Abstract
Transforaminal epidural steroid injections are commonly used for the treatment of radicular pain. Some providers opt for an antero-superior approach and others a postero-inferior approach. In this retrospective cohort study, we evaluated MRI evident anatomic differences between the antero-superior and postero-inferior neural foramen at L5-S1 that may be relevant when choosing an approach for injections. A total of 29 L5-S1 neural foramina that were targeted for transforaminal epidural steroid injections were included. Pre-procedure MRIs were assessed for the distribution of the fat within the foramen. Additionally, the presence of foraminal vessels and foraminal stenosis and the presence/absence of anterolisthesis was also observed. Final imaging data were obtained by majority opinion of three or four radiologists. There was a statistically significant difference in the distribution of foraminal fat between the postero-inferior foramen and the antero-superior foramen (p < 0.001), with more fat generally in the postero-inferior foramen. Foraminal vessels were not consistently visualized. There was weak inter-reader reliability for the presence of vessels. In conclusion, this study suggests that there is a difference in the distribution of foraminal epidural fat between the postero-inferior and antero-superior foramen at L5-S1. Through MRI, vessels are inconsistently visualized and cannot be reliably detected on conventional MRI between readers.
1. Introduction
Transforaminal epidural steroid injections (TFESI) are common non-surgical interventions for the management of radicular pain [1]. The choice of injection location for TFESI is up for debate. Classically, a supra-neural approach, targeting the antero-superior foramen or the “safe triangle”, was recommended. This approach has been recommended because the needle traverses the foramen supero-laterally, minimizing the risk of encountering the nerve root or dural sleeve [2,3]. An infra-neural approach, targeting the postero-inferior foramen or “Kambin’s triangle”, is another alternative which has gained popularity [4].
There is variability in the degree of foraminal fat between patients/spinal levels. Foraminal fat fills the space in the foramen that is not occupied by nerve root and thus is more likely to be absent, diminished, or displaced within stenosed foramina. If all else is equal, an operator may opt to target the portion of the foramen with the most space—and in turn, fat—to ensure a safe needle position to minimize risks and pain associated with nerve root or dural sleeve puncture.
In this study, we aim to retrospectively assess the relevant anatomy of the affected neural foramina at L5-S1 in patients presenting for TFESI at L5-S1. Specifically, we used magnetic resonance imaging (MRI) to characterize the pattern of foraminal fat and the presence/location and/or absence of MR-visible foraminal vessels.
2. Materials and Methods
2.1. Sample Selection
IRB approval was obtained. A total of 29 L5-S1 TFESI performed at our institution between 1 February 2023, and 31 July 2023, were randomly selected. All injections were performed in an out-patient setting with a C-arm for fluoroscopic guidance. Patients were excluded if they did not have an MRI within the picture archiving and communication system (PACS) available for review, had surgical hardware which limited MRI assessment, had transitional lumbosacral 46 anatomy, or were younger than 19 years old. No patients were randomly selected younger than 19 or with transitional lumbosacral anatomy, but two patients were excluded due to the presence of hardware. In total, there were 26 patients because bilateral injections were performed in three.
2.2. Data Collection and MRI Review Methods
Demographic data were obtained from the electronic medical record (EMR), and the side of injection was obtained from the documented procedure within the PACS. The most recent MRI was reviewed for each patient, all of which were performed on a 1.5 T or 3 T scanner.
A semi-quantitative method was used to grade foraminal fat in the antero-superior and postero-inferior aspects of the neural foramen, the presence/absence/location of foraminal vessels, and the degree of foraminal stenosis. The amount and direction of listhesis and the presence/absence of lateral recess stenosis at the L4-L5 level were also included as additional internal metrics of inter-reader reliability of commonly assessed lumbar spine pathology. The MRI images were reviewed independently by 3 fellowship-trained musculoskeletal radiologists with 1+, 2+, and 15+ years of experience. A consensus was determined by majority agreement of the reviewers. In the absence of majority, a 4th reviewer, a musculoskeletal radiologist with 10+ years of experience, acted as an arbitrator.
The semi-quantitative method for determining the amount of fat in both the antero-superior and the postero-inferior aspects of the neural foramina was based on the diameter of a 22-gauge needle, which is ~0.7 mm. With this as the standard, the reviewers were instructed to grade the fat as “None” if there was less than 0.8 mm of distance in any direction between nerve and bone/disc material, “Minimal” if there was 0.8 mm to 2.5 mm of distance, and “Substantial” if there was more than 2.5 mm of distance. These numbers reflect, in essence, a space too small to accommodate a 22-gauge needle, a space that is 1–3 times the size of a 22-gauge needle, or a space that can accommodate more than 3 times the diameter of a 22-gauge needle (Figure 1).
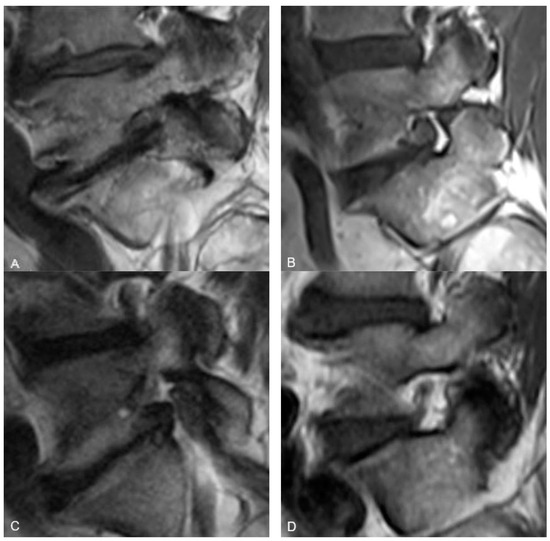
Figure 1.
Representative sagittal T1w MR images at the level of the L5-S1 neural foramen demonstrating the foraminal fat grading system. (A) demonstrates “No fat” in either the antero-superior or postero-inferior portions of the foramen. (B) demonstrates “Minimal fat” in the antero-superior foramen and “Substantial fat” in the postero-inferior foramen. (C) demonstrates “No fat” in the antero-superior foramen and “Minimal fat” in the postero-inferior foramen. (D) demonstrates “Substantial fat” in both the antero-superior and postero-inferior foramen.
For qualitatively determining the presence and location of vessels within the neural foramen, the reviewers were instructed to use the same MRI slice that they used to judge the foraminal fat. A convincing punctate low signal intensity structure next to the nerve root (either antero-superiorly or postero-inferiorly) on the sagittal T1 sequence was considered a vessel (Figure 2). Vessels were considered present when seen but considered “Not Applicable” when not seen because in cases where there was no fat (“None”) in the neural foramen, it would not be possible to see a vessel, and because many vessels may be too small to see within the resolution limits of the MRI.
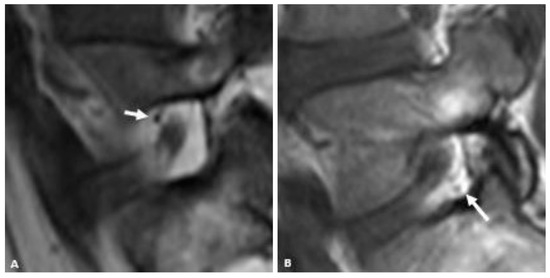
Figure 2.
Representative sagittal T1w MR images of the L5-S1 neural foramen demonstrating foraminal vessels (arrows) in the antero-superior foramen (A) and postero-inferior foramen (B).
Foraminal stenosis was graded with a qualitative binary system as either “None to mild” or “Moderate to severe”. “None to mild” was defined as the range of no encroachment of the foraminal fat to some distortion of the foraminal fat without substantial mass effect or effacement of nerve root. “Moderate to severe” was defined as substantial encroachment of the foraminal fat with or without mass effect/distortion of the nerve root in the foramen.
2.3. Statistical Analysis
Inter-reader reliability between raters was evaluated using Fleiss’ kappa for categorical measures. Inter-reader reliability was described as “None”, “Minimal” “Weak”, “Moderate”, “Strong”, or “Almost perfect”, using the interpretation of kappa described by McHugh, where kappa expresses agreement beyond chance [5]. Continuous data are presented as mean and standard deviation, and categorical data are presented as frequency and percentage. Normality of continuous data were evaluated using the Shapiro–Wilk test. Categorical data were compared between groups using Fisher’s exact test, and continuous data were compared using a one-wayanalysis of variance (ANOVA). A two-tailed p < 0.05 was considered statistically significant. Statistical analysis was performed using Stata (v17.1, StataCorp LLC, College Station, TX, USA).
A post hoc power calculation was performed to ensure this study was appropriately powered, with the goal of achieving 80% power between the compared groups [6]. The achieved sample size of 29 foramina—with 19 foramina with “None to mild” stenosis, and 10 foramina with “Moderate to severe” stenosis—provided 80% power for detecting clinically meaningful differences between these two groups (0.5 absolute difference in proportions), based on Fisher’s exact test and assuming a two-tailed 5% alpha. Power calculations were performed using G*Power software (version 3.1.9.6, University of Dusseldorf, Germany).
3. Results
3.1. Demographics and Foraminal Stenosis
A total of 26 patients were included in this study. The average age of each patient for each assessed foramen at the time of their MRI was 61.1 years (range: 21.2–90.5; SD: 16.3). There was a near-even distribution of men and women with 15/29 (51.7%) of foramen in women and 14/29 (48.3%) of 100 foramen in men. There was a similarly even distribution of laterality with 14/29 (48.3%) left-sided and 15/29 (51.7%) right-sided. A total of 19/29 (65.5%) foramina were deemed to have “None to mild” foraminal stenosis and 10/29 (34.5%) were assessed as “Moderate to severe” foraminal stenosis.
3.2. Foraminal Fat Distribution
In all, there was a statistically significant difference in fat in the postero-inferior foramen compared to the antero-superior foramen (p < 0.001). There was more often no fat (“None”) and less often “Substantial” fat in the antero-superior foramen compared to the postero-inferior foramen (Table 1). The quantity of fat showed a statistically significant correlation to the degree of foraminal stenosis both in the antero-superior and postero-inferior foramen. This effect was more dramatic within the antero-superior foramen than in the postero-inferior foramen. For instance, in patients with “Moderate-severe” foraminal stenosis, 40% had “Substantial” postero-inferior foraminal fat, while not one had “Substantial” fat in the antero-superior foramen. All the patients with “None-Mild” foraminal stenosis had “Substantial” postero-inferior foraminal fat, while less than a third of the patients with “None-Mild” foraminal stenosis had “Substantial” antero-superior foraminal fat (Table 2). The amount and direction of listhesis and patient age had no statistically significant correlation with the amount of fat within either the antero-superior or postero-inferior portion of the foramen.

Table 1.
Consensus grade of foraminal fat in the antero-superior and postero-inferior portions of the L5-S1 neural foramen.

Table 2.
Distribution of the grade foraminal fat in the antero-superior and postero-inferior portions of the L5-S1 foramen in the setting of “None-Mild” and “Moderate-severe” foraminal stenosis.
3.3. Foraminal Vessels
Foraminal vessels were not seen in either the antero-superior or postero-inferior foramen in 12/29 (41.4%), were seen in both portions of the foramen in 9/29 (31.0%), only in the postero-inferior foramen in 7/29 (24.1%) and only in the antero-superior foramen in 1/29 (3.5%). There was a statistically significant relationship between the visualization of vessels in the neural foramen and the degree of foraminal stenosis (p = 0.005). Vessels were more often seen when there was “None-mild” foraminal stenosis than in the setting of “Moderate-severe” foraminal stenosis (Table 3).

Table 3.
Distribution of the visualized foraminal vessels in the L5-S1 foramen in the setting of “None-mild” and “Moderate-severe” foraminal stenosis.
3.4. Inter-Rater Reliability
There was moderate inter-rater reliability beyond chance (K = 0.60–0.79) for the postero-inferior foraminal fat, foraminal stenosis, and listhesis variables. There was weak inter-rater reliability beyond chance (K = 0.40–0.59) for the antero-superior foraminal fat and the presence of vessels (Table 4).

Table 4.
Inter-rater reliability of the measured variables.
4. Discussion
In this study, we demonstrated a difference in the distribution of foraminal fat in the postero-inferior and antero-superior foramen. In this population, 80% of L5-S1 foramina with moderate to severe stenosis had at least minimal postero-inferior epidural fat while just 40% of these same foramina had at least minimal fat in the antero-superior neural foramen. In fact, none of the foramina with moderate to severe stenosis had more than minimal foraminal fat in the antero-superior foramen. Even in patients without stenosis, more than 10% had no fat (“None”) in the antero-superior foramen, and only 31.6% had more than minimal fat in this location. Postero-inferiorly, all patients without foraminal stenosis had substantial foraminal fat. These findings for the L5-S1 foramen may support the notion that the postero-inferior approach (“Kambin’s triangle) for L5-S1 TFESI may be more advantageous than one in which the antero-superior foramen (“Safe triangle”) is targeted (Figure 3).
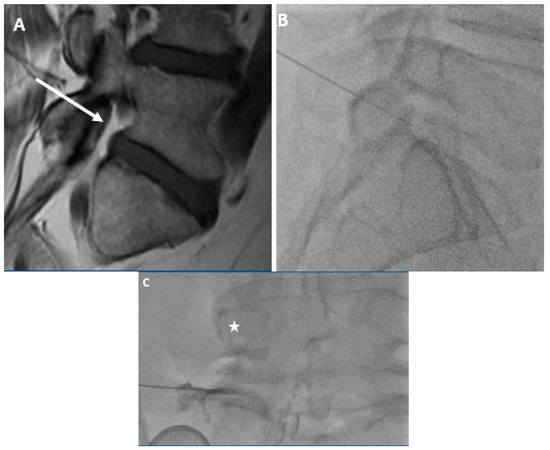
Figure 3.
59-year-old gentleman with L5 radiculopathy. (A) Sagittal T1w MR image demonstrates substantial fat in the postero-inferior foramen. The arrow corresponds to the needle trajectory for an infra-neural approach. Lateral (B) and frontal (C) fluoroscopic images during transforaminal epidural steroid injection show the needle projecting over the postero-inferior aspect of the foramen. The star is over the pedicle in (C). Contrast can be seen moving centrally towards the spinal canal within the inferior portion of the foramen.
Anatomical studies support the imaging findings in this study. The L5 nerve root has been shown to enter the foramen from a more cranial position than any of the other lumbar nerve roots [7]. The nerve root has also been shown to be further anterior and closer to the intervertebral disc within the neural foramen than the other lumbar nerve roots [8]. While the lumbar neural foramina are all similar in height, L5-S1 is the widest, which allows for more epidural fat to occupy the space posterior to the anteriorly positioned nerve [8].
In this study, vessels were visualized less often by MRI within the foramen in the setting of stenosis. The presence of vessels also had the lowest inter-rater reliability of all the variables recorded. The presence of vessels is important for a few reasons. For one, an embolic phenomenon related to intra-arterial injection or direct needle trauma to a dominant radiculo-medullary artery has been implicated in TFESI-related paralysis. Secondly, if the anti-inflammatory/anesthetic is administered intra-vascularly, it will not offer the local tissue effects desired from the procedure.
The inability to see vessels in the setting of stenosis is unsurprising. We identified vessels as punctate low-signal-intensity foci on T1-weighted images which may not be visible without a background of foraminal fat, as is often the case in the setting of foraminal stenosis. Vessels were more often seen in the postero-inferior foramen (16/29) than in the antero-superior foramen (10/29). This finding may simply reflect a secondary marker of the increased foraminal fat postero-inferiorly. There is a known venous plexus within the foramen, including in the postero-inferior portion of the foramen, which specifically includes the dorsal ascending vertebral vein and emissary veins, which may account for the vessels seen posteriorly by MRI [9].
Cases of paralysis relating to TFESI preempted the 2015 publication of “Safeguards to Prevent Neurologic Complications After Epidural Steroid Injections: Consensus Opinions from a Multidisciplinary Working Group and National Organizations” [10]. These events are believed to be related to an embolic phenomenon from either direct needle trauma or inadvertent injection of a dominant radiculo-medullary spinal artery—also known as an artery of Adamkiewicz—with a particulate steroid. The consensus from the working group to minimize the risk of paralysis focused on minimizing the risk of embolic phenomena but did not specifically make recommendations on the procedural approach to avoid encountering vessels [10]. However, anatomic studies have identified that dominant radiculo-medullary arteries arise within the antero-superior foramen and have implied that an infra-neural approach, rather than a supra-neural approach, may minimize risk of an inadvertent intra-arterial injection [11].
Even in the lower lumbar spine, there may still be risk of encountering the artery of Adamkiewicz as its origin is highly variable [12]. Though it most commonly arises within the T8-L1 neural foramina from left-sided intercostal or lumbar arteries, it has been seen to arise from intercostal arteries as cranial as T3 and as caudal as L4 [13,14,15,16]. When present in the neural foramen, this artery is typically located within the antero-superior portion of the neural foramen [11]. The L5 nerve roots lack a lumbar artery, and to our knowledge, only one study has ever reported a dominant radiculo-medullary artery arising at the L5-S1 level [9,17]. Despite this, a potentially significant supporting radiculo-medullary vessel arising from the iliolumbar trunk coursing alongside the L5 or S1 nerve roots has been described in the antero-superior L5-S1 foramen [18]. This vessel, also known as the artery of Desproges–Grotteron, has been implicated in cases of conus medullary syndrome or sciatica after L5-S1 TFESI [18,19].
There was moderate inter-rater reliability for characterizing the postero-inferior foraminal fat compared to weak inter-rater reliability for characterizing the fat in the antero-superior foramen. The inter-rater reliability for the postero-inferior foraminal fat was similar to that of both listhesis and L4-L5 lateral recess stenosis. The reason for the difference in inter-reader reliability for the foraminal fat between the antero-superior and postero-inferior foramen is unclear but may be because there was more often greater epidural fat in the postero-inferior foramen. With more foraminal fat in the postero-inferior foramen, the interpreter would less often need to decide between “Minimal” or “None”, which they would have to do more regularly in their assessment of the antero-superior foramen.
This study was limited by the retrospective design and small sample size. This study was also limited by utilization of a non-validated, semi-quantitative system for assessing the anatomic characteristics of the neural foramen on MRI which showed less-than-perfect inter-reader reliability across all studied variables. In particular, the weak inter-reader reliability of assessment of the fat in the antero-superior foramen may limit its ability for clinical implementation. It may be that the findings of this study should influence practitioners to default to a postero-inferior approach when performing TFESI at L5-S1, and only stray from this approach if there are findings on MRI that contraindicate this approach. Such findings that may preclude a postero-inferior approach that might be seen on MRI include posterior disc herniations occupying the postero-inferior foramen or facet joint synovial cysts and/or osteophytes obstructing access to the postero-inferior foramen.
For confident clinical implementation, future studies should directly compare the safety and clinical efficacy of the two different approaches to TFESI. The safety concerns associated with the supra-neural approach have been discussed at length, though there are also risks that may be increased with an infra-neural approach. The primary concern unique to the infra-neural approach is inadvertent disc puncture, which generally cannot occur with a supra-neural approach [20]. Fortunately, at the time of this manuscript preparation, there is reportedly a single-blinded, non-inferiority clinical trial underway in the United Kingdom, funded by the British Journal of Anaesthesia/Royal College of Anaesthetists, aiming to prospectively compare the clinical efficacy and safety between the supra-neural and infra-neural approaches to lumbar TFESI [21].
Future studies may aim to establish a validated method for assessing the foraminal fat by MRI. It is possible that this could be achieved through prospective studies with intra-operative correlation. Alternatively, this could potentially be accomplished through the development of automated software capable of more precise quantification of the foraminal fat by MRI. If developed, this technological innovation could allow for assessment of the neural foraminal fat on a much larger scale.
5. Conclusions
In conclusion, in this population presenting for L5-S1 TFESI, there was a statistically significant difference in the distribution of epidural fat between the postero-inferior foramen and the antero-superior foramen, with generally more fat present postero-inferiorly than antero-superiorly. This effect appears to be more dramatic in the setting of neural foraminal stenosis.
Knowledge of the variable distribution of fat and the variable presence of vessels in the L5-S1 neural foramen can help to inform the proceduralist’s approach when performing an L5-S1 TFESI. Specifically, since the foraminal epidural fat represents the target for needle positioning and the conduit for the transforaminal flow of injectate, the main finding in this study—the presence of more foraminal fat postero-inferiorly than antero-superiorly—may encourage proceduralists to opt for the infra-neural (postero-inferior) approach over the supra-neural (antero-superior) approach when performing TFESI at L5-S1. Future prospective clinical trials directly comparing the safety and efficacy of these two approaches may provide further clarity in guiding clinical decision-making.
Author Contributions
Conceptualization, Z.E.S.; Methodology, Z.E.S. and S.S.; Formal analysis, S.S.; Investigation, Z.E.S., R.W.M., F.J.S. and A.J.H.; Writing—original draft, Z.E.S.; Writing—review & editing, Z.E.S., R.W.M., S.S., F.J.S. and A.J.H.; Visualization, Z.E.S. and A.J.H.; Supervision, Z.E.S.; Project administration, Z.E.S. and R.W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Massachusetts General Hospital (Institution: Massachusetts General Hospital Protocol #: 2023P002329).
Informed Consent Statement
Patient consent was waived due to the use of retrospective chart reviews in this study. There was no more than minimal risk to the included patients, and no protected health information was disclosed.
Data Availability Statement
The de-identified raw data supporting the conclusions of this article can be made available by the corresponding author on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Helm, S.; Harmon, P.C.; Noe, C.; Calodney, A.K.; Abd-Elsayed, A.; Knezevic, N.N.; Racz, G.B. Meta-Analysis Transforaminal Epidural Steroid Injections: A Systematic Review and Meta-Analysis of Efficacy and Safety. Pain Physician 2021, 24, S209–S232. Available online: www.painphysicianjournal.com (accessed on 12 April 2024).
- Slipman, C.W.; Chow, D.W. Therapeutic spinal corticosteroid injections for the management of radiculopathies. Phys. Med. Rehabil. Clin. N. Am. 2002, 13, 697–711. [Google Scholar] [CrossRef]
- Botwin, K.P.; Gruber, R.D.; Bouchlas, C.G.; Torres-Ramos, F.M.; Sanelli, J.T.; Freeman, E.D.; Slaten, W.K.; Rao, S. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: An outcome study. Am. J. Phys. Med. Rehabil. 2002, 81, 898–905. [Google Scholar] [CrossRef]
- Mandell, J.C.; Czuczman, G.J.; Gaviola, G.C.; Ghazikhanian, V.; Cho, C.H. The lumbar neural foramen and transforaminal epidural steroid injections: An anatomic review with key safety considerations in planning the percutaneous approach. Am. J. Roentgenol. 2017, 209, W26–W35. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Arslan, M.; Cömert, A.; Açar, H.I.; Özdemir, M.; Elhan, A.; Tekdemir, I.; Tubbs, S.R.; Attar, A.; Uğur, H. Lumbosacral intrathecal nerve roots: An anatomical study. Acta Neurochir. 2011, 153, 1435–1442. [Google Scholar] [CrossRef]
- Arslan, M.; Cömert, A.; Açar, H.I.; Özdemir, M.; Elhan, A.; Tekdemir, I.; Tubbs, R.S.; Uğur, H. Nerve root to lumbar disc relationships at the intervertebral foramen from a surgical viewpoint: An anatomical study. Clin. Anat. 2012, 25, 218–223. [Google Scholar] [CrossRef]
- Uchikado, H.; Nishimura, Y.; Hattori, G.; Ohara, Y. Micro-anatomical structures of the lumbar intervertebral foramen for full-endoscopic spine surgery: Review of the literatures. J. Spine Surg. 2020, 6, 405–414. [Google Scholar] [CrossRef]
- Rathmell, J.P.; Benzon, H.T.; Dreyfuss, P.; Huntoon, M.; Wallace, M.; Baker, R.; Riew, K.D.; Rosenquist, R.W.; Aprill, C.; Rost, N.S.; et al. Safeguards to Prevent Neurologic Complications after Epidural Steroid Injections. Anesthesiology 2015, 122, 974–984. [Google Scholar] [CrossRef]
- Gregg, L.; Sorte, D.E.; Gailloud, P. Intraforaminal location of thoracolumbar radicular arteries providing an anterior radiculomedullary artery using flat panel catheter angiotomography. Am. J. Neuroradiol. 2017, 38, 1054–1060. [Google Scholar] [CrossRef]
- Taterra, D.; Skinningsrud, B.; Pękala, P.A.; Hsieh, W.C.; Cirocchi, R.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A.; Henry, B.M. Artery of Adamkiewicz: A meta-analysis of anatomical characteristics. Neuroradiology 2019, 61, 869–880. [Google Scholar] [CrossRef]
- Yamada, N.; Okita, Y.; Minatoya, K.; Tagusari, O.; Ando, M.; Takamiya, M.; Kitamura, S. Preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography in patients with descending or thoracoabdominal aortic aneurysms. Eur. J. Cardiothorac. Surg. 2000, 18, 104–111. [Google Scholar] [CrossRef]
- Koshino, T.; Murakami, G.; Morishita, K.; Mawatari, T.; Abe, T. Does the Adamkiewicz artery originate from the larger segmental arteries? J. Thorac. Cardiovasc. Surg. 1999, 117, 898–905. [Google Scholar] [CrossRef]
- Biglioli, P.; Roberto, M.; Cannata, A.; Parolari, A.; Fumero, A.; Grillo, F.; Maggioni, M.; Coggi, G.; Spirito, R. Upper and lower spinal cord blood supply: The continuity of the anterior spinal artery and the relevance of the lumbar arteries. J. Thorac. Cardiovasc. Surg. 2004, 127, 1188–1192. [Google Scholar] [CrossRef]
- Gailloud, P. The artery of von Haller: A constant anterior radiculomedullary artery at the upper thoracic level. Neurosurgery 2013, 73, 1034–1043. [Google Scholar] [CrossRef]
- Reis, C.; Rocha, J.A.; Chamadoira, C.; Pereira, P.; Fonseca, J. Foraminal L5-S1 disc herniation and conus medullaris syndrome: A vascular etiology? Acta Neurochir. 2007, 149, 533–535. [Google Scholar] [CrossRef]
- Chou, D.; Narsinh, K. Editorial. The relevance of the artery of Adamkiewicz in the 21st century. J. Neurosurg. Spine 2023, 38, 230–231. [Google Scholar] [CrossRef]
- Masson, C.; Bardin, T. Desproges-Gotteron artery, spinal cord infarction, and transforaminal glucocorticoid injections. Jt. Bone Spine 2009, 76, 120–121. [Google Scholar] [CrossRef]
- Levi, D.; Horn, S.; Corcoran, S. The Incidence of Intradiscal, Intrathecal, and Intravascular Flow during the Performance of Retrodiscal (Infraneural) Approach for Lumbar Transforaminal Epidural Steroid Injections. Pain Med. 2016, 17, 1416–1422. [Google Scholar] [CrossRef]
- Galley, H.F.; Adam, R.; Columb, M.O.; Onyeakazi, U.M.; Kanakarajan, S. Supraneural versus Infraneural Approach to transforaMinal Epidural StEroid injection for unilateral lumbosacral radicular pain (SIAMESE): A study protocol for a randomised non-inferiority trial. BJA Open 2023, 5, 100126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).