Abstract
Jamoytius kerwoodi, is a primitive, eel-like jawless vertebrate found uniquely in an Early Silurian (Llandovery epoch; 444–433 Ma) horizon near Lesmahagow, Scotland. This species is a rare component of a low-diversity dominantly nektonic detritus-feeding and herbivorous fauna living over an anoxic bottom and is found at the transition from a marine-influenced, probably brackish-water, deep-water basin to a shallower-water, less saline and likely freshwater basin. In the absence of true teeth, Jamoytius was probably a detritivore or herbivore feeding on Dictyocaris. Jamoytius may have a common ancestor with living lampreys, especially as their ectoparasitic mode of life might have evolved from ancestral detritivores or herbivores.
1. Introduction
Jamoytius kerwoodi White was a primitive, eel-like jawless fish that lived in the Llandovery epoch (444–433 Ma) of the Early Silurian period [1] (Figure 1). The fossil is preserved as rare carbonized films on bedding planes in one laminated siltstone horizon in the bank of the Logan water in the Lesmahagow inlier of Lanarkshire, SW Scotland [2]. It was once considered the most primitive known vertebrate [1], but with additional studies, its affinities are now debatable [3,4,5,6,7,8]. Because the interpretations of such exceptionally preserved soft-bodied fossils is difficult, observed features can be interpreted in different ways [9,10,11] (Figure 2). Various cladistic analyses of Jamoytius with other jawless vertebrates, using different character codings, give divergent results [7,12,13,14,15,16,17,18,19,20]. Choice of the in-group taxa affects its placement [17,21,22]. The position of Jamoytius on cladograms has consequently not stabilized, though it often appears as a sister taxon to euphaneropids, and/or lampreys, and/or anaspids [5,15,17,23,24,25] (Figure 3).
As a sister taxon to the lampreys, Jamoytius has been compared with parasitic lampreys which attack fish. But, only 18 of the 38 known species of lamprey, are carnivorous [26]. Living nonparasitic lampreys appear to be derived from parasitic species with heterochronic shifts in metamorphosis [27]. Adult non-parasitic lampreys tend to be somewhat smaller (10 to 20 cm long) than adult parasitic lampreys (15 to 120 cm long) (Figure 4), whose size is related to life history trade offs; either become a parasite/predator with high growth potential, fecundity and mortality, or remain a detritivore with lower growth rates, fecundities and mortality [26,28], Size is, however, not diagnostic and cannot be used to distinguish parasitic from non-parasitic forms [29], and the supposed parasitic Paleozoic forms may not have been such, but grazers or scavengers (see below).
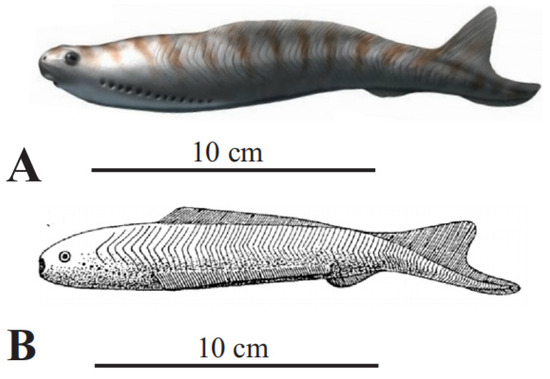
Figure 1.
Jamoytius reconstructions: (A) with ventral “lamprey” mouth (with permission from Nobu Tamura); (B) with terminal suspension/detritus feeding mouth ([2], plate 11).
Figure 1.
Jamoytius reconstructions: (A) with ventral “lamprey” mouth (with permission from Nobu Tamura); (B) with terminal suspension/detritus feeding mouth ([2], plate 11).
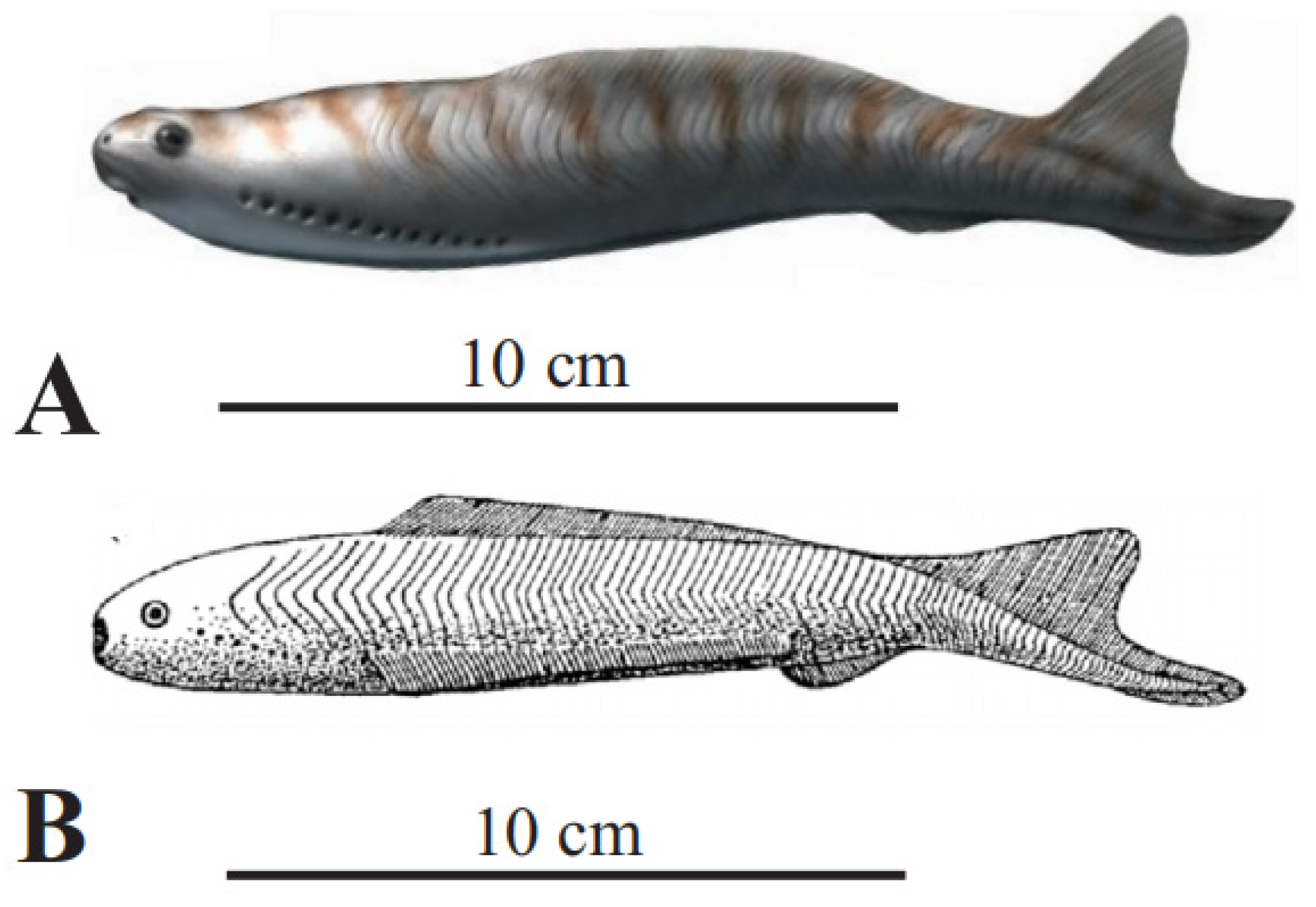
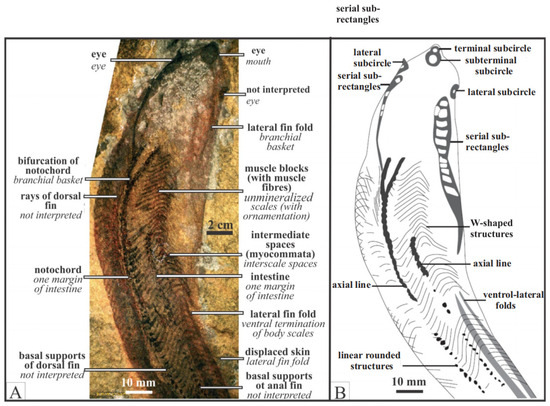
Figure 2.
Jamoytius fossil and inferred features: (A) with the conflicting interpretations of White [1], in bold, and Ritchie [2,18,25,30] in plain italics (modified from Sansom et al. [31] (Plate 1); (B) body parts and topological interpretation of the holotype (NHM P11284a), from [31] (text Figure 5).
Figure 2.
Jamoytius fossil and inferred features: (A) with the conflicting interpretations of White [1], in bold, and Ritchie [2,18,25,30] in plain italics (modified from Sansom et al. [31] (Plate 1); (B) body parts and topological interpretation of the holotype (NHM P11284a), from [31] (text Figure 5).
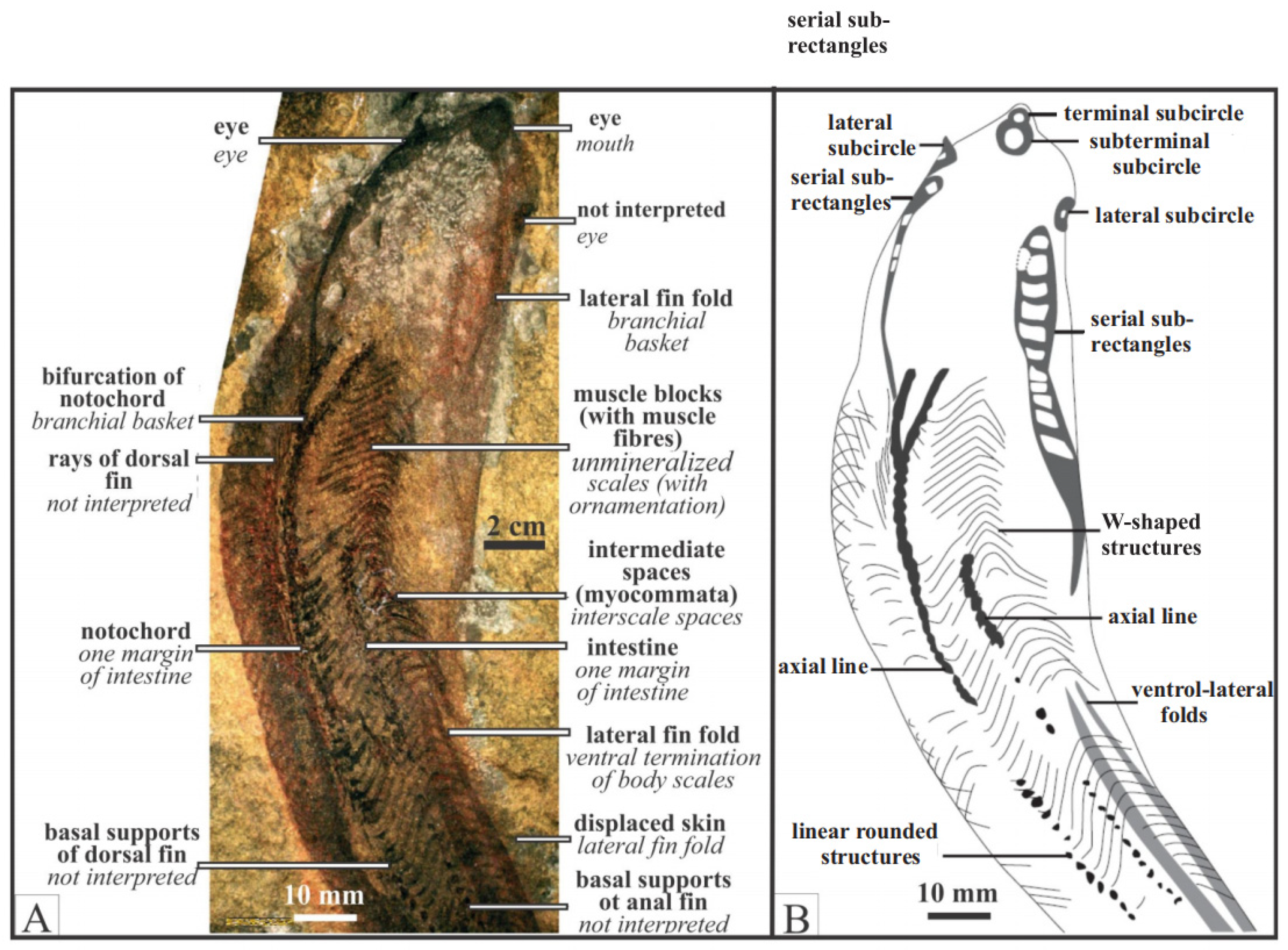
Figure 3.
Examples of cladistic analyses showing three interpretations for Jamoytius. (A): Shu et al. (1999) [19], (B): Donoghue et al. (2001) [21], where Ostraco/Jawed represents other ostracoderms and jawed vertebrates, (C): Sansom et al. (2010) [31].
Figure 3.
Examples of cladistic analyses showing three interpretations for Jamoytius. (A): Shu et al. (1999) [19], (B): Donoghue et al. (2001) [21], where Ostraco/Jawed represents other ostracoderms and jawed vertebrates, (C): Sansom et al. (2010) [31].
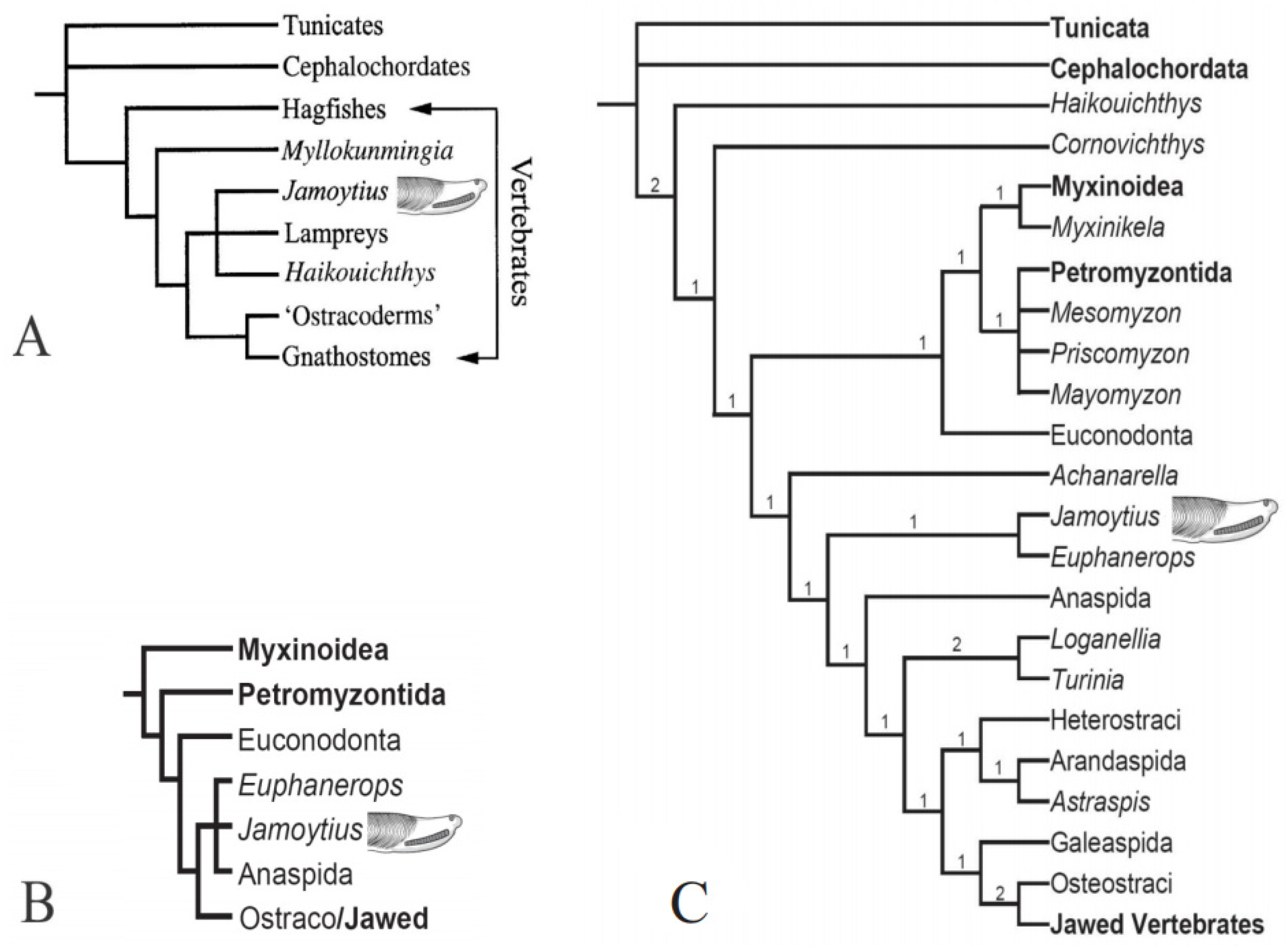
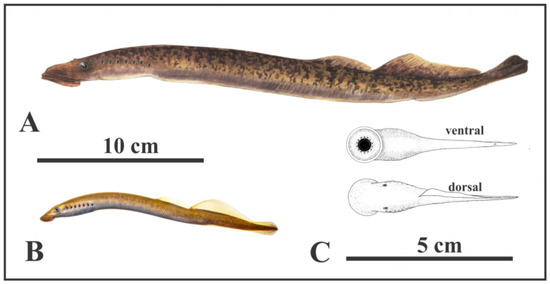
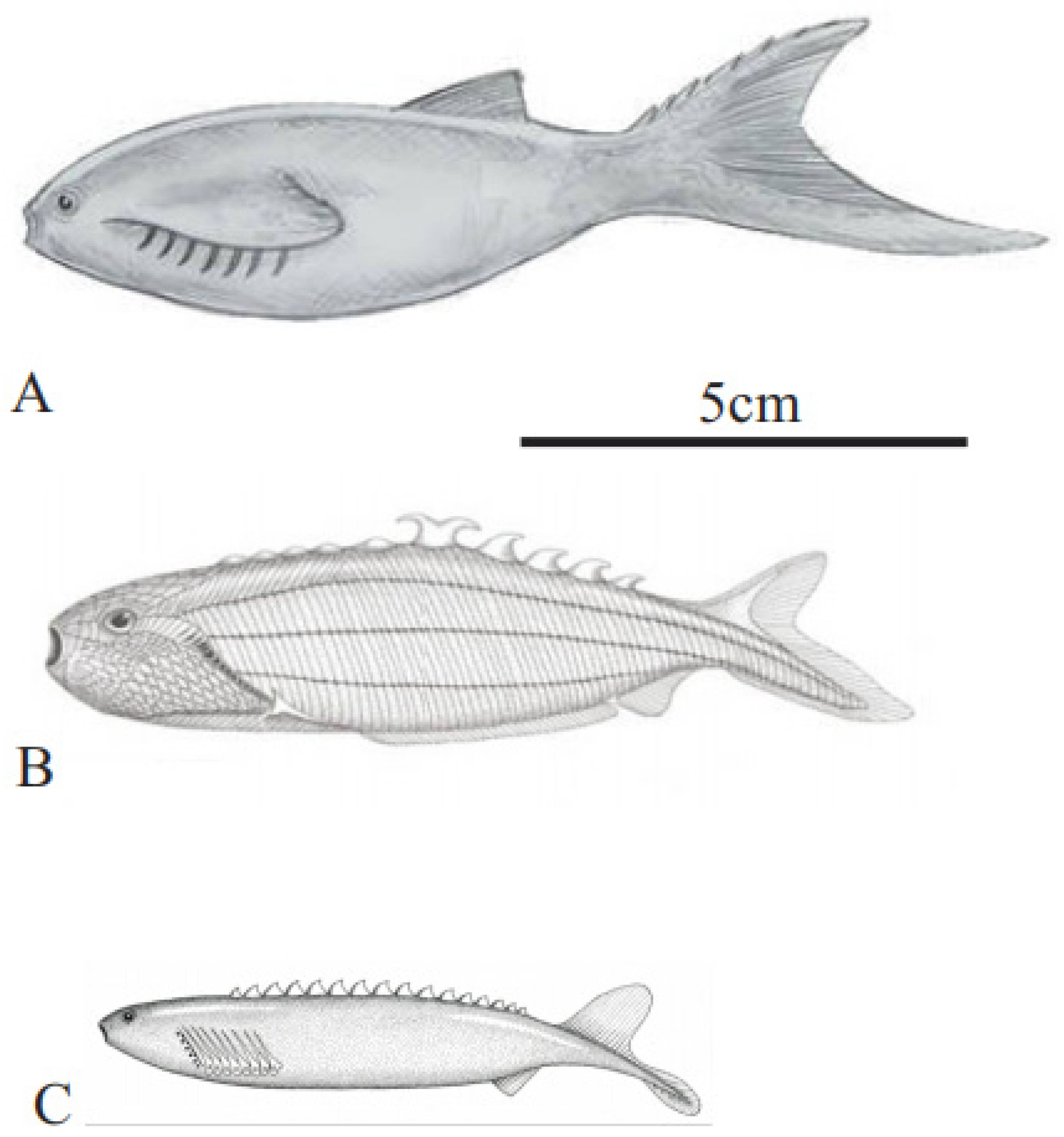
Figure 4.
Living and oldest fossil (Devonian) lampreys. (A) Parasitic sea lamprey (Petromyzon marinus Linnaeus), 35–60 cm long; (B) American brook lamprey (Lethenteron appendix DeKay) 15–25 cm long (A,B) courtesy of North Carolina Wildlife Resources Commission; (C) Devonian parasitic fossil lamprey (Priscomyzon riniensis Gess et al. [22], ~5 cm long (public domain).
Figure 4.
Living and oldest fossil (Devonian) lampreys. (A) Parasitic sea lamprey (Petromyzon marinus Linnaeus), 35–60 cm long; (B) American brook lamprey (Lethenteron appendix DeKay) 15–25 cm long (A,B) courtesy of North Carolina Wildlife Resources Commission; (C) Devonian parasitic fossil lamprey (Priscomyzon riniensis Gess et al. [22], ~5 cm long (public domain).
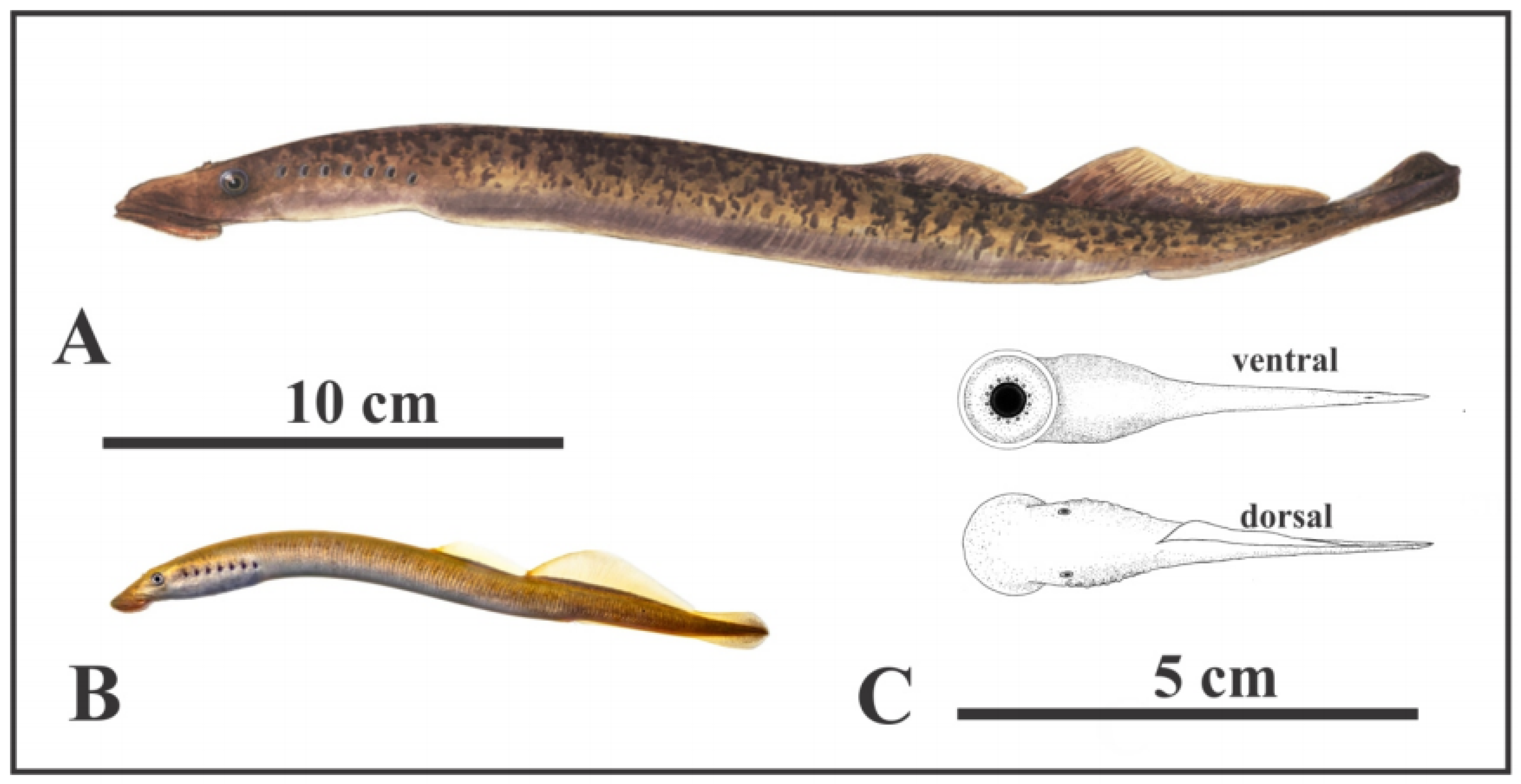
The fossil evidence for early evolution of lampreys is limited. Based on both morphological and molecular evidence, Brownstein and Near [32] estimated that 90% of living lamprey clades originated only since the late Cretaceous. As reconstructed by Reeves and Sansom [11], carnivorous lampreys evolved from non-carnivorous early Paleozoic forms in the Jurassic, when innovations of their feeding apparatus may underlie their evolutionary increase of the body size and the ‘modernization’ of their life-history during the Jurassic period [33], and then radiated from the late Cretaceous times (~100 Ma) and especially from Miocene times (~25 Ma) onwards into many both carnivorous and non-carnivorous forms.
Only four undoubted Palaeozoic lamprey species have been recorded, the Devonian (419–359 Ma) Priscomyzon riniensis, from South Africa considered the oldest parasitic lamprey [22]. Priscomyzon, and three from the Carboniferous (359–299 Ma) [8]. These Paleozoic lampreys might not, however, be parasitic as conventionally assumed, as they have tiny dentition and a small buccal cavity (which accommodates the anti-coagulant secreting glands and food processing in living parasitic lampreys) and lack an ammocoete stage [8,34]. Wu et al. [33] speculated that the well-developed oral discs and attaching skills of these early lampreys might be adaptations to grazing algal mats, which would fit with the mode of life proposed here for Jamoytius and other similar forms like Euthanerops.
As a sister taxon to the anaspids (Figure 3B), Jamoytius resembles the genera, Loganellia, Birkenia, and, especially, Lasanius [35] (Figure 5). Loganellia also occurs in the Jamoytius bed, while Birkenia and Lasanius occur in slightly younger fish beds in the Lesmahagow succession [36,37].
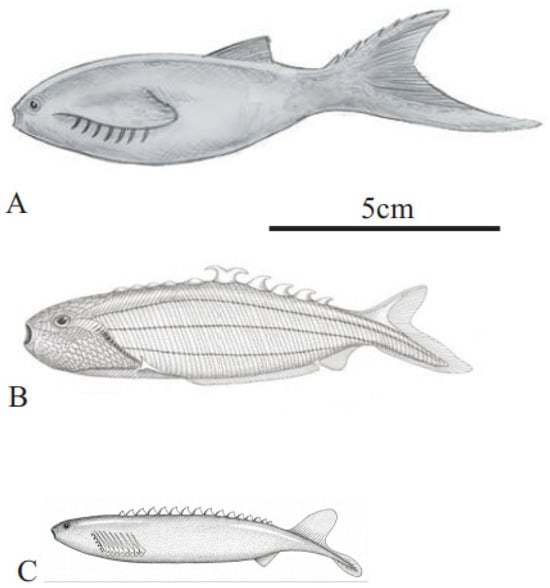
Figure 5.
Agnathan reconstructions: (A) Thelodont Loganellia scotica (https://creativecommons.org/licenses/by-sa/4.0/, permission Norbu Tamura); (B) Anaspid Birkenia (creative common, Highlander Fossils; (C) Anaspid? Lasanius (creative commons, permission Rob Van Assen).
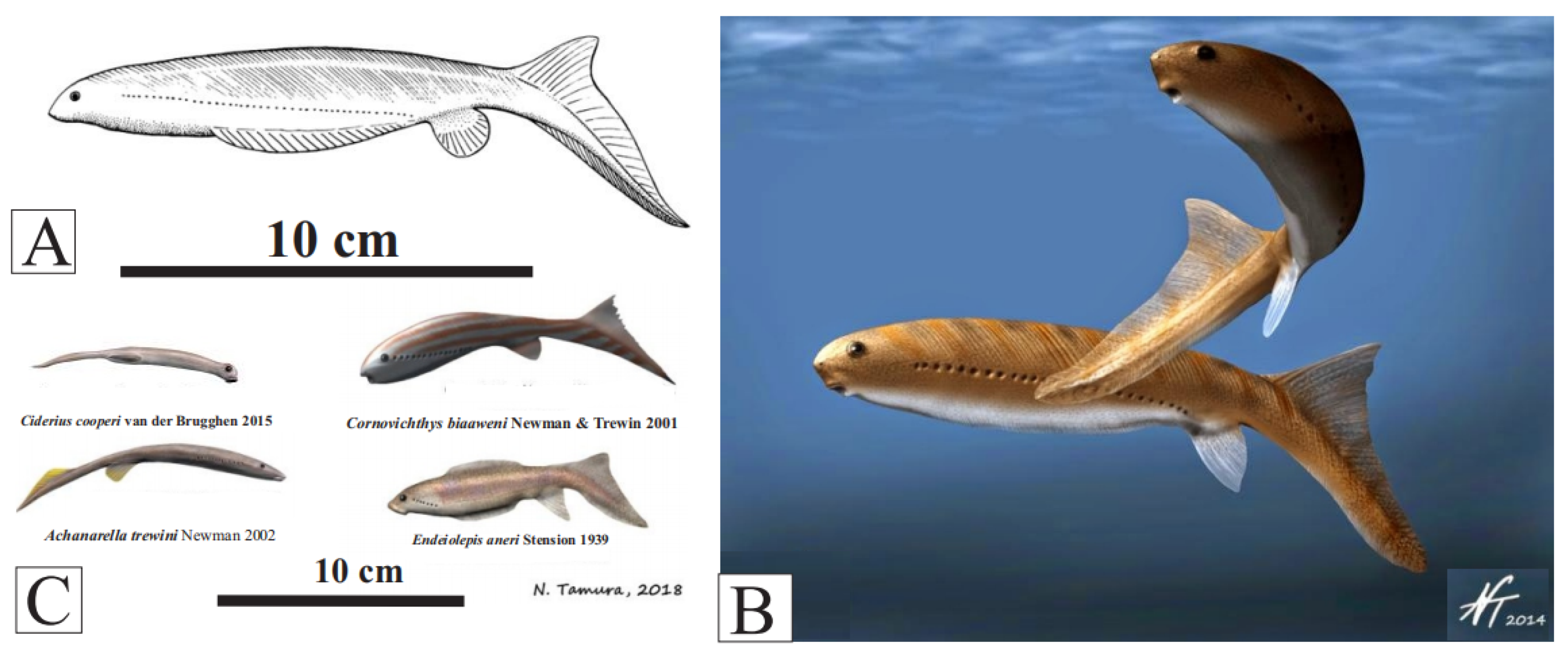
Jamoytius is often classified as a sister taxon to the Upper Devonian fish, Euphanerops, originally grouped together in the Jamoytiforms [38] (Figure 3C), though many of the structures in the available fossils remain unexplained [24]. Several other euphaneropids have now been recognized: one, Ciderius cooperi van der Brugghen from the fish beds above the Jamoytius bed at Lesmahagow [39]. These are similar to Jamoytius, both in anatomy and possibly mode of life (Figure 6).
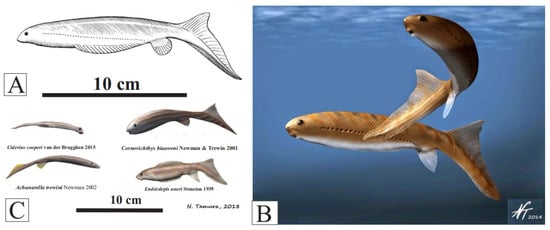
Figure 6.
(A) Euphanerops longaevus reconstruction (Upper Devonian, Canada) https://www.miguasha.ca/mig-en/euphanerops.php (public domain) (consulted 7 August 2023); (B) Euphanerops longaevus as a swimming nektonic detritivore/herbivore (with permission from Nobu Tamura); (C) various euphaneropids; Ciderius couperi, (Lower Silurian), Achanarella trewinii, Cornovichthys blaaeweni (Middle Devonian), Endiolepis aneri (Upper Devonian) (with permission from Nobu Tamura).
The mode of life of Jamoytius kerwoodi is thus unresolved; even its life orientation is still not certain [31]. In this paper I am not particularly concerned with its affinities, but with its mode of life as inferred from its anatomy (which bears, of course, on its affinities), adaptative morphology and palaeoenvironment based on the sedimentology of the enclosing strata and the lifestyles of it and its associated biota.
2. Anatomy
Because the preservation of soft-bodied organism like Jamoytius is so variable, and because there are often so few fossils of them preserved, then even their basic anatomy is subject to different interpretations, leading to radically different reconstructions and affinities [11].
Jamoytius had an elongated body, ranging from 14–18 cm long by 3–4 cm wide, a cartilaginous skeleton, akin to the branchial basket of lampreys, and weakly mineralized scales [31]. Earlier reconstructions show side-fins running the length of its body, but these are now interpreted as artifacts formed as a corpse was squashed post-burial. A ring-like stain, interpreted as cartilage, encircles the very small ‘mouth’ (seen as 0.5 to 0.7 mm in diameter in Figure 2), which suggested to Ritchie [2,18,25] that it was an ancestral parasitic lamprey. Jamoytius, however, apparently had no true teeth or teeth-like structures, in its ‘mouth’ [31], If Jamoytius had rasping keratin teeth like living parasitic lampreys, as Stensiö [40] inferred for Norwegian anapsids, then these should probably be preserved carbonized, as is much of the rest of the animal (Figure 2). The third most abundant vertebrate fossils (after bones and teeth) are keratin-derived materials such as skin and feathers [41,42].
The controversy about whether this ‘mouth’ was anterior terminal, or subterminal ventral, seems to be resolved in favour of the latter [31]. Towards the anterior end, many specimens preserve a pair of linear features composed of serially repeated, contiguous, sub-rectangular shapes, interpreted as branchial openings [31]. The anterior of Jamoytius has room for a piston-like tongue comparable with living parasitic lampreys [34]. In living parasitic lampreys, this holds the biting and cutting plates used to parasitize fish, which are not present in Jamoytius. On the other hand, such plates would not be required to eat soft vegetation, which is a possibility considering the holes in associated Dictyoocaris (see Section 5), and Jamoytius does not have the lamprey lips used for suction [43].
Most specimens do not preserve the posterior portion of Jamoytius, and where they do, it is too faint to be seen clearly [31]. So, the inferred hypocercal tail is reconstructed only by analogy with other near-contemporary anaspids, like Birkenia and Lasanius [17,44].
3. Mode of Life of Jamoytius
Jamoytius has been compared with parasitic lampreys which attack fish [2] (Figures 2A and 3). But, only 18 of the 38 known species of lamprey, are carnivorous. The ancestral crown lamprey was probably a freshwater nonparasitic species, some of which evolved into parasites [32]. Living non-parasitic lampreys are smaller (less than 40 cm long) than parasitic sea lampreys (35–120 cm long), and all inhabit freshwater [26]. The non-carnivorous lampreys do not eat at all, since they have a nonfunctional intestine, only live for four to six months on the energy stored when young; as a result, they typically have small mouths and poorly-developed teeth, useless for attaching to a host, and die after spawning [45]. For example, Lethenteron appendix, the American brook lamprey, has small larvae (1–2 cm long) that feed on algae and detritus for between three and seven years, before metamorphosing into sexually mature adults (15–25 cm long) [46]. The size and anatomy of Jamoytius is more compatible with non-carnivorous living lampreys, though the Devonian inferred parasitic lamprey, Priscomyzon riniensis, is also very small [22] (Figure 4C).
The comparable agnathans, have terminal anterior mouths which do not appear to be protrusible (Figure 5). Such mouths are often found among omnivorous mid-water feeders, which eating anything available, by grabbing bits of food as they move [47].
4. Paleoenvironments
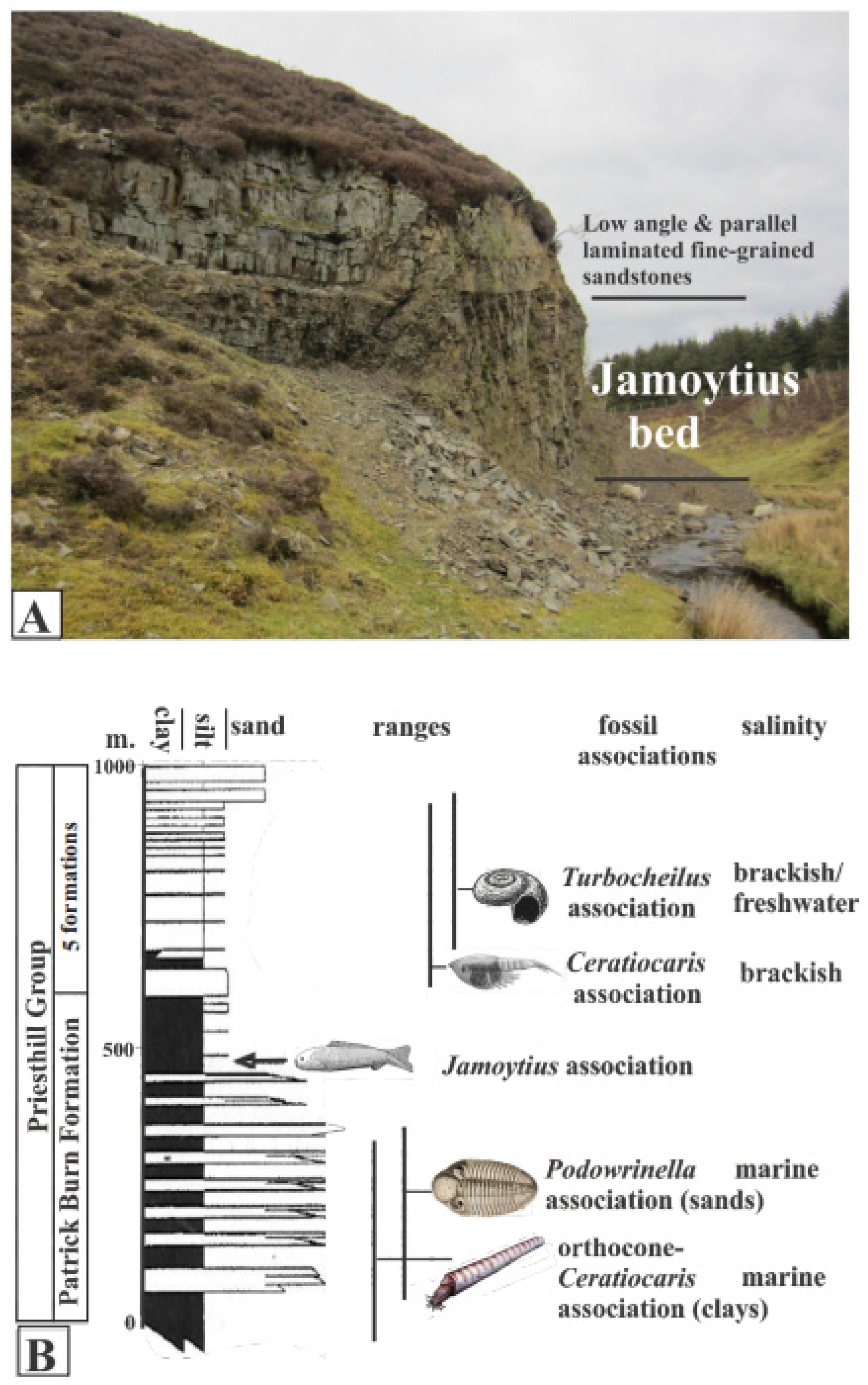
The Jamoytius-bearing horizon is one of several eurypterid- and fish-bearing units in the Silurian (Llandoverian; 444–433 Ma) Priesthill Group of the Lesmahagow inlier in the Midland Valley of Scotland [18,30,48]. It is exposed at Birk Knowes in a cliff next to the Logan Water (NS737346) (Figure 7A) near the top of the Patrick Burn Formation, an over 500-m-thick section of alternating grey sandstones, siltstones, and mudstones [37,49] (Figure 7B). The sediments of the Patrick Burn Formation change gradually upwards from deeper water interbedded mudstone/turbidite sandstone facies into shallower water interbedded mudstone/cross-bedded and laminated sandstone facies [37,49]. This is accompanied by changes in the taxonomy and ecology of the fossil biotas from marine to freshwater [30,37,49] (Figure 7B).
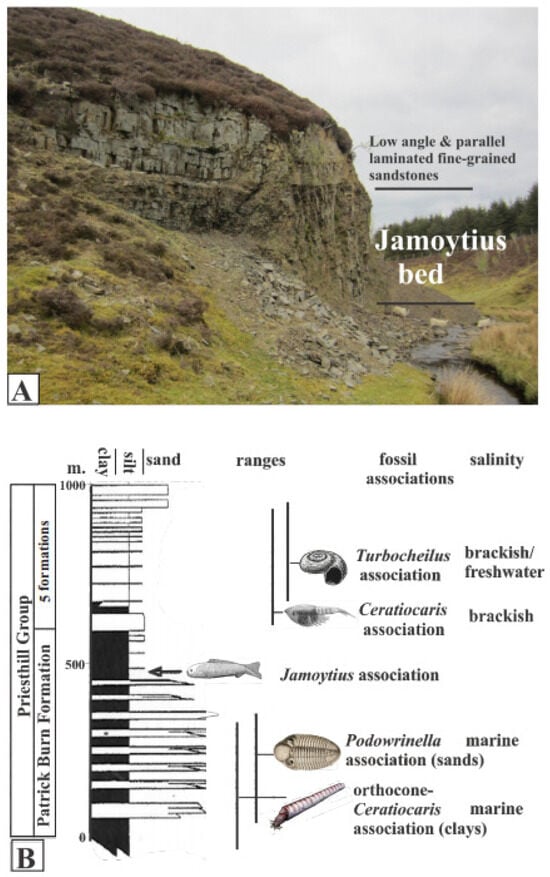
Figure 7.
(A) Birk Knowes exposure of Jamoytius beds. (B) Section of Priesthill Group with fossil associations, ranges, and inferred salinity (modified from Lovelock, 1998 [22]).
Throughout the Patrick Burn Formation, and in the Jamoytius bed itself, there is a complete absence of burrowing organisms and there are no tracks or trails on the bedding plane surfaces [37] The undisturbed nature of the sediments, together with abundant pyrite and organic matter, indicates anaerobic bottom conditions in very quiet water subject to periodic underflows [30]; in keeping with the abundance of Ceratiocaris. Geochemical evidence indicates a gradual salinity drop through the upper part of the Priesthill Group, which contains all the fish beds [49].
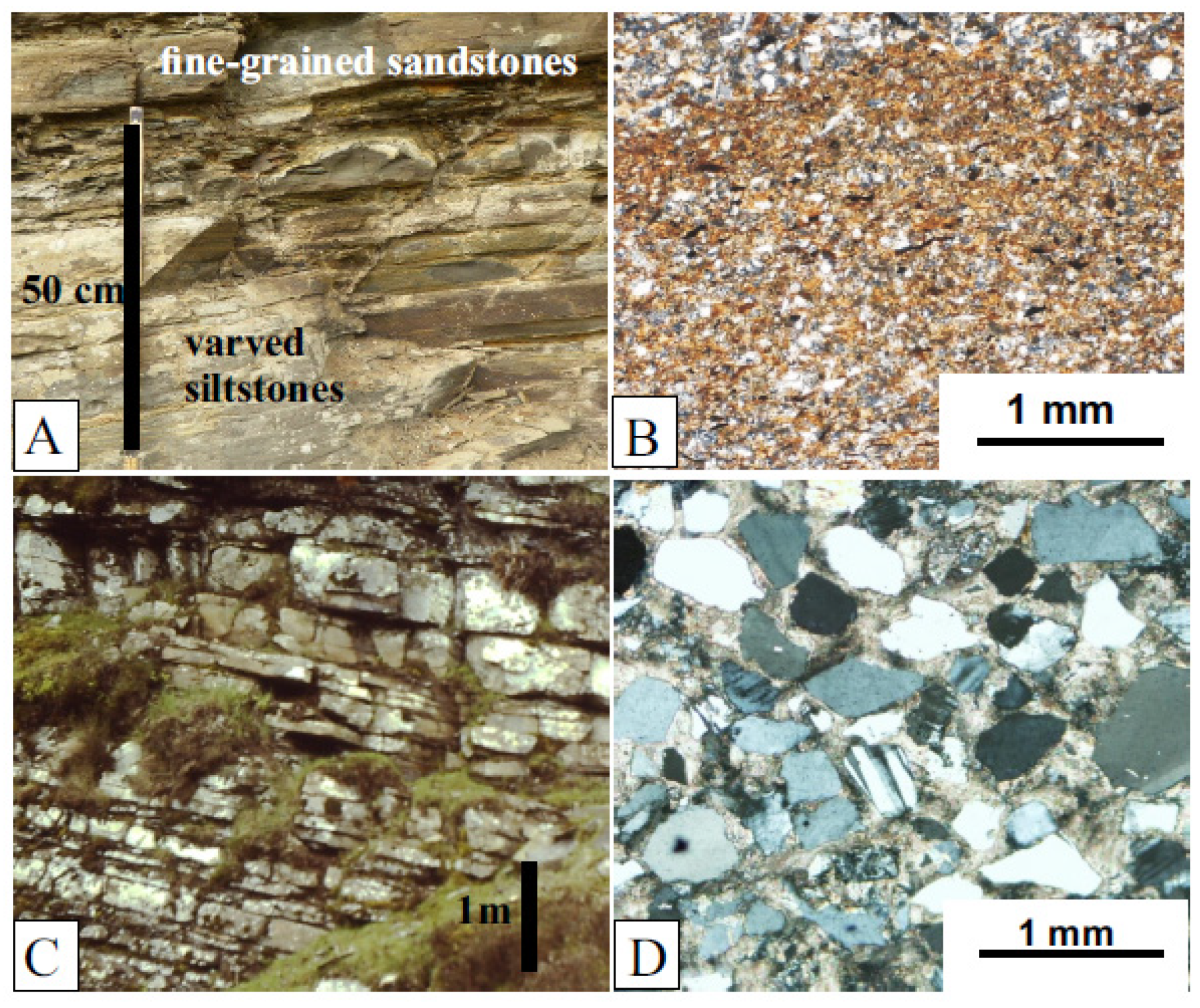
The Jamoytius bed itself is about 10 m thick and consists of alternating fine-grained thin sandstones, deposited by turbidity currents, and varved siltstones in which the varves consist of a lower graded siltstone overlain by an organic-rich muddy layer: the latter enclose most of the fossils [30,49] (Figure 8A,B). The bounding sandstones are low-angle tabular cross-bedded fine-grained mature muddy quartz- and felspathic-sandstones [49], deposited by sand waves in shallow water by storms [37,50] (Figure 8C,D). The deeper water Jamoytius bed with its turbidite sandstones and laminated siltstone contrasts markedly with the enclosing shallow water cross-bedded fine sandstones and testify to great fluctuations in water levels and climate at millennial scales typical of marginal marine and semi-arid lake basins such as those of Lake Cariaco in northern Venezuela and Lake Chad in the central Sahara [51,52].
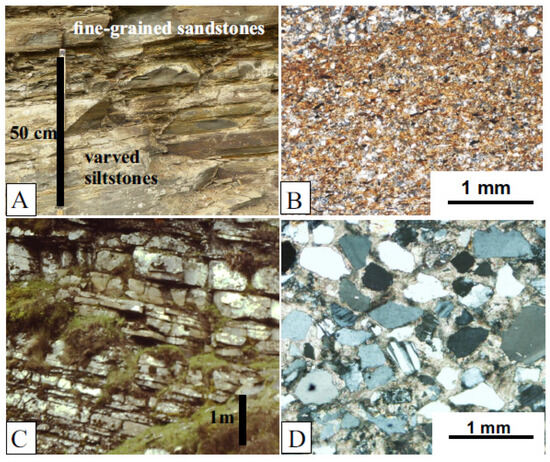
Figure 8.
Sediments; (A) view of part of Jamoytius bed showing varved siltstones alternating with graded fine-grained sandstones; (B) photomicrograph of Jamoytius bed varved siltstone showing lower graded silt layer overlain by organic-rich silt cap, then coarser basal silt layer of overlying varve; (C) view of outcrop of fine-grained quartz-sandstones overlying Jamoytius bed; (D) photomicrograph of fine-grained sandstone from (C), dominated by subangular quartz grains with rare plagioclase felspar, in carbonate cement.
Both the Jamoytius bed, and higher fish-bearing beds at Lesmahagow, have alternations of fine silt-clay couplets (varves) which contain the biota, and olive-grey massive mudstones which are barren [53]. In the Jamoytius horizon, itself, the alternations of laminated siltstones and graded quartz sandstones are almost identical to those of Cariaco basin in Venezuela [54,55]. Water circulation inside this partially isolated basin is restricted, which, combined with high annual primary productivity, causes it to be permanently anoxic at depth, where alternating light and dark coloured varved sediments correspond respectively to the winter-spring dry season and the summer-fall rainy season [56].
The tectonic situation of the Midland basin Silurian inliers is also comparable with that of the Cariaco and related basins along the northern Venezuelan coast. Both are complex basins between ocean and continent, which evolved through time from marine to freshwater continental conditions due to shortening and inversion caused by major strike-slip faulting, occupying about he same ~100 Ma time period, Cretaceous to Recent for Venezuela, and Silurian through Devonian for the Midland Valley [57,58].
5. Palaeoecology
Though the lower Partick Burn Formation has transported marine, or brackish water, fossils in turbidite sandstone, which shows source connections with the sea, the lack of normal marine planktonic organisms above these basal beds is clear evidence that the oceanic connection was tenuous at best [37].
The Jamoytius bed lies above the Podowrinella (sands) and orthocone-Ceratiocaris (clays) biofacies, between shallow water unfossiliferous sandstones [37] (Figure 6). The Podowrinella biofacies is in turbidite sandstones and has been transported from shallower water. It has benthonic scavengers (4 trilobite species, 1 ostracod), attached filter feeders (3 brachiopods, 1 bivalve, crinoid ossicles, bryozoa), herbivores (1 gastropod), free living filter feeders (Tentaculites, Cornulitids). This fauna suggests living conditions in shallow turbulent marine, possibly slightly brackish, water [37]. The orthocone-Ceratiocaris biofacies is in the clays and has only the podshrimp, Ceratiocaris papilio, rare orthocones and the occasional patch of thelodont scales. The orthocones are upright in the sediment and have floated in and settled with decomposition gas in chambers holding them upright as they settled though the water. The Ceratiocaris and thelodonts, in the absence of marine fossils in situ, indicate brackish to freshwater environments [37].
For the fossil biota of the Jamoytius bed, I use the list of Lovelock [37], which list only those fossils from the actual laminated siltstones. Peach and Horne [59] believed the Birk Knowes outcrop to be equivalent to those at Shank Castle, which was later shown to be incorrect [49]. Unfortunately, this mis-correlation has led to confusion over the attribution of some fossils to the Jamoytius bed [37] (pp. 166–167), an error repeated through successive editions of ‘The Geology of Scotland’ [60]. The single example of the blind “horseshoe crab”, Cyamocephalus loganensis Currie 1927, is a museum specimen, attributed to the Jamoytius bed only on similar lithology [61]. Hunter [62] never recorded from where he got his single specimen of the scorpion Palaeophonus caledonicus, though this might be a plant (Ritchie, 1963), and it was dubiously assigned to the Jamoytius horizon by Peach and Horne [59] (p. 574).
The actual fossil biota of the Jamoytius bearing laminated siltstone is dominated by the crustacean Ceratiocaris papilio, accompanied by the thelodont, Loganellia scotica, the enigmatic thylacocephalan crustacean? Aniktozoon loganense [63], Dictyocaris slimoni, most likely a plant thallus [30], and disc- and stem-shaped plants. Other members are rare to very rare. Rare members are the eurypterids Slimonia acuminata, Jamoytius kerwoodi itself and the molluscs. Very rare members are the eurypterids, Erretopterus bilobus, Hughmilleria sp., the ostracod, Beyrichia sp. (one specimen), and the problematica, Taitia catena and Striatuncus scoticus [30] (Table 1).

Table 1.
Taxa, ecology, and abundance of Jamoytius association biota.
The phyllocarid, Ceratiocaris, or pod shrimp, is up to 30 cm long, and is the most abundant fossil. Living phyllocarids (leptostracans) are little know, but seem to prefer low energy conditions over mud bottoms, and are tolerant of low oxygen concentrations [64,65]. Although usually considered filter feeders, they can like shrimps, be opportunistic scavengers, eating plants, organic detritus, and any living or dead organism that does not eat them first [66,67,68].
Loganellia scoticus is up to 30 cm long, and was originally reconstructed as a bottom detritus feeder with heterocercal tail [69]: but it more likely lived as a nektonic feeder with hypocercal tail, as supposed for the anaspid Birkenia, especially considering the anoxic bottom over which it lived [30,70,71]. Indirect evidence comes from fossil scroll coprolites assigned to the anaspids Birkenia and Loganellia, which occur in post-Llandoverian varved siltstones in Northern Ireland, and are ascribed to detritus feeders [72].
Ainiktozoon, is about 12 cm long, and though originally described as an early chordate [73], is now more plausibly an arthropod, more precisely a thylacocephalan crustacean [63]. Its mode of life is unknown, though its abundance suggests an herbivore or detritus feeder rather than a carnivore as postulated for other thylacocephalans [74].
Other arthropods, which are sometimes attributed to the Jamoytius bed, come from the overlying Kip Burn Formation, now mostly covered by the waters of the Logan reservoir, including the millipede Archidesmus loganensis Peach, 1899 [59].
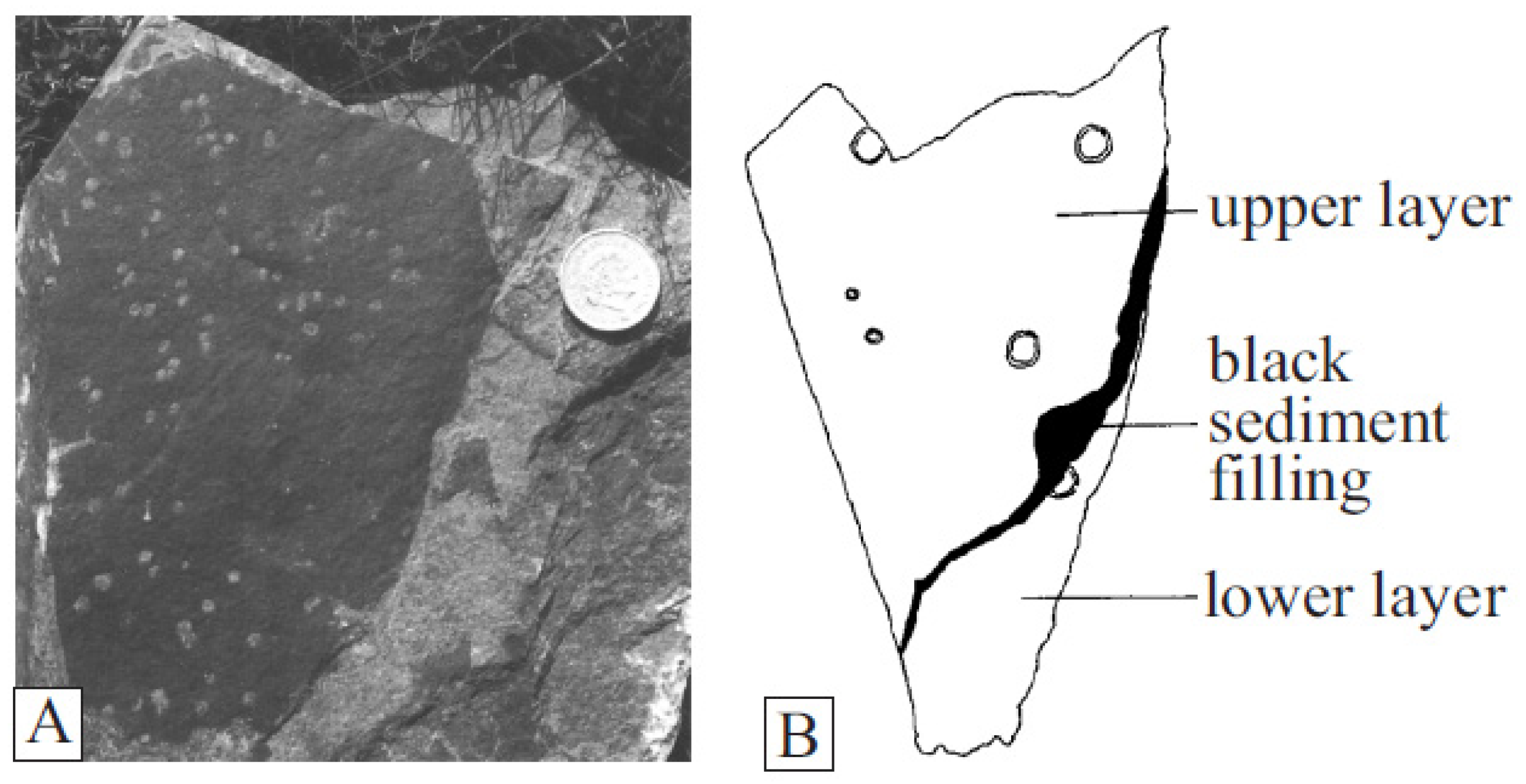
Dictyocaris slimoni, forms large carbonaceous sheets up to 30 cm in diameter, commonly pierced by variably sized circular holes up to 5 mm across, with raised rims [75,76] (Figure 9). Dictyocaris specimens were originally thought to be fragments of large arthropod carapaces, with the holes as parasitic injuries from Jamoytius mouths [30]. Dictyocaris is, however, never found even partially articulated, despite its association with articulated Ceratiocaris specimens which have no similar holes. And the large number of holes on the illustrated specimen also seems too many to be the results of parasitism, considering the size of Jamoytius (14–18 cm). Dictyocaris thus is likely a plant [30].
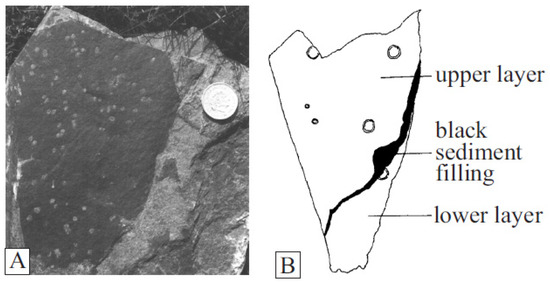
Figure 9.
Dictyocaris: (A) thallus with holes; (B) drawing showing holes of varying sizes and raised rims (both from van der Brugghen, 1995 [76]).
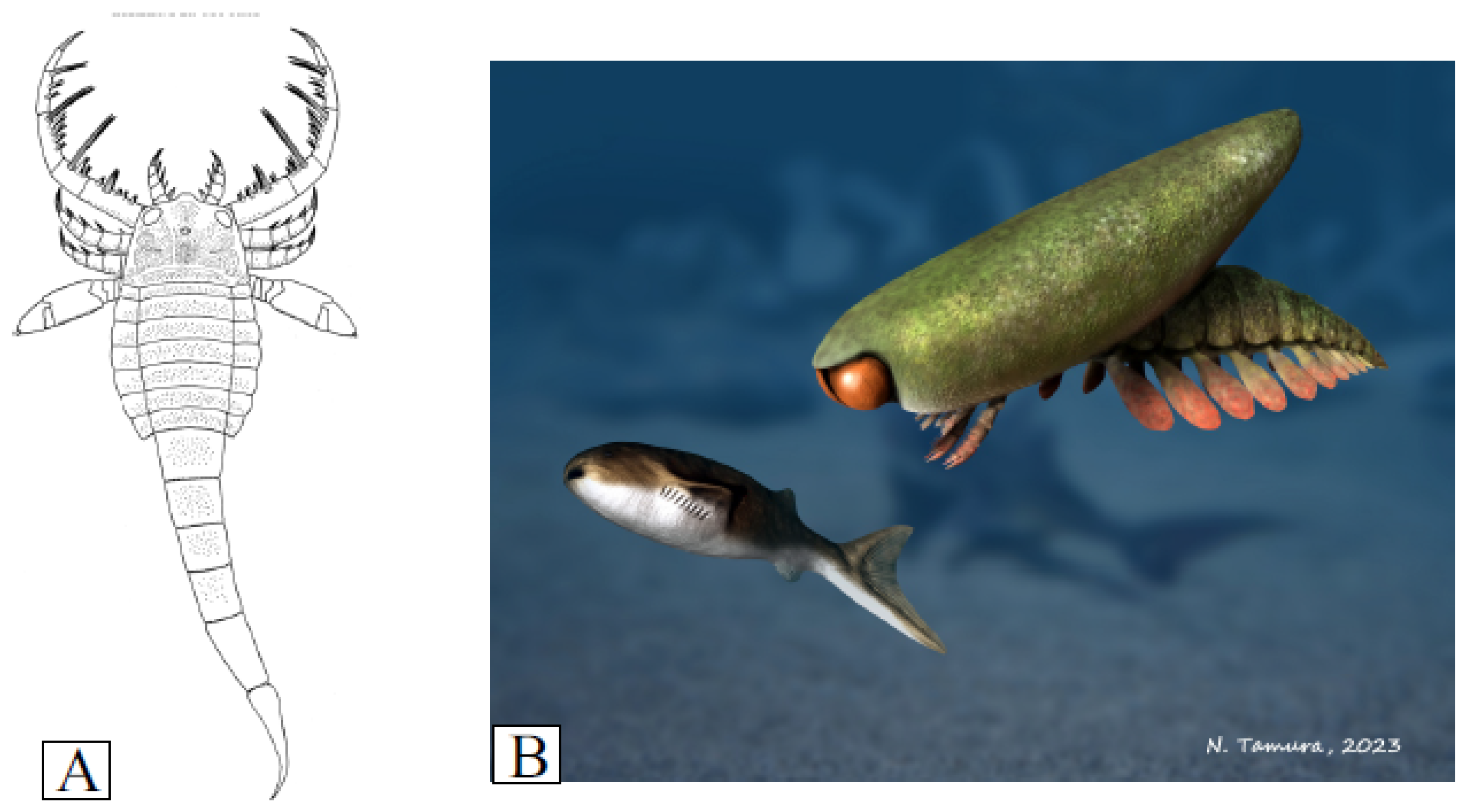
The morphologies of the rare eurypterids in the Jamoytius bed are more easily interpreted as those of nektonic scavengers, because they have none of the specialized adaptations for catching prey found in eurypterids in higher fish beds at Lesmahagow, such as the large spiny grasping arms of the mixopterid Lanarkopterus dolichoschelus [77,78] (Figure 10A). For example, Erettopterus with its small pincers and compound eyes was probably a predator/scavenger with high visual acuity, but it was not as highly specialized or active as other eurypterids [79]. Similarly, Ainiktozoon had likely neither the speed, nor the appendages, to catch a fast-moving Loganellia (Figure 10B).
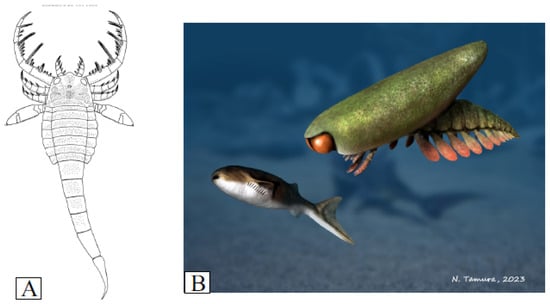
Figure 10.
(A) Reconstruction of Lanarkopterus dolichelus Ritchie 1968 (after Ritchie, 1968b [2]); (B) Ainitkozoon chasing a Loganellia (with permission from Nobu Tamura).
Of the rare molluscs and the small orthocone cephalopod recorded by Ritchie [30] in the Jamoytius horizon, the bivalve Pterinea is a byssally attached suspension feeder. Small clusters of Pterinea occur with carbonaceous patches, which may be floating plants to which they attached [30] (p. 149); such Pteriniids often attach to floating vegetation [80], The low-spired gastropod Platyschisma is most likely a grazing or scavenging form [81]. The small orthocones are not part of the Jamoytius bed biota, and were transported in from another shallower water environment, as they occur in the turbidite sandstones interbedded with the laminated siltstones that have the vertebrates and the eurypterids (personal observations in 2022).
6. Discussion
The Jamoytius association is dominated by supposed omnivores and herbivore/detritus feeders, with primary production represented by phytoplankton and land plant spores and? algal thalluses (? Dictyocaris) that contribute the dark laminae within the siltstone beds (Figure 8B). There is no evidence for large scale transportation of the fossils after death: they simply settled into the anoxic bottom.
The Jamoytius reconstructions with a terminal mouth suggest a filter-feeder or a detritus-feeder, analogous to larval lampreys [82] (Figure 1C,E) and possibly to the Loganelliform thelodonts with which it is associated [83,84]: the latter are interpreted as pelagic slow swimmers in open water [85]. Larval lampreys can feed on highly concentrated food suspensions so thick that they border on organic deposits [86,87]. Jamoytius, however, lacks any obvious adaptations to suspension feeding [88], and the more likely anterior ventral position of the mouth indicates particulate feeding or grazing [47]. The mouth-sized holes in the possible plant Dictyocaris suggest its soft tissues (there are no signs of cuticles) was grazed by Jamoytius without teeth, which may have been later evolved in younger forms like the Devonian Priscomyzon.
A bottom detritus feeding life style proposed by Parrington (1958) [89] is unlikely given the anoxic bottom inferred from sedimentology, and the hypocercal tail which would give lift to a fish whose morphology also suggests an active lifestyle, like the related euphaneropids [90]. Like euphaneropids, Jamoytius resembles elongate arrow-like bony fish, like pike (Esox spp.) and barracuda (Sphyraena spp.), with posterior dorsal and anal fins, which assist the tail in bursts of rapid acceleration, but are inefficient at steady swimming [91]. Ritchie (1968) considered that its highly developed metamerism (a linear series of body segments fundamentally similar in structure) and large eyes of Jamoytius indicated a very active, fast-swimming vertebrate.
Sedimentological and palaeoecological characteristics of the Jamoytius-associated organisms indicate that Jamoytius lived in a brackish water environment in which the bottom waters and sediments were anoxic, and inhospitable to benthos and a predominantly planktonic and nektonic biota lived only in the overlying oxic waters (Figure 11). A benthonic mode of life for any of the Jamoytius association organisms is unlikely. Jamoytius and its likely euphaneropid sister taxa, despite the latter’s autapomorphic, elongated branchial basket, could plausibly be stem lampreys [5], especially considering that the earliest lampreys are interpreted as non-parasitic [32]. Lampreys may initially have evolved as herbivorous organisms and only later developed ectoparasitic modes of life [34,86,92]. The tiny preserved dentition in Devonian Priscomyzon riniensis might be an evolutionary advance for algal grazing from inefficient toothless Jamoytius.
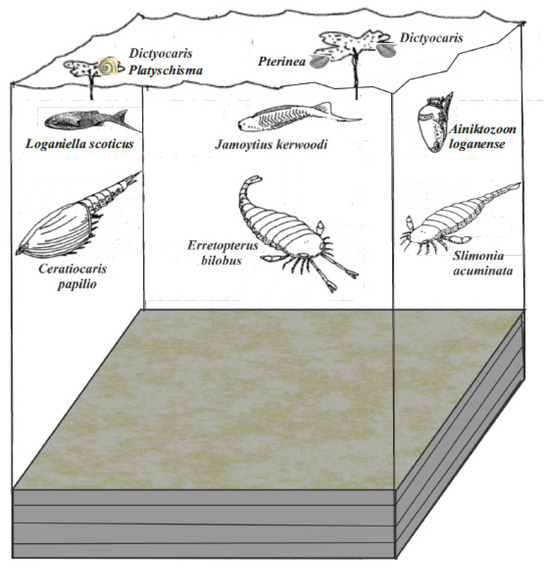
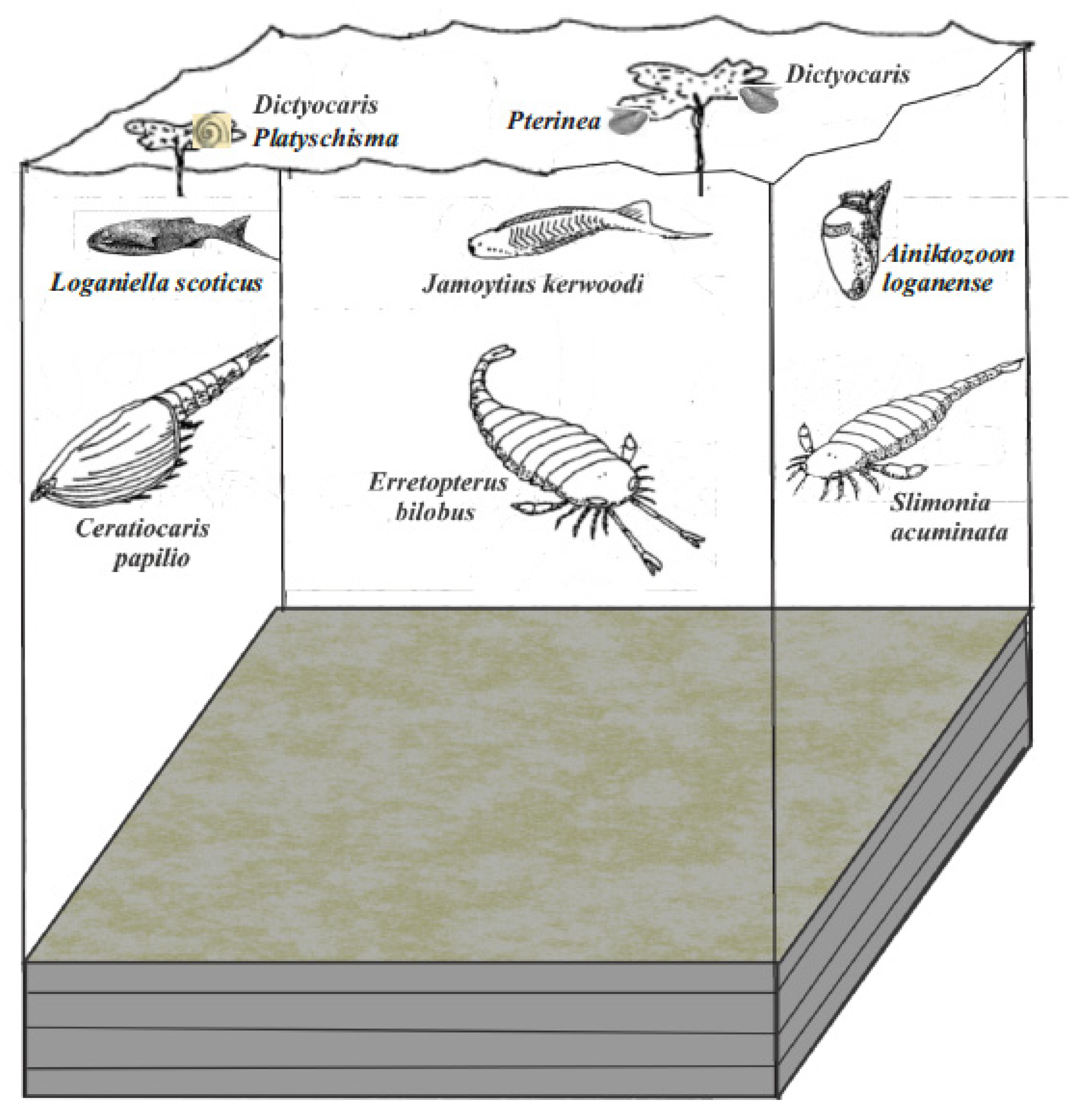
Figure 11.
Palaeoecological sketch of the Jamoytius association living over an anoxic bottom (modified from Lovelock, 1998, [22], Figure 4.3).
7. Conclusions
Jamoytius is associated with a low-diversity dominantly nektonic detritus and herbivorous fauna living over an anoxic bottom, at the transition from a marine-influenced, probably brackish-water and deep-water basin to a shallower-water, less saline and likely freshwater basin. Jamoytius was likely a free-living surface feeding detritivore/herbivore.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This study is part of an ongoing study on the palaeoenvironments and ages of the Silurian inliers of the Midland Valley of Scotland. I thank my colleagues, Elizabeth Catlos and Hector Garza for help during joint field and Susan Turner for a detailed review. I also thank Reviewer 3 for an outstandingly helpful and constructive review. This paper is dedicated to Alex Ritchie, who sadly died on 16 November 2023 at the age of 88.
Conflicts of Interest
The author declares no conflicts of interest.
References
- White, E.I. Jamoytius kerwoodi, a new chordate from the Silurian of Lanarkshire. Geol. Mag. 1946, 83, 89–97. [Google Scholar] [CrossRef]
- Ritchie, A. New evidence on Jamoytius kerwoodi White, an important ostracoderm from the Silurian of Lanarkshire, Scotland. Palaeontology 1968, 11, 21–39. [Google Scholar]
- Chevrinais, M.; Johanson, Z.; Trinajstic, K.; Long, J.; Morel, C.; Renaud, C.; Cloutier, R. Evolution of vertebrate postcranial complexity: Axial skeleton regionalization and paired appendages in a Devonian jawless fish. Palaeontology 2018, 61, 949–961. [Google Scholar] [CrossRef]
- Janvier, P. Early Vertebrates. Oxford Monographs on Geology and Geophysics 33; Clarendon Press: Oxford, UK, 1996; 393p. [Google Scholar] [CrossRef]
- Janvier, P. Early Jawless Vertebrates and Cyclostome Origins. Zool. Sci. 2008, 25, 1045–1056. [Google Scholar] [CrossRef]
- Keating, J.N.; Donaghue, P.C.J. Histology and affinity of anaspids, and the early evolution of the vertebrate dermal skeleton. Proc. R. Soc. 2016, B283, 20152917. [Google Scholar] [CrossRef]
- Miyashita, T.; Coates, M.; Farrar, R.; Larson, P.; Manning, P.; Wogelius, R.; Edwards, N.; Anné, J.; Bergmann, U.; Palmer, A.; et al. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proc. Natl. Acad. Sci. USA 2019, 116, e2146–e2151. [Google Scholar] [CrossRef]
- Miyashita, T.; Gess, R.W.; Tietjen, K.; Coates, M.I. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 2021, 591, 408–412. [Google Scholar] [CrossRef]
- Donoghue, P.C.J.; Purnell, M.A. Distinguishing heat from light in debate over controversial fossils. BioEssays 2009, 31, 178–189. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. The Vertebrate Integument: Origin and Evolution, Volume 1; Springer: Berlin/Heidelberg, Germany, 2014; 268p. [Google Scholar]
- Reeves, J.C.; Sansom, R.S. Multivariate mapping of ontogeny, taphonomy and phylogeny to reconstruct problematic fossil taxa. Proc. R. Soc. B 2023, 290, 20230333. [Google Scholar] [CrossRef]
- Forey, P.L. Yet more reflections on agnatha-gnathostome relationships. J. Vertebr. Paleontol. 1984, 4, 330–343. [Google Scholar] [CrossRef]
- Forey, P.L. Agnathans recent and fossil, and the origin of jawed vertebrates. Rev. Fish Biol. Fish. 1995, 5, 267–303. [Google Scholar] [CrossRef]
- Forey, P.L.; Janvier, P. Agnathans and the origin of jawed vertebrates. Nature 1993, 361, 129–134. [Google Scholar] [CrossRef]
- Janvier, P. The phylogeny of the Craniata, with reference to the significance of fossil ‘agnathans’. J. Vertebr. Paleontol. 1981, 1, 121–159. [Google Scholar] [CrossRef]
- Kuraku, S.; Kuratani, S. Time Scale for Cyclostome Evolution Inferred with a Phylogenetic Diagnosis of Hagfish and Lamprey cDNA Sequences. Zool. Sci. 2006, 23, 1053–1064. [Google Scholar] [CrossRef]
- Reeves, J.C.; Wogelius, R.; Keating, J.; Sansom, R.S. Lasanius, an exceptionally preserved Silurian jawless fish from Scotland. Palaeontology 2023, 66, e12643. [Google Scholar] [CrossRef]
- Ritchie, A. A new interpretation of Jamoytius kerwoodi White. Nature 1960, 188, 647–649. [Google Scholar] [CrossRef]
- Shu, D.; Luo, H.-L.; Conway-Morris, S.; Zhang, X.; Hu, S.; Han, J.; Zhu, M.; Li, Y.; Chen, L.-Z. A Lower Cambrian vertebrate from South China. Nature 1999, 402, 42–46. [Google Scholar] [CrossRef]
- Turner, S. Early vertebrates: Analysis from microfossil evidence. Recent Adv. Orig. Early Radiat. Vertebr. 2004, 65, 67–94. [Google Scholar]
- Donoghue, P.C.J.; Smith, M.P.; Sansom, I.J. The origin and early evolution of chordates: Molecular clocks and the fossil record. In Telling the Evolutionary Time: Molecular Clocks and the Fossil Record; Donoghue, P.C.J., Smith, M.P., Eds.; CRC Press: London, UK, 2003; pp. 190–223. [Google Scholar]
- Gess, R.W.; Coates, M.I.; Rubidge, B.S. A lamprey from the Devonian period of South Africa. Nature 2006, 443, 981–984. [Google Scholar] [CrossRef]
- Donoghue, P.C.J.; Keating, J.N. Early vertebrate evolution. Palaeontology 2014, 57, 879–893. [Google Scholar] [CrossRef]
- Janvier, P.; Arsenault, M. The anatomy of Euphanerops longaevus Woodward, 1900, an anaspid-like jawless vertebrate from the Upper Devonian of Miguasha, Quebec, Canada. Godiversitas 2007, 29, 143–216. [Google Scholar]
- Ritchie, A. Conflicting interpretations of the Silurian agnathan, Jamoytius. Scott. J. Geol. 1984, 20, 249–256. [Google Scholar] [CrossRef]
- Potter, I.C.; Gill, H.S.; Renaud, C.B.; Haoucher, D. The Taxonomy, Phylogeny, and Distribution of Lampreys. In Lampreys: Biology, Conservation and Control; Docker, M.F., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 35–73. [Google Scholar] [CrossRef]
- Docker, M.F. A review of the evolution of nonparasitism in lampreys an update of the paired species concept. In Biology, Management, and Conservation of Lampreys in North America; Brown, L., Chase, S., Mesa, M., Beamish, R., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 71–114. [Google Scholar]
- Evans, T.M.; Limburg, K.E. Parasitism offers large rewards but carries high risks: Predicting parasitic strategies under different life history conditions in lampreys. J. Evol. Biol. 2019, 32, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.A.; Quintella, B.R.; Almeida, P.R.; Treble, A.J.; Jolley, J.C. The ecology of larval and metamorphosing lampreys. In Lampreys: Biology, Conservation and Control; Docker, M.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1, pp. 75–138. [Google Scholar]
- Ritchie, A. Palaeontological Studies on Scottish Silurian Fish Beds. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1963; 170p. [Google Scholar]
- Sansom, R.S.; Freedman, K.; Gabbott, S.E.; Aldridge, R.J.; Purnell, M.A. Taphonomy and affinity of an enigmatic Silurian vertebrate, Jamoytius kerwoodi White. Palaeontology 2010, 53, 1393–1409. [Google Scholar] [CrossRef]
- Brownstein, C.D.; Near, T.J. Phylogenetics and the Cenozoic Radiation of lampreys. Curr. Biol. 2023, 33, 397–404. [Google Scholar] [CrossRef]
- Wu, F.; Janvier, P.; Zhang, C. The rise of predation in Jurassic lampreys. Nat. Commun. 2023, 14, 6652. [Google Scholar] [CrossRef] [PubMed]
- Mallatt, J. Vertebrate origins are informed by larval lampreys (ammocoetes): A response to Miyashita et al., 2021. Zool. J. Linn. Soc. 2023, 197, 287–321. [Google Scholar] [CrossRef]
- Blom, H.; Märss, T. The interrelationships and evolutionary history of anaspid fishes. In Morphology, Phylogeny, and Paleobiogeography of Fossil Fishes; Elliott, D.K., Maisey, J.G., Yu, X., Miao, D., Eds.; Verlag Dr. F. Pfeil, Munich: Munich, Germany, 2010; pp. 45–58. [Google Scholar]
- Dineley, D. Silurian fossils fish sites of Scotland. In Fossil Fishes of Great Britain; Chapter 2; Geological Conservation Review Series; Dineley, D., Metcalf, S., Eds.; Joint Nature Conservation Committee: Peterborough, UK, 1999; Volume 16, pp. 33–62. [Google Scholar]
- Lovelock, C.E. Sedimentary Environments and Biofacies of the Silurian Inlier at Lesmahagow Midland Valley of Scotland. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1998; 448p. [Google Scholar]
- Tarlo, L.B.H. Agnatha. In The Fossil Record; Harland, W.B., Ed.; London Geological Society: London, UK, 1967; pp. 629–636. [Google Scholar]
- van der Brugghen, G. Ciderius cooperi gen. nov., sp. nov., the earliest known euphaneropid from the Lower Silurian of Scotland. Neth. J. Geosci. 2015, 94, 279–288. [Google Scholar] [CrossRef]
- Stensiö, E.A. Les cyclostomes fossiles ou ostracodermes. In Traite de Zoologie 13; Grasse, P.-P., Ed.; Masson: Paris, France, 1958; pp. 173–425. [Google Scholar]
- Moyer, A.E.; Zheng, W.; Schweitzer, M.H. Keratin durability has implications for the fossil record: Results from a 10-year feather degradation experiment. PLoS ONE 2016, 11, e0157699. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Moyer, A.E.; Sjövall, P.; Lindgren, J. Preservation potential of keratin in deep time. PLoS ONE 2018, 13, e0206569. [Google Scholar] [CrossRef]
- Richardson, M.K.; Admiraal, J.; Wright, G.M. Developmental anatomy of lampreys. Biol. Rev. 2010, 85, 1–206. [Google Scholar] [CrossRef] [PubMed]
- Blom, H. New Birkeniid anaspid from the Lower Devonian of Scotland and its phylogenetic implications. Palaeontology 2012, 55, 641–652. [Google Scholar] [CrossRef]
- Cochran, P.A. Observations on giant American Brook Lamprey (Lampetra appendix). J. Freshw. Ecol. 2008, 23, 161–164. [Google Scholar] [CrossRef]
- Fuller, P.; Neilson, M. Lethenteron appendix; USGS Nonindigenous Aquatic Species Database: Gainesville, FL, USA, 2015. Available online: http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=835 (accessed on 5 December 2023).
- Oleh, M. Laboratory Manual on General and Special Ichthyology. World News Nat. Sci. 2018, 18, 1–51. [Google Scholar]
- Clarkson, E.N.K.; Harper, D.T. Silurian of the Midland Valley of Scotland and Ireland. Geol. Today 2016, 32, 195–200. [Google Scholar] [CrossRef]
- Jennings, J.S. The geology of the Eastern Part of the Lesmahagow Inlier. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 1961; 306p. [Google Scholar]
- Harms, J.C.; Southard, J.B.; Spearing, D.R.; Walker, R.G. Depositional Environments as Interpreted from Primary Sedimentary Structures and Stratification Sequences; Society for Economic Petrology and Mineralogy: Tulsa, OK, USA, 1975; 161p. [Google Scholar] [CrossRef]
- Armitage, S.J.; Bristow, C.S.; Drake, N.A. West African monsoon dynamics inferred from abrupt fluctuations of Lake Mega-Chad. Proc. Natl. Acad. Sci. USA 2015, 112, 8543–8548. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Dupont, L.M.; Behling, H.; Wefer, G. Neotropical vegetation response to rapid climate changes during the last glacial period: Palynological evidence from the Cariaco Basin. Quat. Res. 2008, 69, 217–230. [Google Scholar] [CrossRef]
- Zolitschka, B.; Francus, P.; Antti, E.K.O.; Schimmelmann, A. Varves in lake sediments a review. Quat. Sci. Rev. 2015, 117, 1–41. [Google Scholar] [CrossRef]
- Athearn, W.D. Sediment Cores from the Cariaco Trench, Venezuela; Unpublished Technical Report; Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 1965; pp. 65–73. 20p. [Google Scholar]
- Hughen, K.A.; Overpeck, J.T.; Peterson, L.C.; Anderson, R.F. The nature of varved sedimentation in the Cariaco Basin, Venezuela, and its paleoclimatic significance. Geol. Soc. Spec. Publ. 1996, 116, 171–183. [Google Scholar] [CrossRef]
- Muller-Karger, F.E.; Varela, R.; Thunell, R.; Scranton, M.; Bohrer, R.; Taylor, G.; Capelo, J.; Astor, Y.; Tappa, E.; Ho, T.Y.; et al. Annual cycle of primary production in the Cariaco Basin: Response to upwelling and implications for vertical export. J. Geophys. Res. 2001, 106, 4527–4542. [Google Scholar] [CrossRef]
- Dewey, J.F.; Strachan, R.A. Changing Silurian–Devonian relative plate motion in the Caledonides: Sinistral transpression to sinistral transtension. J. Geol. Soc. 2003, 160, 219–229. [Google Scholar] [CrossRef]
- James, K. The Venezuelan Hydrocarbon Habitat. Geol. Soc. Lond. Spec. Publ. 1990, 50, 9–35. [Google Scholar] [CrossRef]
- Peach, B.N.; Horne, J. The Silurian Rocks of Britain; Vol.1 Scotland. (with Petrological Chapters and Notes by J.J.H. Teall); Memoirs of the Geological Survey of the United Kingdom; HMSO: Glasgow, UK, 1899; 749p.
- Walton, E.K.; Oliver, G.J.H. Lower Palaeozoic stratigraphy. In Geology of Scotland, 3rd ed.; Craig, G.Y., Ed.; Scottish Academic Press: Edinburgh, UK, 1991; pp. 161–193. [Google Scholar]
- Anderson, L.I. A new specimen of the Silurian synziphosurine arthropod Cyamocephalus. Proc. Geol. Assoc. 1999, 110, 211–216. [Google Scholar] [CrossRef]
- Hunter, J.R.S. Notes on a new fossil scorpion (Palaeophonus caledonicus) from the Upper Silurian Shales, Logan Water, Lesmahagow. Trans. Edinb. Geol. Soc. 1884, 5, 187–191. [Google Scholar] [CrossRef]
- van der Brugghen, G.; Schram, F.R.; Marthill, D.M. The Fossil Ainiktozoon is an arthropod. Nature 1997, 385, 589–591. [Google Scholar] [CrossRef][Green Version]
- Hessler, R.R.; Schram, F.R. Leptostraca as Living Fossils. In Living Fossils. Casebooks in Earth Sciences; Eldredge, N., Stanley, S.M., Eds.; Springer: New York, NY, USA, 1984; pp. 187–191. [Google Scholar] [CrossRef]
- Rolfe, W.D.I.; Beckett, E.C.M. Autecology of Silurian Xiphosurida, Scorpionida, Cirripedia, and Phyllocarida. Spec. Pap. Paleontol. 1984, 32, 27–37. [Google Scholar]
- Albertoni, E.F.; Palma-Silva, C.; Esteves, F.A. Overlap of dietary niche and electivity of three shrimp species (Crustacea, Decapoda) in a tropical coastal lagoon (Rio de Janeiro, Brazil). Rev. Bras. Zool. 2003, 20, 135–140. [Google Scholar] [CrossRef][Green Version]
- Rolfe, W.D.I. Phyllocarida and the origin of the Malacostraca. Geobios 1981, 14, 17–26. [Google Scholar] [CrossRef]
- Walker, I. Omnivory and resource-sharing in nutrient-deficient Rio Negro waters: Stabilization of biodiversity? Acta Amaz. 2009, 39, 617–626. [Google Scholar] [CrossRef]
- Traquair, R.H. Report on fossil fishes collected by the Geological Survey of Scotland in the Silurian rocks of the south of Scotland. Trans. R. Soc. Edinb. 1899, 39, 827–864. [Google Scholar] [CrossRef]
- Turner, S. A new articulated thelodont (Agnatha) from the Early Devonian of Britain. Palaeontology 1982, 25, 879–889. [Google Scholar]
- Turner, S. Early Silurian to Early Devonian thelodont assemblages and their possible ecological significance. In Palaeocommunities: A Case Study from the Silurian and Lower Devonian-International Geological Correlation Programme 53, Project Ecostratigraphy, Final Report; Boucot, A.J., Lawson, J., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 42–78. [Google Scholar]
- Gilmore, B. Scroll coprolites from the Silurian of Ireland and the feeding of early invertebrates. Palaeontology 1992, 35, 319–333. [Google Scholar]
- Scourfield, D.J. An anomalous fossil organism, possibly a new type of chordate, from the Upper Silurian of Lesmahagow, Lanarkshire—Ainiktozoon loganense, gen. et sp. nov. Proc. R. Soc. B 1937, 121, 533–547. [Google Scholar] [CrossRef]
- Haug, C.; Briggs, D.E.G.; Mikulic, D.G.; Kluessendorf, J.; Huag, J.T. The implications of a Silurian and other thylacocephalan crustaceans for the functional morphology and systematic affinities of the group. BMC Evol. Biol. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Störmer, L. Dictyocaris salter, a large crustacean from the Upper Silurian and Downtonian. Nor. Geol. Tidsskr. 1935, 15, 267–298. [Google Scholar]
- van der Brugghen, G. Dictyocaris, een enigmatisch fossiel uit het Silur. Grondboor Hamer 1995, 1, 18–22. [Google Scholar]
- Ritchie, A. Lanarkopterus dolichoschelus (Störmer) gen. nov., a mixopterid eurypterid from the Upper Silurian of the Lesmahagow and Hagshaw Hills inliers, Scotland. Scott. J. Geol. 1968, 4, 317–338. [Google Scholar] [CrossRef]
- Schmidt, M.; Melzer, R.R.; Plotnick, R.E.; Bicknell, R.D.C. Spines and baskets in apex predatory sea scorpions uncover unique feeding strategies using 3D-kinematics. Science 2022, 25, 103662. [Google Scholar] [CrossRef] [PubMed]
- Plotnick, R.E. Habitat of Llandoverian-Lochkovian eurypterids. In Paleocommunities—A Case Study from the Silurian and Lower Devonian; Boucot, A.J., Lawson, J.D., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 106–136. [Google Scholar]
- Stanley, S.M. Functional morphology and evolution of byssally attached bivalves. J. Paleontol. 1972, 46, 165–212. [Google Scholar]
- Linsley, R.M. Some “laws” of gastropod shell form. Paleobiology 1977, 3, 196–206. [Google Scholar] [CrossRef]
- Denison, R.N. Feeding mechanisms of agnatha and early gnathostomes. Am. Zool. 1961, 1, 177–182. [Google Scholar] [CrossRef]
- Donoghue, P.C.J.; Smith, M.P. The anatomy of Turinia pagei (Powrie), and the phylogenetic status of the Thelodonti. Trans. R. Soc. Edinb. Earth Sci. 2001, 92, 15–37. [Google Scholar] [CrossRef]
- Donoghue, P.C.J.; Forey, P.L.; Aldridge, R.J. Conodont affinity and chordate phylogeny. Biol. Rev. 2000, 75, 191–251. [Google Scholar] [CrossRef] [PubMed]
- Ferrón, H.G.; Botella, H. Squamation and ecology of thelodonts. PLoS ONE 2017, 12, e0172781. [Google Scholar] [CrossRef]
- Mallatt, J. Feeding ecology of the earliest vertebrates. Zool. J. Linn. Soc. 1984, 82, 261–272. [Google Scholar] [CrossRef]
- Mallatt, J. Reconstructing the Life Cycle and the Feeding of Ancestral Vertebrates. In Evolutionary Biology of Primitive Fishes; NATO ASI Series; Foreman, R.E., Gorbman, A., Dodd, J.M., Olsson, R., Eds.; Springer: Boston, MA, USA, 1985; Volume 103, pp. 59–68. [Google Scholar] [CrossRef]
- Lammons, M.L. Mud and Mucus: Feeding Selectivity in a Suspension-Feeding Detritivorous Fish. Master’s Thesis, The College of William and Mary, Williamsburg, VA, USA, 2009; 101p. [Google Scholar]
- Parrington, F.R.; Westoll, T.S. On the nature of the Anaspida. In Studies on Fossil Vertebrates; Westoll, T.S., Ed.; Athlone Press: London, UK, 1958; pp. 108–128. [Google Scholar]
- Kermack, K.A. The functional significance of the hypocercal Tail in Pteraspis rostrata. J. Exp. Biol. 1943, 20, 23–27. [Google Scholar] [CrossRef]
- Fletcher, T.; Altringham, J.; Peakall, J.; Wignall, P.; Dorrell, R. Hydrodynamics of fossil fishes. Proc. R. Soc. 2014, B281, 20140703. [Google Scholar] [CrossRef]
- Strahan, A. The behaviour of Mixinoids. Acta Zool. Stockh. 1963, 44, 73–102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).