Abstract
The analysis of metabolite mediators has allowed a broader understanding of disease mechanisms. Experimental evidence indicates that metabolic rewiring is a key feature of inflammatory cells to restore tissue homeostasis upon damage. Over the last two decades, next-generation sequencing techniques have offered the possibility of looking at the genome-wide effect of the exposure of inflammatory cells to external stimuli. During gout flares, monosodium urate crystals activate a distinct metabolic profile and inflammatory transcriptional program in inflammatory cells. The extracellular signals are transduced through distinct signalling pathways, which are regulated by non-coding RNA and DNA sequences, and modification of histones. During response to inflammatory stimuli, changes in the abundance of metabolic mediators can regulate the activation of histones and of chromatin remodellers. The interplay between metabolic changes by MSUc, the regulation of epigenetic changes and the activation of transcription factor networks in inflammatory cells remains unknown. A better understanding of the interplay between metabolites and how it alters inflammatory response may provide novel insights into disease mechanisms during gout. In this review, we aim to provide a deeper understanding of the current view of how metabolic deregulation could alter the epigenetic landscape of inflammatory cells during gout.
1. Introduction
Over the last five decades, the working hypothesis has been that most illnesses have a genetic disorder in their origin [1,2,3]. Vigorous efforts in large-scale, population-wise studies have aimed to find gene susceptibility to disease by genome-wide association studies (GWAS). Even though GWAS have generated essential insights to understand the underlying mechanisms of some diseases [4,5], they have resulted in only a few susceptibility genes being identified [6,7]. This indicates that additional factors such as chronic environmental causes [8,9,10], an altered epigenome, and the contribution of metabolites and microbiome could take part in the onset and progression of human diseases [8,9,10].
Gout is the most common inflammatory arthritis worldwide, and its incidence is rising in developed and underdeveloped countries [11]. Gout is caused by the deposition of monosodium urate crystals in the joints in patients with persistent hyperuricaemia (HU) [12,13,14,15,16]. Besides the local clinical manifestations in the joints, gout is associated with many other systemic complications, from renal disease [17,18] to cardiovascular disease [19,20,21,22], diabetes [23], and metabolic syndrome [24,25]. Since the deregulation of urate metabolism is at the heart of gout, it is vital to understand genetic conditions [26,27] and environmental or behavioural exposures such as diet [28,29,30,31,32,33] that modify blood urate levels and underlying molecular mechanisms [28,29,31].
Together with the acute inflammatory reaction, gout attacks are accompanied by an altered local and systemic metabolomic profile [34,35,36]. Diverse significantly dysregulated pathways have been described in individuals with hyperuricaemia and patients with gout compared to normouricaemic controls, among which arginine metabolism [37] and other amino acids [38] appeared to play a critical role. Lipid and carbohydrate metabolism are other proposed dysregulated pathways [39,40]. Finally, a significant increase in leukotriene B4 (LTB4) in plasma associated to an increased transcriptional level of 5-lipoxygenase in whole blood cells was described in patients with acute gout flares [41].
Metabolites are the substrate, intermediate, or final products of metabolic reactions that drive the function of a given cell in a particular time and context. Therefore, metabolites provide essential information about the connection between gene expression and the environment, and, as such, they are elegant disease biomarkers [42]. Over the last decade, metabolomics has extensively characterized metabolites and metabolic pathways in many biological systems, providing novel opportunities to understand disease mechanisms and untangle the cause of complex diseases such as cancer [43,44], diabetes [45], cardiovascular [46,47,48], and other types of inflammatory arthritis [49,50,51]. In addition, it has been suggested that the metabolic deregulation observed during gout could contribute to kidney and cardiovascular disease [24,52,53]. Metabolomics offers, combined with other omics (genomics, transcriptomics, proteomics), an increasing number of biomedical applications, from disease diagnosis to patient monitoring, personalized drug treatments, and predicting drug response [54]. Recently, the application of machine learning to perform automated identification and quantification of novel metabolites has provided a road map of metabolic deregulation in various biological scenarios [55,56]. In this Review, we will provide a general overview of how metabolic changes affects histone lactylation and acetylation macrophages during response to external stimuli. We also explain how metabolic changes affect AP-1 transcription factor binding in macrophages by MSUc. Finally, we will present a hypothetical novel mechanism of inflammation resolution by changes in lipid metabolism during gouty inflammation by MSUc.
2. From Metabolomics to Epigenetics and Transcription Factor Binding: Coupling Environmental Changes to Molecular Phenotypes
The biochemical actions of metabolites go far beyond their role in biosynthesis and bioenergetic processes. In the last decade, metabolomics has shed light on how metabolites alter gene expression and contribute to dictating biological phenotypes [54]. The well-characterized role of metabolites in regulating the epigenetic landscape of embryonic stem (ES) cells [57,58,59,60] was followed by an increasing interest in how metabolites alter the immune cell phenotype, also named immunometabolism. The field of immunometabolism has emerged as a critical tool for understanding how metabolic changes can modulate immune cell response [61,62,63]. Moreover, we propose that the connection between epigenetics and metabolism will provide new avenues to understand disease predisposition and to develop personalised treatment [64,65]. The assembly of eukaryotic genomes is accomplished by a complex of octamers of histones that bind to the DNA. The segment of DNA wrapped around the histone octamer in the nucleosomes presents a barrier for the binding of transcription factors (TFs), transcription initiation complexes, and other transcriptional regulators. Therefore, the interaction of TFs with histone modifications plays a crucial role in integrating a finely tuned gene expression program [66,67,68]. Some post-translational modifications of histones and epigenetic regulators impact the inflammatory reaction during response to external stimuli [69,70]. For instance, whereas genome-wide remodelling of acetylation of lysine 27 of H3 (H3K27ac) is seen specifically in distal regions, changes in methylation of lysine 4 of H3 (H3K4me3) are mainly detected in genomic regions close to the transcription start site and regulate macrophages’ response to microenvironmental signals [69,70,71,72,73]. During the last decades, the development of next-generation sequencing (NGS) techniques has allowed profiling the histone landscape and transcription factor binding genome-wide to understand the dynamic mechanism of gene expression during homeostasis and disease [74,75]. In addition, NGS techniques have allowed an advance which links genetics with environment and disease, revolutionizing the way we carry out science [76,77].
Macrophages represent an elegant model for understanding histone dynamics, transcription factor recruitment, and changes in gene expression during signal transduction by environmental signals [69,78,79]. These environmental signals are integrated with intracellular signalling pathways that regulate non-coding DNA regulatory elements (RE), termed enhancers, and promoters, to control macrophage phenotypes. The accessibility of DNA binding sites in the regulatory elements controls the ability of TFs to produce a spatiotemporal-specific and context-specific transcriptional output. Macrophage phenotypes are regulated by activation of distinct inflammatory programs determined by the response of various TF to external signals from the cellular context [69,78,79].
2.1. Histone Lactylation Contributes to Establishing an Inflammation Resolving Program in Macrophages
In the immunometabolism field, there has been emerging interest in understanding the molecular consequences of metabolic imbalance in regulating histone activity through changes in post-translational modifications (PTMs) [80]. Histone lactylation is one of the most compelling cases of epigenetic modification by changes in metabolic balance in macrophages. The Warburg effect was first described in cancer cells, where glycolysis is highly upregulated; therefore, cancer cells produce large amounts of lactate [81,82,83]. Initially, lactate was considered a by-product of the glycolytic activity of the cell. However, more evidence suggests that lactate is involved in various cellular processes in health and disease [84,85,86], including activating the Krebs cycle [87,88] and acting as an extracellular signalling molecule allowing the intercommunication of neighbouring cells [89]. In 2019, Zhang and colleagues demonstrated that lactate could modify histones in macrophages, a process named histone lactylation [90]. During the response to a pro-inflammatory perturbation, macrophages produce larger amounts of lactate that, among other molecular consequences, leads to histone lactylation over a subset of inflammatory genes associated with establishing an anti-inflammatory gene expression program and genes related to facilitating the resolution of inflammation [90]. Zhang’s work has been followed up by other studies supporting the hypothesis that dynamic histone lactylation by lactate is a hallmark of metabolic rewiring and a crucial mechanism of gene expression in macrophages [91,92]. Interestingly, histone lactylation is not observed over the promoter regions of inflammatory genes and does not alter the expression of cytokines and other proinflammatory genes, indicating specificity for a subset of anti-inflammatory genes. The current view is that to react against an inflammatory insult the cells need to use glucose via anaerobic glycolysis to activate an inflammatory gene expression program at early time points. The sustained activation of glycolysis leads to increased intracellular lactate levels, which induces lactylation of the histones recruited over the promoter of anti-inflammatory/resolution genes. Histone lactylation is a hallmark of an anti-inflammatory phenotype in macrophages that establishes resolution of inflammation. Importantly, we and others have demonstrated that stimulation of macrophages with MSUc leads to increased glycolytic metabolism, including higher intracellular lactate levels [34,35,36]. Thus, histone lactylation to activate anti-inflammatory genes could be part of the underlying mechanism observed during gout flares that leads to resolution. In addition, we speculate that the imprinting of an anti-inflammatory epigenetic and transcriptomic signature during gout flare must occur to avoid the apoptosis of inflammatory cells and the destruction of the synovial membrane of the joint tissue MSUc and promote resolution of the flare. This mechanistic role for the so-called “reparative inflammation” has been extensively studied in highly proliferative tissues such as gut and liver epithelial cells [93], but common molecular behaviour highlights its importance in other tissues. As they are involved in any inflammatory disease, there has been great interest in understanding the role of innate immune cells, mainly macrophages, in regulating resolution of inflammation [94,95,96,97]. Therefore, we hypothesise that histone lactylation would have a crucial role in regulating macrophage response to MSUc. However, supporting experimental data are required to confirm this hypothesis.
2.2. Histone Acetylation Takes Charge of the Dynamic Enhancer Activation in Macrophages in Response to External Stimuli
Besides glycolysis, another important metabolic pathway is the Krebs cycle. The Krebs cycle is fuelled by acetyl-CoA produced from glucose or fatty acids and can lead to acetylation. Interestingly, MSUc leads to increased glycolysis, with accumulation of citrate and succinate [35], which together with itaconate can modify proteins in the context of immunity and inflammation [98,99]. For instance, itaconate was shown to modify cysteines on a range of target proteins, with the modification being linked to a functional change [98]. In addition, acetyl-CoA can produce acetylation and deacetylation of histones and it represents one of the most widely used histone modifications to understand the epigenetic activation of macrophages during inflammation [69,70,79]. Two main protein families, the histone acetyltransferases (HATs) and the histone deacetylases (HDACs), dynamically and reversibly control the acetylation state of histones and, in consequence, are important regulators of the context-specific responses of macrophages [100], and help to decipher molecular phenotypes of macrophages during disease. This dynamic epigenetic response has been largely studied in enhancer regions by using H3K27ac as a read-out of enhancer activity in the context of macrophage activation by TLR agonists and other ligands [70,78,101], but the enhancer landscape induced by MSUc in macrophages remains unknown. In vitro studies of mouse macrophages indicate that most enhancers are recognised by a combination of TFs that collaboratively interact with each other to bind to specific DNA sequence motifs in regulatory regions of the genome. Of these TFs, the current view is that the transcriptional output of a repertoire of enhancers in macrophages is dictated by the contribution of lineage-determining transcription factors (LDTFs), including PU.1 and the AP-1 family, and signal-dependent transcription factors (SDTFs), including nuclear receptors (NR) and NFKB and interferon regulatory factors (IRFs), among others [78,79,102]. Of note, interestingly, whereas the promoter of genes induced by LPS in macrophages is enriched in IRFs, NFKB and STAT motifs, the promoter of genes induced by MSUc is enriched in DNA motifs for AP-1, MITF/TFE, NR, and circadian clock regulators, indicating that a different combination of TFs takes charge of the epigenetic activation of macrophages by MSUc [35]. Moreover, the genes uniquely upregulated by MSUc belong to signalling pathways related to NRs signalling and transcription and activation of circadian clock regulators. However, this in silico prediction of putative binding of TFs to the DNA requires empirical validation to identify the motif enrichment in regions of open chromatin with increased enhancer activity. However, the changes in the epigenetic landscape of macrophages by MSUc and its contribution to altering inflammatory programs of gene expression remain to be elucidated.
2.3. Differential Recruitment of Transcription Factor Binding to Genomic Regulatory Regions Regulates the Response of Macrophages to MSUc: The Case of the AP-1 Family
The integration of histone landscape and TF binding by ChIP-Seq analysis with gene expression by RNA-Seq is fundamental to dissecting macrophage cell phenotypes during gout flares. Our work and that of others demonstrate that in macrophages signalling pathways regulated by inflammatory molecules such as MSUc during gout flares are coupled to a battery of TFs whose ability to induce specific gene expression programs is dictated by the accessibility (“openness”) of the chromatin and the presence of particular motifs in the macrophage DNA genome [35,69,103]. Our in silico analyses of DNA motifs define the putative transcription factor families to regulate the transcriptional output of macrophages and offer candidates to understand the interplay between MSUc and the control of macrophage function. In line with this, we found that the promoter region of genes induced by MSUc in unprimed bone marrow derived (BMDM) mouse and human monocyte-derived (MDM) macrophages are mainly enriched in motifs for activator protein 1 (AP-1). The AP-1 is a dimeric family that includes members of the JUN, FOS, activating transcription factor (ATF), and musculoaponeurotic fibrosarcoma (MAF) protein families [104,105]. The different dimer compositions of AP-1 complexes determine the biological output of the process controlled by AP-1 [106,107]. Historically described during oncogenic transformation [104,108,109], AP-1 members are abundant in macrophages, regulating molecular phenotypes and contributing to macrophage function. Increased JUN upregulation and phosphorylation by MSUc via JNK and increased JUN recruitment to the promoter of metabolic genes are required for the response of macrophages to MSUc. Molecular or pharmacological reduction in JNK-JUN activity modifies the epigenetic landscape; it ameliorates the induction of metabolic genes and metabolic changes, including a reduction in lactate, indicating that JUN’s role during gouty inflammation by MSUc goes beyond its role in activating an inflammatory gene expression program. The sustained activation of JUN and JNK and the altered metabolic program when JNK-JUN activity is compromised could indicate that JUN and its downstream target genes are involved in the later stages of the response of macrophages to MSUc by regulating metabolic mediators.
2.4. Epigenetic Changes by Higher Soluble Urate Levels in Myeloid Cells
Hyperuricaemia is the main risk factor for gout flares [12,13,14,15,16]. High levels of urate induce IL1b and IL6 production by monocytes and reduced levels of IL-1 receptor antagonist (IL-1Ra) [110]. Moreover, ChIP-Seq data on H3K27ac and H3K4me3 show that some inflammatory genes such as Il1a, Il1b displayed increased enrichment in H3K27ac and H3K4me3 by urate [110], which indicates that soluble urate can alter the epigenetic landscape of inflammatory genes in myeloid cells [111]. Together with changes in histone modifications, elevated serum urate alters the DNA methylation profile of circulating inflammatory cells including the glucose transporter SLC2A9, the amino acid transporter SLC7A11 and the amino acid biosynthesis gene PHGDH [112]. Interestingly, SLC2A9 is a known urate transporter that regulates serum urate concentration and excretion during gout [113], indicating that epigenetic gene deregulation may provide information about genetic traits in hyperuricaemia and gout.
3. Lipidomics and Gout, Signalling Pathways in the Resolution of Inflammation by Macrophages
The deposition of MSUc in the joints causes a self-limited, acute inflammatory reaction. The effect MSUc during gouty inflammation offers a suitable system to understand anti-inflammatory programs of gene expression in macrophages. The original view was that biological systems resolve inflammation by diluting proinflammatory mediators that eventually restore tissue function. This view has been surpassed, thanks to the work of Dr Charles N. Serhan and others, by a more active notion where macrophages and other cell types produce specialized pro-resolving mediators (SPMs) and other anti-inflammatory oxylipins to counterbalance the initial wave of proinflammatory signals to prevent surplus inflammation and subsequent tissue damage [114,115,116,117,118,119]. Even though SPMs are oxylipins widely studied in the context of inflammation and have been primarily studied in other inflammatory diseases, including lung disease [118,120] and cancer [121], the role of SPMs in the resolution of gout flares remains unknown. SPMs, resolvins, protectins, and maresins are derived mostly from alpha linolenic acid (α-LA), which is an omega-3 essential fatty acid (EFA) from green leafy vegetables, flax and chia seeds, and walnuts. Omega-6 EFA are generally generated by linoleic acid (LA) from vegetable oils, meats, and eggs. Some omega-6 lipids, such as lipoxins, PGJ2, and PGB2, are also considered anti-inflammatory molecules [122].
The central catalytic enzymes involved in the generation of STMs are phospholipases (PLA)2 and lipoxygenases (LOX). The time course of biosynthesis and bioavailability of SPMs dictates their molecular function to ensure a cell type and context-specific response. In macrophages, lipoxin A4 (LXA4), protectin D1 (PD1) and resolvin D1 (RvD1) are involved in the clearance of apoptotic neutrophils and other polymorphonuclear cells [123,124,125]. Regardless of the subtype, SPMs exert their biological activity upon binding with high affinity to specific cognate receptors. Over the last years, the receptors for some of the SPMs have been characterized. LXA4 binds and signals through the LXA4 receptor (ALX or formyl peptide receptor(FPR2)) [126], RvE1 through chemokine-like receptor 1 (CMKLR1) [127], RvD1 through G protein-coupled receptor GPR32, and RvD2 through GPR18 [128,129]. Therefore, changes in EFA, an altered expression of the enzymatic cascade that bio-converts EFA to SPMs and other anti-inflammatory oxylipins, or changes in the expression and availability of any of the receptors will impact the activity of SPMs during the resolution of inflammation. Interestingly, the treatment of mice with MSUc results in an increased production of prostaglandins and other oxylipins suggesting that oxylipin metabolism could be also involved in limiting the duration of gouty inflammation by MSUc [130,131]. Below we will review some of the mechanisms that could contribute to regulate SPM production in macrophages during gout flares.
3.1. Phospholipases A2
Phospholipase A2 (PLA2) encompasses a superfamily of enzymes with more than 50 members, whose expression and activity dictate a cell-specific and temporal response [132,133,134,135]. PLA2 is the first enzymatic machinery in the metabolism of SPMs. Therefore, extensive work has been put into understanding PLA2 regulation during inflammatory processes in macrophages [136]. PLA2 enzymes can act as degradative, biosynthetic (when coupled to an acetyltransferase) or as a signalling enzyme. This versatility of action, the high degree of functional redundancy, and their dynamic expression have made the PLA2 family challenging to ascribe to specific regulatory signalling programs. It is widely accepted that many different mechanisms, including increased [Ca2+] [137], ceramide phosphate [138], phosphatidylinositol [139,140], bisphosphate [141], and phosphorylation [142] activate PLA2. In addition, the transcription of the endogenous secretory phospholipase A2 group IIA (sPLA2-IIA) gene is regulated by the direct binding of CCAAT/Enhancer Binding Protein (C/EBP), NFKB, and ETS proto-oncogene TF (ETS) transcription factors to the PLA2 regulatory region [143,144]. Interestingly, whereas Pla2g4a and Pla2g5 are upregulated, Pla2g15 is downregulated, suggesting a role of Pla2 transcriptional regulation in macrophages during gout.
3.2. COX and ALOX5/ALOX5AP
The next step in the formation of oxylipins associated to the resolution of inflammation involves the conversion of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and some products derived from AA by cyclooxygenases (COX) and lipoxygenases. MSUc stimulates COX-2 expression in peripheral monocytes, which correlated with the synthesis of pro-inflammatory oxylipins such as prostaglandin E2 (PGE2) and thromboxane A2 (TXA2) [145]. Leukotriene B4 (LTB4) was also relevant in the MSUc-induced maturation of IL-1b [146]. Of interest, PGD2 and 15d-PGJ2 had an anti-inflammatory role in animal models of MSUc-induced inflammation [147,148]. Of the genes coding for lipoxygenases, ALOX5/Alox5 and Alox5 activating protein ALOX5AP/Alox5ap are the two most expressed in unprimed MDM and BMDM. The significance of ALOX5/ALOX5AP during gout is supported by a study by Luo and colleagues where after performing metabolomics of PUFA of patients with acute gout plasma validated in two independent cohorts, they found a higher increase in leukotriene B4 (LTB4), accounting for altered activity of lipoxygenase 5 [41]. Notably, stimulation with MSUc leads to downregulation of ALOX5 in unprimed MDM and BMDM and downregulation of Alox5ap in unprimed BMDM [35], which is in accordance with a negative feedback mechanism of metabolic networks to regulate active metabolic pathways [149,150]. Importantly, ALOX5/ALOX5AP are JUN target genes, and treatment with JNK inhibitor SP600125 ameliorates the downregulation by MSUc, providing further evidence that ALOX5 and ALOX5AP repression by JUN could contribute to the formation of oxylipins during gouty inflammation. Interestingly, the expression of the main LXA4 receptor, FPR2, is downregulated in unprimed MDM stimulated with MSUc. Of interest, besides the participation of 5-LOX in inflammation by promoting the biosynthesis of leukotrienes, this enzyme possesses other non-canonical functions as transcriptional regulator in monocytic cells including the interaction with β-catenin, p53, and chromatin [151,152]. These results provide substantial evidence to suggest a role of signalling by LOX products and their downstream signalling during the resolution of gout flares.
3.3. Activation of Enzymatic Pathways by Damaged Subcellular Organelles
During the early stages of the acute phase of gout flares, ingested MSUc induces the rupture of lysosome in leukocytes and the release of the lysosomal content into the surrounding medium, which is a hallmark of damage induced by MSUc [153,154,155,156,157]. Aberrant lysosomal compartment leads to increased intracellular and extracellular [Ca2+] [158,159], which can activate PLA2 to release free fatty acids that fuel the synthesis of new pro- and anti-inflammatory oxylipins.
4. Conclusions
In this review, we have provided extensive evidence demonstrating the importance of metabolomic analyses in gouty inflammation. Although well implemented in the study of other pathologies, the knowledge of the metabolic contribution during the acute phase of gout flares is relatively scarce. We have decided to focus the scope of this review on the some of the possible epigenetic mechanisms underlying the activation of innate immune cells, mainly macrophages, by MSUc during the acute phase of gouty inflammation. Undeniably, other cell types are involved in regulating the response to MSUc. However, the degree of plasticity of macrophage epigenetic phenotypes [69,79], their bona fide cell ability to be involved in the phagocytosis of MSUc, and their involvement in the resolution phase of inflammatory processes make macrophages an elegant target to dissect molecular mechanisms in pursuit of novel therapeutical regimens [153]. In addition, the extensive knowledge of the epigenetics and transcriptomics of macrophages after perturbation makes it easier to ascribe epigenetic programs of gene expression associated with the stimulation by MSUc.
We have placed significant emphasis on the epigenetic activation of transcriptional programs by changes in metabolite composition as in the production of specialised molecules involved in the resolution of inflammation. Our view is summarised in Figure 1. In summary, during gout flares, macrophages respond to MSUc, activating first a cascade of pro-inflammatory signalling and then likely a more robust cascade engaged in the resolution of the inflammation. Whereas the “pro-inflammatory” phase enables macrophages to recruit other inflammatory cell types, the resolution phase might activate a vital signalling cascade to induce the phagocytosis of apoptotic cells and produce pro-resolution lipids to restore tissue homeostasis. In our view, the activation of a pro-inflammatory program by MSUc lies in the early activation of JNK-JUN and other AP-1 members [35]. The role of AP-1 in activating inflammatory programs is well known, and our data demonstrate that treatment with a JNKi ameliorates severe inflammation by MSUc in vitro and in vivo [35].
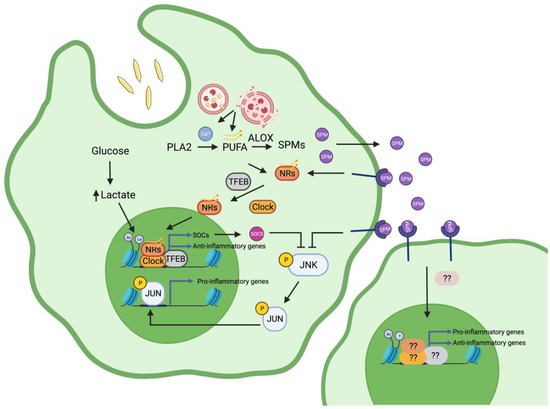
Figure 1.
Mechanisms of macrophage activation during gouty inflammation. During gout flares, MSUc leads to a cell autonomous response in macrophages that is mediated by activation of transcription factors (TFs) including activation of nuclear receptors (NRs) upon binding to fatty acids, AP-1 via JNK signalling, MITF/TFEB, and circadian clock regulators (Clock). The recruitment of these TFs to genomic regions with altered histone landscape marked with histone lactylation (La), due to increased intracellular lactate, or H3K27ac (Ac) dictates the transcriptional response of macrophages by MSUc. On the other hand, local activation by MSUc leads to a non-cell autonomous activation of neighbouring macrophages mediated by a yet-unknown transcriptional and epigenetic mechanism. We hypothesise that the production of specialised pro-resolving mediators (SPM) by activated metabolism of polyunsaturated fatty acids (PUFA) via arachidonate lipoxygenases (ALOX) and intracellular calcium levels plays a crucial role in in activating a resolution program in macrophages during gout flares. Figure created with BioRender.com.
In parallel with the activation of inflammatory gene expression, we hypothesise that in macrophages during gouty inflammation, the resolution of inflammation is regulated at different levels. Increased lactate production through anaerobic glycolysis lactylates histone H3 and histone H4 could open the chromatin of the regulatory regions of anti-inflammatory genes and promote their transcription. The family of suppressors of cytokine signalling proteins (SOCS), including Socs1, Socs3, Socs4, Socs5, and Socs7, which are upregulated by MSUc [35], ameliorates the production of inflammatory cytokines and is a well-known negative regulator of JNK signalling [160,161,162,163]. The SOCS gene family is an example of many other anti-inflammatory genes induced by MSUc. Our data using a JNKi suggest that JNK and AP-1 also regulate the levels of some metabolites [35], including lactate, possibly through JUN binding to their promoter region, and could act as a TF effector downstream of histone lactylation. We acknowledge that the high levels of urate in patients with hyperuricaemia and gout can contribute to the deregulation of the epigenetic landscape of myeloid cells during gout flares. However, given the lack of strong deregulation of H3K4me3 or H3K27ac enrichment in monocytes exposed to urate [110], we hypothesise that the majority of epigenetic changes will be driven by the deposition of MSUc. On the contrary, we hypothesise that exposure to urate will impact the capacity of myeloid cells to respond to MSUc, priming myeloid cells to more exacerbated changes induced by MSUc. Moreover, it has been proposed that urate can induce immune memory in inflammatory cells [111], which is in accordance with our view of more dramatic epigenetic changes of macrophages by MSUc during gout. The proposed mechanisms of macrophage activation during gouty inflammation are summarised in Figure 1.
However, perhaps the most challenging thing will be to relate metabolites to their biological roles in regulating the response to MSUc. It is true that with machine learning techniques we have been able to narrow down the spectrum of action of a specific metabolite, but this is an ongoing area of research and needs to be improved. Integrating metabolomics with epigenomics, transcriptomics, and proteomics could help determine the relationship between gene expression, metabolite concentration, and biological function. Applying orthogonal approaches, including silencing gene expression by CRISPR-mediated knock-down, inhibiting enzymatic activity using chemical blockers or anti-metabolites, or targeting the immune response of macrophages, could help to provide novel mechanistic insights.
Author Contributions
Conceptualization, I.C., M.G., J.M.-S., M.A.; investigation, I.C., M.G., J.M.-S., M.A.; draft preparation, I.C., M.G.; writing—review and editing, I.C., M.G., J.M.-S., M.A.; visualization, I.C., M.G., J.M.-S., M.A.; supervision, M.G.; project administration, I.C., M.G.; funding acquisition, I.C., M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Arthritis National Research Foundation (ANRF) to I.C. and National Institute of Health (AR073324) to M.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Botstein, D.; Risch, N. Discovering genotypes underlying human phenotypes: Past successes for mendelian disease, future approaches for complex disease. Nat. Genet. 2003, 33, 228–237. [Google Scholar] [CrossRef]
- Stranger, B.E.; Stahl, E.A.; Raj, T. Progress and Promise of Genome-Wide Association Studies for Human Complex Trait Genetics. Genetics 2011, 187, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Shastry, B.S. SNPs in disease gene mapping, medicinal drug development and evolution. J. Hum. Genet. 2007, 52, 871–880. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef]
- Hall, S.S. Revolution Postponed. Sci. Am. 2010, 303, 60–67. [Google Scholar] [CrossRef]
- Maher, B. Personal genomes: The case of the missing heritability. Nature 2008, 456, 18–21. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Barupal, D.K.; Wishart, D.; Vineis, P.; Scalbert, A. The Blood Exposome and Its Role in Discovering Causes of Disease. Environ. Health Perspect. 2014, 122, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Dhimal, M.; Neupane, T.; Dhimal, M.L. Understanding linkages between environmental risk factors and noncommunicable diseases—A review. FASEB BioAdv. 2021, 3, 287–294. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Van Deventer, E.; Mudu, P.; Campbell-Lendrum, D.; Vickers, C.; Ivanov, I.; Forastiere, F.; Gumy, S.; Dora, C.; Adair-Rohani, H.; et al. Environmental risks and non-communicable diseases. BMJ 2019, 364, l265. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, M.; Ou, J.; Lv, Q.; Wei, Q.; Chen, Z.; Wu, J.; Tu, L.; Jiang, Y.; Zhang, X.; et al. Identification of the urine and serum metabolomics signature of gout. Rheumatology 2020, 59, 2960–2969. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Shipley, M. Hyperuricaemia and gout. J. R. Coll. Physicians Edinb. 2011, 41, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Farquhar, H.; Pisaniello, H.L.; Vargas-Santos, A.B.; Fisher, M.; Mount, D.B.; Choi, H.K.; Terkeltaub, R.; Hill, C.L.; Gaffo, A.L. Management of gout in chronic kidney disease: A G-CAN Consensus Statement on the research priorities. Nat. Rev. Rheumatol. 2021, 17, 633–641. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.-J.; Wu, C.-Y.; Yang, C.-W.; Yang, H.-Y. Hyperuricemia and Progression of Chronic Kidney Disease: A Review from Physiology and Pathogenesis to the Role of Urate-Lowering Therapy. Diagnostics 2021, 11, 1674. [Google Scholar] [CrossRef]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef]
- Everett, B.M.; MacFadyen, J.G.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial. J. Am. Coll. Cardiol. 2020, 76, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, N. Anti-Interleukin-1 Therapy in the Management of Gout. Curr. Rheumatol. Rep. 2014, 16, 398. [Google Scholar] [CrossRef]
- Solomon, D.H.; Glynn, R.J.; MacFadyen, J.G.; Libby, P.; Thuren, T.; Everett, B.M.; Ridker, P.M. Relationship of Interleukin-1β Blockade With Incident Gout and Serum Uric Acid Levels: Exploratory Analysis of a Randomized Controlled Trial. Ann. Intern. Med. 2018, 169, 535–542. [Google Scholar] [CrossRef]
- Lv, Q.; Meng, X.-F.; He, F.-F.; Chen, S.; Su, H.; Xiong, J.; Gao, P.; Tian, X.-J.; Liu, J.-S.; Zhu, Z.-H.; et al. High Serum Uric Acid and Increased Risk of Type 2 Diabetes: A Systemic Review and Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2013, 8, e56864. [Google Scholar] [CrossRef]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and Metabolic Syndrome: A Tangled Web. Curr. Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef]
- Puig, J.G.; Martínez, M.A. Hyperuricemia, gout and the metabolic syndrome. Curr. Opin. Rheumatol. 2008, 20, 187–191. [Google Scholar] [CrossRef]
- Major, T.J.; Dalbeth, N.; Stahl, E.A.; Merriman, T.R. An update on the genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol. 2018, 14, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Stamp, L.K.; Merriman, T.R. The genetics of gout: Towards personalised medicine? BMC Med. 2017, 15, 108. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Yuan, M.; Xu, Y.; Xu, H. Gout and Diet: A Comprehensive Review of Mechanisms and Management. Nutrients 2022, 14, 3525. [Google Scholar] [CrossRef]
- Li, R.; Yu, K.; Li, C. Dietary factors and risk of gout and hyperuricemia: A meta-analysis and systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Vedder, D.; Walrabenstein, W.; Heslinga, M.; de Vries, R.; Nurmohamed, M.; van Schaardenburg, D.; Gerritsen, M. Dietary Interventions for Gout and Effect on Cardiovascular Risk Factors: A Systematic Review. Nutrients 2019, 11, 2955. [Google Scholar] [CrossRef] [PubMed]
- Yokose, C.; McCormick, N.; Choi, H.K. The role of diet in hyperuricemia and gout. Curr. Opin. Rheumatol. 2021, 33, 135–144. [Google Scholar] [CrossRef]
- Wu, X.; You, C. The biomarkers discovery of hyperuricemia and gout: Proteomics and metabolomics. PeerJ 2023, 11, e14554. [Google Scholar] [CrossRef]
- Albrecht, E.; Waldenberger, M.; Krumsiek, J.; Evans, A.M.; Jeratsch, U.; Breier, M.; Adamski, J.; Koenig, W.; Zeilinger, S.; Fuchs, C.; et al. Metabolite profiling reveals new insights into the regulation of serum urate in humans. Metabolomics 2014, 10, 141–151. [Google Scholar] [CrossRef]
- Shao, T.; Shao, L.; Li, H.; Xie, Z.; He, Z.; Wen, C. Combined Signature of the Fecal Microbiome and Metabolome in Patients with Gout. Front. Microbiol. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Cobo, I.; Cheng, A.; Murillo-Saich, J.; Coras, R.; Torres, A.; Abe, Y.; Lana, A.J.; Schlachetzki, J.; Liu-Bryan, R.; Terkeltaub, R.; et al. Monosodium urate crystals regulate a unique JNK-dependent macrophage metabolic and inflammatory response. Cell Rep. 2022, 38, 110489. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann. Rheum. Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef]
- Shen, X.; Wang, C.; Liang, N.; Liu, Z.; Li, X.; Zhu, Z.; Merriman, T.R.; Dalbeth, N.; Terkeltaub, R.; Li, C.; et al. Serum Metabolomics Identifies Dysregulated Pathways and Potential Metabolic Biomarkers for Hyperuricemia and Gout. Arthritis Rheumatol. 2021, 73, 1738–1748. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, L.; Liu, X.-Y.; Chen, X.; Song, Y.-X.; Li, X.-H.; Jiang, C.; Peng, A.; Liu, J.-Y. Plasma profiling of amino acids distinguishes acute gout from asymptomatic hyperuricemia. Amino Acids 2018, 50, 1539–1548. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Chang, D.; Guo, F.; Pan, H.; Yang, Y. Metabolomics approach by 1H NMR spectroscopy of serum reveals progression axes for asymptomatic hyperuricemia and gout. Arthritis Res. Ther. 2018, 20, 111. [Google Scholar] [CrossRef]
- Guma, M.; Dadpey, B.; Coras, R.; Mikuls, T.R.; Hamilton, B.; Quehenberger, O.; Thorisdottir, H.; Bittleman, D.; Lauro, K.; Reilly, S.M.; et al. Xanthine oxidase inhibitor urate-lowering therapy titration to target decreases serum free fatty acids in gout and suppresses lipolysis by adipocytes. Arthritis Res. Ther. 2022, 24, 175. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, L.; Peng, A.; Liu, J.-Y. Metabolic profiling of human plasma reveals the activation of 5-lipoxygenase in the acute attack of gouty arthritis. Rheumatology 2019, 58, 345–351. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Beger, R.D. A Review of Applications of Metabolomics in Cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.M.N.; Lesner, N.P.; Sabatier, M.; Ubellacker, J.M.; Tasdogan, A. Emerging metabolomic tools to study cancer metastasis. Trends Cancer 2022, 8, 988–1001. [Google Scholar] [CrossRef]
- Pallares-Méndez, R.; Aguilar-Salinas, C.A.; Cruz-Bautista, I.; del Bosque-Plata, L. Metabolomics in diabetes, a review. Ann. Med. 2016, 48, 89–102. [Google Scholar] [CrossRef] [PubMed]
- McGranaghan, P.; Kirwan, J.A.; Garcia-Rivera, M.A.; Pieske, B.; Edelmann, F.; Blaschke, F.; Appunni, S.; Saxena, A.; Rubens, M.; Veledar, E.; et al. Lipid Metabolite Biomarkers in Cardiovascular Disease: Discovery and Biomechanism Translation from Human Studies. Metabolites 2021, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- McGranaghan, P.; Saxena, A.; Rubens, M.; Radenkovic, J.; Bach, D.; Schleußner, L.; Pieske, B.; Edelmann, F.; Trippel, T.D. Predictive value of metabolomic biomarkers for cardiovascular disease risk: A systematic review and meta-analysis. Biomarkers 2020, 25, 101–111. [Google Scholar] [CrossRef]
- Ojanen, X.; Cheng, R.; Törmäkangas, T.; Rappaport, N.; Wilmanski, T.; Wu, N.; Fung, E.; Nedelec, R.; Sebert, S.; Vlachopoulos, D.; et al. Towards early risk biomarkers: Serum metabolic signature in childhood predicts cardio-metabolic risk in adulthood. eBioMedicine 2021, 72, 103611. [Google Scholar] [CrossRef]
- Hur, B.; Gupta, V.K.; Huang, H.; Wright, K.A.; Warrington, K.J.; Taneja, V.; Davis, J.M., 3rd; Sung, J. Plasma metabolomic profiling in patients with rheumatoid arthritis identifies biochemical features predictive of quantitative disease activity. Arthritis Res. Ther. 2021, 23, 164. [Google Scholar] [CrossRef]
- Bartikoski, B.J.; De Oliveira, M.S.; Santo, R.C.D.E.; Dos Santos, L.P.; Dos Santos, N.G.; Xavier, R.M. A Review of Metabolomic Profiling in Rheumatoid Arthritis: Bringing New Insights in Disease Pathogenesis, Treatment and Comorbidities. Metabolites 2022, 12, 394. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Z.; Huang, S.; Lon, J.R.; Xie, S. Metabolic Profiling of Serum for Osteoarthritis Biomarkers. Dis. Markers 2022, 2022, 1800812. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Liebal, U.W.; Phan, A.N.T.; Sudhakar, M.; Raman, K.; Blank, L.M. Machine Learning Applications for Mass Spectrometry-Based Metabolomics. Metabolites 2020, 10, 243. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, X.; Deng, X.; Kong, Y.; Wang, W.; Zhou, Y. Machine learning of plasma metabolome identifies biomarker panels for metabolic syndrome: Findings from the China Suboptimal Health Cohort. Cardiovasc. Diabetol. 2022, 21, 288. [Google Scholar] [CrossRef]
- Sperber, H.; Mathieu, J.; Wang, Y.; Ferreccio, A.; Hesson, J.; Xu, Z.; Fischer, K.A.; Devi, A.; Detraux, D.; Gu, H.; et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat. Cell Biol. 2015, 17, 1523–1535. [Google Scholar] [CrossRef]
- Yanes, O.; Clark, J.; Wong, D.M.; Patti, G.J.; Sánchez-Ruiz, A.; Benton, H.P.; Trauger, S.A.; Desponts, C.; Ding, S.; Siuzdak, G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 2010, 6, 411–417. [Google Scholar] [CrossRef]
- Brunet, A.; Rando, T.A. Interaction between epigenetic and metabolism in aging stem cells. Curr. Opin. Cell Biol. 2017, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- D’aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Metabolic–Epigenetic Axis in Pluripotent State Transitions. Epigenomes 2019, 3, 13. [Google Scholar] [CrossRef]
- Voss, K.; Hong, H.S.; Bader, J.E.; Sugiura, A.; Lyssiotis, C.A.; Rathmell, J.C. A guide to interrogating immunometabolism. Nat. Rev. Immunol. 2021, 21, 637–652. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Chavakis, T. Immunometabolism: Where Immunology and Metabolism Meet. J. Innate Immun. 2022, 14, 1–3. [Google Scholar] [CrossRef]
- Petersen, A.-K.; Zeilinger, S.; Kastenmüller, G.; Römisch-Margl, W.; Brugger, M.; Peters, A.; Meisinger, C.; Strauch, K.; Hengstenberg, C.; Pagel, P.; et al. Epigenetics meets metabolomics: An epigenome-wide association study with blood serum metabolic traits. Hum. Mol. Genet. 2014, 23, 534–545. [Google Scholar] [CrossRef]
- Wong, C.C.; Qian, Y.; Yu, J. Interplay between epigenetics and metabolism in oncogenesis: Mechanisms and therapeutic approaches. Oncogene 2017, 36, 3359–3374. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Zoghbi, H.Y.; Beaudet, A.L. Epigenetics and Human Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, a019497. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Spann, N.J.; Heinz, S.; Romanoski, C.E.; Allison, K.A.; Stender, J.D.; Chun, H.B.; Tough, D.F.; Prinjha, R.K.; Benner, C.; et al. Remodeling of the Enhancer Landscape during Macrophage Activation Is Coupled to Enhancer Transcription. Mol. Cell 2013, 51, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.S.; Troutman, T.D.; Sakai, M.; Gola, A.; Spann, N.J.; Bennett, H.; Bruni, C.M.; Ouyang, Z.; Li, R.Z.; Sun, X.; et al. Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity 2020, 52, 1057–1074.e7. [Google Scholar] [CrossRef]
- Da Lin, D.; Xu, W.; Hong, P.; Wu, C.; Zhang, Z.; Zhang, S.; Xing, L.; Yang, B.; Zhou, W.; Xiao, Q.; et al. Decoding the spatial chromatin organization and dynamic epigenetic landscapes of macrophage cells during differentiation and immune activation. Nat. Commun. 2022, 13, 5857. [Google Scholar] [CrossRef]
- Zhang, P.; Amarasinghe, H.E.; Whalley, J.P.; Tay, C.; Fang, H.; Migliorini, G.; Brown, A.C.; Allcock, A.; Scozzafava, G.; Rath, P.; et al. Epigenomic analysis reveals a dynamic and context-specific macrophage enhancer landscape associated with innate immune activation and tolerance. Genome Biol. 2022, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef]
- Qin, D. Next-generation sequencing and its clinical application. Cancer Biol. Med. 2019, 16, 4–10. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.E.; Wilson, R.K.; Mardis, E.R. The Next-Generation Sequencing Revolution and Its Impact on Genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- Heinz, S.; Romanoski, C.E.; Benner, C.; Glass, C.K. The selection and function of cell type-specific enhancers. Nat. Rev. Mol. Cell Biol. 2015, 16, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.D.; Kofman, E.; Glass, C.K. Exploiting dynamic enhancer landscapes to decode macrophage and microglia phenotypes in health and disease. Mol. Cell 2021, 81, 3888–3903. [Google Scholar] [CrossRef]
- Diskin, C.; Ryan, T.A.J.; O’Neill, L.A.J. Modification of Proteins by Metabolites in Immunity. Immunity 2021, 54, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Waste Not, Want Not: Lactate Oxidation Fuels the TCA Cycle. Cell Metab. 2017, 26, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, F.; Afonso, J.; Costa, M.; Granja, S. Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy. Front. Oncol. 2020, 10, 231. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Zhou, X. Lactylation, an emerging hallmark of metabolic reprogramming: Current progress and open challenges. Front. Cell Dev. Biol. 2022, 10, 972020. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Day, E.A.; O’Neill, L.A. Protein targeting by the itaconate family in immunity and inflammation. Biochem. J. 2022, 479, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Peace, C.G.; O’Neill, L.A. The role of itaconate in host defense and inflammation. J. Clin. Investig. 2022, 132, e148548. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, M.A.; Shen, Z.; Holtman, I.R.; Zheng, A.; Spann, N.J.; Cobo, I.; Gymrek, M.; Glass, C.K. Mechanisms underlying divergent responses of genetically distinct macrophages to IL-4. Sci. Adv. 2021, 7, eabf9808. [Google Scholar] [CrossRef]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef]
- Glass, C.K.; Natoli, G. Molecular control of activation and priming in macrophages. Nat. Immunol. 2016, 17, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, G.; Liu, J.; Li, Q.; Wang, Y. Revealing transcription factor and histone modification co-localization and dynamics across cell lines by integrating ChIP-seq and RNA-seq data. BMC Genom. 2018, 19, 914. [Google Scholar] [CrossRef]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Chinenov, Y.; Kerppola, T.K. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 2001, 20, 2438–2452. [Google Scholar] [CrossRef]
- Bakiri, L.; Matsuo, K.; Wisniewska, M.; Wagner, E.F.; Yaniv, M. Promoter Specificity and Biological Activity of Tethered AP-1 Dimers. Mol. Cell. Biol. 2002, 22, 4952–4964. [Google Scholar] [CrossRef]
- Bakiri, L.; Hasenfuss, S.C.; Wagner, E.F. A FATal AP-1 dimer switch in hepatosteatosis. Cell Cycle 2014, 13, 1218–1219. [Google Scholar] [CrossRef]
- Jochum, W.; Passegué, E.; Wagner, E.F. AP-1 in mouse development and tumorigenesis. Oncogene 2001, 20, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Badii, M.; Gaal, O.I.; Cleophas, M.C.; Klück, V.; Davar, R.; Habibi, E.; Keating, S.T.; Novakovic, B.; Helsen, M.M.; Dalbeth, N.; et al. Urate-induced epigenetic modifications in myeloid cells. Arthritis Res. Ther. 2021, 23, 202. [Google Scholar] [CrossRef]
- Cabău, G.; Crișan, T.O.; Klück, V.; Popp, R.A.; Joosten, L.A.B. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.; Schlosser, P.; Matias-Garcia, P.R.; Thio, C.H.L.; Joehanes, R.; Liu, H.; Yu, Z.; Weihs, A.; Hoppmann, A.; Grundner-Culemann, F.; et al. Epigenome-wide association study of serum urate reveals insights into urate co-regulation and the SLC2A9 locus. Nat. Commun. 2021, 12, 7173. [Google Scholar] [CrossRef] [PubMed]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.A.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Sommer, C.; Birklein, F. Resolvins and inflammatory pain. F1000 Med. Rep. 2011, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Julliard, W.A.; Myo, Y.P.A.; Perelas, A.; Jackson, P.D.; Thatcher, T.H.; Sime, P.J. Specialized pro-resolving mediators as modulators of immune responses. Semin. Immunol. 2022, 59, 101605. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef]
- Isopi, E.; Mattoscio, D.; Codagnone, M.; Mari, V.C.; Lamolinara, A.; Patruno, S.; D’aurora, M.; Cianci, E.; Nespoli, A.; Franchi, S.; et al. Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Front. Immunol. 2020, 11, 581. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef]
- Coras, R.; Murillo-Saich, J.D.; Singh, A.G.; Kavanaugh, A.; Guma, M. Lipidomic Profiling in Synovial Tissue. Front. Med. 2022, 9, 857135. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Gemperle, C.; Rimann, N.; Hersberger, M. Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J. Immunol. 2016, 196, 3429–3437. [Google Scholar] [CrossRef]
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72. [Google Scholar] [CrossRef]
- Romano, M.; Recchia, I.; Recchiuti, A. Lipoxin Receptors. Sci. World J. 2007, 7, 1393–1412. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.-H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef]
- Margalit, A.; Duffin, K.L.; Shaffer, A.F.; Gregory, S.A.; Isakson, P.C. Altered arachidonic acid metabolism in urate crystal induced inflammation. Inflammation 1997, 21, 205–222. [Google Scholar] [CrossRef]
- Rae, S.A.; Davidson, E.M.; Smith, M.J. Leukotriene B4, an inflammatory mediator in gout. Lancet 1982, 320, 1122–1124. [Google Scholar] [CrossRef]
- Vasquez, A.M.; Mouchlis, V.D.; Dennis, E.A. Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 2018, 67, 212–218. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kudo, I. Phospholipase A2. J. Biochem. 2002, 131, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef]
- Dabral, D.; van den Bogaart, G. The Roles of Phospholipase A2 in Phagocytes. Front. Cell Dev. Biol. 2021, 9, 673502. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Hsu, Y.-H.; Deems, R.A.; Li, S.; Woods, V.L., Jr.; Dennis, E.A. A Phospholipid Substrate Molecule Residing in the Membrane Surface Mediates Opening of the Lid Region in Group IVA Cytosolic Phospholipase A2. J. Biol. Chem. 2008, 283, 31227–31236. [Google Scholar] [CrossRef]
- Stahelin, R.V.; Subramanian, P.; Vora, M.; Cho, W.; Chalfant, C.E. Ceramide-1-phosphate Binds Group IVA Cytosolic Phospholipase a2 via a Novel Site in the C2 Domain. J. Biol. Chem. 2007, 282, 20467–20474. [Google Scholar] [CrossRef]
- Seilhamer, J.J.; Pruzanski, W.; Vadas, P.; Plant, S.; Miller, J.A.; Kloss, J.; Johnson, L.K. Cloning and Recombinant Expression of Phospholipase A2 Present in Rheumatoid Arthritic Synovial Fluid. J. Biol. Chem. 1989, 264, 5335–5338. [Google Scholar] [CrossRef]
- Kramer, R.M.; Hession, C.; Johansen, B.; Hayes, G.; McGray, P.; Chow, E.P.; Tizard, R.; Pepinsky, R.B. Structure and Properties of a Human Non-pancreatic Phospholipase A2. J. Biol. Chem. 1989, 264, 5768–5775. [Google Scholar] [CrossRef] [PubMed]
- Six, D.A.; Dennis, E.A. Essential Ca2+-independent Role of the Group IVA Cytosolic Phospholipase A2 C2 Domain for Interfacial Activity. J. Biol. Chem. 2003, 278, 23842–23850. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef]
- Jaulmes, A.; Janvier, B.; Andreani, M.; Raymondjean, M. Autocrine and Paracrine Transcriptional Regulation of Type IIA Secretory Phospholipase A2 Gene in Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2005, 25, 1161–1167. [Google Scholar] [CrossRef]
- Antonio, V.; Brouillet, A.; Janvier, B.; Monne, C.; Bereziat, G.; Andreani, M.; Raymondjean, M. Transcriptional regulation of the rat type IIA phospholipase A2 gene by cAMP and interleukin-1β in vascular smooth muscle cells: Interplay of the CCAAT/enhancer binding protein (C/EBP), nuclear factor-κB and Ets transcription factors. Biochem. J. 2002, 368, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, M.; James, M.J.; McColl, S.R.; Naccache, P.H.; Cleland, L.G. Monosodium urate microcrystals induce cyclooxygenase-2 in human monocytes. Blood 1998, 91, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Costa, V.V.; Tavares, L.D.; Sachs, D.; Coelho, F.M.; Fagundes, C.T.; Soriani, F.M.; Silveira, T.N.; Cunha, L.D.; Zamboni, D.S.; et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B4 in a murine model of gout. Arthritis Rheum. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Murakami, Y.; Akahoshi, T.; Hayashi, I.; Endo, H.; Hashimoto, A.; Kono, S.; Kondo, H.; Kawai, S.; Inoue, M.; Kitasato, H. Inhibition of monosodium urate monohydrate crystal-induced acute inflammation by retrovirally transfected prostaglandin D synthase. Arthritis Rheum. 2003, 48, 2931–2941. [Google Scholar] [CrossRef]
- Ruiz-Miyazawa, K.W.; Staurengo-Ferrari, L.; Pinho-Ribeiro, F.A.; Fattori, V.; Zaninelli, T.H.; Badaro-Garcia, S.; Borghi, S.M.; Andrade, K.C.; Clemente-Napimoga, J.T.; Alves-Filho, J.C.; et al. 15d-PGJ2-loaded nanocapsules ameliorate experimental gout arthritis by reducing pain and inflammation in a PPAR-gamma-sensitive manner in mice. Sci. Rep. 2018, 8, 13979. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Andersson, A.M.C.; Semsey, S.; Sneppen, K. Structure and function of negative feedback loops at the interface of genetic and metabolic networks. Nucleic Acids Res. 2006, 34, 2455–2462. [Google Scholar] [CrossRef]
- Chubukov, V.; Zuleta, I.A.; Li, H. Regulatory architecture determines optimal regulation of gene expression in metabolic pathways. Proc. Natl. Acad. Sci. USA 2012, 109, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Häfner, A.-K.; Kahnt, A.S.; Steinhilber, D. Beyond leukotriene formation—The noncanonical functions of 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 2019, 142, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kreiß, M.; Oberlis, J.H.; Seuter, S.; Bischoff-Kont, I.; Sürün, D.; Thomas, D.; Göbel, T.; Schmid, T.; Rådmark, O.; Brandes, R.P.; et al. Human 5-lipoxygenase regulates transcription by association to euchromatin. Biochem. Pharmacol. 2022, 203, 115187. [Google Scholar] [CrossRef]
- Weissmann, G.; Rita, G.A. Molecular Basis of Gouty Inflammation: Interaction of Monosodium Urate Crystals with Lysosomes and Liposomes. Nat. New Biol. 1972, 240, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fenando, A.; Rednam, M.; Widrich, J. Gout. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Hoffstein, S.; Weissmann, G. Mechanisms of lysosomal enzyme release from leukocytes. IV. Interaction of monosodium urate crystals with dogfish and human leukocytes. Arthritis Rheum. 1975, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, T.; Cohen, A.S. Ultrastructural evidence for leakage of lysosomal contents after phagocytosis of monosodium urate crystals. A mechanism of gouty inflammation. Am. J. Pathol. 1974, 76, 501–520. [Google Scholar]
- Rajan, K.T. Lysosomes and Gout. Nature 1966, 210, 959–960. [Google Scholar] [CrossRef]
- Höglinger, D.; Haberkant, P.; Aguilera-Romero, A.; Riezman, H.; Porter, F.D.; Platt, F.M.; Galione, A.; Schultz, C. Intracellular sphingosine releases calcium from lysosomes. eLife 2015, 4, e10616. [Google Scholar] [CrossRef] [PubMed]
- Kiselyov, K.; Yamaguchi, S.; Lyons, C.W.; Muallem, S. Aberrant Ca2+ handling in lysosomal storage disorders. Cell Calcium 2010, 47, 103–111. [Google Scholar] [CrossRef]
- Yoshimura, A.; Suzuki, M.; Sakaguchi, R.; Hanada, T.; Yasukawa, H. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012, 3, 20. [Google Scholar] [CrossRef]
- Sobah, M.L.; Liongue, C.; Ward, A.C. SOCS Proteins in Immunity, Inflammatory Diseases, and Immune-Related Cancer. Front. Med. 2021, 8, 727987. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Hsieh, S.-C.; Chen, W.-Y.; Li, K.-J.; Wu, C.-H.; Wu, P.-C.; Tsai, C.-Y.; Yu, C.-L. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGF 1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann. Rheum. Dis. 2011, 70, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Orji, O.C.; López-Domínguez, M.B.; Sandoval-Plata, G.; Guetta-Baranes, T.; Valdes, A.M.; Doherty, M.; Morgan, K.; Abhishek, A. Upregulated expression of FFAR2 and SOC3 genes is associated with gout. Rheumatology 2023, 62, 977–983. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).