Abstract
Our study aimed to quantify the pollen trapped by the seeds and relate it with the airborne pollen concentrations. Individual Populus spp. tuft-like seeds were sampled while suspended twice a day in 2017 and 2018 during the seed dispersal season. The seeds were submitted to laboratory treatment for pollen extraction, which was quantified using an optical microscope. Airborne pollen was monitored using a seven-day Hirst-type volumetric spore sampler. A correlation analysis was performed between the airborne pollen, the pollen on the seeds, and the meteorological parameters. A total of 26 pollen grains/mg was counted in the airborne tuft-like seeds, with 26 different taxa being identified, compared with the 18 pollen taxa identified in the airborne samples. Quercus, Poaceae, Urticaceae, Pinus, and Platanus were the most frequent pollen found on the seeds, while in the atmosphere, pollen from Urticaceae, Quercus, and Cupressaceae were the most representative. A tendency of higher pollen concentrations found in the afternoon samples, both airborne and on the seeds, was observed. Correlations between the meteorological parameters and pollen concentration found airborne and in the seeds were overall not significant. Thus, airborne poplar tuft-like seeds can trap and transport pollen, most of which has been recognized to induce respiratory allergies.
1. Introduction
In most cities, trees occupy a large proportion of the urban greenery [1,2,3] and play a crucial role in the quality of life of urban communities (human and non-human) [4]. According to the World Health Organization, urban residents should be able to access public green spaces of at least 0.5–1 hectare within 300 m of their homes’ linear distance (around 5 min walk) [5]. The urban green structures comprise urban woodlands, large and small urban parks, green fields/gardens surrounding public/historical buildings, street trees, private green spaces, and railway and road corridors [6].
One aspect that should be taken into consideration in urban ecosystems is the careful selection of appropriate tree species, not only those meeting parameters such as a dense crown, easy maintenance, or adaptability to local climate stress [7] but also the allergenicity level of the pollen produced [8,9], once pollen-related respiratory allergies have been increasing in number and severity in the last decades, particularly in urbanized areas [10]. In fact, during the pollination season of anemophilous trees, a large quantity of pollen is released into the atmosphere, being among the ecosystem disservices with the greatest impact [8]. So, in urban ecosystems, interdisciplinary involvement in the implementation of the different planning and maintenance stages of tree populations is important to maintain the natural quality and sustainability of the city environment [6,11].
Trees of the genus Populus L., popularly known as poplars, are common trees present in urban green spaces, particularly the species P. alba L., P. nigra L., P. tremula L., P. × canadensis Moench., and P. × canescens (Aiton) Sm. [12]. Some of these species are typical of riparian formations associated with watercourses that run through many urban environments [13]. These are deciduous species, dioicous, present in temperate regions in the Northern Hemisphere with fast growth and easy adaption to adverse terrain [14]. They are considered to have high net CO2 sequestration, moderate-to-high drought tolerance, erosion control, and air pollution mitigation [12]. Also, the Populus commercial value has been increasing in recent years due to the growing demands for industrial purposes, such as biomass and biodiesel production, particularly in Europe, North America, and Asia [15].
The poplar male trees flower in early spring [16], being entirely wind-pollinated and producing a high amount of pollen, scoring maximum points for pollen emission [8]. The female trees produce fruits that are capsules grouped in catkins that, when mature, around two months after pollination, open and release the seeds [14]. These seeds have specialized trichomes on their outside, allowing them to float in the air and facilitating their transport by wind. These trichomes are similar to cotton fibers and are composed of pure cellulose [17]. The tuft-like seeds are released daily for about 2 to 12 weeks during the seed dispersion season and can be windblown miles from their origin [18,19]. This characteristic is viewed as a disservice and results in a dislike from the inhabitants, who associate it with health problems [20].
The population perceives that the Populus seeds can cause respiratory allergies; however, it has been argued that the tuft from the seeds is allergen-free, and its allergenicity is null [21]. Nevertheless, it was reported by Hu et al. [19] that the tuft-like seeds collected from the ground contain pollen. However, it is not possible to ascertain from this study if the pollen present in the seeds is collected during the seed dispersion, while airborne, when the seeds contact the ground, or both.
Since the dispersion of the cotton seeds coincides with the pollination period of many plants, the seeds, which can be innocuous in terms of their allergenicity, might become responsible for allergy symptoms or play a role in the scavenging of airborne allergenic pollen, contributing to their removal from the atmosphere. Therefore, the aim of our study was to identify and quantify the pollen trapped by the poplar tuft-like seeds while airborne and compare it with the amount and species-variations of airborne pollen concentrations in the days of seed dispersal.
2. Materials and Methods
2.1. Study Area
The present study was carried out in 2017 and 2018 in the second largest Portuguese city, Porto (41°8′58 N and 8°36′39 W), an urbanized area located in northwest Portugal with 238,000 inhabitants and a population density of 5736 inhabitants/km2. It is delimited by the Atlantic Ocean at the west and the Douro River at the south and has a Mediterranean climate with Atlantic influence, presenting warm winters and mild summers. The annual average, maximum, and minimum temperatures are 15.2 °C, 19.6 °C, and 10.7 °C, respectively; the annual average air humidity is 80% and the total annual precipitation ranges from 690 mm and 1780 mm, mainly concentrated during the winter and early spring months. Prevailing winds are from W and NW in the summer and E and SE in the winter [22].
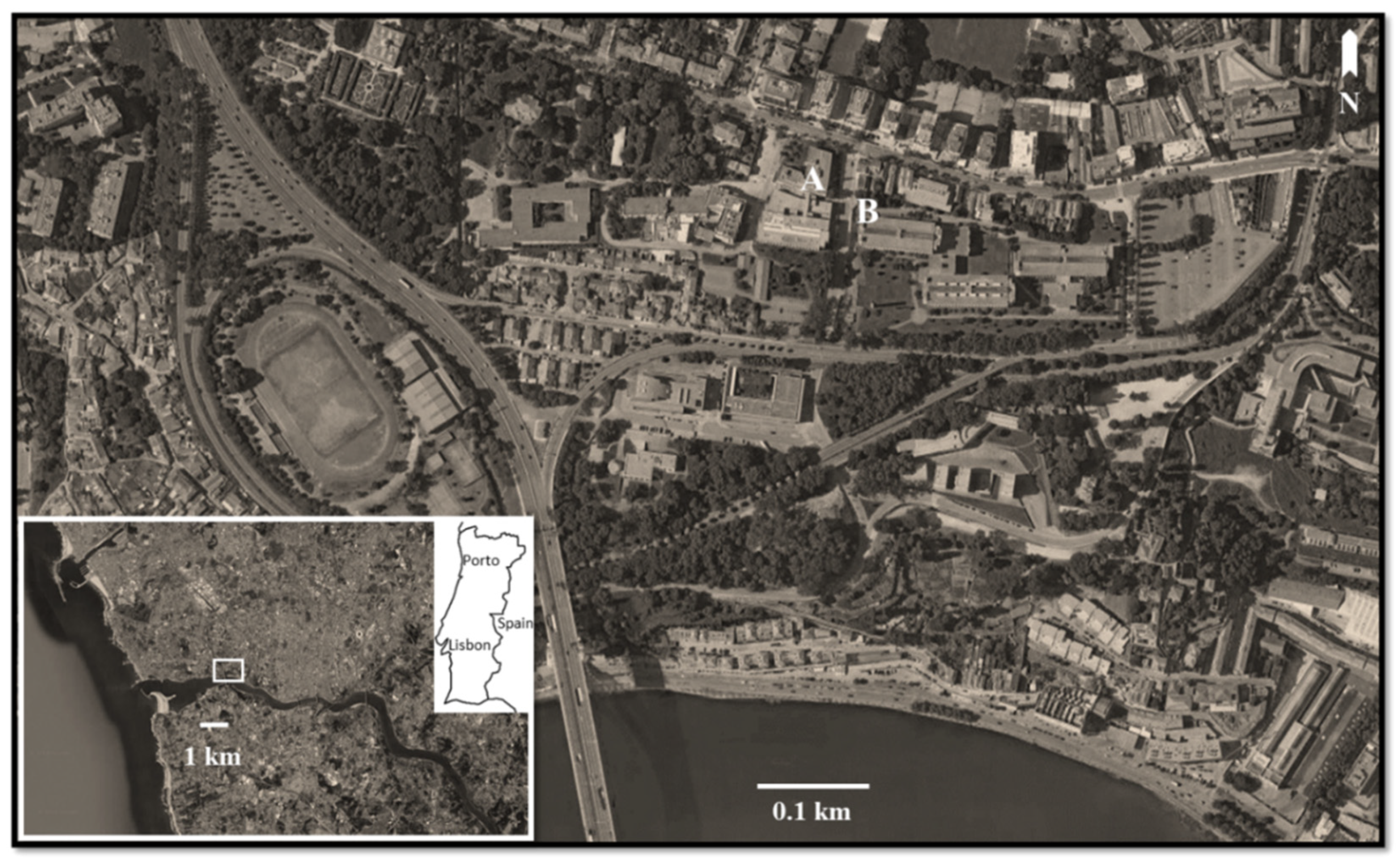
The sampling site was the surroundings of the Faculty of Sciences of the University of Porto, located within a residential zone, near the ocean and the Douro River and close to a highway road that is one of the main entrances of the city, with frequent traffic congestion and jams during the rush hours (Figure 1).
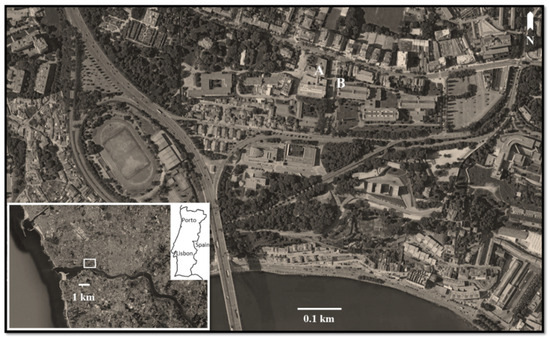
Figure 1.
Sampling area of Populus spp. tuft-like seeds. Location of the spore sampler (A) and meteorological station (B) (Google, Digital Globe images).
The landscape around the study area is characterized by public parks, small gardens around the faculty campus buildings, the Botanical Garden, and several avenues and street walks ornamented with different trees. In all these spaces, Populus trees are represented primarily by the species Populus alba and Populus nigra, but Populus × canadensis, Populus deltoides W. Bartram ex Marshall, and Populus nigra var. italica Münchh. can also be found in lower proportions.
2.2. Airborne Sampling
Sampling was conducted on the 3 and 4 of May in 2017 and in 2018 during four consecutive days from 15 to 18 May, which coincided with the main period of Populus seed dispersal and absence of rainfall.
2.2.1. Populus spp. Seed Sampling
Individual Populus spp. tuft-like seeds were hand-picked from the air while in suspension in a sterile glass bottle using disposable sterile gloves. The sampling was made twice daily (at 11 a.m. and 4 p.m.) for 10 min. The seeds collected in each sampling period were stored in separate glass containers, sealed, identified, and returned to the laboratory to be submitted to laboratorial treatment for pollen extraction.
For each sample, a sub-sample fraction of 30 mg of seeds was used and submitted to several physical-chemical treatments to concentrate the pollinic content. The treatment methodology includes adding sulfuric acid to destroy the tuft, filtration through a disposable nylon mesh, and acetolysis to enable the destruction of the pollen cytoplasmatic content for better identification [23]. The residue obtained was diluted and stored in phenolated glycerin. For pollen identification and quantification, slides were mounted and observed using an optical microscope (Leica DM/LS) at a magnification of 400× along latitudinal sections, allowing for the scanning of all the slide area. Pollen grains were classified by morphological characteristics using our palynology laboratory slide reference collection and by comparison with a published atlas [24,25,26]. Pollen concentration was expressed into pollen/mg of sample.
Control samples corresponding to closed seed capsules were collected directly from the tree (Figure 2A). In the laboratory, under a controlled and pollen-free environment, the capsules were naturally opened (Figure 2B), and the tuft-like seeds released were collected and submitted to the same laboratorial treatment for pollen extraction and quantification as the airborne sampled seeds.

Figure 2.
Closed seed capsules collected from a Populus spp. tree branch (A) and naturally opened seed capsules in the laboratory under a controlled environment (B).
2.2.2. Pollen Sampling
Airborne pollen was continuously monitored using a seven-day Hirst-type volumetric spore sampler (Lanzoni VPPS-2000, Bologna, Italy) placed on the roof of the Faculty of Sciences of the University of Porto, approximately 18 m above the ground level (Figure 1).
This sampler is calibrated to sample air at a rate of 10 L min−1, which is accelerated by passing through a narrow intake orifice (2 × 14 mm) and impacts onto a clock-driven drum covered with a Melinex tape, where airborne pollen is retained. The drum rotates once every seven days, and after exposure, the tape is cut into seven 48 mm daily segments and mounted on microscopic slides with a mounting medium. The identification and quantification of the airborne pollen concentrations were determined using an optical microscope at a magnification of 400× along four complete lengthwise traverses, divided into 24 latitudinal sections of 2 mm intervals (each representing one h), which allows for the quantification of the hourly variation in airborne pollen concentration [27]. Pollen concentration was expressed as the number of pollen grains per cubic meter of air recorded during the morning (7 a.m. to 11 a.m.) and afternoon (11 a.m. to 4 p.m.) hours.
2.3. Data Analysis
The normality of the data was assessed by the Shapiro–Wilk test. A correlation analysis was performed using the Pearson’s correlation coefficient (significance level of 99%, 95%, and 90%) between (i) airborne pollen and pollen trapped in the Populus spp. airborne tuft-like seeds and (ii) the mean meteorological parameters (temperature, relative humidity, and wind velocity) and the pollen concentration (airborne and present in the seeds). The meteorological data were obtained from a meteorological station located at the Faculty of Sciences of the University of Porto, 7 m apart from the pollen sampler at approximately the same height, being managed by the Palynology laboratory.
These analyses were performed using the IBM SPSS Statistics 24 software.
3. Results
In our study, it was observed that all samples of airborne Populus spp. tuft-like seeds, collected twice a day each day, presented pollen content. A total of 26 different pollen taxa were identified on the tuft-like seeds, while only 18 were observed on the airborne pollen samples, but apart from Juglans pollen type, all the others were concordant with the ones observed on the tuft-like seed samples (Table 1). No pollen was observed in the closed seed capsules naturally opened in the laboratory under a controlled environment (control samples).

Table 1.
Pollen taxa identified on the airborne Populus spp. tuft-like seed samples and on the atmosphere during the 2017 and 2018 Populus spp. seed dispersal season. Bold names are pollen recognized to induce allergic respiratory symptoms [28].
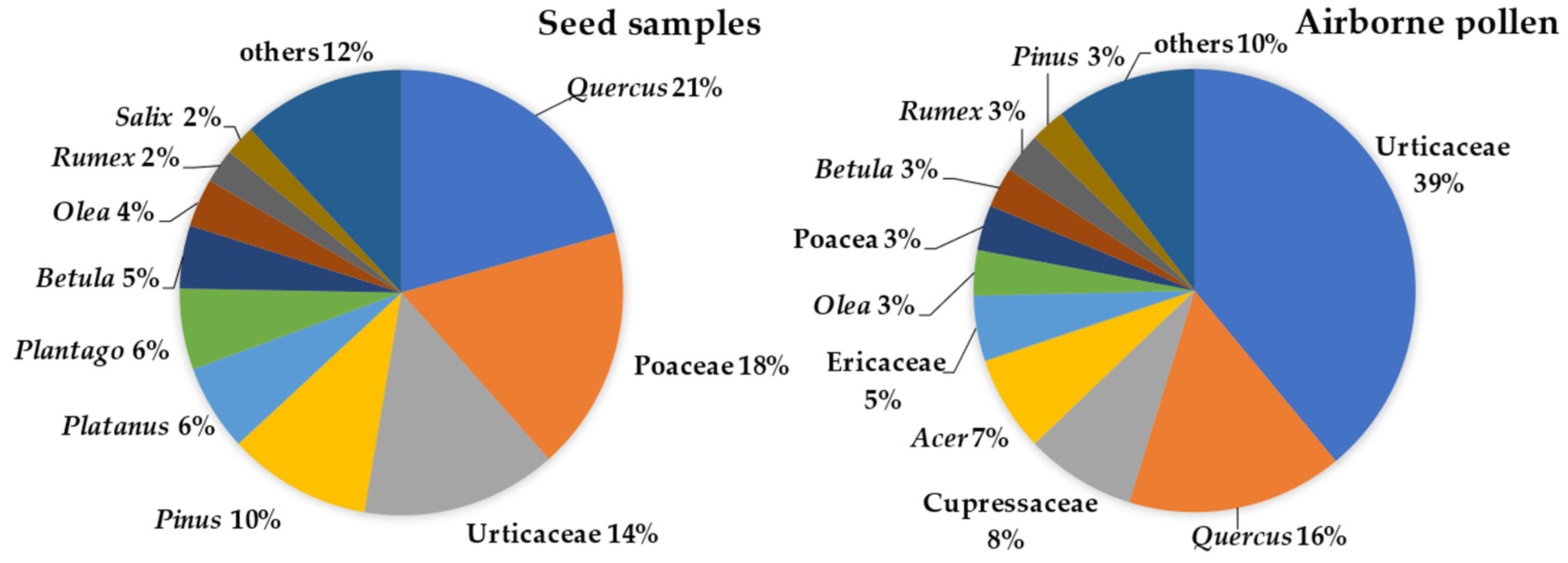
The most frequent pollen taxa found on the airborne tuft-like seeds were Quercus, Poaceae, Urticaceae (mostly Parietaria), Pinus, Platanus, and Plantago, accounting for 75% of the total pollen concentration. In the atmosphere, pollen from Urticaceae, Quercus and Cupressaceae, and Acer and Ericaceae provided 75% of the total pollen concentration, with the other frequently observed taxa representing less than 5%, namely, Olea, Poaceae, Rumex, and Betula (Figure 3). We also observed, with a representation lower than 2%, the presence on the tuft-like seed sample of untypical airborne pollen in Porto such as Cyperaceae and Liliaceae.
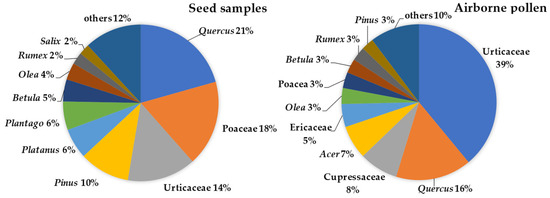
Figure 3.
Most representative pollen taxa, as a percentage (more than 2%), identified on the airborne Populus spp. tuft-like seed samples and in the atmosphere during the 2017 and 2018 Populus spp. seed dispersal season.
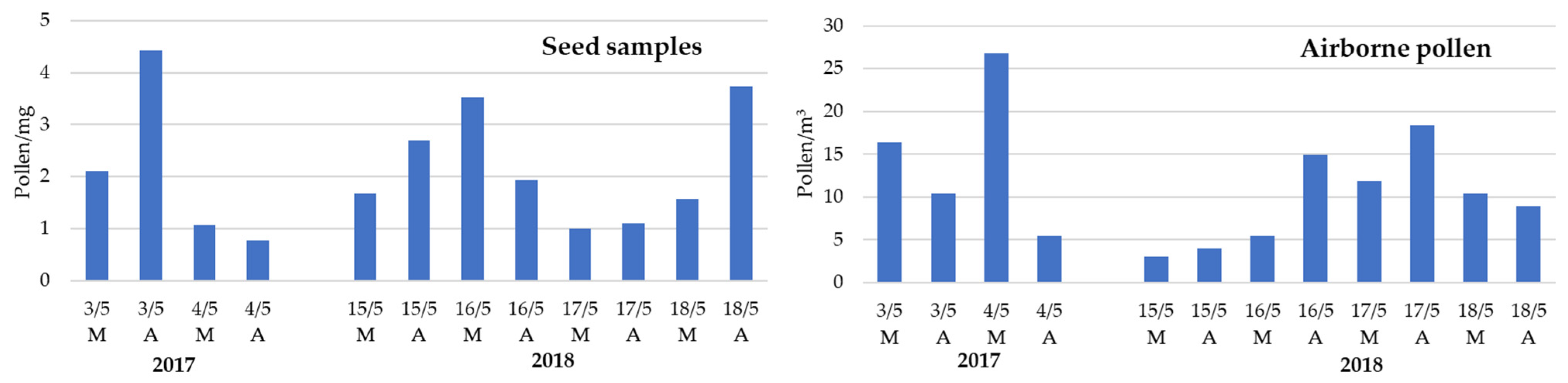
Daily and inter-daily total pollen concentration variations were registered in the atmosphere and on Populus spp. tuft-like seed samples (Figure 4). Airborne pollen concentration was at its minimum on 15 May 2018 and at its maximum on 4 May 2017. Curiously, these days coincided, respectively, with one of the highest and lowest pollen concentrations found on the tuft-like seed, with an observed negative linear correlation between the daily total airborne pollen and the pollen on the seed samples, although this was not statistically significant (Table 2). Also, a tendency towards more days with higher pollen concentration in the afternoon compared with the morning one was observed on airborne and tuft-like seeds.
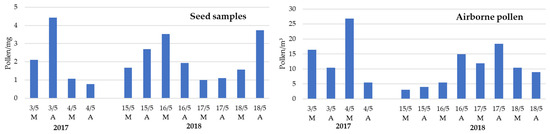
Figure 4.
Diurnal variation of total pollen identified on the airborne Populus spp. tuft-like seed samples and on the atmosphere during the 2017 and 2018 Populus spp. seed dispersal season. Airborne pollen is expressed as the sum of pollen grains per cubic meter of air recorded during the morning (M: 7 a.m. to 11 a.m.) and afternoon (A: 11 a.m. to 4 p.m.) hours.

Table 2.
Pearson’s correlation coefficients (significance level of 95% and 90%) calculated between airborne pollen and pollen identified in the Populus spp. tuft-like seed samples, and between the mean meteorological parameters (temperature, relative humidity, and wind velocity) and the pollen concentration (airborne and present in the seeds) during the 2017 and 2018 Populus spp. seed dispersal season. The dashed values presented correspond to significant correlation coefficients.
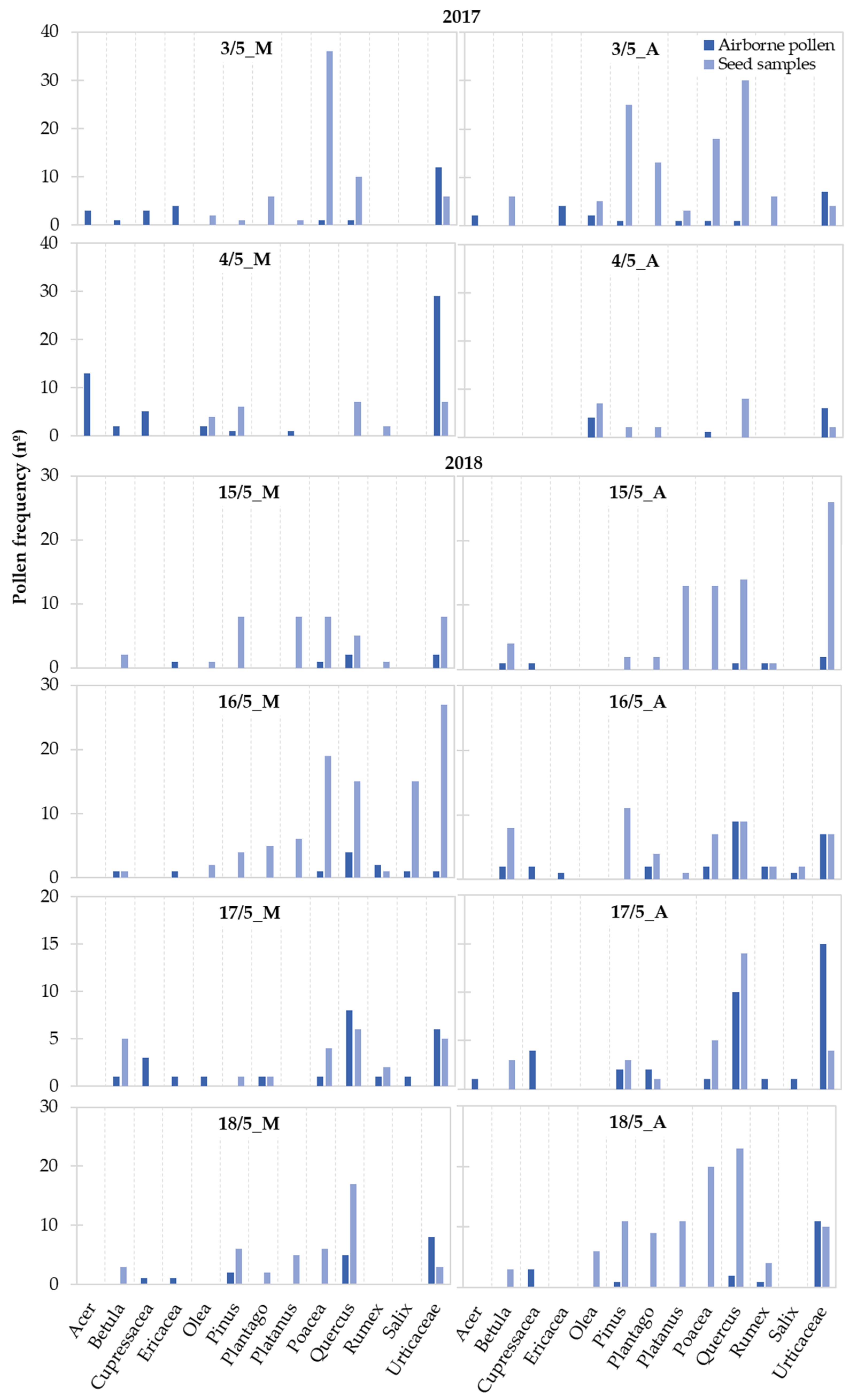
Considering the taxa with a representation higher than 2%, we observed variations in terms of the pollen frequency on airborne and seed samples between the morning and the afternoon hours and along the sampling period (Figure 5). Only the pollen of Urticaceae was continuously present in the atmosphere and on the seed samples during the study period. In terms of the seed samples, pollen from Pinus and Quercus were also always present, and Poaceae pollen was only absent on 4 May 2017. Overall, for each taxon, a greater number of mornings and/or afternoons were observed where no pollen was present in the atmosphere compared with the seed samples.
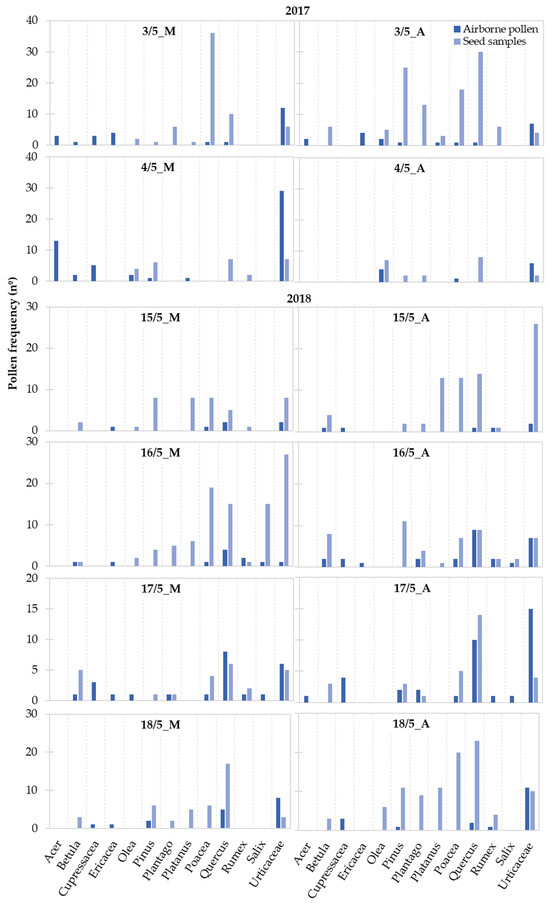
Figure 5.
Frequency variation along the study period of the pollen taxa presenting a total representation higher than 2% identified on the airborne Populus spp. tuft-like seed samples and on the atmosphere during the 2017 and 2018 Populus spp. seed dispersal season. Airborne pollen is expressed in the number of pollen grains recorded during the morning (M: 7 a.m. to 11 a.m.) and afternoon (A: 11 a.m. to 4 p.m.) hours.
Correlation analysis showed that a statistically significant influence was only observed between wind speed and airborne pollen concentration (Table 2). However, it was possible to notice a positive relationship between temperature parameters and pollen concentration in the air (particularly maximum values) and on the seeds (considerable for mean temperature).
The influence of relative humidity and wind speed showed a distinct pattern. Although not significant, correlation coefficients were positive in the case of airborne pollen and negative with pollen in the seed samples. On the contrary, a negative significant correlation was observed between wind speed and airborne pollen, while for the seeds, it was positive.
4. Discussion
Poplars can contribute to clean air by discharging oxygen, storing carbon, and capturing atmospheric contaminants [29,30], helping in water and soil conservation, having the role of balancing the city’s natural urban environment (temperature, noise, biodiversity) [12], and having great commercial value in the wood and biomass industries [15]. Aside from these Populus traits, the female trees also release wind-dispersed seeds that the population views as litter and, therefore, as a disservice from these trees [20,31,32]. Moreover, as demonstrated in our study, these tuft-like seeds transport pollen trapped during airborne seed dispersion since no pollen content was observed in the closed seed capsules directly collected from the tree.
In our study, of the 26 different pollen taxa identified on the seed samples, 14 are recognized to induce allergic respiratory symptoms, 8 of them from trees and the remaining from herbaceous vegetation, particularly Poaceae and Urticaceae that are of the most concern [28]. This points to a possible allergenic impact of the poplar seeds, not because of the seed itself but due to its ability to trap, transport, and accumulate pollen in the tufts, which can be in contact with atopic individuals when the seeds enter in people’s houses, offices, and public spaces.
On the other hand, a curious negative linear relationship (although not statistically significant) between the daily total airborne pollen and the pollen on the seed samples was observed. This makes us question if the seeds could have a role in the natural pollen cleaning out of the atmosphere since thousands to millions of seeds may be released by a single mature female Poplar tree [31,32]. However, this hypothesis should be further investigated, inclusive in in vitro simulations.
Indeed, in our study site, the maximum dispersion period of the Poplar seeds was only four days. This can be due to the reduced number of female trees found within the FCUP campus, which were phenologically synchronized, mainly Populus alba and Populus nigra that have been reported to have shorter seed dispersal seasons of 2–3-weeks [18,31] and also to the occurrence of rainfall in other days that did not allow the proper sampling. However, in areas with more trees, the presence of airborne seeds is higher and prolonged, and therefore there is a longer contact time for airborne pollen to be trapped by the seeds and transported. In the case of tree pollen, the pollination season is described as short, characterized by a rapid release of a great quantity of pollen at the beginning of the main pollen season, with the maximum airborne concentration achieved a few days later [8,16,33]. This characteristic diminishes the interaction period between pollen and airborne seeds. However, Poaceae and Urticaceae pollen can be airborne for many weeks, including months, and therefore it can be trapped and accumulated by the tuft-like seeds.
Our study showed higher pollen taxa diversity in the seeds compared to airborne samples. Also, a greater number of days were observed where pollen from a particular taxon was present on the seed samples but absent from the atmosphere. This may be related to the seed residence time in the atmosphere. The seed’s large tuft-like plumage induces a decrease in its terminal velocity and therefore promotes its buoying behavior [34], remaining in the air for a longer time. A percentage of the seeds sampled bi-daily may have been released on the previous day(s), being airborne for longer periods, with particular emphasis in the afternoon sampling, where high total pollen concentrations were observed. Also, the presence of pollen on the seeds not common in the atmosphere of Porto [16], such as Liliaceae pollen with big size and less aerodynamic, can point out the existence of the resuspension phenomenon of the seeds that can also catch pollen from the ground or very close to it.
The dominance of Urticaceae, Quercus, and Poaceae pollen was observed on both the airborne tuft-like seeds and on airborne samples. The presence of pollen from Betula, Cupressaceae, Pinus, Plantago, Rumex, and Salix was coincident in the seeds and airborne samples, pointing to a local origin of the pollen found on the seed samples. In fact, looking at the long-term airborne pollen calendar for Porto City, during May, high concentrations of these taxa are traditionally observed in the atmosphere [16].
Furthermore, more than 50% of the pollen taxa counted belonged to trees. Porto airborne pollen spectrum is much dominated by an arboreal contribution (total annual around 48%) [16], being the reflection of its urbanization level with parks, city avenues, and streets-walks mainly ornamented with trees [20].
Only statistically significant correlations were found between the airborne pollen data and wind speed. This negative relationship can indicate that the airborne pollen sampled can originate mostly from the vicinity area [35]. However, a positive relationship between air temperature and airborne pollen was found. This is widely described in the literature, with increasing temperatures facilitating the opening of flowers and synchronization of anthers’ dehiscent [36,37].
On the other hand, the positive association between air temperature, wind speed, and the amount of pollen collected in the seeds can be associated with higher temperatures facilitating the suspension of the seeds that, together with wind, allow them to be transported to longer distances. The negative relationship with relative humidity can be related to the cotton fibers of the seed, which in higher humidity conditions could absorb water and become heavier, facilitating sedimentation. Nonetheless, the absence of a significant correlation could indicate that the pollen trapped could be more determined by the atmospheric content of the different pollen types and their amount.
5. Conclusions
We observed pollen on the poplar tuft-like seeds that were captured while airborne. Higher concentrations were found in the afternoon compared to the morning. Twenty-six different pollen taxa were identified on the seeds, and 54% of them were recognized to induce allergic respiratory symptoms. A higher pollen taxa diversity was observed in the seed samples compared to airborne.
This study can contribute knowledge to the decision-making process of urban green space maintenance and management systems, since some measures can be taken such as the selection of poplar species with low seed dispersal season, implementing a frequent street cleaning routine during these periods, and increasing the awareness of the population suffering from pollen-related allergies for the need of a regular cleaning of indoor work and house spaces.
Author Contributions
Conceptualization, H.R. and I.A.; methodology, P.C.; formal analysis, P.C.; data curation, P.C. and H.R.; writing—H.R.; writing—review and editing, P.C. and I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Institute of Earth Sciences under the projects UIDB/04683/2020 and UIDP/04683/2020 funded by the FCT—Foundation for Science and Technology, I.P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are shown in the article.
Acknowledgments
We would like to thank J. Pereira and R. Lamas for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feng, Y.; Tan, P.Y. Imperatives for Greening Cities: A Historical Perspective. In Greening Cities: Forms and Functions; Tan, P.Y., Jim, C.Y., Eds.; Springer: Singapore, 2017; pp. 41–70. [Google Scholar]
- Leitão, I.A.; Ferreira, C.S.S.; Ferreira, A.J.D. Assessing long-term changes in potential ecosystem services of a peri-urbanizing Mediterranean catchment. Sci. Total Environ. 2019, 660, 993–1003. [Google Scholar] [CrossRef]
- Song, X.P.; Tan, P.Y.; Edwards, P.; Richards, D. The economic benefits and costs of trees in urban forest stewardship: A systematic review. Urban For. Urban Green. 2018, 29, 162–170. [Google Scholar] [CrossRef]
- Mullaney, J.; Lucke, T.; Trueman, S.J. A review of benefits and challenges in growing street trees in paved urban environments. Landsc. Urban Plan. 2015, 134, 157–166. [Google Scholar] [CrossRef]
- WHO. Urban Green Spaces: A Brief for Action; WHO/Europe: Copenhagen, Denmark, 2017.
- Anguluri, R.; Narayanan, P. Role of green space in urban planning: Outlook towards smart cities. Urban For. Urban Green. 2017, 25, 58–65. [Google Scholar] [CrossRef]
- Gerstenberg, T.; Hofmann, M. Perception and preference of trees: A psychological contribution to tree species selection in urban areas. Urban For. Urban Green. 2016, 15, 103–111. [Google Scholar] [CrossRef]
- Cariñanos, P.; Casares-Porcel, M.; Quesada-Rubio, J.-M. Estimating the allergenic potential of urban green spaces: A case-study in Granada, Spain. Landsc. Urban Plan. 2014, 123, 134–144. [Google Scholar] [CrossRef]
- Charalampopoulos, A.; Lazarina, M.; Tsiripidis, I.; Vokou, D. Quantifying the relationship between airborne pollen and vegetation in the urban environment. Aerobiologia 2018, 34, 285–300. [Google Scholar] [CrossRef]
- Bunne, J.; Moberg, H.; Hedman, L.; Andersson, M.; Bjerg, A.; Lundbäck, B.; Rönmark, E. Increase in Allergic Sensitization in Schoolchildren: Two Cohorts Compared 10 Years Apart. J. Allergy Clin. Immunol. Pract. 2017, 5, 457–463.e1. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Hochuli, D.F. Defining greenspace: Multiple uses across multiple disciplines. Landsc. Urban Plan. 2017, 158, 25–38. [Google Scholar] [CrossRef]
- Samson, R.; Ningal, T.F.; Tiwary, A.; Grote, R.; Fares, S.; Saaroni, H.; Hiemstra, J.A.; Zhiyanski, M.; Vilhar, U.; Cariñanos, P.; et al. Species-Specific Information for Enhancing Ecosystem Services. In The Urban Forest: Cultivating Green Infrastructure for People and the Environment; Pearlmutter, D., Calfapietra, C., Samson, R., O’Brien, L., Krajter, S., Sanesi, G., Alonso del Amo, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 111–144. [Google Scholar]
- Przepióra, F.; Ciach, M. Tree microhabitats in natural temperate riparian forests: An ultra-rich biological complex in a globally vanishing habitat. Sci. Total Environ. 2022, 803, 149881. [Google Scholar] [CrossRef]
- Rae, A.M.; Street, N.R.; Rodriguez-Acosta, M. Populus trees. In Forest Trees; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–28. [Google Scholar]
- Gordon, J.C. Poplars: Trees of the people, trees of the future. For. Chron. 2001, 77, 217–219. [Google Scholar] [CrossRef]
- Ribeiro, H.; Abreu, I. A 10-year survey of allergenic airborne pollen in the city of Porto (Portugal). Aerobiologia 2014, 30, 333–344. [Google Scholar] [CrossRef]
- Ye, M.; Chen, Z.; Su, X.; Ji, L.; Wang, J.; Liao, W.; Ma, H.; An, X. Study of seed hair growth in Populus tomentosa, an important character of female floral bud development. BMC Genom. 2014, 15, 475. [Google Scholar] [CrossRef]
- Guilloy-Froget, H.; Muller, E.; Barsoum, N.; Hughes, F.M.R. Dispersal, germination, and survival of Populus nigra L. (Salicaceae) in changing hydrologic conditions. Wetlands 2002, 22, 478–488. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Ferguson, D.K.; Bera, S.; Li, C.-S. Seed hairs of poplar trees as natural airborne pollen trap for allergenic pollen grains. Grana 2008, 47, 241–245. [Google Scholar] [CrossRef]
- Fernandes, C.O.; Martinho da Silva, I.; Patoilo Teixeira, C.; Costa, L. Between tree lovers and tree haters. Drivers of public perception regarding street trees and its implications on the urban green infrastructure planning. Urban For. Urban Green. 2019, 37, 97–108. [Google Scholar] [CrossRef]
- Kadocsa, E.; Bittera, I.; Juhász, M. Aeropollinologic and allergologic studies for the clarification of “poplar tree hay fever”. Orvosi Hetil. 1993, 134, 2081–2083. [Google Scholar]
- Miranda, P.; Coelho, F.E.S.; Tomé, A.R.; Valente, M.A.; Carvalho, A.; Pires, C.; Pires, H.O.; Pires, V.C.; Ramalho, C. 20th Century Portuguese Climate and Climate Scenarios. In Climate Change in Portugal. Scenarios, Impacts and Adaptation Measures—SIAM; Santos, F.D., Forbes, K., Moita, R., Eds.; Executive Summary and Conclusions; Gradiva: Lisbon, Portugal, 2001; pp. 23–84. [Google Scholar]
- Cour, P. Nouvelles technique de détection des flux et des retombées polliniques: Étude de la sedimentation des pollens et des spores à la surface du sol. Pollen Spores 1974, 16, 103–141. [Google Scholar]
- Reille, M. Pollen et Spores d’Europe et d’Afrique du Nord; Laboratoire de Botanique Historique et Palynologie: Marseille, France, 1992. [Google Scholar]
- Reille, M. Pollen et Spores d’Europe et d’Afrique du Nord—Supplément 2; Laboratoire de Botanique Historique et Palynologie: Marseille, France, 1995. [Google Scholar]
- Reille, M. Pollen et Spores d’Europe et d’Afrique du Nord—Supplément 3; Laboratoire de Botanique Historique et Palynologie: Marseille, France, 1998. [Google Scholar]
- Galán, C.; Carinanos, P.; Alcázar, P.; Domínguez-Vilches, E. Spanish Aerobiology Network (REA): Management and Quality Manual; Servicio Publicaciones Universidad de Córdoba: Córdoba, Spain, 2007. [Google Scholar]
- Galán, C.; Dahl, A.; Frenguelli, G.; Gehrig, R. Airborne pollen in Europe. In Allergy and Allergen Immunotherapy: New Mechanisms and Strategies; Singh, A.D., Ed.; Apple Academic Press: Palm Bay, FL, USA; CRC Press: Boca Raton, FL, USA, 2017; pp. 127–162. [Google Scholar]
- Donovan, G.H.; Butry, D.T.; Michael, Y.L.; Prestemon, J.P.; Liebhold, A.M.; Gatziolis, D.; Mao, M.Y. The relationship between trees and human health: Evidence from the spread of the emerald Ash Borer. Am. J. Prev. Med. 2013, 44, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.M.A. Urban green spaces and an integrative approach to sustainable environment. J. Environ. Prot. 2011, 2, 601–608. [Google Scholar] [CrossRef]
- González, E.; Comín, F.A.; Muller, E. Seed dispersal, germination and early seedling establishment of Populus alba L. under simulated water table declines in different substrates. Trees 2010, 24, 151–163. [Google Scholar] [CrossRef]
- Karrenberg, S.; Suter, M. Phenotypic trade-offs in the sexual reproduction of Salicaceae from flood plains. Am. J. Bot. 2003, 90, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Picornell, A.; Buters, J.; Rojo, J.; Traidl-Hoffmann, C.; Damialis, A.; Menzel, A.; Bergmann, K.C.; Werchan, M.; Schmidt-Weber, C.; Oteros, J. Predicting the start, peak and end of the Betula pollen season in Bavaria, Germany. Sci. Total Environ. 2019, 690, 1299–1309. [Google Scholar] [CrossRef]
- Leadem, C.L.; Gilles, S.L.; Yearsley, H.K.; Sit, V.; Spittlehouse, D.L.; Burton, P.J. Field Studies of Seed Biology; Crown Publications Inc.: Victoria, BC, Canada, 1997. [Google Scholar]
- Pérez-Badia, R.; Rapp, A.; Vaquero, C.; Fernandez-Gonzalez, F. Aerobiological study in east-central Iberian Peninsula: Pollen diversity and dynamics for major taxa. Ann. Agric. Environ. Med. 2011, 18, 99–111. [Google Scholar] [PubMed]
- Dahl, Å.; Galán, C.; Hajkova, L.; Pauling, A.; Sikoparija, B.; Smith, M.; Vokou, D. The Onset, Course and Intensity of the Pollen Season. In Allergenic Pollen; Sofiev, M., Bergmann, K.C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 29–70. [Google Scholar]
- Fernández-González, M.; Ribeiro, H.; Pereira, J.R.S.; Rodríguez-Rajo, F.J.; Abreu, I. Assessment of the potential real pollen related allergenic load on the atmosphere of Porto city. Sci. Total Environ. 2019, 668, 333–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).