Pilot Study of Intensive Pain Rehabilitation, Sleep, and Small-World Brain Networks in Adolescents with Chronic Pain
Abstract
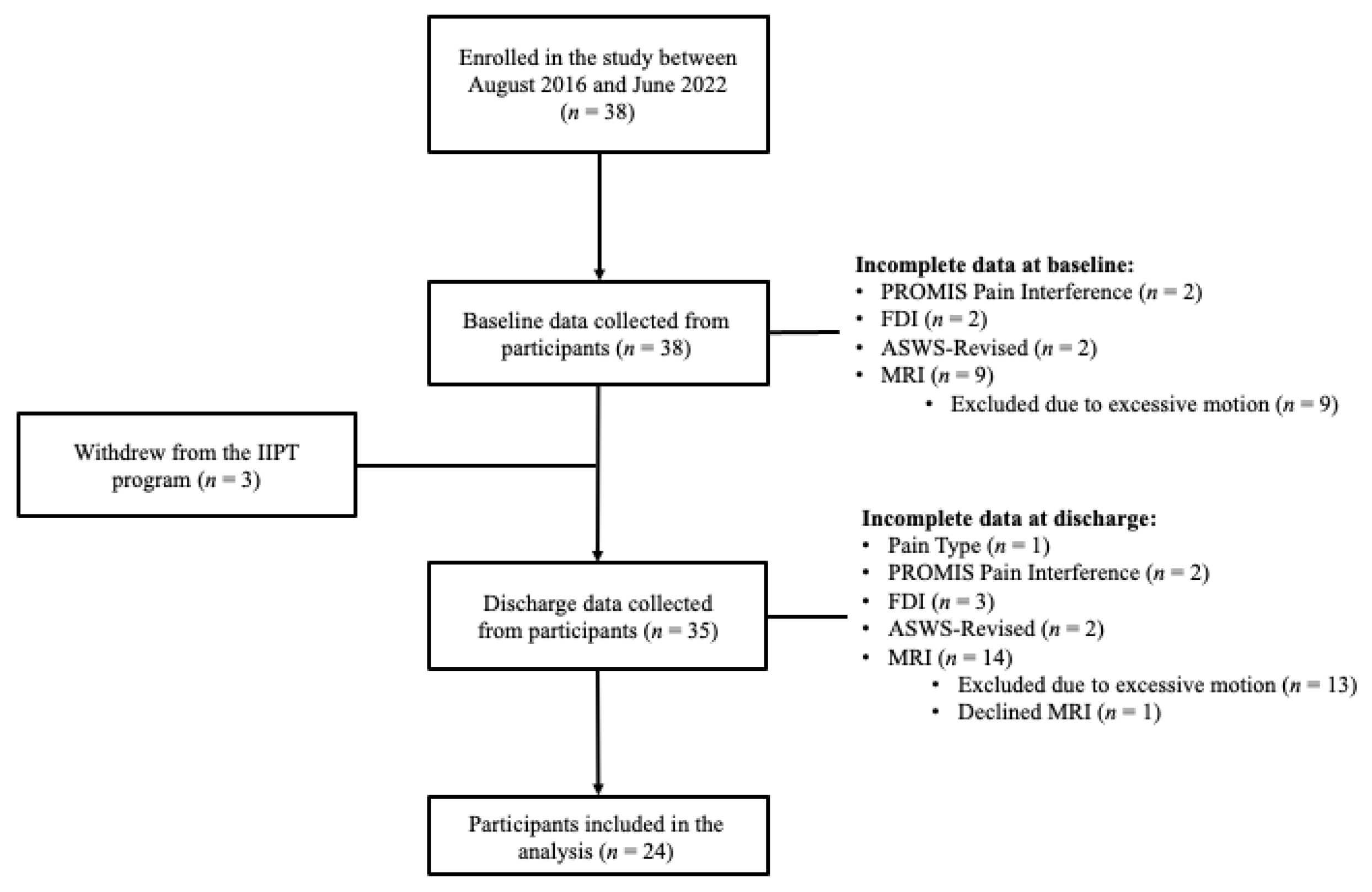
:1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. The Intensive Pain Rehabilitation Program
2.3. Measures
2.3.1. Demographics
2.3.2. Functional Disability Questionnaire
2.3.3. Sleep Questionnaires
2.4. Brain Imaging
2.4.1. Acquisition Parameters
2.4.2. Image Preprocessing
2.4.3. Functional Connectome Construction
2.4.4. Calculating Graph-Theory-Based Metrics
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Total Sleep Quality, Small-World Brain Networks, and Functional Disability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, S.; Chambers, C.T.; Huguet, A.; MacNevin, R.C.; McGrath, P.J.; Parker, L.; MacDonald, A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Perquin, C.W.; Hazebroek-Kampschreur, A.; Hunfeld, J.A.M.; Bohnen, A.M.; van Suijlekom-Smit, L.W.A.; Passchier, J.; van der Wouden, J.C. Pain in children and adolescents: A common experience. Pain 2000, 87, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, B.N.; Rabbitts, J.A.; Palermo, T.M. A developmental perspective on the impact of chronic pain in late adolescence and early adulthood: Implications for assessment and intervention. Pain 2017, 158, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Hechler, T.; Kanstrup, M.; Holley, A.L.; Simons, L.E.; Wicksell, R.; Hirschfeld, G.; Zernikow, B. Systematic Review on Intensive Interdisciplinary Pain Treatment of Children with Chronic Pain. Pediatrics 2015, 136, 115–127. [Google Scholar] [CrossRef]
- Hurtubise, K.; Blais, S.; Noel, M.; Brousselle, A.; Dallaire, F.; Rasic, N.; Camden, C. Is It Worth It? A Comparison of an Intensive Interdisciplinary Pain Treatment and a Multimodal Treatment for Youths With Pain-related Disability. Clin. J. Pain 2020, 36, 833–844. [Google Scholar] [CrossRef]
- Argaman, Y.; Granovsky, Y.; Sprecher, E.; Sinai, A.; Yarnitsky, D.; Weissman-Fogel, I. Resting-state functional connectivity predicts motor cortex stimulation-dependent pain relief in fibromyalgia syndrome patients. Sci. Rep. 2022, 12, 17135. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Liu, X.; Zhuo, Z.; Wei, J.; Du, M.; Chan, Q.; Wang, X.; Wang, D. Altered small-world, functional brain networks in patients with lower back pain. Sci. China Life Sci. 2018, 61, 1420–1424. [Google Scholar] [CrossRef]
- Li, K.; Liu, L.; Yin, Q.; Dun, W.; Xu, X.; Liu, J.; Zhang, M. Abnormal rich club organization and impaired correlation between structural and functional connectivity in migraine sufferers. Brain Imaging Behav. 2017, 11, 526–540. [Google Scholar] [CrossRef]
- Erpelding, N.; Simons, L.; Lebel, A.; Serrano, P.; Pielech, M.; Prabhu, S.; Becerra, L.; Borsook, D. Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct. Funct. 2016, 221, 1095–1111. [Google Scholar] [CrossRef]
- Palermo, T.M.; Wilson, A.C.; Lewandowski, A.S.; Toliver-Sokol, M.; Murray, C.B. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain 2011, 152, 89–94. [Google Scholar] [CrossRef]
- Lewin, D.S.; Dahl, R.E. Importance of sleep in the management of pediatric pain. J. Dev. Behav. Pediatr. 1999, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Boggero, I.A.; Krietsch, K.N.; Pickerill, H.M.; Byars, K.C.; Homan, K.J.; Williams, S.E.; King, C.D. Improvements in Sleep Correlate With Improvements in Clinical Outcomes Among Adolescents Undergoing Intensive Interdisciplinary Pain Treatment. Clin. J. Pain 2021, 37, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Pigott, T.; McPeak, A.; de Chastelain, A.; DeMayo, M.M.; Rasic, N.; Rayner, L.; Noel, M.; Miller, J.V.; Harris, A.D. Changes in Brain GABA and Glutamate and Improvements in Physical Functioning Following Intensive Pain Rehabilitation in Youth with Chronic Pain. J. Pain 2023, 24, 1288–1297. [Google Scholar] [CrossRef]
- Epp, S.; Walker, A.; Boudes, E.; Bray, S.; Noel, M.; Rayner, L.; Rasic, N.; Miller, J.V. Brain Function and Pain Interference After Pediatric Intensive Interdisciplinary Pain Treatment. Clin. J. Pain 2024, 40, 393–399. [Google Scholar] [CrossRef]
- Long, R.D.; Walker, A.; Pan, S.C.; Miller, J.V.; Rayner, L.; Vallely, J.; Rasic, N. Baseline Factors Associated with Pain Intensity, Pain Catastrophizing, and Pain Interference in Intensive Interdisciplinary Pain Treatment for Youth. Children 2023, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Greene, J.W. The functional disability inventory: Measuring a neglected dimension of child health status. J. Pediatr. Psychol. 1991, 16, 39–58. [Google Scholar] [CrossRef]
- Claar, R.L.; Walker, L.S. Functional assessment of pediatric pain patients: Psychometric properties of the functional disability inventory. Pain 2006, 121, 77–84. [Google Scholar] [CrossRef]
- Essner, B.; Noel, M.; Myrvik, M.; Palermo, T. Examination of the Factor Structure of the Adolescent Sleep-Wake Scale (ASWS). Behav. Sleep Med. 2015, 13, 296–307. [Google Scholar] [CrossRef]
- Sufrinko, A.M.; Valrie, C.R.; Lanzo, L.; Bond, K.E.; Trout, K.L.; Ladd, R.E.; Everhart, D.E. Empirical validation of a short version of the Adolescent Sleep-Wake Scale using a sample of ethnically diverse adolescents from an economically disadvantage community. Sleep Med. 2015, 16, 1204–1206. [Google Scholar] [CrossRef]
- Long, X.; Kar, P.; Gibbard, B.; Tortorelli, C.; Lebel, C. The brain’s functional connectome in young children with prenatal alcohol exposure. Neuroimage Clin. 2019, 24, 102082. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L.; Brain Development Cooperative, G. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011, 54, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Mueller, B.A.; Mattson, S.N.; Coles, C.D.; Kable, J.A.; Jones, K.L.; Boys, C.J.; Lim, K.O.; Riley, E.P.; Sowell, E.R.; et al. Functional connectivity abnormalities and associated cognitive deficits in fetal alcohol Spectrum disorders (FASD). Brain Imaging Behav. 2017, 11, 1432–1445. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 386. [Google Scholar] [CrossRef]
- IBM. IBM SPSS Statistics for Macintosh, 28th ed.; IBM: Armonk, NY, USA, 2021. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 3rd ed.; Guilford Publications: New York, NY, USA, 2022. [Google Scholar]
- Logan, D.E.; Sieberg, C.B.; Conroy, C.; Smith, K.; Odell, S.; Sethna, N. Changes in sleep habits in adolescents during intensive interdisciplinary pediatric pain rehabilitation. J. Youth Adolesc. 2015, 44, 543–555. [Google Scholar] [CrossRef]
- Krietsch, K.N.; Beebe, D.W.; King, C.; Homan, K.J.; Williams, S.E. Sleep among Youth with Severely Disabling Chronic Pain: Before, during, and after Inpatient Intensive Interdisciplinary Pain Treatment. Children 2021, 8, 42. [Google Scholar] [CrossRef]
- Eadie, J.; van de Water, A.T.; Lonsdale, C.; Tully, M.A.; van Mechelen, W.; Boreham, C.A.; Daly, L.; McDonough, S.M.; Hurley, D.A. Physiotherapy for sleep disturbance in people with chronic low back pain: Results of a feasibility randomized controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 2083–2092. [Google Scholar] [CrossRef]
- Fales, J.; Law, E.; Claar, R.; Palermo, T. Sleep outcomes in adolescents with chronic pain: Findings from a multi-site randomized clinical trial of web-based cognitive behavioral therapy for pediatric chronic pain. J. Pain 2013, 14, S99. [Google Scholar] [CrossRef]
- Valrie, C.R.; Bromberg, M.H.; Palermo, T.; Schanberg, L.E. A systematic review of sleep in pediatric pain populations. J. Dev. Behav. Pediatr. 2013, 34, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Fulong, X.; Spruyt, K.; Chao, L.; Dianjiang, Z.; Jun, Z.; Fang, H. Resting-state brain network topological properties and the correlation with neuropsychological assessment in adolescent narcolepsy. Sleep 2020, 43, zsaa018. [Google Scholar] [CrossRef]
- Farahani, F.V.; Fafrowicz, M.; Karwowski, W.; Douglas, P.K.; Domagalik, A.; Beldzik, E.; Oginska, H.; Marek, T. Effects of Chronic Sleep Restriction on the Brain Functional Network, as Revealed by Graph Theory. Front. Neurosci. 2019, 13, 1087. [Google Scholar] [CrossRef]
- Smith, R.; Sanova, A.; Alkozei, A.; Lane, R.D.; Killgore, W.D.S. Higher levels of trait emotional awareness are associated with more efficient global information integration throughout the brain: A graph-theoretic analysis of resting state functional connectivity. Soc. Cogn. Affect. Neurosci. 2018, 13, 665–675. [Google Scholar] [CrossRef]
- Mohanty, R.; Sethares, W.A.; Nair, V.A.; Prabhakaran, V. Rethinking Measures of Functional Connectivity via Feature Extraction. Sci. Rep. 2020, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.E.; Carpino, E.A.; Chiang, G.; Condon, M.; Firn, E.; Gaughan, V.J.; Hogan, M.; Leslie, D.S.; Olson, K.; Sager, S.; et al. A day-hospital approach to treatment of pediatric complex regional pain syndrome: Initial functional outcomes. Clin. J. Pain 2012, 28, 766–774. [Google Scholar] [CrossRef]
- Becerra, L.; Sava, S.; Simons, L.E.; Drosos, A.M.; Sethna, N.; Berde, C.; Lebel, A.A.; Borsook, D. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 2014, 6, 347–369. [Google Scholar] [CrossRef]

| Characteristic | Baseline (n = 24) | Discharge (n = 24) | p-Value | Cohen’s d or Effect Size |
|---|---|---|---|---|
| Age, Median (IQR), y | 17.00 (16.00–17.00) | - | - | - |
| Gender (female), n (%) | 20 (83.3) | - | - | - |
| Pain Type, n (%) | 0.977 | 0.1 | ||
| Abdominal | 1 (4.2) | 1 (4.3) | ||
| Nerve/Neuropathic | 9 (37.5) | 9 (39.1) | ||
| Headache | 8 (33.3) | 6 (26.1) | ||
| Musculoskeletal | 4 (16.7) | 4 (17.4) | ||
| Other | 2 (8.3) | 3 (13.0) | ||
| Functional Disability, M (SD) | 27.33 (12.77) | 23.14 (13.24) | 0.105 | 0.36 |
| Sleep Quality, M (SD) | 3.11 (0.89) | 3.41 (0.90) | 0.035 | 0.47 |
| Normalized Clustering Coefficient, M (SD) | 1.84 (0.13) | 1.82 (0.14) | 0.63 | 0.11 |
| Normalized Characteristic Path Length, Median (IQR) | 1.26 (1.23–1.28) | 1.25 (1.23–1.30) | 0.9 | 0.14 |
| Small-Worldness, M (SD) | 1.46 (0.10) | 1.44 (0.10) | 0.39 | 0.19 |
| Functional Disability | |||
|---|---|---|---|
| Factors | Coefficient | Confidence Interval | p-Value |
| Total Sleep Quality | −0.51 | −0.73, −0.28 | <0.001 |
| Small-Worldness | −0.25 | −0.50, −0.01 | 0.044 |
| Small-Worldness X Total Sleep Quality * | 0.41 | 0.13, 0.70 | 0.005 |
| R2 Value | 0.38 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, S.A.; Farag, S.; Cobos, K.L.; Long, X.; Rasic, N.; Rayner, L.; Lebel, C.; Noel, M.; Walker, A.; Miller, J.V. Pilot Study of Intensive Pain Rehabilitation, Sleep, and Small-World Brain Networks in Adolescents with Chronic Pain. Anesth. Res. 2024, 1, 193-203. https://doi.org/10.3390/anesthres1030018
Miller SA, Farag S, Cobos KL, Long X, Rasic N, Rayner L, Lebel C, Noel M, Walker A, Miller JV. Pilot Study of Intensive Pain Rehabilitation, Sleep, and Small-World Brain Networks in Adolescents with Chronic Pain. Anesthesia Research. 2024; 1(3):193-203. https://doi.org/10.3390/anesthres1030018
Chicago/Turabian StyleMiller, Samantha A., Salma Farag, Karen L. Cobos, Xiangyu Long, Nivez Rasic, Laura Rayner, Catherine Lebel, Melanie Noel, Andrew Walker, and Jillian V. Miller. 2024. "Pilot Study of Intensive Pain Rehabilitation, Sleep, and Small-World Brain Networks in Adolescents with Chronic Pain" Anesthesia Research 1, no. 3: 193-203. https://doi.org/10.3390/anesthres1030018
APA StyleMiller, S. A., Farag, S., Cobos, K. L., Long, X., Rasic, N., Rayner, L., Lebel, C., Noel, M., Walker, A., & Miller, J. V. (2024). Pilot Study of Intensive Pain Rehabilitation, Sleep, and Small-World Brain Networks in Adolescents with Chronic Pain. Anesthesia Research, 1(3), 193-203. https://doi.org/10.3390/anesthres1030018






