis of carmofur. Can. J. Chem. 2023, 101, 518–524. [Google Scholar] [CrossRef]
- Udgaonkar, A.; Wu, J.; Rao, A.; Njoo, E. Deuterated Solvent Effects in the Kinetics and Thermodynamics of Keto-Enol Tautomerization of ETFAA. J. Emerg. Investig. 2022, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Singh, P.; Su, S.; Kocalar, S.; Wang, X.; Mandava, N.; Venkatesan, S.; Ferguson, A.; Rao, A.; Le, E.; et al. Benchtop 19F Nuclear Magnetic Resonance (NMR) Spectroscopy Provides Mechanistic Insight into the Biginelli Condensation toward the Chemical Synthesis of Novel Trifluorinated Dihydro- and Tetrahydropyrimidinones as Antiproliferative Agents. ACS Omega 2023, 8, 10545–10554. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.G.; Feltman, P.M. Correction to Determination of Solvent Effects on Keto−Enol Equilibria of 1,3-Dicarbonyl Compounds Using NMR. J. Chem. Educ. 2010, 87, 678–679. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.; Gibson, M.; Osman, Y.; Edgar, M.; Molinari, M.; Mather, M.L.; Casanova, F.; Wilson, P.B. Progress in Low-Field Benchtop NMR Spectroscopy in Chemical and Biochemical Analysis. Anal. Chim. Acta 2019, 1067, 11–30. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Myoung, S.; Ahn, S. Recent Applications of Benchtop Nuclear Magnetic Resonance Spectroscopy. Magnetochemistry 2021, 7, 121. [Google Scholar] [CrossRef]
- Archambault, C.M.; Leadbeater, N.E. A Benchtop NMR Spectrometer as a Tool for Monitoring Mesoscale Continuous-Flow Organic Synthesis: Equipment Interface and Assessment in Four Organic Transformations. RSC Adv. 2016, 6, 101171–101177. [Google Scholar] [CrossRef]
- Giberson, J.; Scicluna, J.; Legge, N.; Longstaffe, J. Chapter Three—Developments in benchtop NMR spectroscopy 2015–2020. Annu. Rep. NMR Spectrosc. 2021, 102, 153–246. [Google Scholar] [CrossRef]
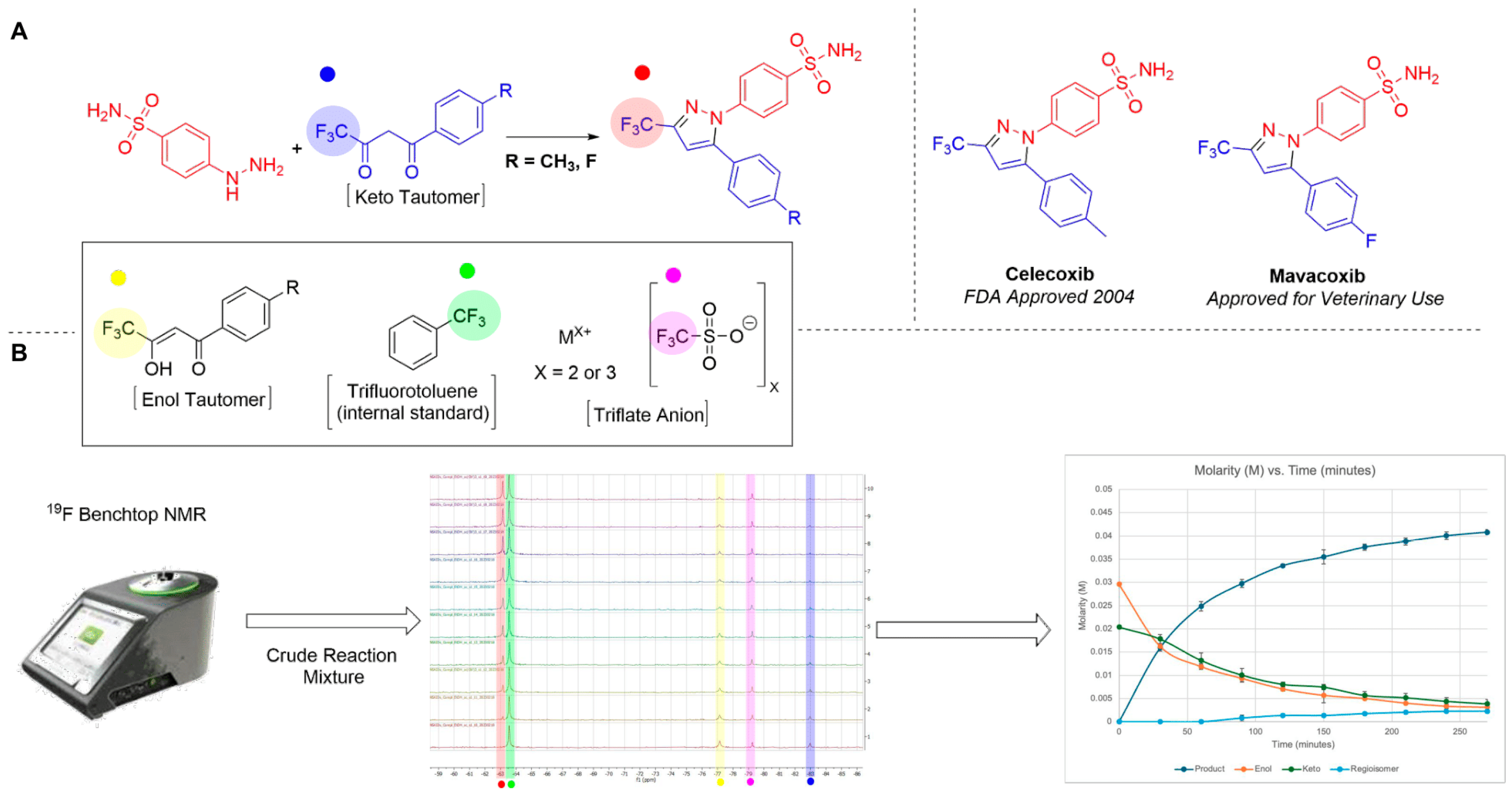
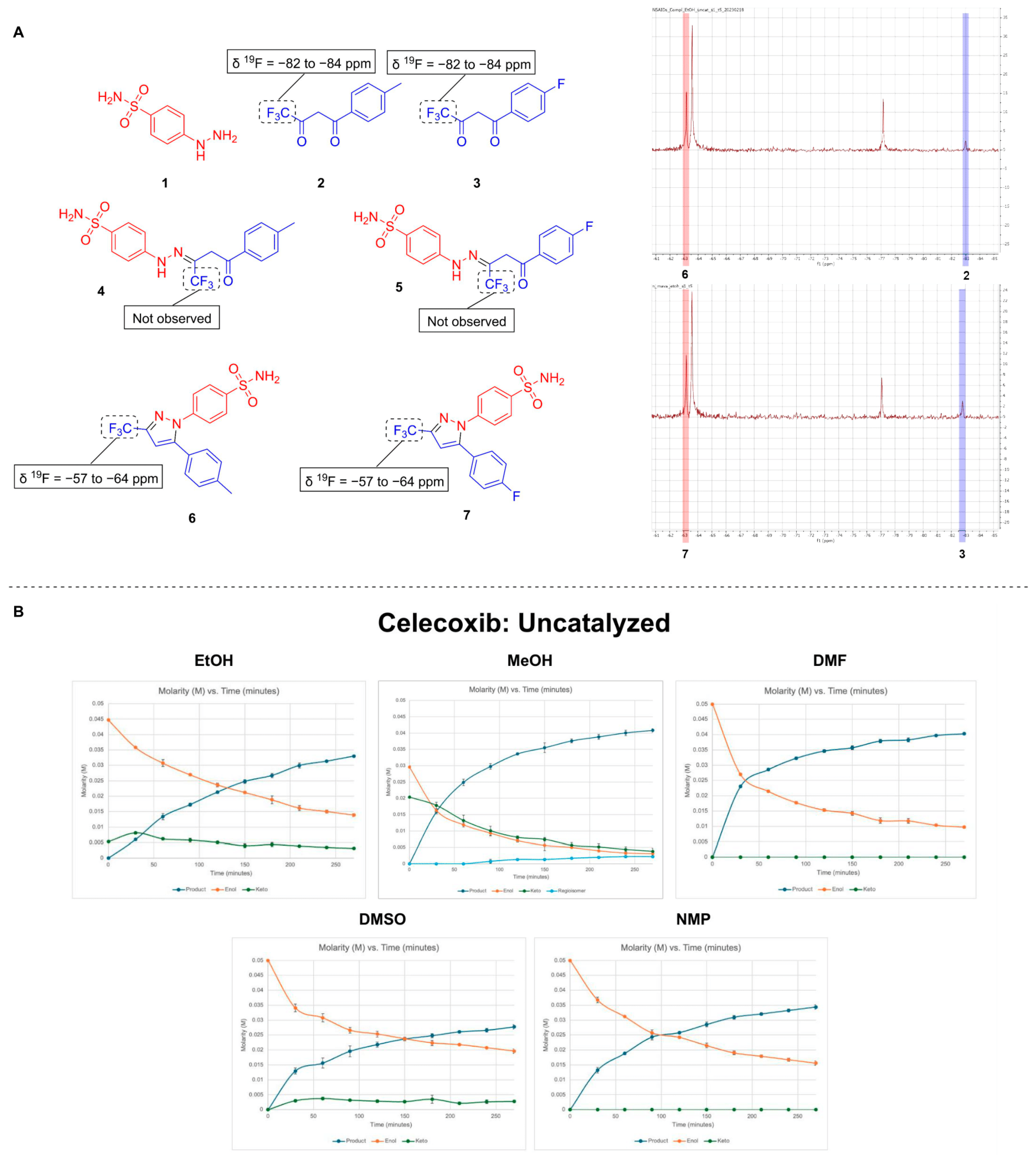
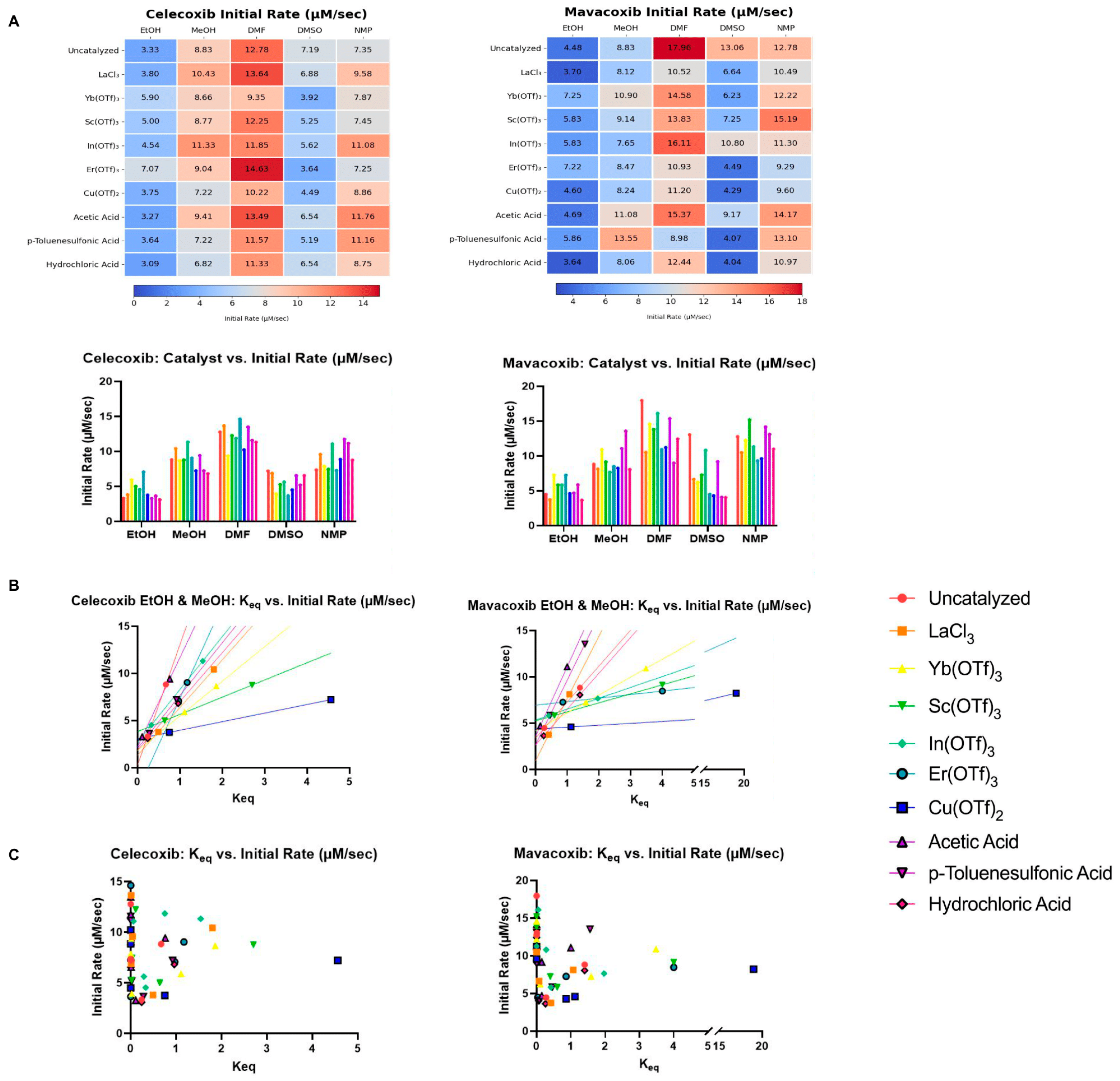
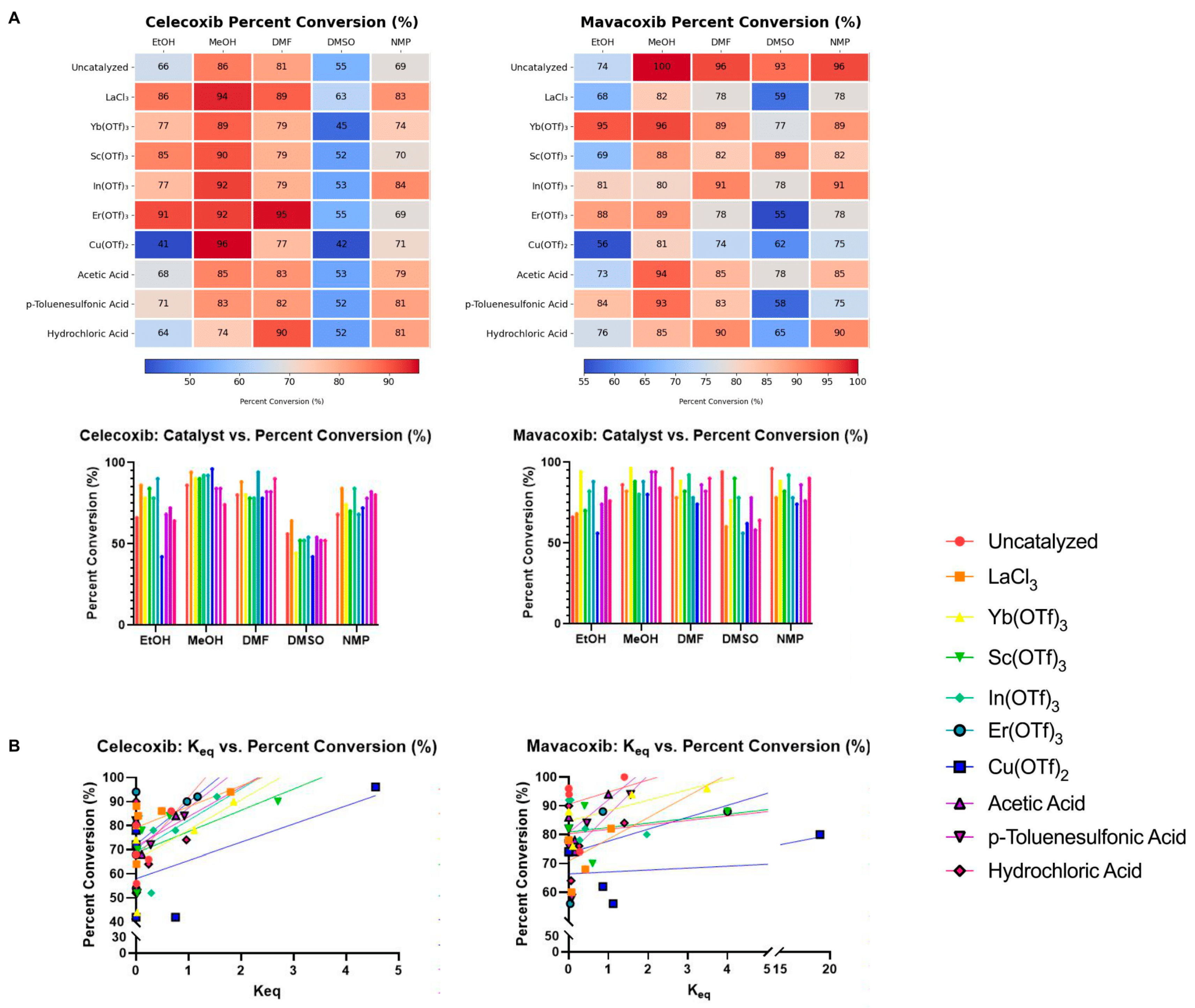

| Catalyst | Celecoxib | Mavacoxib |
|---|---|---|
| Uncatalyzed | 4.5% | 0.0% |
| LaCl3 | 3.2% | 2.8% |
| Yb(OTf)3 | 2.2% | 3.0% |
| Sc(OTf)3 | 3.9% | 4.0% |
| In(OTf)3 | 4.4% | 3.2% |
| Er(OTf)3 | 3.4% | 3.3% |
| Cu(OTf)2 | 2.5% | 2.7% |
| Acetic acid | 4.4% | 3.3% |
| p-toluenesulfonic acid | 3.9% | 3.7% |
| Hydrochloric acid | 2.7% | 3.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chyu, A.; Xi, S.; Kim, J.; Liu, G.; Chan, I.; Hong, S.; Ke, A.; Lavery, T.; Marimuthu, A.; Akula, A.; et al. Benchtop 19F Nuclear Magnetic Resonance (NMR) Spectroscopy-Optimized Knorr Pyrazole Synthesis of Celecoxib and Mavacoxib, 3-(Trifluoromethyl) Pyrazolyl Benzenesulfonamides, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Spectrosc. J. 2024, 2, 206-215. https://doi.org/10.3390/spectroscj2040014
Chyu A, Xi S, Kim J, Liu G, Chan I, Hong S, Ke A, Lavery T, Marimuthu A, Akula A, et al. Benchtop 19F Nuclear Magnetic Resonance (NMR) Spectroscopy-Optimized Knorr Pyrazole Synthesis of Celecoxib and Mavacoxib, 3-(Trifluoromethyl) Pyrazolyl Benzenesulfonamides, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Spectroscopy Journal. 2024; 2(4):206-215. https://doi.org/10.3390/spectroscj2040014
Chicago/Turabian StyleChyu, Andrew, Selina Xi, Joshua Kim, Galen Liu, Indalina Chan, Seoyeon Hong, Allen Ke, Thomas Lavery, Anushree Marimuthu, Arjun Akula, and et al. 2024. "Benchtop 19F Nuclear Magnetic Resonance (NMR) Spectroscopy-Optimized Knorr Pyrazole Synthesis of Celecoxib and Mavacoxib, 3-(Trifluoromethyl) Pyrazolyl Benzenesulfonamides, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)" Spectroscopy Journal 2, no. 4: 206-215. https://doi.org/10.3390/spectroscj2040014
APA StyleChyu, A., Xi, S., Kim, J., Liu, G., Chan, I., Hong, S., Ke, A., Lavery, T., Marimuthu, A., Akula, A., & Njoo, E. (2024). Benchtop 19F Nuclear Magnetic Resonance (NMR) Spectroscopy-Optimized Knorr Pyrazole Synthesis of Celecoxib and Mavacoxib, 3-(Trifluoromethyl) Pyrazolyl Benzenesulfonamides, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Spectroscopy Journal, 2(4), 206-215. https://doi.org/10.3390/spectroscj2040014







