Endocannabinoid System in Sepsis: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Information Sources
2.3. Eligibility Criteria and Study Selection
2.4. Data Charting
3. Results
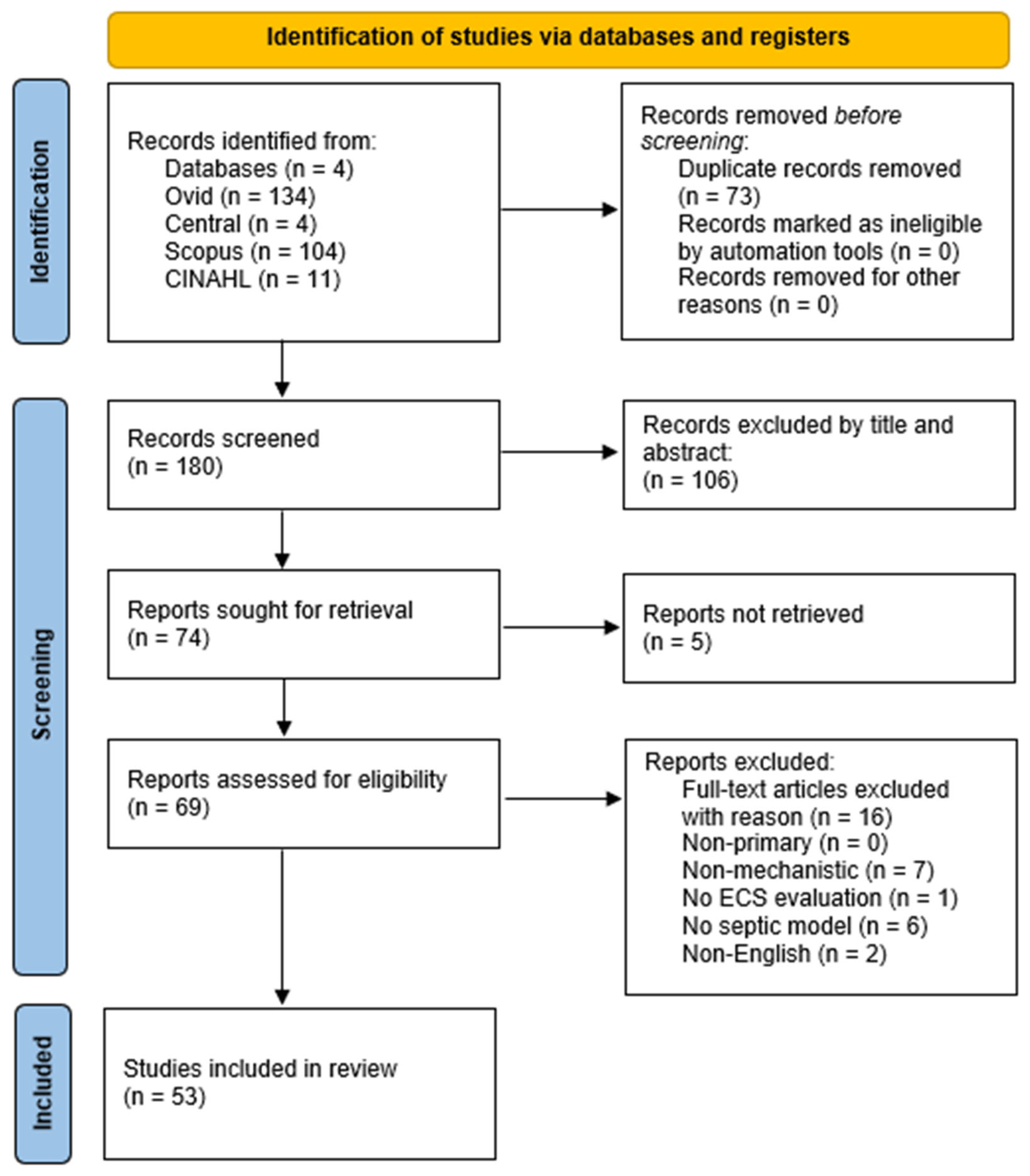
3.1. Study Identification and Inclusion
3.2. Characteristics of Sources
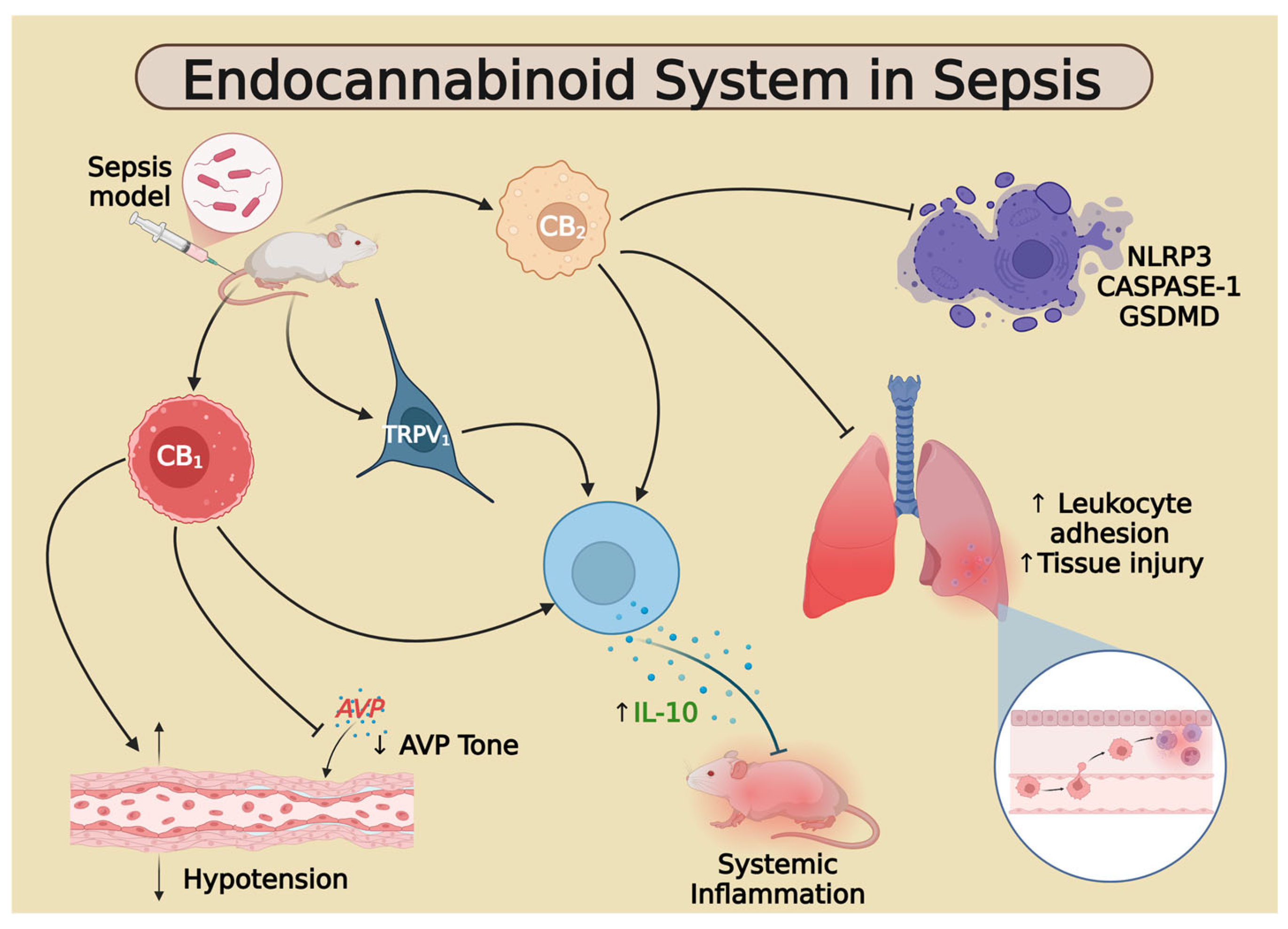
3.3. Study Results and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Ovid(Medline): Search ran on 6/13/25 and produced 134 results. 1. exp Sepsis/or (sepsis or “bloodstream infection *” or septicemia * or “blood poisoning *” or pyemia * or pyaemia * or pyohemia * or septic *).mp. 2. exp Endocannabinoids/or (endocannabinoid * or endo-cannabinoid *).mp. 3. exp Receptors, Cannabinoid/or “cannabinoid receptor *”.mp. 4. 2 or 3 5. 1 and 4 |
| Cochrane Trials (CENTRAL): Search ran on 6/13/25 and produced 4 results. 1. [mh Sepsis] OR (sepsis:ti,ab,kw OR (“bloodstream” NEXT infection *):ti,ab,kw OR septicemia *:ti,ab,kw OR (“blood” NEXT poisoning *):ti,ab,kw OR pyemia *:ti,ab,kw OR pyaemia *:ti,ab,kw OR pyohemia *:ti,ab,kw OR septic *:ti,ab,kw) 2. [mh Endocannabinoids] OR (endocannabinoid*:ti,ab,kw OR endo-cannabinoid *:ti,ab,kw) 3. [mh “Receptors, Cannabinoid”] OR (“cannabinoid” NEXT receptor *):ti,ab,kw 4. #2 OR #3 5. #1 AND #4 |
| Scopus: Search ran on 6/13/25 and produced 164 results. (INDEXTERMS (sepsis) OR TITLE-ABS-KEY (sepsis OR “bloodstream infection *” OR septicemia * OR “blood poisoning *” OR pyemia * OR pyaemia * OR pyohemia * OR septic *) AND (INDEXTERMS (endocannabinoids) OR TITLE-ABS-KEY (endocannabinoid * OR endo-cannabinoid *) OR INDEXTERMS (“Receptors, Cannabinoid”) OR TITLE-ABS-KEY (“cannabinoid receptor *”))) |
| CINAHL: Search ran on 6/13/25 and produced 11 results. (MH “Sepsis+” OR (sepsis OR “bloodstream infection *” OR septicemia * OR “blood poisoning *” OR pyemia * OR pyaemia * OR pyohemia * OR septic *)) AND ((endocannabinoid * OR endo-cannabinoid * OR “cannabinoid receptor *”)) |
| Study (1st Author, Year) | Model System | ECS Component | Intervention/Comparison | Key Mechanistic Finding(s) | Outcome(s) | Quick Summary |
|---|---|---|---|---|---|---|
| Caraceni, 2009 [83] | Sprague-Dawley rats; partial hepatic ischemia–reperfusion (IR) + LPS; in vivo | CB1R | CB1 antagonist (Rimonabant) vs. vehicle at 3 and 10 mg/kg | CB1 antagonism reduced neutrophil infiltration, modulated cytokines (↓ TNF-α, ↑ IL-6, IFN-γ), and improved STAT3 signaling | Reduced liver injury (↓ ALT, necrosis), improved hemodynamics, reduced oxidative stress | Rimonabant mitigated LPS-enhanced hepatic IR injury via CB1 antagonism, modulating inflammation and oxidative stress. |
| Gardiner et al., 2005 [78] | Sprague-Dawley rats; 24 h LPS infusion (150 µg kg−1 h−1); conscious | CB1R | CB1 antagonist AM251 (3 mg kg−1) pretreatment | AM251 inhibited LPS-induced tachycardia & hind-quarter vasodilation, an effect mimicked by β-adrenoceptor blockade | Partial normalization of regional vascular conductance | Suggests ECS amplifies sympathetic beta-adrenergic vasodilator drive during sustained endotoxemia. |
| Joffre 2020 [64] | C57BL/6 mice, LPS endotoxemia, in vivo | CB1R | Δ9-THC (agonist) ± SR141716 (CB1 inverse agonist) | CB1 activation sharply increased IL-10 from Mo-MDSCs and suppressed IL-6/CCL-2 and NF-κB activation | Lower clinical sepsis score, reduced lung & spleen injury | Cannabinoid agonism can be protective when it drives early IL-10 via CB1R. |
| Kadoi et al., 2005 [46] | Male Wistar rats; bolus LPS (10 mg kg−1 i.v.); in vivo | CB1R | AM281 antagonist (1 mg kg−1 i.v.) given with LPS | Prevented systemic hypotension and carotid blood-flow fall; moderated TNF-α/IL-1β rise | Improved hemodynamics & halved 12 h mortality | CB1 blockade stabilized circulation and enhanced short-term survival during endotoxic shock. |
| Kadoi, 2005 [75] | Male Wistar rats; cecal ligation & puncture (CLP); | CB1R | CB1 antagonist AM281 (1 mg kg−1 i.p.) vs. vehicle | AM281 blunted caspase-3 activation in hippocampus and preserved brainstem reflexes | ↓ Neurologic dysfunction & mortality after CLP | Blocking CB1 improved survival and neurological outcomes in septic rats. |
| Kadoi, 2008 [44] | Streptozotocin-induced diabetic and non-diabetic rats; LPS-induced endotoxemia; in vivo | CB1R | CB1 antagonist (AM281) vs. vehicle | AM281 reduced hypotension in diabetic rats more effectively; affected survival rates | Improved blood pressure and survival in diabetic rats | CB1 antagonism by AM281 improved hemodynamics and survival in diabetic endotoxemia model. |
| Kianian 2014 [93] | Male Lewis rats, LPS-induced endotoxemia—in vivo | CB1R | AM281 (CB1 antagonist) ± ACEA (CB1 agonist) | CB1 blockade lowered leukocyte adhesion in intestinal venules and restored functional capillary density (FCD) | Improved gut microcirculation; no hypotension reported | Blocking CB1 during early endotoxemia rapidly normalized intestinal microvascular perfusion, suggesting CB1 drives microvascular inflammation. |
| Leite-Avalca 2016 [47] | Male Wistar rats, CLP (severe, 3 punctures); central i.c.v. cannula (in vivo) | CB1R | CB1 antagonist Rimonabant (oral or i.c.v.) vs. vehicle | CB1 blockade boosted plasma vasopressin and dropped core temperature without altering peritoneal bacterial load | ↑ 12-h and 5-day survival after CLP | Inhibiting CB1 increases AVP release and improves survival in late septic shock. |
| Leite-Avalca 2019 [48] | Wistar male rats, CLP (3 × 16 G punctures), in vivo | CB1R | Rimonabant 10 mg kg−1 p.o. at 4 h post-CLP (antagonist) vs. vehicle | Blockade did not change NOx but normalized aortic AVP reactivity and tempered AVP hyper-responsiveness | ↓ plasma lactate, LDH, CK-MB; attenuated tail-artery vasoconstriction; improved organ-dysfunction panel | Late-phase CB1 antagonism limits metabolic/vascular dysfunction in sepsis. |
| Varga 1998 [57] | Sprague-Dawley rats, LPS 15 mg kg−1 i.v.; plus adoptive transfer of LPS-stimulated macrophages/platelets; in vivo | CB1R | LPS or transfer ± CB1 antagonist SR141716A 3 mg kg−1 i.v. | LPS ↑ 2-AG in platelets & anandamide in macrophages → CB1-mediated vasodilation; SR141716A blocks hypotension | SR141716A prevents hypotension & improves survival after LPS | Macrophage anandamide + platelet 2-AG paracrine drive septic hypotension via CB1; antagonist rescues BP and survival. |
| Vercelli 2009 [94] | Pregnant mouse uterine explants; LPS challenge; in vitro | CB1R | CB1 antagonist AM-251 vs. vehicle (±exogenous AEA) | LPS raises uterine AEA (↓ FAAH) and drives iNOS-derived NO; blockade of CB1 abolishes NO spike and tissue injury | AEA–CB1 promotes NO-mediated uterine damage in septic abortion model | Endotoxin uses local ECS tone (AEA/CB1) to amplify inflammatory NO production |
| Villanueva 2009 [45] | Sprague-Dawley rats; i.v. LPS endotoxemia; in vivo | CB1R | I.c.v. rimonabant (CB1 antagonist) vs. vehicle | CB1 blockade prevents early & late hypotension and blunts LPS-evoked NE overflow in pre-optic anterior hypothalamus, plus lowers plasma TNF-α | Maintained mean arterial pressure; ↓ systemic TNF-α | Central CB1 activation is required for endotoxic hypotension |
| Çakır 2020 [36] | Sprague-Dawley rats + CLP sepsis | CB2R | JWH-133 (agonist) 0.2–5 mg kg−1 | CB2 activation lowers NF-κB, caspase-3 and pro-inflammatory cytokines in brain, lung, liver & heart | ↓ Multi-organ histopathology; ↑ IL-10 | Systemic CB2 agonism shields multiple organs from CLP-induced damage. |
| Chen 2022 [53] | C57BL/6 mice; CLP sepsis; in vivo lung (plus CD4-specific Cnr2 knock-out) | CB2R | HU-308 (CB2 agonist) vs. vehicle; CD4-Cre Cnr2^fl/fl vs. WT; | CD4-T-cell CB2R signaling suppresses IL-10; loss of CB2R switches the effect | ↓ Survival, ↑ lung injury with HU-308; protection in CD4-CB2-KO | CB2 activation on CD4 T cells hampers IL-10 and worsens CLP sepsis. |
| Chen 2025 [42] | C57BL/6 mice, CLP sepsis; BV-2 microglia in vitro, brain | CB2R | HU-308 agonist ± AM-630 inverse agonist; Nogo-B over-expression | CB2R activation ↓ Nogo-B, shifts microglia M1→M2, ↓ IL-1β/IL-6/TNF-α; neuroprotection | ↑ Cognitive scores; Nogo-B over-expression blunted benefits | CB2 activation represses Nogo-B, steering microglia to a pro-repair M2 state and easing septic encephalopathy. |
| Csóka 2009 [82] | CB2-KO vs. WT mice; CLP polymicrobial sepsis; in vivo | CB2R | Genetic knockout of CB2 | Loss of CB2 → ↓ NF-κB activation, ↓ IL-10/IL-6/MIP-2, less lymphoid apoptosis, more immune cells | Lower mortality, ↓ bacteremia & organ (kidney, muscle) injury | CB2 signaling impairs antibacterial defense; antagonism could be beneficial |
| Gui 2013 [81] | C57BL/6 mice, high-dose LPS endotoxemia—in vivo & ex vivo splenocytes/macrophages | CB2R | GW405833 (CB2 agonist) and CB2-/- knockout | Agonist inhibited ERK1/2, STAT3 and NF-κB activation in macrophages; CB2-/- mice showed exaggerated cytokine storm | GW405833 improved 72 h survival and lowered serum TNF-α/IL-6/HMGB1 | Genetic and pharmacologic evidence that CB2 signaling restrains pro-inflammatory pathways and improves survival in acute LPS sepsis. |
| Kapellos 2017 [17] | C57BL/6 WT vs. CB2−/− mice, LPS 10 mg kg−1 i.p., in vivo | CB2R | Genetic deletion | CB2 deficiency significantly increased rolling/adhesion and neutrophil influx into lung & liver (pro-recruitment) | Heightened neutrophil tissue accumulation; no mortality difference reported. | CB2 signaling restrains early neutrophil recruitment during endotoxemia. |
| Lehmann 2012 [34] | Lewis rats, LPS endotoxemia & CASP peritonitis models—in vivo | CB2R | HU308 (CB2 agonist) or AM630 (CB2 antagonist) | CB2 activation reduced intestinal leukocyte adhesion and lowered systemic cytokines in both sepsis models | Decreased inflammatory mediator release; microvascular protection | Pharmacologic CB2 stimulation dampens early hyper-inflammation in sepsis, pointing to CB2 as a therapeutic target. |
| Liu 2014 [37] | Wistar rats, CLP sepsis; lung tissue (in vivo) | CB2R | Oral Melilotus extract vs. vehicle (2 h pre-CLP) | Extract up-regulated CB2R and impeded NF-κB activation in PBMCs/lung | ↓ TNF-α, ↓ IL-6, fewer BAL neutrophils, attenuated histologic lung injury | Herbal up-regulation of CB2 breaks NF-κB-driven lung injury in polymicrobial sepsis. |
| Liu 2020 [38] | C57BL/6 mice + CLP; RAW264.7 macrophages + LPS | CB2R | HU-308 (agonist) ± 3-MA (autophagy blocker) | HU-308 triggers protective autophagy; 3-MA abrogates the effect, linking CB2 → autophagy → anti-inflammation | ↓ Lung pathology, ↓ TNF-α/IL-6, ↑ cell viability | CB2-driven autophagy is central to limiting septic lung injury. |
| Sardinha 2014 [56] | C57BL/6 mice, i.v. LPS endotoxemia; intestinal microcirculation (in vivo) | CB2R | CB2 agonist (HU-308), antagonist (AM630), FAAH inhibitor (URB597), MAGL inhibitor (JZL184) | HU-308, URB597 & JZL184 each lowered leukocyte rolling/adhesion; AM630 maintained adherent leukocyte levels | ↓ Leukocyte endothelial interactions; preserved capillary density | Multiple pharmacologic routes that raise CB2 activation reduce gut microvascular inflammation in sepsis. |
| Souza 2023 [39] | Swiss mice (♂/♀), K. pneumoniae pneumonia-sepsis (in vivo, lung) | CB2R | AM-1241 agonist (0.3/3 mg kg−1 i.p.) | CB2R activation ↓ neutrophil influx, NOS-2, MPO, protein leak; plasma IL-1β ↓, IL-10 ↑ | ↓ Lung bacterial load, injury score & 7-day mortality | Peripheral CB2 agonism tempers lung neutrophilia and systemic cytokines, boosting survival in pneumonia-sepsis. |
| Tschöp 2009 [55] | CB2-KO & WT mice; CLP; plus WT + CB2 agonist (GP1a); in vivo | CB2R | KO (loss-of-function) & agonist (GP1a) (gain-of-function) | CB2-KO → ↑ IL-6, bacteremia, lung injury, neutrophil influx & ↓ activation; GP1a reverses in WT, boosts p38 in neutrophils | KO: ↓ survival (22%); GP1a: longer survival, ↓ IL-6, ↓ bacteremia | Here CB2 supports antibacterial immunity; precise role is host-context dependent |
| Yang 2022 [43] | Male C57BL/6 mice; CLP-induced sepsis-associated encephalopathy; in vivo | CB2R | HU-308 (agonist) vs. vehicle | CB2 activation curbs microglial over-activation and neuronal pyroptosis (↓ NLRP3, GSDMD-NT) | Improved cognition (OFT, NORT, MWM) & histology | CB2 agonism safeguards the brain from CLP-induced neuro-inflammation and pyroptotic damage. |
| Zhang 2021 [16] | C57BL/6 mice + CLP (in vivo); BMDM + LPS/ATP (in vitro) | CB2R | HU-308 (agonist) vs. AM-630 (antagonist) and CB2-knock-down | CB2 activation dampens NLRP3-caspase-1-GSDMD pyroptosis cascade | ↓Pro-inflammatory cytokines, ↓ lung injury, ↑survival | CB2 agonism curbs pyroptosis-driven inflammation and protects septic mice. |
| Zhang 2023 [65] | C57BL/6 mice, CLP sepsis; heart tissue | CB2R | HU-308 agonist vs. CLP vehicle | CB2R activation ↓ NLRP3, caspase-1, GSDMD and myocardial pyroptosis; ↓ IL-1β, LDH, CK-MB | Improved myocardial histology & injury markers | HU-308-driven CB2 signaling weakens cardiac pyroptosis and biochemical injury after CLP. |
| Zhao 2023 [40] | C57BL/6 mice, LPS-induced SA-ALI; DCs in vitro/in vivo | CB2R | HU-308 agonist, SR144528 antagonist, DC-specific CNR2 KO | CB2R signaling limits dendritic-cell maturation & pro-cytokines, mitigating lung pathology | ↓ Histologic lung injury & cytokines; antagonist/KO reversed protection | CB2 signaling in DCs dampens cytokine storm and rescues LPS-driven acute lung injury. |
| Zhou 2020 [51] | Mouse peritoneal macrophages + LPS (in vitro) | CB2R | GW-405833 (agonist); 3-MA vs. MG-132 | CB2 activation enhances autophagy-lysosome flux → Cathepsin-B-dependent degradation of HMGB1 | ↓ Extracellular HMGB1 and downstream cytokines | CB2 signaling clears danger-signal HMGB1 via autophagy, tempering inflammation. |
| Braile 2021 [67] | Human neutrophils (PMNs) + LPS (in vitro) | CB1R/CB2R | ACEA (CB1 agonist)/JWH-133 (CB2 agonist) ± AM-251(CB1 antagonists)/AM630(CB2 antagonist) | Low-dose CB agonists selectively inhibit LPS-induced VEGF-A transcription & release without affecting CXCL8/HGF | ↓ Endothelial permeability & tube formation (VEGF-A-driven) | Cannabinoid signaling tempers neutrophil-driven angiogenic leakage relevant to septic vasculopathy. |
| Godlewski et al., 2004 [49] | Pithed, vagotomised Wistar rats; LPS (0.4–4 mg kg−1); in vivo | CB1R/CB2R | CB1 antagonist SR141716A; CB2 antagonist; VR1 & H3 antagonists | LPS suppressed neurogenic vasopressor response via presynaptic CB1—not CB2/VR1/H3—receptors | CB1 blockade restored sympathetic vasopressor tone in early septic shock | Endocannabinoids acting on presynaptic CB1 dampen sympathetic vasoconstriction during sepsis. |
| Li 2010 [77] | Rat jejunal myoelectrical activity & mouse charcoal transit; LPS septic ileus; in vivo | CB1R/CB2R | Agonists HU210 (CB1) & JWH133 (CB2); antagonists AM251 (CB1) & AM630 (CB2) | LPS lowers spike amplitude/frequency & GI transit; CB1/CB2 agonists mimic this, while antagonists prevent motility loss and cytokine rise | Antagonists restore GI transit and jejunal activity | Blocking either receptor averts LPS-induced gut stasis—CB1/CB2 antagonists may treat septic ileus |
| Matias 2023 [66] | Wistar rats, CLP (1- or 3-puncture) sepsis; CNS focus | CB1R/CB2R | CB1 antagonist (AM-251), CB2 antagonist (AM-630) 4 h post-CLP | Early CB1 blockade ↑ survival & prevented long-term fear-memory generalization; CB2 blockade or minocycline prevented fear generalization without ↑ survival, linking early ECS & neuro-inflam. to PTSD-like phenotype | Survival, hyperalgesia, contextual-fear tests; hippocampal TNF-α levels ↓ with minocycline | Early central CB1 (±CB2) antagonism raises survival and blocks long-term fear generalization via dampened neuro-inflammation. |
| Smith 2000 [76] | BDF1 mice (±C. parvum priming) → LPS endotoxemia (E. coli O55:B5); in vivo | CB1R/CB2R | i.p. agonists (0.05–0.4 mg HU-210; 3.1–50 mg WIN 55 212-2) ± SR141716A/SR144528 | CB1 activation ↓ TNF-α & IL-12, ↑ IL-10; effects abolished by CB1—but not CB2—antagonism | Protected primed mice from lethal LPS challenge (↑ survival) | Synthetic CB1 agonists blunt the cytokine storm and rescue survival in endotoxemic, C. parvum–primed mice. |
| Smith 2001 [68] | BDF1 mice (±C. parvum priming) → LPS endotoxemia (E. coli O55:B5); in vivo | CB1R/CB2R | Low-dose i.c.v. agonists (≈¼ i.p. dose) ± central SR141716A | Central CB1 activation suppresses TNF-α & IL-12 and elevates IL-10 at much lower doses; blockade by SR141716A, not SR144528 | Demonstrated potent cytokine modulation; survival not assessed | Brain CB1 receptors are a sensitive switch that controls systemic cytokine output during endotoxemia. |
| Steiner 2011 [58] | Conscious rats, systemic LPS (25–100 µg kg−1)—in vivo | CB1R/CB2R | Rimonabant (CB1 antagonist), SLV319, CB2/TRPV1 antagonists, ICV AEA | Central CB1 blockade abolished LPS-induced hypothermia; CB2/TRPV1 antagonism had no effect; ICV AEA enhanced hypothermia | Body-temperature drop during severe sepsis is CB1-dependent | Endocannabinoids acting on brain CB1 receptors drive the hypothermic phase of systemic inflammation, distinguishing CB1 from CB2/TRPV1 roles. |
| Bányai 2023 [71] | Wistar rats, LPS (5 mg kg−1 i.v.) endotoxemia; aorta & heart ex vivo | Mixed CB1/CB2 (THC) | Δ9-THC 10 mg kg−1 i.p. | THC ↓ 4-HNE, 3-nitrotyrosine & COX-2; preserved endothelial-dependent relaxation & ventricular volumes | Maintained cardiac output; mitigated vascular dysfunction | THC safeguards cardiovascular function in endotoxemic rats by cutting oxidative-nitrative stress and COX-2. |
| Angelina 2022 [52] | BALB/c female mice + LPS sepsis; human monocyte-DC/T-cell co-culture | WIN55,212-2 (mixed CB1/CB2 agonist) | WIN55-212-2 (mixed CB1/CB2 agonist) ± autophagy, CB1 or PPAR-α blockers | CB1/PPAR-α-driven autophagy reprograms DC metabolism → tolerogenic DCs → FOXP3+ Tregs; blocking autophagy or receptors reverses benefit | ↑ 78 h survival from 0% → 90% in lethal LPS; ↑ splenic Tregs; ↓ IL-6 | Cannabinoids re-educate immunity through autophagy-dependent DC-Treg axis, rescuing mice from endotoxic shock. |
| He 2022 [35] | MH-S alveolar-macrophage line (LPS) & C57BL/6 LPS-sepsis mice; in vitro + in vivo lung | WIN55,212-2 (mixed CB1/CB2 agonist) | WIN (0.625–40 µM in vitro; dosing in mice) vs. LPS; miR-29b-3p/FOXO3 gain–loss | WIN ↑ miR-29b-3p → ↓ FOXO3 → ↓ PFKFB3 glycolysis → shifts M1 → M2 phenotype | ↓ BALF protein, cells, lactate; better lung histology | WIN blocks macrophage glycolysis via a miR-29b-3p axis, easing LPS-induced acute lung injury. |
| Toguri 2015 [80] | Lewis rats, LPS endotoxemia; (in vivo) | WIN55,212-2 (mixed CB1/CB2 agonist) | WIN55,212-2 ± CB1 antagonist (AM281) or CB2 antagonist (AM630) | CB2 (but not CB1) activation curtailed leukocyte-endothelium adhesion and normalized micro-vascular flow | ↓ Adherent leukocytes; ↑ capillary perfusion | Systemic CB2 stimulation rapidly rescues microcirculatory dysfunction in endotoxemia. |
| Wilhelmsen 2014 [79] | Primary human lung microvascular ECs (HMVEC-lung) stimulated with LPS/FSL-1/TNF-α (in vitro) | WIN55,212-2 (mixed CB1/CB2 agonist) | NADA or WIN vs. vehicle ± CB1/CB2 antagonists | WIN and NADA dose-dependently cut IL-6/IL-8 release & neutrophil adhesion; NADA effect is CB1/CB2-dependent; | ↓ Endothelial cytokines & leukocyte binding; no effect on permeability | Endothelial ECS (via NADA or WIN) is a brake on cytokine-driven vascular activation relevant to sepsis. |
| Singh 2018 [50] | Swiss-albino mice, CLP (6 h & 20 h), ex vivo aortic rings | 2-AG/CB1R | DAGL inhibitor (KT-109), MAGL inhibitor (JZL-184), CB1 antagonist (AM-251) | Sepsis up-regulated CB1R & reduced MAGL mRNA; blocking DAGL or CB1 restored NA-induced vasoconstriction | KT-109 or AM-251 normalized vascular reactivity; improved mean arterial tone (survival not assessed). | Endocannabinoid 2-AG acting on CB1 underlies septic vasoplegia. |
| Sultan 2021 [41] | Female C57BL/6 Mice; SEB-induced ARDS (systemic super-antigen sepsis); in vivo lung | Anandamide (AEA) ligand | Exogenous AEA 40 mg kg−1 vs. vehicle | AEA down-regulates miR-23a-3p/miR-34a-5p → ↑ ARG1, TGF-β2, FOXP3 → expansion of MDSCs & Tregs; ↓ IL-2, IFN-γ, TNF | ↑ Lung function, ↓ cell infiltrates, cytokines | AEA re-programs miRNA networks to tilt immunity toward MDSC/Treg-driven resolution of SEB-ARDS. |
| Murakami 2009 [69] | C57BL/6 mice sensitized with D-galactosamine + LPS (endotoxin shock, in vivo) ± RAW264.7 cells (in vitro) | Anandamide (ligand) | Cationic antimicrobial peptide CAP11 vs. scrambled control | CAP11 prevents LPS-induced rise in AEA, HMGB1, TNF-α & IL-6 in macrophages and in serum | ↓ systemic mediators, improved survival in shock model; suppresses ECS ligand generation | CAP11’s protection is partly via curbing endocannabinoid (AEA) over-production |
| Liu, 2006 [73] | RAW264.7 macrophages with/without LPS; mouse brain extracts; in vitro | AEA biosynthetic enzymes (NAPE-PLD, PLC, PTPN22) | LPS stimulation; siRNA knock-down; overexpression; enzyme inhibitors | LPS up-regulates a PLC–phosphatase pathway (involving PTPN22) that converts NAPE→phospho-AEA→AEA, bypassing NAPE-PLD | ↑ AEA synthesis under endotoxin challenge; identifies alternate biosynthetic route | Defined a novel PLC/PTPN22 route for LPS-induced anandamide production in macrophages. |
| De Filippis, 2008 [74] | Swiss OF1 mice; LPS-induced sepsis; in vivo + intestinal muscle strips | CB1 receptor, FAAH (indirectly through cannabidiol) | Cannabidiol alone and with CB1 antagonist (AM251) | Sepsis upregulated CB1 and FAAH in intestine; cannabidiol reversed FAAH increase but worsened motility via CB1 | Cannabidiol reduced motility in septic mice; CB1 antagonist reversed this effect | Cannabidiol worsened sepsis-induced ileus through CB1 signaling; reversed by CB1 antagonist. |
| Szafran 2015 [70] | Female C57BL/6 mice, single i.p. LPS (6–24 h); spleen & splenocytes (in vivo & ex vivo) | Ces2g (2-AG-hydrolyzing carboxylesterase) | Endotoxin vs. saline | LPS selectively suppressed Ces2g activity → ↓ 2-AG hydrolysis in spleen & splenocytes | Potential ↑ 2-AG levels (not directly measured)—suggests feedback dampening of inflammation | Endotoxin shuts down a 2-AG catabolic enzyme, potentially prolonging anti-inflammatory eCB signaling during systemic inflammation. |
| Wolfson 2013 [59] | Murine peripheral blood mononuclear cells (PBMCs) from non-pregnant vs. pregnant mice—in vitro/in vivo LPS | FAAH | Progesterone (PR agonist) ± PR antagonist | LPS lowered FAAH activity in PBMCs; progesterone restored FAAH via progesterone-receptor signaling | Normalized FAAH prevents excess anandamide buildup linked to pregnancy failure | Hormonal regulation can counter LPS-driven suppression of FAAH, highlighting cross-talk between endocrine and ECS during systemic inflammation. |
| Kianian 2013 [54] | Lewis rats, LPS endotoxemia—in vivo | FAAH & CB2 | URB597 (FAAH inhibitor) ± AM630 (CB2 antagonist) | FAAH inhibition raised endocannabinoids, cutting leukocyte adhesion and increasing FCD; CB2 blockade reversed adhesion benefit | Enhanced intestinal perfusion; reduced microvascular inflammation | Elevating endogenous cannabinoids via FAAH inhibition protects gut microcirculation during endotoxemia, largely through CB2. |
| Chen 2022 [60] | C57BL/6 mice + CLP sepsis; serum from septic patients (observational) | GPR55 (non-classical ECS GPCR) | CID16020046 (antagonist) 20 mg kg−1 | GPR55 blockade suppresses ROCK1/2 signaling, inflammation and apoptosis in kidney | ↓ BUN/Creatinine, ↓ KIM-1/NGAL, ↓ TNF-α/IL-6, no off-target toxicity | Targeting GPR55 mitigates sepsis-induced AKI via anti-inflammatory/anti-apoptotic routes. |
| Joffre 2022 [61] | C57BL/6 mice, LPS i.v./i.t. endotoxemia; S. aureus i.t. pneumonia/sepsis; in vivo; plus BMDM, HMVEC, astrocytes in vitro | TRPV1; OLDA agonist | OLDA 5–10 mg/kg i.v. given at 0–2 h post-challenge vs. vehicle; WT vs. Trpv1−/−; cell-specific TRPV1 knockdowns; monocyte/macrophage depletion | Pan-neuronal (CNS) TRPV1 triggers an early surge in IL-10, suppresses IL-6/TNF/chemokines; reversed with knockdown, not with PNS or myeloid TRPV1 knockdown. Monocyte depletion blocks OLDA-induced IL-10. | ↓ Lung injury, ↑ Lung IL-10; improved mouse sepsis scores; bacterial load unchanged in S. aureus model. | CNS Neuronal TRPV1 activation by ODA results in a neuro-immune pathway that drives peripheral monocyte IL-10; attenuates systemic inflammation; reduces clinical severity |
| Lawton 2017 [95] | Mice, LPS i.v., CLP; ex vivo hematopoietic | TRPV1; NADA agonist | NADA 1–10 mg/kg i.v. (often 5–10 mg/kg) ± LPS/CLP; WT vs. Trpv1−/−; bone-marrow chimeras | Non-hematopoietic cell TRPV1 (pointing to neuronal) reduces systemic pro-inflammatory cytokines and PAI-1; raises IL-10. NADA alters neuropeptides (↓ CGRP, ↑ substance P) during LPS. | ↑ Survival in endotoxemic mice; ↓ inflammatory mediator levels across models; ↑ IL-10, ↓ IL-6, CCL2, PAI-1 | Nonhematopoietic TRPV1 activation attenuates systemic inflammation and improves survival in endotoxemic mice |
| Orliac 2007 [96] | Rat, i.p. endotoxemia (6 h); isolated mesenteric vascular bed; in vivo + ex vivo | TRPV1; AEA agonist | LPS vs. saline; ex vivo anandamide (0.01–10 μM) and capsaicin; TRPV1 antagonist capsazepine; PKC activator PMA | LPS increases TRPV1 and CGRP-positive nerve density in mesenteric arteries; ↑ AEA mediated CGRP and vasorelaxation (TRPV1 dependent) post-LPS; PKC activation ↑ AEA efficacy | Upregulation of TRVP1 during endotoxemia implicated in septic hypotension | Early endotoxemia upregulates TRPV1/CGRP signaling in perivascular sensory nerves, amplifying AEA-driven CGRP release and vasodilation; indicates an ECS–TRPV1 contribution to vascular changes in sepsis. |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Chechulina, V.; Sheikh, F.; Lóser, M.; Englesakis, M.; Barrett, K.; Canada, S. Healthcare costs after sepsis: A systematic review. Crit. Care 2025, 29, 381. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Prescott, H.C. Sepsis and Septic Shock. N. Engl. J. Med. 2024, 391, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Kissoon, N.; Reinhart, K.; Daniels, R.; Machado, M.F.R.; Schachter, R.D.; Finfer, S. Sepsis in Children: Global Implications of the World Health Assembly Resolution on Sepsis. Pediatr. Crit. Care Med. 2017, 18, e625–e627. [Google Scholar] [CrossRef]
- Robey, R.C.; Logue, C.; Caird, C.A.; Hansel, J.; Hellyer, T.P.; Simpson, J.; Dark, P.; Mathioudakis, A.G.; Felton, T. Immunomodulatory drugs in sepsis: A systematic review and meta-analysis. Anaesthesia 2024, 79, 869–879. [Google Scholar] [CrossRef]
- Liu, D.; Langston, J.C.; Prabhakarpandian, B.; Kiani, M.F.; Kilpatrick, L.E. The critical role of neutrophil-endothelial cell interactions in sepsis: New synergistic approaches employing organ-on-chip, omics, immune cell phenotyping and. Front. Cell. Infect. Microbiol. 2023, 13, 1274842. [Google Scholar]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.-C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Singer, M.; Leone, M. Sepsis: Key insights, future directions, and immediate goals. A review and expert opinion. Intensiv. Care Med. 2024, 50, 2043–2049. [Google Scholar] [CrossRef]
- Cuddihey, H.; Macnaughton, K.W.; Sharkey, A.K. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92. [Google Scholar] [CrossRef]
- Gong, F.; Zheng, X.; Xu, W.; Xie, R.; Liu, W.; Pei, L.; Zhong, M.; Shi, W.; Qu, H.; Mao, E.; et al. H3K14la drives endothelial dysfunction in sepsis-induced ARDS by promoting SLC40A1/transferrin-mediated ferroptosis. MedComm 2025, 6, e70049. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hong, Z.; Huang, L.S.; Tsukasaki, Y.; Nepal, S.; Di, A.; Zhong, M.; Wu, W.; Ye, Z.; Gao, X.; et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020, 130, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yu, Y.; Kang, R.; Zhu, S.; Yang, L.; Zeng, L.; Sun, X.; Yang, M.; Billiar, T.R.; Wang, H.; et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 2016, 7, 13280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, W.; Wen, T.; Zheng, G.; Qiu, G.; Qian, H.; Zhang, R.; Xia, J.; Hu, Y.; Huang, R.; et al. Extracellular vesicle-bound S100A8/A9 is differentially expressed in septic shock and prompts acute lung injury. Respir. Res. 2025, 26, 107. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, F.; Liu, A.; Li, Z.; Zheng, F.; Liu, Q.; Yang, L.; Chen, K.; Wang, Y.; Zhang, Z.; et al. Activation of CB2 receptor inhibits pyroptosis and subsequently ameliorates cecal ligation and puncture-induced sepsis. Int. Immunopharmacol. 2021, 99, 108038. [Google Scholar] [CrossRef]
- Kapellos, S.T.; Recio, C.; Greaves, R.D.; Iqbal, J.A. Cannabinoid Receptor 2 Modulates Neutrophil Recruitment in a Murine Model of Endotoxemia. Mediat. Inflamm. 2017, 2017, 4315412. [Google Scholar] [CrossRef]
- Tortora, C.; Di Paola, A.; Argenziano, M.; Creoli, M.; Marrapodi, M.M.; Cenni, S.; Tolone, C.; Rossi, F.; Strisciuglio, C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines 2022, 10, 874. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Martínez-Torres, S.; Bergadà-Martínez, A.; Ortega, J.E.; Galera-López, L.; Hervera, A.; Reyes-Ramírez, L.d.L.; Ortega-Álvaro, A.; Remmers, F.; Muñoz-Moreno, E.; Soria, G.; et al. Peripheral CB1 receptor blockade acts as a memory enhancer through a noradrenergic mechanism. Neuropsychopharmacology 2023, 48, 341–350. [Google Scholar] [CrossRef]
- Nogueiras, R.; Veyrat-Durebex, C.; Suchanek, P.M.; Klein, M.; Tschōp, J.; Caldwell, C.; Woods, S.C.; Wittmann, G.; Watanabe, M.; Liposits, Z.; et al. Peripheral, but Not Central, CB1 Antagonism Provides Food Intake–Independent Metabolic Benefits in Diet-Induced Obese Rats. Diabetes 2008, 57, 2977–2991. [Google Scholar] [CrossRef] [PubMed]
- Contino, M.; Mccormick, J.P. Editorial: The Canonical and Non-Canonical Endocannabinoid System as a Target in Cancer and Acute and Chronic Pain. Front. Pharmacol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Haugh, O.; Penman, J.; Irving, A.; Campbell, V. The Emerging Role of the Cannabinoid Receptor Family in Peripheral and Neuro-immune Interactions. Curr. Drug Targets 2016, 17, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Kasatkina, A.L.; Rittchen, S.; Sturm, M.E. Neuroprotective and Immunomodulatory Action of the Endocannabinoid System under Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5431. [Google Scholar] [CrossRef]
- Jaiswal, S.; Ayyannan, R.S. Anticancer Potential of Small-Molecule Inhibitors of Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase. ChemMedChem 2021, 16, 2172–2187. [Google Scholar] [CrossRef]
- Kouchi, Z. Physiological Role of Endocannabinoid Hydrolyzing Enzymes in Brain Development and Neurodegeneration. Biochem. Physiol. 2015, 4, 2. [Google Scholar]
- Antcliffe, D.B.; Burnham, K.L.; Al-Beidh, F.; Santhakumaran, S.; Brett, S.J.; Hinds, C.J.; Ashby, D.; Knight, J.C.; Gordon, A.C. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 980–986. [Google Scholar] [CrossRef]
- Ma, H.; Wang, W.; Tang, B.; Zhang, H.; Tan, C.; Yang, Y. Inflammatory Protein Signatures of Sepsis Risk and Mortality: A Mendelian Randomization Study. Shock 2025, 64, 148–153. [Google Scholar] [CrossRef]
- Reyes, M.; Filbin, R.M.; Bhattacharyya, P.R.; Billman, K.; Eisenhaure, T.; Hung, T.D.; Levy, B.D.; Baron, R.M.; Blainey, P.C.; Goldberg, M.B.; et al. An immune-cell signature of bacterial sepsis. Nat. Med. 2020, 26, 333–340. [Google Scholar] [CrossRef]
- Deng, H.; Li, J.; Shah, A.A.; Lin, G.; Chen, H.; Ouyang, W. Commonly expressed key transcriptomic profiles of sepsis in the human circulation and brain via integrated analysis. Int. Immunopharmacol. 2022, 104, 108518. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Thai, B.; Yamamoto, H.; Koutrouvelis, A.; Yamamoto, S. Endocannabinoid system in sepsis: A scoping review protocol. Open Sci. Framew. 2025. [Google Scholar] [CrossRef]
- Lehmann, C.; Kianian, M.; Zhou, J.; Küster, I.; Kuschnereit, R.; Whynot, S.; Hung, O.; Shukla, R.; Johnston, B.; Cerny, V.; et al. Cannabinoid receptor 2 activation reduces intestinal leukocyte recruitment and systemic inflammatory mediator release in acute experimental sepsis. Crit. Care 2012, 16, R47. [Google Scholar] [CrossRef]
- He, Q.; Yin, J.; Zou, B.; Guo, H. WIN55212-2 alleviates acute lung injury by inhibiting macrophage glycolysis through the miR-29b-3p/FOXO3/PFKFB3 axis. Mol. Immunol. 2022, 149, 119–128. [Google Scholar] [CrossRef]
- Cakir, M.; Tekin, S.; Okan, A.; Cakan, P.; Doganyigit, Z. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture-induced sepsis model in rats. Int. Immunopharmacol. 2020, 78, 105978. [Google Scholar] [CrossRef]
- Liu, M.-W.; Su, M.-X.; Wang, Y.-H.; Wei, W.; Qin, L.-F.; Liu, X.; Tian, M.-L.; Qian, C.-Y. Effect of melilotus extract on lung injury by upregulating the expression of cannabinoid CB2 receptors in septic rats. BMC Complement. Altern. Med. 2014, 14, 94. [Google Scholar] [CrossRef]
- Liu, A.; Yuan, Q.; Zhang, B.; Yang, L.; He, Q.; Chen, K.; Liu, Q.; Li, Z.; Zhan, J. Cannabinoid receptor 2 activation alleviates septic lung injury by promoting autophagy via inhibition of inflammatory mediator release. Cell. Signal. 2020, 69, 109556. [Google Scholar] [CrossRef]
- Souza, C.F.; Borges, L.B.; Oliveira, F.R.M.B.; Silva, P.C.d.S.; Patricio, D.O.; Rosales, T.O.; Souza, N.F.; Spiller, F.; Mansur, D.S.; Assreuy, J.; et al. Cannabinoid CB2 receptor agonist reduces local and systemic inflammation associated with pneumonia-induced sepsis in mice. Eur. J. Pharmacol. 2023, 959, 176092. [Google Scholar] [CrossRef]
- Zhao, F.-Z.; Gu, W.-J.; Li, L.-Z.; Qu, Z.-K.; Xu, M.-Y.; Liu, K.; Zhang, F.; Liu, H.; Xu, J.; Yin, H.-Y. Cannabinoid receptor 2 alleviates sepsis-associated acute lung injury by modulating maturation of dendritic cells. Int. Immunopharmacol. 2023, 123, 110771. [Google Scholar] [CrossRef]
- Sultan, M.; Alghetaa, H.; Mohammed, A.; Abdulla, O.A.; Wisniewski, P.J.; Singh, N.; Nagarkatti, P.; Nagarkatti, M. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating miRNA that Target Inflammatory Pathways. Front. Pharmacol. 2021, 12, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Z.; Yang, L.; Xu, Z.; Liu, A.; He, Q.; Xiao, F.; Zhan, J. Cannabinoid Receptor-2 Alleviates Sepsis-Induced Neuroinflammation by Modulating Microglia M1/M2 Subset Polarization Through Inhibiting Nogo-B Expression. Mol. Neurobiol. 2025, 62, 9258–9270. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Z.; Xu, Z.; Zhang, B.; Liu, A.; He, Q.; Zheng, F.; Zhan, J. Protective Effects of Cannabinoid Type 2 Receptor Activation Against Microglia Overactivation and Neuronal Pyroptosis in Sepsis-Associated Encephalopathy. Neuroscience 2022, 493, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kadoi, Y.; Hinohara, H.; Kunimoto, F.; Saito, S. Effects of the cannabinoid antagonist AM281 on systemic hemodynamics and mortality rate in streptozotocin-induced diabetic rats with endotoxic shock: Comparison between non-diabetic and diabetic rats. Acta Anaesthesiol. Scand. 2008, 52, 664–672. [Google Scholar] [CrossRef]
- Villanueva, A.; Yilmaz, S.M.; Millington, W.R.; Cutrera, R.A.; Stouffer, D.G.; Parsons, L.H.; Cheer, J.F.; Feleder, C. Central cannabinoid 1 receptor antagonist administration prevents endotoxic hypotension affecting norepinephrine release in the preoptic anterior hypothalamic area. Shock 2009, 32, 614–620. [Google Scholar] [CrossRef]
- Kadoi, Y.; Hinohara, H.; Kunimoto, F.; Kuwano, H.; Saito, S.; Goto, F. Effects of AM281, a cannabinoid antagonist, on systemic haemodynamics, internal carotid artery blood flow and mortality in septic shock in rats. Br. J. Anaesth. 2005, 94, 563–568. [Google Scholar] [CrossRef]
- Leite-Avalca, C.G.M.; Lomba, A.L.; Bastos-Pereira, L.A.; Brito, O.H.; Fraga, D.; Zampronio, R.A. Involvement of Central Endothelin ETA and Cannabinoid CB1 Receptors and Arginine Vasopressin Release in Sepsis Induced by Cecal Ligation and Puncture in Rats. Shock 2016, 46, 290–296. [Google Scholar] [CrossRef]
- Leite-Avalca, M.C.G.; Staats, F.T.; Verona, D.; de Souza, P.; Almeida, M.C.; Silva-Santos, J.E.; Zampronio, A.R. Cannabinoid CB1 Receptor Antagonist Rimonabant Decreases Levels of Markers of Organ Dysfunction and Alters Vascular Reactivity in Aortic Vessels in Late Sepsis in Rats. Inflammation 2019, 42, 618–627. [Google Scholar] [CrossRef]
- Godlewski, G.; Malinowska, B.; Schlicker, E. Presynaptic cannabinoid CB1 receptors are involved in the inhibition of the neurogenic vasopressor response during septic shock in pithed rats. Br. J. Pharmacol. 2004, 142, 701–708. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P.; Nakade, U.P.; Sharma, A.; Gari, M.; Choudhury, S.; Shukla, A.; Garg, S.K. Endocannabinoid-mediated modulation of Gq protein-coupled receptor mediates vascular hyporeactivity to nor-adrenaline during polymicrobial sepsis. Pharmacol. Rep. 2018, 70, 1150–1157. [Google Scholar] [CrossRef]
- Zhou, H.; Du, R.; Li, G.; Bai, Z.; Ma, J.; Mao, C.; Wang, J.; Gui, H. Cannabinoid receptor 2 promotes the intracellular degradation of HMGB1 via the autophagy-lysosome pathway in macrophage. Int. Immunopharmacol. 2020, 78, 106007. [Google Scholar] [CrossRef]
- Angelina, A.; Pérez-Diego, M.; López-Abente, J.; Rückert, B.; Nombela, I.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.; Palomares, O. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming. Mucosal Immunol. 2022, 15, 96–108. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Zhang, S.; Lin, Q.; Xu, H.; Zhu, T.; Peng, L.; Cen, F.; Li, F.; Wang, Z.; et al. Activation of CD4+ T Cell–Derived Cannabinoid Receptor 2 Signaling Exacerbates Sepsis via Inhibiting IL-10. J. Immunol. 2022, 208, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Kianian, M.; Al-Banna, A.N.; Kelly, E.M.M.; Lehmann, C. Inhibition of endocannabinoid degradation in experimental endotoxemia reduces leukocyte adhesion and improves capillary perfusion in the gut. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, J.; Kasten, K.R.; Nogueiras, R.; Goetzman, H.S.; Cave, C.M.; England, L.G.; Dattilo, J.; Lentsch, A.B.; Tschöp, M.H.; Caldwell, C.C. The Cannabinoid Receptor 2 Is Critical for the Host Response to Sepsis. J. Immunol. 2009, 183, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, J.; Kelly, M.E.M.; Zhou, J.; Lehmann, C. Experimental Cannabinoid 2 Receptor-Mediated Immune Modulation in Sepsis. Mediat. Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef]
- Varga, K.; Wagner, A.J.; Bridgen, T.D.; Kunos, G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998, 12, 1035–1044. [Google Scholar] [CrossRef]
- Steiner, A.A.; Molchanova, Y.A.; Dogan, D.M.; Patel, S.; Pétervári, E.; Balaskó, M.; Wanner, S.P.; Eales, J.; Oliveira, D.L.; Gavva, N.R.; et al. The hypothermic response to bacterial lipopolysaccharide critically depends on brain CB1, but not CB2 or TRPV1, receptors. J. Physiol. 2011, 589, 2415–2431. [Google Scholar] [CrossRef]
- Wolfson, L.M.; Aisemberg, J.; Salazar, I.A.; Rubio, D.P.A.; Vercelli, A.C.; Franchi, M.A. Progesterone reverts LPS-reduced FAAH activity in murine peripheral blood mononuclear cells by a receptor-mediated fashion. Mol. Cell. Endocrinol. 2013, 381, 97–105. [Google Scholar] [CrossRef]
- Chen, R.; Xu, H.; Guo, Z.; Zhang, P.; Chen, J.; Chen, Z. CID16020046, a GPR55 antagonist, attenuates sepsis-induced acute kidney injury. Mol. Med. Rep. 2022, 25, 155. [Google Scholar] [CrossRef]
- Joffre, J.; Wong, E.; Lawton, S.; Lloyd, E.; Nguyen, N.; Xu, F.; Sempio, C.; Kobzik, L.; Zlatanova, I.; Schumacher, M.; et al. N-Oleoyl dopamine induces IL-10 via central nervous system TRPV1 and improves endotoxemia and sepsis outcomes. J. Neuroinflamm. 2022, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Pommerolle, L.; Arif, M.; Meliton, A.Y.; Udofia, I.; Wu, D.; Mutlu, G.M.; Gochuico, B.R.; Summer, R.; Adegunsoye, A.; et al. Elevated blood anandamide levels in acute COVID-19 pneumonia with respiratory failure. Am. J. Med. Sci. 2025, 370, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Termine, A.; Fabrizio, C.; Gimenez, J.; Panuccio, A.; Balsamo, F.; Passarello, N.; Caioli, S.; Saba, L.; De Bardi, M.; Della Valle, F.; et al. Transcriptomic and Network Analyses Reveal Immune Modulation by Endocannabinoids in Approach/Avoidance Traits. Int. J. Mol. Sci. 2022, 23, 2538. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Yeh, C.-C.; Wong, E.; Thete, M.; Xu, F.; Zlatanova, I.; Lloyd, E.; Kobzik, L.; Legrand, M.; Hellman, J. Activation of CB1R Promotes Lipopolysaccharide-Induced IL-10 Secretion by Monocytic Myeloid-Derived Suppressive Cells and Reduces Acute Inflammation and Organ Injury. J. Immunol. 2020, 204, 3339–3350. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Chen, S.; Xu, Z.; Zhang, B.; Liu, A.; He, Q.; Zhan, J. Activation of cannabinoid receptors 2 alleviates myocardial damage in cecal ligation and puncture-induced sepsis by inhibiting pyroptosis. Immunol. Lett. 2023, 264, 17–24. [Google Scholar] [CrossRef]
- Matias, E.M.; Radulski, R.D.; Silva, D.R.T.; Raymundi, M.A.; Stern, J.A.C.; Zampronio, R.A. Involvement of cannabinoid receptors and neuroinflammation in early sepsis: Implications for posttraumatic stress disorder. Int. Immunopharmacol. 2023, 123, 110745. [Google Scholar] [CrossRef]
- Braile, M.; Cristinziano, L.; Marcella, S.; Varricchi, G.; Marone, G.; Modestino, L.; Ferrara, A.L.; De Ciuceis, A.; Scala, S.; Galdiero, M.R.; et al. LPS-mediated neutrophil VEGF-A release is modulated by cannabinoid receptor activation. J. Leukoc. Biol. 2021, 109, 621–631. [Google Scholar] [CrossRef]
- Smith, R.S.; Terminelli, C.; Denhardt, G. Modulation of cytokine responses in Corynebacterium parvum-primed endotoxemic mice by centrally administered cannabinoid ligands. Eur. J. Pharmacol. 2001, 425, 73–83. [Google Scholar] [CrossRef]
- Murakami, T.; Obata, T.; Kuwahara-Arai, K.; Tamura, H.; Hiramatsu, K.; Nagaoka, I. Antimicrobial cathelicidin polypeptide CAP11 suppresses the production and release of septic mediators in D-galactosamine-sensitized endotoxin shock mice. Int. Immunol. 2009, 21, 905–912. [Google Scholar] [CrossRef]
- Szafran, B.; Borazjani, A.; Lee, H.J.; Ross, K.M.; Kaplan, L.F.B. Lipopolysaccharide suppresses carboxylesterase 2g activity and 2-arachidonoylglycerol hydrolysis: A possible mechanism to regulate inflammation. Prostaglandins Other Lipid Mediat. 2015, 121, 199–206. [Google Scholar] [CrossRef]
- Bányai, B.; Répás, C.; Miklós, Z.; Johnsen, J.; Horváth, M.E.; Benkő, R. Delta 9-tetrahydrocannabinol conserves cardiovascular functions in a rat model of endotoxemia: Involvement of endothelial molecular mechanisms and oxidative-nitrative stress. PLoS ONE 2023, 18, e0287168. [Google Scholar] [CrossRef]
- Antcliffe, D.B.; Mi, Y.; Santhakumaran, S.; Burnham, K.L.; Prevost, A.T.; Ward, J.K.; Marshall, T.J.; Bradley, C.; Al-Beidh, F.; Hutton, P.; et al. Patient stratification using plasma cytokines and their regulators in sepsis: Relationship to outcomes, treatment effect and leucocyte transcriptomic subphenotypes. Thorax 2024, 79, 515–523. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Harvey-White, J.; Osei-Hyiaman, D.; Razdan, R.; Gong, Q.; Chan, A.C.; Zhou, Z.; Huang, B.X.; Kim, H.Y.; et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA 2006, 103, 13345–13350. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Iuvone, T.; D’Amico, A.; Esposito, G.; Steardo, L.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y.; De Man, J.G. Effect of cannabidiol on sepsis-induced motility disturbances in mice: Involvement of CB1 receptors and fatty acid amide hydrolase. Neurogastroenterol. Motil. 2008, 20, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Kadoi, Y.; Hinohara, H.; Kunimoto, F.; Saito, S.; Goto, F. Cannabinoid antagonist AM 281 reduces mortality rate and neurologic dysfunction after cecal ligation and puncture in rats. Crit. Care Med. 2005, 33, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Terminelli, C.; Denhardt, G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000, 293, 136–150. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, Y.-N.; Ni, J.-B.; Chen, C.-J.; Lv, S.; Chai, S.-Y.; Wu, R.-H.; Yüce, B.; Storr, M. Involvement of cannabinoid-1 and cannabinoid-2 receptors in septic ileus. Neurogastroenterol. Motil. 2010, 22, 350-e88. [Google Scholar] [CrossRef]
- Gardiner, M.S.; March, E.J.; Kemp, A.P.; Bennett, T. Involvement of CB1-receptors and β-adrenoceptors in the regional hemodynamic responses to lipopolysaccharide infusion in conscious rats. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H2280–H2288. [Google Scholar] [CrossRef]
- Wilhelmsen, K.; Khakpour, S.; Tran, A.; Sheehan, K.; Schumacher, M.; Xu, F.; Hellman, J. The Endocannabinoid/Endovanilloid N-Arachidonoyl Dopamine (NADA) and Synthetic Cannabinoid WIN55,212-2 Abate the Inflammatory Activation of Human Endothelial Cells. J. Biol. Chem. 2014, 289, 13079–13100. [Google Scholar] [CrossRef]
- Toguri, J.T.; Moxsom, R.; Szczesniak, A.M.; Zhou, J.; Kelly, M.E.M.; Lehmann, C. Cannabinoid 2 receptor activation reduces leukocyte adhesion and improves capillary perfusion in the iridial microvasculature during systemic inflammation. Clin. Hemorheol. Microcirc. 2015, 61, 237–249. [Google Scholar] [CrossRef]
- Gui, H.; Sun, Y.; Luo, Z.-M.; Su, D.-F.; Dai, S.-M.; Liu, X. Cannabinoid Receptor 2 Protects against Acute Experimental Sepsis in Mice. Mediat. Inflamm. 2013, 2013, 741303. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Németh, Z.H.; Mukhopadhyay, P.; Spolarics, Z.; Rajesh, M.; Federici, S.; Deitch, E.A.; Bátkai, S.; Pacher, P.; Haskó, G. CB2 Cannabinoid Receptors Contribute to Bacterial Invasion and Mortality in Polymicrobial Sepsis. PLoS ONE 2009, 4, e6409. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Pertosa, A.M.; Giannone, F.; Domenicali, M.; Grattagliano, I.; Principe, A.; Mastroleo, C.; Perrelli, M.G.; Cutrin, J.; Trevisani, F.; et al. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia-reperfusion injury complicated by endotoxaemia. Gut 2009, 58, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Weis, S.; Sauer, A.; Wendel-Garcia, P.; David, S. Targeting the host response in sepsis: Current approaches and future evidence. Crit. Care 2023, 27, 478. [Google Scholar] [CrossRef]
- Leite-Avalca, M.C.G.; Zampronio, A.; Lehmann, C. Cannabinoid Receptor 1 and 2 Signaling Pathways Involved in Sepsis. Shock 2021, 56, 673–681. [Google Scholar] [CrossRef]
- Chun, K.; Syndergaard, C.; Damas, C.; Trubey, R.; Mukindaraj, A.; Qian, S.; Jin, X.; Breslow, S.; Niemz, A. Sepsis Pathogen Identification. J. Lab Autom. 2015, 20, 539–561. [Google Scholar] [CrossRef]
- Piomelli, D.; Mabou Tagne, A. Endocannabinoid-Based Therapies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 483–507. [Google Scholar] [CrossRef]
- Ciaramellano, F.; Fanti, F.; Scipioni, L.; Maccarrone, M.; Oddi, S. Endocannabinoid Metabolism and Transport as Drug Targets. Methods Mol. Biol. 2023, 2576, 201–211. [Google Scholar]
- ClinicalTrials.gov. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of CB1 Antagonist ANEB-001 in a THC Challenge Test. 2023. Available online: https://clinicaltrials.gov/study/NCT05282797 (accessed on 18 September 2025).
- ClinicalTrials.gov. Evaluation of the Relative Bioavailability of PF-04457845 Tablet to Solution Formulation and Food Effect in Healthy Subjects. 2009. Available online: https://clinicaltrials.gov/study/NCT00918164 (accessed on 18 September 2025).
- López-Sendón Moreno, J.L.; García Caldentey, J.; Trigo Cubillo, P.; Ruiz Romero, C.; García Ribas, G.; Alonso Arias, M.A.; García de Yébenes, M.J.; Tolón, R.M.; Galve-Roperh, I.; Sagredo, O.; et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J. Neurol. 2016, 263, 1390–1400. [Google Scholar] [CrossRef]
- Peball, M.; Werkmann, M.; Ellmerer, P.; Stolz, R.; Valent, D.; Knaus, H.-G.; Ulmer, H.; Djamshidian, A.; Poewe, W.; Seppi, K. Nabilone for non-motor symptoms of Parkinson’s disease: A randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (The NMS-Nab Study). J. Neural Transm. 2019, 126, 1061–1072. [Google Scholar] [CrossRef]
- Kianian, M.; Kelly, M.E.; Zhou, J.; Hung, O.; Cerny, V.; Rowden, G.; Lehmann, C. Cannabinoid receptor 1 inhibition improves the intestinal microcirculation in experimental endotoxemia. Clin. Hemorheol. Microcirc. 2014, 58, 333–342. [Google Scholar] [CrossRef]
- Vercelli, C.; Aisemberg, J.; Billi, S.; Cervini, M.; Ribeiro, M.; Farina, M.; Franchi, A. Anandamide regulates lipopolysaccharide-induced nitric oxide synthesis and tissue damage in the murine uterus. Reprod. Biomed. Online 2009, 18, 824–831. [Google Scholar] [CrossRef]
- Lawton, S.K.; Xu, F.; Tran, A.; Wong, E.; Prakash, A.; Schumacher, M.; Hellman, J.; Wilhelmsen, K. N-Arachidonoyl Dopamine Modulates Acute Systemic Inflammation via Nonhematopoietic TRPV1. J. Immunol. 2017, 199, 1465–1475. [Google Scholar] [CrossRef]
- Orliac, M.L.; Peroni, R.N.; Abramoff, T.; Neuman, I.; Podesta, E.J.; Adler-Graschinsky, E. Increases in vanilloid TRPV1 receptor protein and CGRP content during endotoxemia in rats. Eur. J. Pharmacol. 2007, 566, 145–152. [Google Scholar] [CrossRef]
| Characteristic | Category | n (%) |
|---|---|---|
| Animal Model * | Mice | 31(58%) |
| Rats | 21 (40%) | |
| Other | 5 (9%) | |
| Sepsis Model * | LPS | 37 (70%) |
| CLP | 17 (32%) | |
| Bacterial Exposure | 2 (4%) | |
| Primary ECS Target | CB1R | 12 (23%) |
| CB2R | 17 (32%) | |
| CB1R + CB2R | 7 (13%) | |
| Receptor Ligand | 8 (15%) | |
| ECS Enzyme | 5 (9%) | |
| Noncanonical Receptor | 4 (8%) | |
| Organ/Tissue Studied * | Lung | 26 (49%) |
| Heart | 15 (28%) | |
| Brain | 10 (19%) | |
| Kidney/Liver | 5 (9%) | |
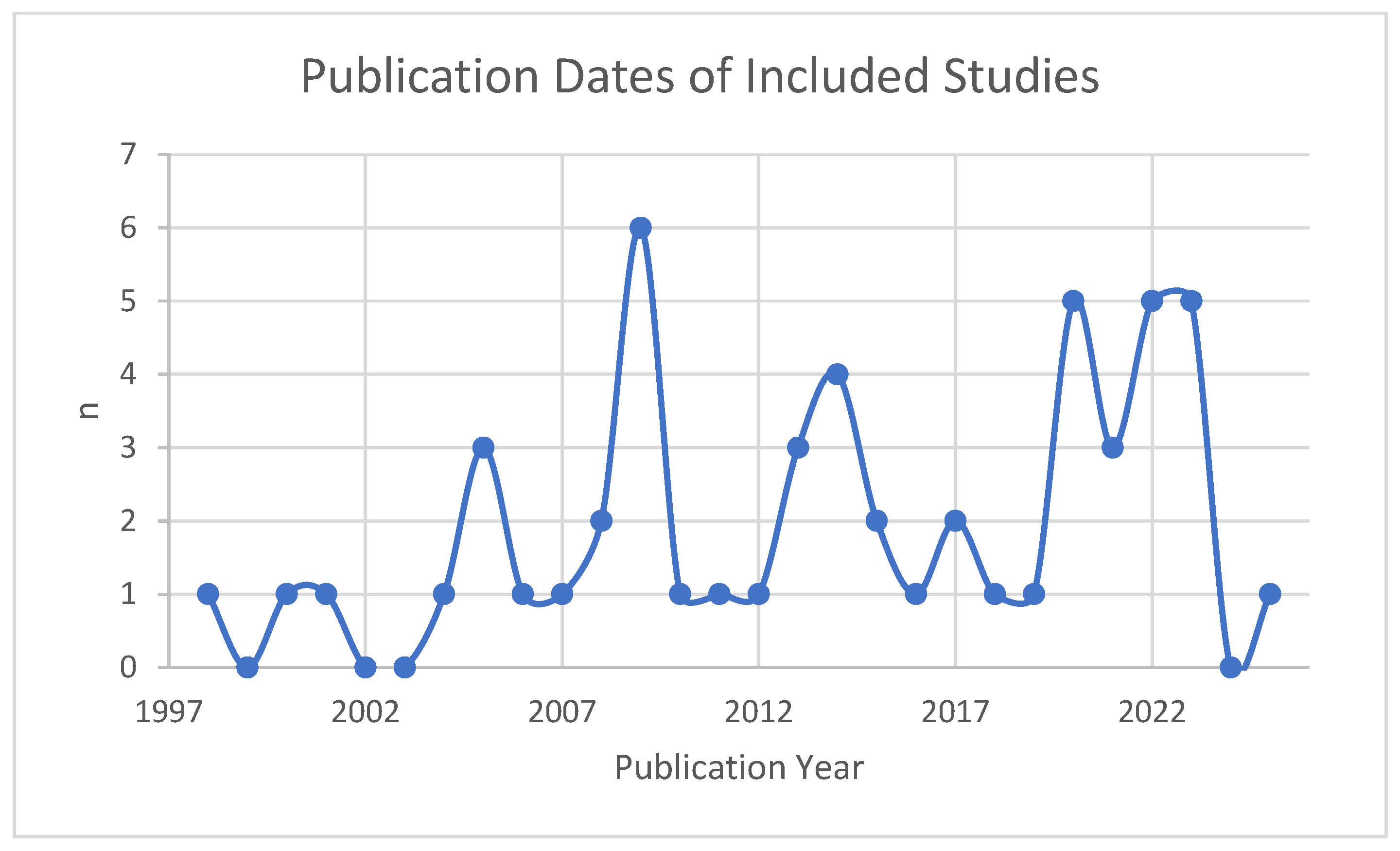
| Publication Year | 1998–2004 | 4 (8%) |
| 2005–2009 | 13 (25%) | |
| 2010–2014 | 10 (19%) | |
| 2015–2019 | 7 (13%) | |
| 2020–2025 | 19 (36%) |
| ECS Component | Modulation Type | Studies (n) | Direction of Effect | Summary of Outcome Trends |
|---|---|---|---|---|
| CB1R | Activation | 4 | ↑ Sepsis Severity | ↑ Mediates hypotension/hypothermia and inflammatory damage |
| Activation | 6 | ↓ Sepsis Severity | ↑ Increases Il-10 production, reduced inflammatory markers (TNF-α and Il-12), increases survival | |
| Inhibition/Knockout | 0 | ↑ Sepsis Severity | N/A | |
| Inhibition/Knockout | 13 | ↓ Sepsis Severity | ↓ Stabilize hemodynamics, through reduction in hypotension/dilation and increases response to vasopressors | |
| CB2R | Activation | 1 | ↑ Sepsis Severity | ↑ Reduces IL-10 production in CD4-T cells, increasing lung injury |
| Activation | 17 | ↓ Sepsis Severity | ↑ Reduces NLRP3 inflammasome related pyroptosis, leukocyte adhesion, pro-inflammatory cytokine (IL-6, TNF-α). Increases autophagy, especially in macrophages | |
| Inhibition/Knockout | 2 | ↑ Sepsis Severity | ↓ Results in increased leukocyte adhesion/influx | |
| Inhibition/Knockout | 2 | ↓ Sepsis Severity | ↓ Decreases mortality and organ damage, improves septic ileus | |
| TRPV1 | Activation | 2 | ↓ Sepsis Severity | ↑ Increases IL-10 production, reduces IL-6 |
| FAAH | Modulation | 2 | Mixed Sepsis Severity | ↑ Reduced septic pregnancy loss, ↓Protected gut microcirculation |
| MAGL | Inhibition | 1 | ↓ Sepsis Severity | Reduced leukocyte adhesion and gut inflammation |
| AEA | Modulation | 2 | Mixed Sepsis Severity | ↑ Reduced lung injury, ↓ Reduced shock survival |
| Δ9-THC | Administration | 1 | ↓ Sepsis Severity | Reduced oxidative-nitrative cardiovascular stress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thai, B.; Yamamoto, H.; Koutrouvelis, A.; Yamamoto, S. Endocannabinoid System in Sepsis: A Scoping Review. Anesth. Res. 2025, 2, 24. https://doi.org/10.3390/anesthres2040024
Thai B, Yamamoto H, Koutrouvelis A, Yamamoto S. Endocannabinoid System in Sepsis: A Scoping Review. Anesthesia Research. 2025; 2(4):24. https://doi.org/10.3390/anesthres2040024
Chicago/Turabian StyleThai, Brandon, Hideaki Yamamoto, Aristides Koutrouvelis, and Satoshi Yamamoto. 2025. "Endocannabinoid System in Sepsis: A Scoping Review" Anesthesia Research 2, no. 4: 24. https://doi.org/10.3390/anesthres2040024
APA StyleThai, B., Yamamoto, H., Koutrouvelis, A., & Yamamoto, S. (2025). Endocannabinoid System in Sepsis: A Scoping Review. Anesthesia Research, 2(4), 24. https://doi.org/10.3390/anesthres2040024







