Abstract
Chylothorax is a rare complication occurring after cardio-thoracic surgical procedures. This condition presents challenges for diagnosis and treatment. Operative ductal ligation is the method of choice for relapsing or refractory cases, and it can be performed through the aid of IGC injection for the identification of chylous leakage. Our report presents the use of ICG fluorescence during VATS to successfully identify and treat a left-sided post-surgical chylothorax. The patient underwent a pulmonary wedge resection for a suspect malignant lesion and developed chylous leakage in the early postoperative period. On postoperative day 7, the patient underwent a revision thoracoscopy for hemostasis and thoracic duct ligation. ICG injections were performed through bilateral inguinal lymph nodes and approximately 15 min after we performed the re-thoracoscopy with effective identification and ligation of the chyle leakage. ICG fluorescence-guided VATS is a valuable and effective method for managing postoperative chylothorax, especially for left-sided leaks.
1. Introduction
Chylothorax is a rare but insidious complication that can occur after thoracic surgery procedures with a range of incidence from 0.5% to 2% [1]. This condition is more frequently associated with esophageal surgery or mediastinal lymphadenectomies. Standard treatment includes conservative measures as the first approach, such as somatostatin administration and a lipid-free diet; however, persistent high-output chylothorax often necessitates more invasive approaches. Lymphangiography can be used both as a diagnostic and a therapeutic tool, as it allows identification the leakage site and embolization of the injured lymphatic vessel. Surgical intervention is reserved for refractory or relapsing cases and its aim is to ligate the thoracic duct from the right hemithorax [2]. However, the presence of accessory lymphatic vessels and the impossibility to properly identify the leakage site may result in the failure of the procedure. Our report presents the use of ICG fluorescence during VATS to successfully identify and treat a left-sided chylothorax, emphasizing the efficacy of this technique in targeted, site-specific interventions.
2. Case Presentation and Methods
We present the case of a 54-year-old female with a pulmonary PET-positive lesion in the left upper lobe. The patient underwent triportal thoracoscopic wide wedge resection of the left upper lobe with adequate macroscopic surgical margins and hilar–mediastinal lymphadenectomy. Intraoperative frozen section analysis was negative for malignancy and did not show any atypia. The surgical procedure took off swiftly without any criticalities. On postoperative day 1, the patient developed chylous drainage from the pleural drain, with outputs of up to 1000 mL daily for the next 4 days. A fat-free diet was immediately initiated and chylous output progressively diminished but did not disappear completely. On postoperative day 7, the patient suffered from an episode of tachycardia and hypotension, associated with cold diaphoresis. We noticed active bleeding from the drainage with a mean of 100 mL/h of hematic output and we decided to take the patient back to the operating room for evacuation of hemothorax and hemostasis. Concurrently, we decided to address the persistent left-sided chylous effusion.
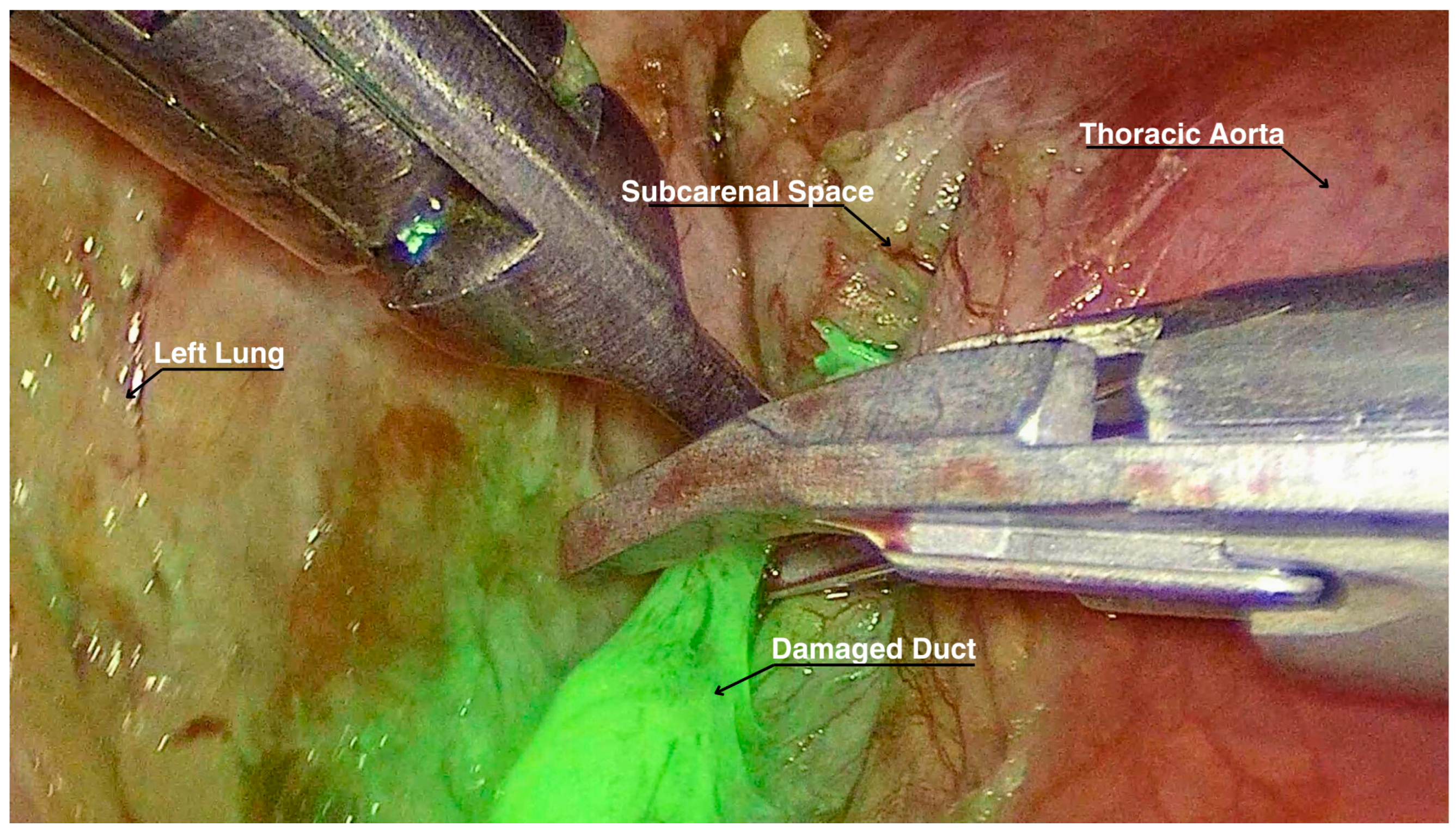
A total of 3 mg of ICG diluted in 5 mL of saline solution was injected bilaterally into the inguinal lymph nodes by our interventional radiologist while the patient was supine and intubated. Approximately 15 min elapsed from the ICG injection to the reopening of the previous surgical incisions. At the exploration in the pleural cavity, we individuated two blood clots adherent to the parenchymal suture, but no active bleeding sources. Eventually, with the dedicated ICG filter in the thoracoscopic optics camera, lymphatic vessels on the left side were clearly visualized, and the leakage point was precisely identified at the level of the previous carinal lymphadenectomy. The leak was successfully ligated with metal clips (Figure 1) and with application of nebulized cyanoacrylate for further tissue sealing. Supplementary Material: The video of the procedure can be found at https://zenodo.org/records/13786288 (accessed on 28 October 2024).
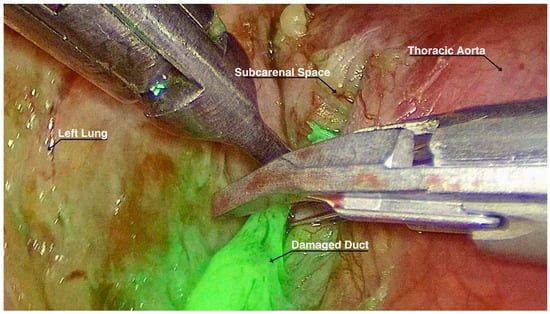
Figure 1.
Lymphatic duct highlighted by ICG while being ligated.
The patient had no further chylous drainage and resumed oral lipid-free intake on postoperative day 1 following the second surgery. She needed oral and intravenous electrolyte supply because of hyponatremia, hypokalemia, hypocalcemia, hypophosphatemia and hypomagnesemia. Thoracic drains were removed on postoperative day 5 due to the absence of air or chylous leakage and the patient was discharged on postoperative day 6 in good overall clinical condition.
3. Discussion
3.1. ICG Fluorescence Use in Surgery
ICG fluorescence is widely used in various surgical fields for the intraoperative localization of lesions and real-time visualization of segmental anatomy, or for tumor and sentinel node identification [3]. Its use can range from general surgery (for instance in the identification of tumors in the liver, pancreas and adrenal glands) to lymphatic mapping in malignant tumors (stomach, breast, colon, rectum, esophagus and skin cancer). Another important utilization is in reconstructive surgery, where ICG fluorescence is used for the evaluation of viable graft implants, mostly in robotic-guided reconstructions [4]. In thoracic surgery, in particular, ICG fluorescence is used for the individuation of the anatomical intersegmental planes for sublobar resections in order to allow the most precise division of the pulmonary parenchyma in complex segmentectomies [5].
3.2. Case Discussion
This case report highlights the effectiveness of using ICG fluorescence-guided VATS lymphatic duct ligation to manage left-sided chylothorax. The technique allowed us the precise identification and ligation of the leaking lymphatic vessels on the left side, which is crucial in preventing complications associated with prolonged chylous drainage. This case deviates from the standard of right-sided thoracic duct ligations, as is mentioned in the next paragraph, showcasing the advantages of targeted intervention when precise localization is feasible.
3.3. Literature Discussion
The use of ICG fluorescence has already been described in the literature, but with different treatment modalities than our case and in a non-standardized fashion. In fact, both Yang et al. [6] and Tzu-Wei Kuo et al. [7] described fluorescence imaging with indocyanine green VATS for chylothorax, but all patients were treated from the right side. Leakage points were actually detected at the 4R lymph node dissection station or at the point of prophylactic ligation of the thoracic duct behind the azygos vein. Another described application for this contrast medium is after esophageal cancer resections [8]. As already mentioned, esophageal surgery is frequently associated with chylothorax occurrence, and the use of ICG fluorescence to identify and ligate the thoracic duct may be of great help for these delicate patients’ management.
4. Conclusions
ICG fluorescence-guided VATS is a valuable and effective method for managing postoperative chylothorax, especially for left-sided leaks. This case report highlights its use as a reliable technique for visualizing and addressing lymphatic leaks during thoracic surgery.
Supplementary Materials
The following supporting information can be downloaded: Video S1: https://zenodo.org/records/13786288. There are no copyright issues for this video.
Author Contributions
Conceptualization, L.L., M.B. and A.B. (Alberto Busetto); methodology, A.B. (Alessandro Bonis); software, V.V. and L.L.; validation, G.C. (Giovanni Comacchio), E.F. and S.N.; formal analysis, G.C. (Giovanni Comacchio); investigation, A.B. (Alberto Busetto); resources, L.L. and A.B. (Alberto Busetto); data curation, A.R.; writing—original draft preparation, L.L and A.B. (Alberto Busetto); writing—review and editing, S.N., A.R. and G.C. (Giorgio Cannone); visualization, E.F.; supervision, F.R.; project administration, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The ethical committee at our center does not require approval for case reports. Written informed consent was obtained from the patient for enrollment in this study.
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martucci, N.; Tracey, M.; Rocco, G. Postoperative Chylothorax. Thorac. Surg. Clin. 2015, 25, 523–528. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.E.; Blades, Z.; Anderson, P.B. Chylothorax: Aetiology, diagnosis and therapeutic options. Respir. Med. 2010, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morales-Conde, S.; Licardie, E.; Alarcón, I.; Balla, A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: Recommendations based on the descriptive review of the literature and the analysis of experience. Cir. Esp. (Engl. Ed.). 2022, 100, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Cadillo-Chávez, R. Utilidad de indocianina verde en cirugía reconstructiva robótica [Usefulness of indocyanine green (ICG) in robotic reconstructive surgery. Arch. Esp. Urol. 2019, 72, 759–764. (In Spanish) [Google Scholar] [PubMed]
- Ng, C.S.; Ong, B.H.; Chao, Y.K.; Wright, G.M.; Sekine, Y.; Wong, I.; Hao, Z.; Zhang, G.; Chaturvedi, H.; Thammineedi, S.R.; et al. Use of Indocyanine Green Fluorescence Imaging in Thoracic and Esophageal Surgery. Ann. Thorac. Surg. 2023, 115, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, J.; Li, H.; Yang, F.; Xiao, R.; Chi, C.; Tian, J.; Wang, J. Near-infrared fluorescence-guided thoracoscopic surgical intervention for postoperative chylothorax. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.W.; Tseng, S.K.; Chou, P.L.; Cheng, C.; Chu, S.Y.; Chao, Y.K. Fluorescence-guided thoracoscopic surgery for postoperative chylothorax: A technical note with video vignette. Asian J. Surg. 2024, 47, 2623–2624. [Google Scholar] [CrossRef] [PubMed]
- Ran, A.; Ma, L.; He, D.; Yibulayin, W.; Abulaiti, A.; Wu, Z.; Xu, K.; Yibulayin, X.; Alimu, P.; Sun, X. Indocyanine green fluorescence imaging technology for the treatment of chylothorax after oesophageal cancer: A case report. Photodiagn. Photodyn. Ther. 2024, 48, 104244. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).