Fungal Exposure and Shelter Assessment in Syrian Refugee Settlements in Lebanon
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Data
2.2. Mold Air Sampling and Enumeration
2.3. Identification of Mold/Fungi
2.4. Moisture Content
2.5. Data Analysis
- Concentration of different mold types
- I/O ratio
- Total indoor count
- Occupancy
3. Results and Discussion
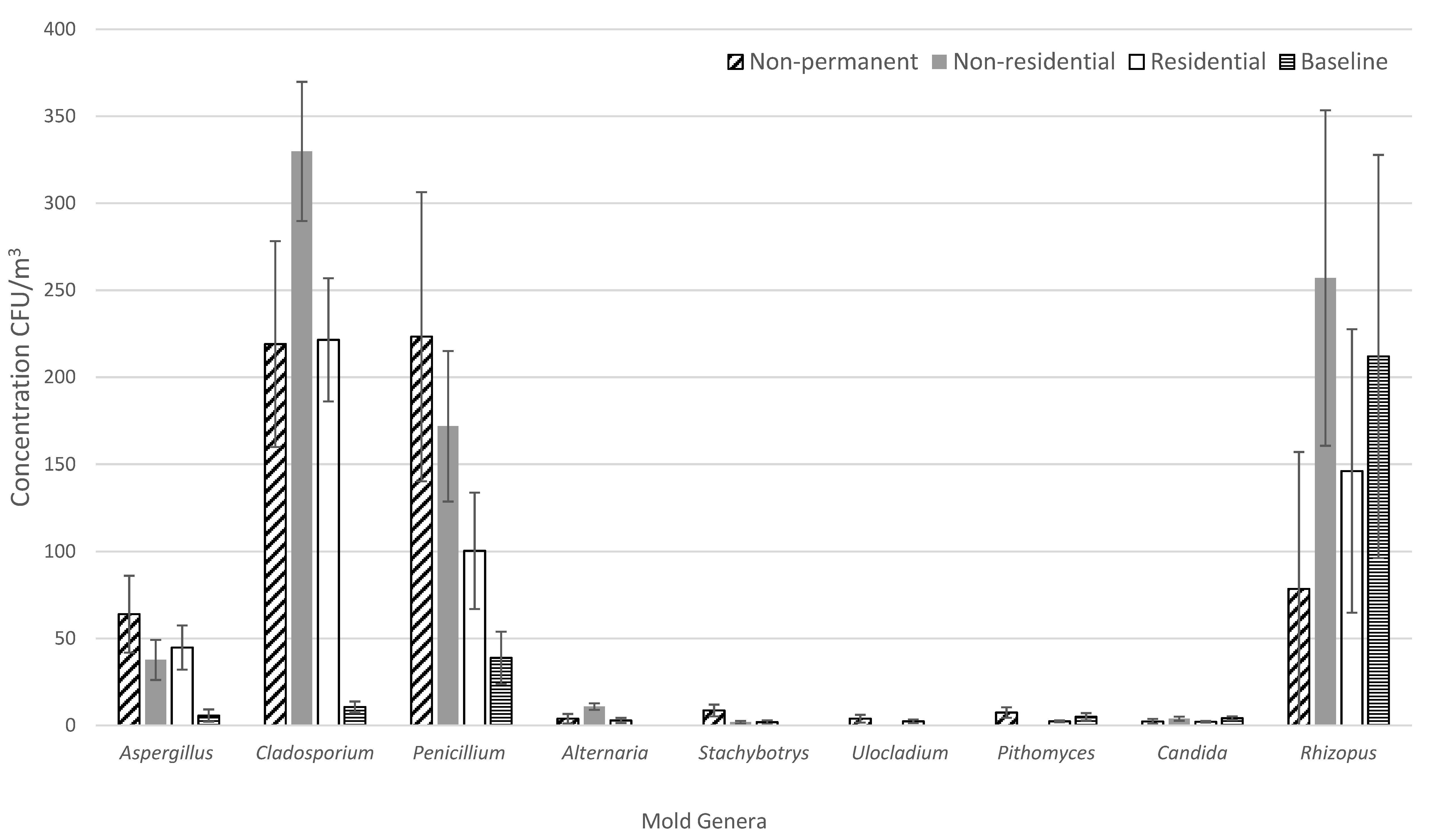
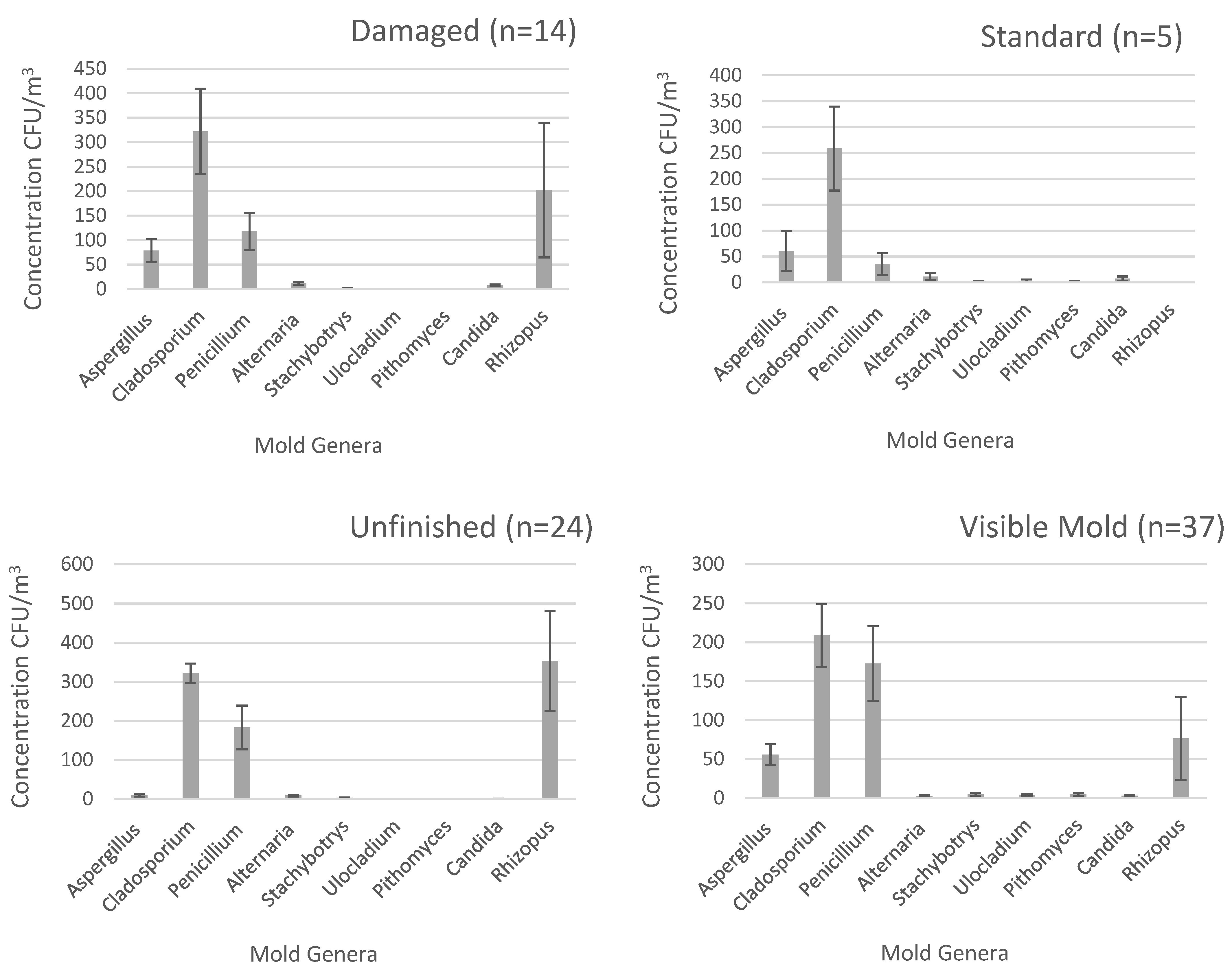
3.1. Mold Concentration
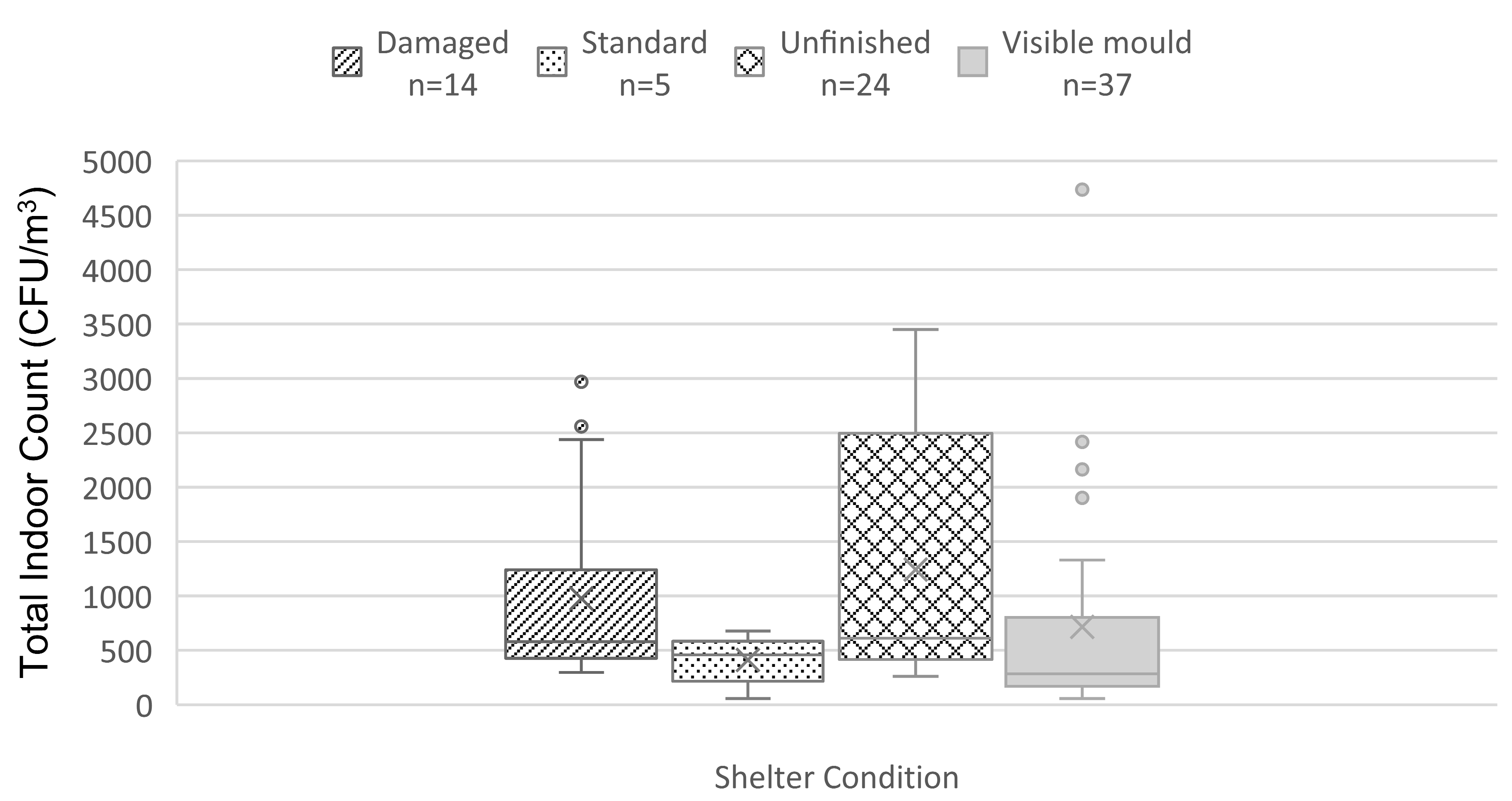
3.2. Total Mold Indoor Count
3.3. I/O Ratio
4. Conclusions
5. Limitations and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNHCR—Refugee Statistics. Available online: https://www.unhcr.org/refugee-statistics/download/?url=2bxU2f (accessed on 17 December 2022).
- Lambert, H. Temporary refuge from war: Customary international law and the Syrian conflict. Int. Comp. Law Q. 2017, 66, 723. [Google Scholar] [CrossRef]
- Regional Refugees and Resilience Plan Regional Strategic Overview. Al-Ahram Weekly. 6 April 2017. Available online: https://www.proquest.com/newspapers/refugee-resilience-plan/docview/1884990887/se-2 (accessed on 10 March 2018).
- Syrian Regional Refugee Response. Available online: https://data2.unhcr.org/en/situations/syria/location/71 (accessed on 10 June 2018).
- John Hopkins Bloomberg School of Public Health; Mèdecins Du Monde; International Medical Corps; American University of Beirut; UNHCR. Syrian Refugee and Affected Host Population Health Access Survey in Lebanon; UNHCR: Beirut, Lebanon, 2015. [Google Scholar]
- El-Khatib, Z.; Scales, D.; Vearey, J.; Forsberg, B.C. Syrian refugees, between rocky crisis in Syria and hard inaccessibility to healthcare services in Lebanon and Jordan. Confl. Health 2013, 7, 6–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanyal, R. A no-camp policy: Interrogating informal settlements in Lebanon. Geoforum 2017, 84, 117–125. [Google Scholar] [CrossRef]
- Choices: Syrian Refugees in Need of Health Care in Lebanon; Amnesty International: London, UK, 2014. Available online: https://www.amnesty.org/en/documents/mde18/001/2014/en/ (accessed on 12 May 2017).
- Allen, E.; Iano, J. Fundamentals of Building Construction: Materials and Methods; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Govender, T.; Barnes, J.M.; Pieper, C.H. Housing conditions, sanitation status and associated health risks in selected subsidized low-cost housing settlements in Cape Town, South Africa. Habitat. Int. 2011, 35, 335–342. [Google Scholar] [CrossRef]
- Bonner, P.C.; Schmidt, W.; Belmain, S.R.; Oshin, B.; Baglole, D.; Borchert, M. Poor Housing Quality Increases Risk of Rodent Infestation and Lassa Fever in Refugee Camps of Sierra Leone. Am. J. Trop. Med. Hyg. 2007, 77, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kysia, R.F. Shelter. In Handbook of Bioterrorism and Disaster Medicine; Antosia, R.E., Cahill, J.D., Eds.; Springer: Boston, MA, USA, 2006; pp. 393–396. [Google Scholar]
- Uhde, E.; Salthammer, T. Impact of reaction products from building materials and furnishings on indoor air quality—A review of recent advances in indoor chemistry. Atmos. Environ. 2007, 41, 3111–3128. [Google Scholar] [CrossRef]
- Krieger, J.; Higgins, D.L. Housing and health: Time again for public health action. Am. J. Public Health 2002, 92, 758–768. [Google Scholar] [CrossRef] [PubMed]
- El-Sharif, N.; Abdeen, Z.; Qasrawi, R.; Moens, G.; Nemery, B. Asthma prevalence in children living in villages, cities and refugee camps in Palestine. Eur. Respir. J. 2002, 19, 1026–1034. [Google Scholar] [CrossRef]
- Abu Mourad, T.A. Palestinian refugee conditions associated with intestinal parasites and diarrhoea: Nuseirat refugee camp as a case study. Public Health 2004, 118, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, I.A.; Arafat, R.N.; Musmar, M. Housing environment and women’s health in a Palestinian refugee camp. Int. J. Environ. Health Res. 2005, 15, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Earl, C.S.; An, S.; Ryan, R.P. The changing face of asthma and its relation with microbes. Trends Microbiol. 2015, 23, 408–418. [Google Scholar] [CrossRef]
- von Mutius, E. The microbial environment and its influence on asthma prevention in early life. J. Allergy Clin. Immunol. 2016, 137, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [PubMed]
- World Health Organization. WHO Guidelines for Indoor Air Quality: Dampness and Mold; World Health Organization: Copenhagen, Denmark, 2009; Volume 33. [Google Scholar]
- Rios, J.L.d.M.; Boechat, J.L.; Gioda, A.; Santos, C.Y.d.; Aquino Neto, F.R.d.; Lapa e Silva, J.R. Symptoms prevalence among office workers of a sealed versus a non-sealed building: Associations to indoor air quality. Environ. Int. 2009, 35, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Chen, Y.; Malkawi, A.; Adamkiewicz, G.; Spengler, J.D. Quantifying the impact of traffic-related air pollution on the indoor air quality of a naturally ventilated building. Environ. Int. 2016, 89–90, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.D.; Chen, Q. Indoor air quality factors in designing a healthy building. Annu. Rev. Energy Environ. 2000, 25, 567–600. [Google Scholar] [CrossRef]
- US Environmental Protection Agency Indoor Air Quality Tools for Schools: Reference Guide; United States Environmental Protection Agency: Washington, DC, USA, 2009. Available online: https://www.epa.gov/iaq-schools (accessed on 8 April 2018).
- Spaul, W.A. Building-related factors to consider in indoor air quality evaluations. J. Allergy Clin. Immunol. 1994, 94, 385–389. [Google Scholar] [CrossRef]
- Nen, O.A.S.; Fisk, W.J.; Mendell, M.J. Association of Ventilation Rates and CO2 Concentrations with Health and Other Responses in Commercial and Institutional Buildings. Indoor Air 1999, 1, 226–252. [Google Scholar]
- Meklin, T.; Hyvärinen, A.; Toivola, M.; Reponen, T.; Koponen, V.; Husman, T.; Taskinen, T.; Korppi, M.; Nevalainen, A. Effect of Building Frame and Moisture Damage on Microbiological Indoor Air Quality in School Buildings. AIHA J. 2003, 64, 108–116. [Google Scholar] [CrossRef]
- Moore, D.; Robson, G.D.; Trinci, A.P.J. 21st Century Guidebook to Fungi with CD; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Burge, H.A. The fungi: How they grow and their effects on human health. Heat. Pip. Air Cond. 1997, 69. Available online: https://www.osti.gov/biblio/538129 (accessed on 8 April 2018).
- Kazemian, N.; Pakpour, S.; Milani, A.S.; Klironomos, J. Environmental factors influencing fungal growth on gypsum boards and their structural biodeterioration: A university campus case study. PLoS ONE 2019, 14, e0220556. [Google Scholar] [CrossRef] [PubMed]
- Storey, E.; Dangman, K.H.; Schenck, P.; Debernardo, R.L.; Chin, Y.S.; Bracker, A.; Hodgson, M.J. Guidance for Clinicians on the Recognition and Management of Health Effects Related to Mold Exposure and Moisture Indoors; University of Connecticut Health Center, Division of Occupational and Environmental Medicine, Center for Indoor Environments and Health: Farmington, CT, USA, 2004. [Google Scholar]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Rea, W.J.; Didriksen, N.; Simon, T.R.; Pan, Y.; Fenyves, E.J.; Griffiths, B. Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch. Environ. Health 2003, 58, 399–405. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15143852 (accessed on 4 April 2018).
- ASHRAE Standard 62.1-2016; Ventilation for Acceptable Indoor Air Quality. American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2016; p. 56.
- Burge, H.A. Allergens and other air pollutants. Monaldi Arch. Chest Dis. = Arch. Monaldi Per Le Mal. Del Torace 1994, 49, 373–374. [Google Scholar]
- Grant, C.; Hunter, C.A.; Flannigan, B.; Bravery, A.F. The Moisture Requirements of Molds Isolated from Domestic Dwellings. Int. Biodeterior. 1989, 25, 259–284. [Google Scholar] [CrossRef]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R.; Roussel, S.; Ashan-Leygonie, C.; Bex, V.; Bretagne, S.; Caillaud, D.; Colleville, A.C.; et al. Indoor mold exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef] [PubMed]
- Quansah, R.; Jaakkola, M.S.; Hugg, T.T.; Heikkinen, S.A.M.; Jaakkola, J.J.K. Residential Dampness and Molds and the Risk of Developing Asthma: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e47526. [Google Scholar] [CrossRef] [PubMed]
- Shultz, A.; Omollo, J.O.; Burke, H.; Qassim, M.; Ochieng, J.B.; Weinberg, M.; Feikin, D.R.; Breiman, R.F. Cholera Outbreak in Kenyan Refugee Camp: Risk Factors for Illness and Importance of Sanitation. Am. J. Trop. Med. Hyg. 2009, 80, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Biran, A.; Schmidt, W.; Zeleke, L.; Emukule, H.; Khay, H.; Parker, J.; Peprah, D. Hygiene and sanitation practices amongst residents of three long-term refugee camps in Thailand, Ethiopia and Kenya. Trop. Med. Int. Health 2012, 17, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.R.; Basma, S.H.; Yeretzian, J.S. Harboring illnesses: On the association between disease and living conditions in a Palestinian refugee camp in Lebanon. Int. J. Environ. Health Res. 2006, 16, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.R.; Mahfoud, Z.; Fawaz, M.; Basma, S.H.; Yeretzian, J.S. Housing quality and ill health in a disadvantaged urban community. Public. Health 2009, 123, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.A.; Shrestha, D.; Spiegel, P.; Gore, F.; Hering, H. Quantifying the burden of disease associated with inadequate provision of water and sanitation in selected sub-Saharan refugee camps. J. Water Health 2009, 7, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.A.; Shrestha, D.; Cornier, N.; Abdalla, F.; Ezard, N.; Aramburu, C. A review of water and sanitation provision in refugee camps in association with selected health and nutrition indicators—The need for integrated service provision. J. Water Health 2008, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Benka-Coker, M.L.; Tadele, W.; Milano, A.; Getaneh, D.; Stokes, H. A case study of the ethanol CleanCook stove intervention and potential scale-up in Ethiopia. Energy Sustain. Dev. J. Int. Energy Initiat. 2018, 46, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Albadra, D.; Vellei, M.; Coley, D.; Hart, J. Thermal comfort in desert refugee camps: An interdisciplinary approach. Build. Environ. 2017, 124, 460–477. [Google Scholar] [CrossRef]
- Everaerts, S.; Lagrou, K.; Vermeersch, K.; Dupont, L.J.; Vanaudenaerde, B.M. Wim Janssens Aspergillus fumigatus Detection and Risk Factors in Patients with COPD-Bronchiectasis Overlap. Int. J. Mol. Sci. 2018, 19, 523. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.H. Fungal Infections in HIV Patients. Ann. N. Y. Acad. Sci. 2018, 616, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Durden, F.M.; Elewski, B. Fungal infections in HIV-infected patients. Semin. Cutan. Med. Surg. 1997, 16, 200–212. [Google Scholar] [CrossRef]
- Downs, S.; Mitakakis, T.; Marks, G.; Car, N.; Belousova, E.; Leüppi, J.; Xuan, W.E.I.; Downie, S.; Tobias, A.; Peat, J. Clinical Importance of Alternaria Exposure in Children. Am. J. Respir. Crit. Care Med. 2001, 164, 455–459. [Google Scholar] [CrossRef] [PubMed]
- BS EN ISO 16000-19; Indoor Air Part 19: Sampling Strategy for Molds. British Standards Institution: London, UK, 2014.
- BS ISO 16000-18; Indoor Air Part 18: Detection and Enumeration of Molds-Sampling by Impaction. British Standards Institution: London, UK, 2011; 29p.
- Thermo Fisher Scientific. Series 10-Instruction Manual; Single Stage Viable Sampler; Thermo Fisher Scientific: Waltham, MA, USA, 2009. [Google Scholar]
- BS ISO 16000-17; Indoor Air Part 17: Detection and Enumeration of Molds—Culture-Based Method. British Standards Institution: London, UK, 2009.
- Yamamoto, N.; Hospodsky, D.; Dannemiller, K.C.; Nazaroff, W.W.; Peccia, J. Indoor Emissions as a Primary Source of Airborne Allergenic Fungal Particles in Classrooms. Environ. Sci. Technol. 1900, 49, 5098–5106. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 3rd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sciortino, C.V. Atlas of Clinically Important Fungi; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2017. [Google Scholar]
- ASTM F2659-10; Standard Guide for Preliminary Evaluation of Comparative Moisture Condition of Concrete, Gypsum Cement and Other Floor Slabs and Screeds Using a Non-Destructive Electronic Moisture Meter. ASTM International: West Conshohocken, PA, USA, 2016; Volume 10, pp. 1–6.
- Lennartsson, P.R.; Taherzadeh, M.J.; Edebo, L. Rhizopus. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 284–290. [Google Scholar]
- Sircar, G.; Bhattacharya, S.G. 221 Allergenic Significance of Airborne Rhizopus Stolonifer (ehrenb.) Vuill, a Common Bread Mold. World Allergy Organ. J. 2012, 5, S73–S90. [Google Scholar] [CrossRef]
- Hurraß, J.; Heinzow, B.; Aurbach, U.; Bergmann, K.; Bufe, A.; Buzina, W.; Cornely, O.A.; Engelhart, S.; Fischer, G.; Gabrio, T.; et al. Medical diagnostics for indoor mold exposure. Int. J. Hyg. Environ. Health 2017, 220, 305–328. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Ghannoum, M.A. Indoor Mold, Toxigenic Fungi, and Stachybotrys chartarum: Infectious Disease Perspective. Clin. Microbiol. Rev. 2003, 16, 144–172. [Google Scholar] [CrossRef]
- Jones, R.; Recer, G.M.; Hwang, S.A.; Lin, S. Association between indoor mold and asthma among children in Buffalo, New York. Indoor Air 2011, 21, 156–164. [Google Scholar] [CrossRef]
- Rea, W.J.; Didriksen, N.; Simon, T.R.; Pan, Y.; Fenyves, E.J.; Griffiths, B. Effects of Toxic Exposure to Molds and Mycotoxins in Building-Related Illnesses. Arch. Environ. Health 2003, 58, 399–405. [Google Scholar] [CrossRef]
- Escriva, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef]
- Bloom, E.; Bal, K.; Nyman, E.; Must, A.; Larsson, L. Mass spectrometry-based strategy for direct detection and quantification of some mycotoxins produced by Stachybotrys and Aspergillus spp. in indoor environments. Appl. Environ. Microbiol. 2007, 73, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Lombardi, E.; Berti, G.; Rusconi, F.; La Grutta, S.; Piffer, S.; Petronio, M.G.; Galassi, C.; Forastiere, F.; Viegi, G. Mold/dampness exposure at home is associated with respiratory disorders in Italian children and adolescents: The SIDRIA-2 Study. Occup. Environ. Med. 2005, 62, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Lehmann, I.; Seiffart, A.; Diez, U.; Wetzig, H.; Borte, M.; Herbarth, O. Increased incidence of allergic sensitisation and respiratory diseases due to mold exposure: Results of the Leipzig Allergy Risk children Study (LARS). Int. J. Hyg. Environ. Health 2002, 204, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, A.; Reboux, G.; Roussel, S.; Grenouillet, F.; Didier-Scherer, E.; Dalphin, J.; Millon, L. Indoor fungal contamination of moisture-damaged and allergic patient housing analysed using real-time PCR. Lett. Appl. Microbiol. 2009, 49, 260–266. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between Fungal Species and Water-Damaged Building Materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, P.V.; Correa, M.V. Analysis of biodeterioration wood estate: Use different techniques to obtain images. Matéria 2018, 23. [Google Scholar] [CrossRef]
- Hyvarinen, A.; Meklin, T.; Vepsalainen, A.; Nevalainen, A. Fungi and actinobacteria in moisture-damaged building materials—Concentrations and diversity. Int. Biodeterior. Biodegrad. 2002, 49, 27–37. [Google Scholar] [CrossRef]
- U.S. Department of Housing and Urban Development Assessing Housing Durability: A Pilot Study. 2001. Available online: https://www.huduser.gov/publications/pdf/housing_durability_0602.pdf (accessed on 10 December 2018).
- Daquisto, D.; Crandell, J.; Lyons, J. Building Moisture and Durability Past, Present and Future Work; Prepared by Newport Partners for Office of Policy Development and Research, Department of Housing and Urban Development; Newport Partners: Irvine, CA, USA, 2004. [Google Scholar]
- Albadra, D.; Kuchai, N.; Acevedo-De-los-Ríos, A.; Rondinel-Oviedo, D.; Coley, D.; da Silva, C.F.; Rana, C.; Mower, K.; Dengel, A.; Maskell, D.; et al. Measurement and analysis of air quality in temporary shelters on three continents. Build. Environ. 2020, 185, 107259. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists. Bioaerosols: Assessment and Control; ACGIH: Washington, DC, USA, 1999. [Google Scholar]
- Jaakkola, M.S.; Quansah, R.; Hugg, T.T.; Heikkinen, S.A.; Jaakkola, J.J. Association of indoor dampness and molds with rhinitis risk: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013, 132, 1099–1110.e18. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (U.S.) Committee on Damp Indoor Spaces and Health. Damp Indoor Spaces and Health; The National Academy Press: Washington, DC, USA, 2004. [Google Scholar]







| Governorate | Area | No. Households | Residential | Non-Residential | Non-Permanent |
|---|---|---|---|---|---|
| Beirut | Bourj Hammoud | 20 | 20 | ||
| Bekaa | Bar Elias | 20 | 2 | 18 | |
| South | Abra | 20 | 7 | 13 | |
| North | Biret Akkar | 20 | 20 |
| Shelter Category | Standard | Damaged | Unfinished | Visible Mold |
|---|---|---|---|---|
| Residential n = 29 | 5 | 1 | 4 | 19 |
| Non-residential n = 33 | 13 | 20 | ||
| Non-permanent n = 18 | 18 |
| Health Effects | Illness/Symptoms |
|---|---|
| Fungal Infections | Flu-like syndrome, interstitial or cavitary pneumonia, meningoencephalitis, tinea cruris, corporis, and pedis. |
| Allergic Rhinitis and Asthma | Upper airway: clear rhinorrhea, nasal congestion, sneezing, post-nasal drip with sore throat, coughing, and hoarseness. Lower airway: bronchospasm, chest tightness, and shortness of breath. |
| Hypersensitivity Pneumonitis and Interstitial Lung Disease | Extrinsic allergic alveolitis, farmer’s lung, Japanese summer-house, cryptogenic fibrosing alveolitis, idiopathic pulmonary fibrosis. |
| Bronchopulmonary Aspergillosis | Eosinophilic pneumonia, mucous plugs, or asthma exacerbations. |
| Allergic Fungal Sinusitis | Polyposis |
| Allergic Dermatitis | Dryness, pruritus, and skin rashes. |
| Irritation | Cough, skin irritation, and burning or itching of the eyes and nose. |
| Organic Dust Toxic Syndrome | Flu-like syndrome with prominent respiratory symptoms and fever. |
| Genus | Occupancy | p-Value | R2 |
|---|---|---|---|
| Aspergillus | 0.075 | 0.461 | 0.6% |
| Cladosporium | 0.215 | 0.032 * | 4.6% |
| Penicillium | −0.015 | 0.879 | 0.02% |
| Alternaria | 0.077 | 0.448 | 0.6% |
| Stachybotrys | 0.337 | <0.001 * | 11.3% |
| Ulocladium | 0.216 | 0.031 * | 4.7% |
| Pithomyces | −0.033 | 0.745 | 0.1% |
| Candida | −0.033 | 0.743 | 0.1% |
| Rhizopus | 0.014 | 0.893 | 0.02% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaouie, M.; Troisi, G.M.; Saliba, N.; Shaib, H.; Hajj, R.; El Hajj, R.; Malak, S.; Jakarian, C.; Jaafar, W. Fungal Exposure and Shelter Assessment in Syrian Refugee Settlements in Lebanon. Aerobiology 2023, 1, 19-36. https://doi.org/10.3390/aerobiology1010003
Alaouie M, Troisi GM, Saliba N, Shaib H, Hajj R, El Hajj R, Malak S, Jakarian C, Jaafar W. Fungal Exposure and Shelter Assessment in Syrian Refugee Settlements in Lebanon. Aerobiology. 2023; 1(1):19-36. https://doi.org/10.3390/aerobiology1010003
Chicago/Turabian StyleAlaouie, Malek, Gera M. Troisi, Najat Saliba, Houssam Shaib, Rayan Hajj, Rawan El Hajj, Sandy Malak, Carla Jakarian, and Wiaam Jaafar. 2023. "Fungal Exposure and Shelter Assessment in Syrian Refugee Settlements in Lebanon" Aerobiology 1, no. 1: 19-36. https://doi.org/10.3390/aerobiology1010003
APA StyleAlaouie, M., Troisi, G. M., Saliba, N., Shaib, H., Hajj, R., El Hajj, R., Malak, S., Jakarian, C., & Jaafar, W. (2023). Fungal Exposure and Shelter Assessment in Syrian Refugee Settlements in Lebanon. Aerobiology, 1(1), 19-36. https://doi.org/10.3390/aerobiology1010003







